Abstract
Introduction
Patients with slowly progressive autosomal dominant polycystic kidney disease (ADPKD) are unlikely to experience outcomes during randomized controlled trials (RCTs). An image classification of ADPKD into typical (diffuse cyst distribution) class 1A to E (by age- and height-adjusted total kidney volume [TKV]) and atypical (asymmetric cyst distribution) class 2 was proposed for prognostic enrichment design, recommending inclusion of only classes 1C to 1E in RCTs.
Methods
A post hoc exploratory analysis was conducted of the TEMPO 3:4 Trial, a prospective, randomized, double-blinded, controlled clinical trial in adult subjects with ADPKD, an estimated creatinine clearance >60 ml/min and total kidney volume >750 ml.
Results
Due to the entry criteria, the study population of TEMPO 3:4 was enriched for classes 1C-E (89.5 % of 1436 patients with baseline magnetic resonance images) compared to unselected populations (e.g., 60.5% of 590 Mayo Clinic patients). The effects of tolvaptan on TKV and eGFR slopes were greater in classes 1C to E than in 1B. In TEMPO 3:4, tolvaptan reduced TKV and eGFR slopes from 5.51% to 2.80% per year and from −3.70 to −2.78 ml/min/1.73 m2 per year, and lowered the risk for a composite endpoint of clinical progression events (hazard ratio = 0.87). Restricting enrollment to classes 1C to E would have reduced TKV and eGFR slopes from 5.78% to 2.91% per year and from −3.93 to −2.82 ml/min/1.73 m2 per year, and the risk of the composite endpoint (hazard ratio = 0.84, P = 0.003), with 10.5% fewer patients.
Discussion
Prognostic enrichment strategies such as the entry criteria used for TEMPO 3:4 or preferably the proposed image classification should be used in RCTs for ADPKD to increase power and to reduce cost.
Keywords: autosomal dominant polycystic kidney disease, clinical trials, disease progression, image classification of ADPKD, kidney volume
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic kidney disease and the fourth leading cause of end-stage renal disease (ESRD) in adults worldwide.1, 2 ADPKD presents with large phenotypic variability, resulting in a wide range of disease severity and progression.3, 4 Patients with mild disease have a good prognosis and may not require therapy to halt or slow down the progression of ADPKD, which would expose them to adverse events without a meaningful benefit. Furthermore, inclusion of patients with a low risk of progression in clinical trials decreases the power to detect a treatment effect. Hence, identifying optimal candidates for enrollment into randomized controlled trials (RCTs), with progressive disease and most likely to benefit from an effective therapy is vital.
Studies have shown that total kidney volume (TKV) predicts renal function decline in patients with ADPKD, qualifying TKV as a prognostic biomarker.3, 5 In fact, kidney volume has been widely used as a primary or secondary endpoint in multiple clinical trials.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, TKV does not always predict change in renal function, as, for example, in patients with few large cysts or in patients with renal atrophy secondary to ischemia or urinary tract obstruction. We have recently developed and validated an image classification of ADPKD in an attempt to more precisely define patients’ risk for disease progression and to optimize the selection of patients for clinical trials.
According to our classification system, class 1 (or typical) patients are those who exhibit classical bilateral distribution of the disease, whereas class 2 (or atypical) patients may exhibit unilateral, segmental, asymmetric, or bilateral atypical presentation (class 2A), but also may exhibit bilateral distribution with acquired unilateral atrophy or bilateral kidney atrophy (class 2B), based on prespecified imaging findings.16 In class 2 patients, TKV did not predict change in estimated glomerular filtration rate (eGFR) over time. Patients in class 2A presented with low risk for disease progression; patients in class 2B without renal enlargement and with atrophic parenchyma were not likely to benefit from therapies directed to slowing kidney growth. In contrast to class 2, TKV and age predicted change in eGFR over time in class 1 patients. Moreover, stratification of class 1 patients into A, B, C, D, and E, based on an increasing height-adjusted total kidney volume (HtTKV) for age, showed that the rate of eGFR decline and renal survival were significantly different, with patients in class C, D, and E being at highest risk for eGFR decline. Thus, we recommended that class 1A, 1B, and 2 patients be excluded for prognostic enrichment.
The aim of this study was to investigate the effects that these exclusions would have had on the results of TEMPO 3:4, a trial already enriched by a maximum age and minimal TKV, to demonstrate a beneficial effect of tolvaptan on the progression of ADPKD.
Materials and Methods
Study Design
This was a post hoc exploratory analysis of the TEMPO 3:4 clinical trial to investigate the performance of a previously developed imaging classification of ADPKD for prognostic enrichment design in clinical trials.
TEMPO 3:4 was a prospective, randomized, double-blinded, controlled clinical trial in adult patients (18−50 years of age) with ADPKD, an estimated creatinine clearance >60 ml/min, and a total kidney volume >750 ml. The participants were randomized in a 2:1 ratio to receive tolvaptan, a V2-receptor antagonist, or placebo. The primary outcome was the annual rate of change in the TKV. Sequential secondary endpoints included a composite of time to clinical progression (defined as worsening kidney function, kidney pain, hypertension, and albuminuria) and rate of kidney function decline. Detailed description of the TEMPO 3:4 study has been published previously.8, 16
All TEMPO 3:4 study participants who met inclusion criteria and underwent randomization (N = 1445) were considered for this post hoc analysis.8 Nine participants were excluded from analysis due to lack of baseline images (n = 6), patient height (n = 2), or incorrect ADPKD diagnosis (n = 1). Baseline magnetic resonance imaging (MRI) studies were used to classify these patients by means of our classification system, which has been previously published.16 The classification into class 1 (typical) and class 2 (atypical) patients was performed blindly. The kidney volumes used to stratify the class 1 patients into the 5 subclasses were the baseline kidney volumes that had been measured for the parent study. The analysts performing these measurements were blinded to treatment allocation.
Classification of Study Participants Into Typical (Class 1) and Atypical (Class 2) Patients
All available baseline magnetic resonance images were transferred to the Mayo Translational PKD Center and later retrieved to a work station for further analysis. Subjects were classified as class 1 (typical) or class 2 (atypical) cases based on prespecified imaging findings.16 Image classifications were performed blinded to clinical data. TKVs were previously measured for the TEMPO 3:4 study.8 Class 1 ADPKD patients were further stratified into 5 subclasses, A to E, based on HtTKV and age, as previously described.16
Primary and Secondary Endpoints
The primary endpoint was the same as for the TEMPO 3:4 study: the annual rate of percentage change in TKV over time. TKVs from TEMPO 3:4 were used for this calculation. The composite secondary endpoint, the time to investigator-assessed clinical progression as defined by worsening of kidney function, clinically significant kidney pain, worsening hypertension, and worsening albuminuria and other secondary endpoints such as change in the slope of kidney function were also the same as for TEMPO 3:4 study.8 GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Patients’ characteristics (i.e., age, sex, race/ethnicity) and laboratory measurements (i.e., serum creatinine, urine albumin excretion) were the same as collected for TEMPO 3:4 study.17
Statistical Analysis
TEMPO 3:4 prespecified primary and secondary endpoints were re-evaluated by image-based classification. Analysis of the primary endpoint compared the average of individual slopes for TKV between groups by fitting log10-transformed data on TKV to a linear mixed-effects Laird-Ware model.18 Analysis of slope of kidney function was similar to slope of TKV. The analysis of the composite secondary endpoint was performed with the use of the Andersen-Gill approach for the extended Cox model, for analysis of time to multiple events. The P value was provided by the Wald test.19
Results
Image Classification and Baseline Clinical, Laboratory, and Genetic Characteristics
The baseline magnetic resonance images of 1436 participants in the TEMPO 3:4 clinical trial were examined. The majority of the patients (96.9%) presented with typical imaging characteristics of ADPKD and were classified as class 1, whereas the remaining 3.1% were classified as atypical or class 2. The main baseline clinical, laboratory, and genetic characteristics are shown in Table 1. Class 1 patients were younger (39 ± 7 vs. 41 ± 8 years, P < 0.01), were more frequently hypertensive (83.2% vs. 60.0%, P < 0.001), and had lower eGFR (81.3 ± 21.6 vs. 90.1 ± 17.6 ml/min/1.73 m2, P < 0.01) at baseline. HtTKV in class 1 and class 2 patients were not significantly different (971 ± 499 vs. 909 ± 483 mL, P = 0.35). The gender distribution was similar in class 1 patients (47.5% female), but the proportion of female patients was higher in class 2 patients (73.3%). Genetic analysis was available in 53.4% of the patients, of whom 85.4% had a mutation in the PKD1 gene, 12.1% a mutation in the PKD2 gene, and 2.5% no mutation detected. The percentages of cases with a PKD2 mutation and no mutation detected were higher in class 2 compared to class 1 patients, whereas that of PKD1 was lower in class 2 compared to class 1 patients.
Table 1.
Clinical, laboratory, and genetic characteristics at baseline, by image-based classification and treatment group
| Class | 1 |
2 |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | B | C | D | E | All | A − Ba | |||||||
| Treatment group | TLV | PLC | TLV | PLC | TLV | PLC | TLV | PLC | TLV | PLC | |||
| Patient number | 1391 | 75 | 31 | 340 | 191 | 332 | 161 | 179 | 82 | 45 | 28 | 17 | 1436 |
| Male, n (%) | 730 (53) | 25 (33) | 12 (39) | 158 (47) | 94 (49) | 189 (57) | 84 (52) | 110 (62) | 58 (71) | 12 (27) | 9 (32) | 3 (18) | 742 (52) |
| Age, yr | 39 | 45 | 45 | 41 | 42 | 38 | 38 | 33 | 31 | 41 | 42 | 42 | 39 |
| Patients w/HTN, n (%) | 1158 (83.2) | 58 (77) | 26 (84) | 269 (79) | 147 (77) | 279 (84) | 145 (90) | 159 (89) | 75 (91) | 27 (60) | 17 (61) | 10 (59) | 1185 (82.5) |
| SBP (mm Hg) | 129 | 127 | 129 | 128 | 127 | 129 | 130 | 130 | 129 | 125 | 127 | 123 | 129 |
| DBP (mm Hg) | 83 | 80 | 83 | 82 | 82 | 83 | 83 | 84 | 83 | 80 | 81 | 80 | 82 |
| eGFR, ml/min/1.73 m2 | 81.3 | 81.5 | 82.4 | 82.3 | 82.8 | 79.8 | 80.5 | 81.6 | 80.5 | 90.1 | 85.8 | 97.3 | 81.6 |
| HtTKV, ml/m | 971 | 509 | 507 | 710 | 732 | 1060 | 1039 | 1513 | 1536 | 909 | 979 | 794 | 969 |
| Genetic analysis, n (%) | 745 (53.6) | 39 (52) | 21 (67.7) | 177 (52.1) | 114 (59.7) | 178 (53.6) | 93 (57.8) | 78 (43,6) | 45 (54.9) | 22 (48.9) | 10 (35.7) | 12 (70.6) | 767 (53.4) |
| NMD, n (%)b | 15 (2.0) | 2 (5.1) | 0 (0) | 4 (2.3) | 3 (2.6) | 2 (1.1) | 0 (0) | 3 (3.8) | 1 (2.2) | 4 (18.2) | 1 (10) | 3 (25) | 19 (2.5) |
| PKD1, n (%)b | 646 (86.7) | 32 (82) | 15 (71.4) | 151 (85.3) | 92 (80.7) | 156 (87.6) | 86 (92.5) | 73 (93.6) | 41 (91.1) | 9 (40.9) | 5 (50) | 4 (33.3) | 655 (85.4) |
| PKD2, n (%)b | 82 (11.3) | 5 (12.8) | 6 (28.6) | 22 (12.4) | 19 (16.7) | 20 (11.2) | 7 (7.5) | 2 (2.6) | 3 (6.7) | 9 (40.9) | 4 (40) | 5 (41.7) | 93 (12.1) |
DBP, diastolic blood pressure, average during clinical trial; eGFR, estimated glomerular filtration rate (estimated by CKD-EPI equation); HTN, patients with a diagnosis of hypertension at baseline; HtTKV, height adjusted total kidney volume; NMD, no mutation detected; PLC, placebo treatment group; SBP, systolic blood pressure, average during clinical trial; TLV, tolvaptan treatment group.
Only 1 patient was classified as class 2B.
Percent frequencies of mutations in the patients who had genetic analysis.
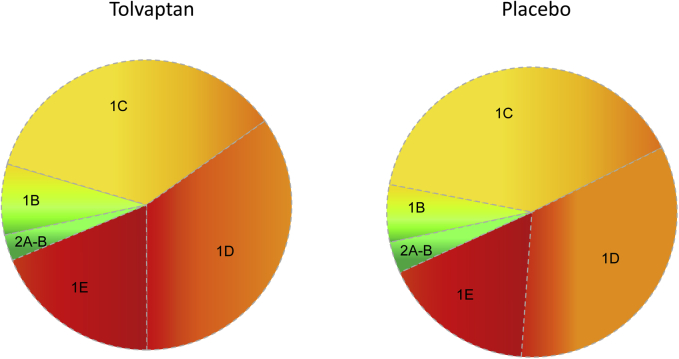
Class 1 patients were further stratified into subclasses (1A−E), as previously described.16 No participants were classified as class 1A, thus reflecting the inclusion criteria for enrollment into TEMPO 3:4 (TKV >750 mL, <50 years of age). Most class 1 patients were classified as 1C = 38.2% or 1D = 35.4%, followed by 1E = 18.8% and 1B = 7.6%. In fact, classes 1C to 1E constituted 89.5% of the total 1436 patients included in the study. In an unselected ADPKD population at the Mayo Clinic (n = 590, age 16−80, eGFR 9−159 ml/min/1.73 m2), classes 1C to E accounted for 60.5% of the patients, confirming the enrichment of TEMPO 3:4 for patients with severe, rapidly progressing disease. The distribution by class 1B to 1E was similar between the tolvaptan and placebo groups (Figure 1). Baseline age decreased from class 1B through 1E in both treatment groups, whereas TKV increased. Estimated GFR was similar in all classes and treatment groups (Table 1). Interestingly, the male/female ratio increased consistently from 1B to 1E in the tolvaptan and placebo groups. The percentage of PKD1 cases increased from class 1B to 1E in tolvaptan- and placebo-treated patients, whereas PKD2 cases decreased from class 1B to 1E. The rate of NMD decreased in classes 1B to 1D and increased slightly in 1E.
Figure 1.
Distribution of subjects based on image classification in the tolvaptan and placebo treatment arms.
Outcome Measures by Class
Primary Endpoint: TKV Slopes
TEMPO 3:4 had shown a reduction in the rate of TKV increase from 5.5% to 2.8% per year over the 3-year duration of the trial. For the current post hoc analysis of the primary endpoint,8 1270 patients had a baseline and 1 postrandomization MRI available. The analysis shows that the TKV slopes increased from class 1B through class 1E in both treatment arms and confirms that the rate of total kidney volume growth was lower in tolvaptan- compared to placebo-treated patients (P = 0.02 for 1B and P < 0.001 for each of 1C−1E) (Table 2). Treatment effects of tolvaptan in classes 1B through 1E were not significantly different. Tolvaptan did not reduce the rate of TKV growth in class 2 patients (P = 0.88).
Table 2.
TKV slopes (percent change per year), by image-based classification and treatment group
| Class | Class 1B | Class 1C | Class 1D | Class 1E | Class 2A/2B | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | PLC n = 29 |
TLV n = 64 |
PLC n = 186 |
TLV n = 288 |
PLC n = 150 |
TLV n = 291 |
PLC n = 76 |
TLV n = 144 |
PLC n = 17 |
TLV n = 25 |
| TKV slope (%/yr) | 3.25 | 1.23 | 5.12 | 1.79 | 5.62 | 3.03 | 7.75 | 4.96 | 2.48 | 2.27 |
| 95% CI | 1.50, 5.03 | 0.30, 2.17 | 4.35, 5.90 | 1.26, 2.32 | 4.58, 6.68 | 2.47, 3.59 | 6.16, 9.35 | 4.03, 5.89 | −0.34, 5.39 | 0.11, 4.47 |
| Treatment effect (%/yr) | −2.00 | −3.27 | −2.52 | −2.66 | −0.21 | |||||
| 95% CI | −3.76, −0.27 | −4.03, −2.52 | −3.54, −1.50 | −4.17, −1.16 | −3.05, 2.55 | |||||
| P value | 0.02 | <0.001 | <0.001 | <0.001 | 0.88 | |||||
CI, confidence interval; PLC, placebo treatment group; TKV, total kidney volume.
Secondary Endpoint: eGFR Slopes
In TEMPO 3:4, tolvaptan slowed the rate of eGFR decline from −3.70 to −2.72 ml/min/1.73 m2 per year. For the post hoc analysis of the secondary endpoint, on treatment slopes were estimated in 1320 patients. The analysis shows that the rates of eGFR decline increased from class 1B to 1E in both tolvaptan and placebo-treated patients. Furthermore, the rates of eGFR decline were significantly lower in the patients randomized to tolvaptan compared to those randomized to placebo in class 1C, 1D, and 1E patients, but not in class 1B patients (P < 0.001, P = 0.007, P = 0.002, and P = 0.64, respectively) (Table 3). The treatment effects of tolvaptan in classes 1C, 1D, and 1E were not significantly different. Tolvaptan did not decrease the rate of eGFR decline in class 2 patients (P = 0.75).
Table 3.
eGFR slopes (ml/min/1.73 m2 per year) by image-based classification and treatment group
| Class | Class 1B | Class 1C | Class 1D | Class 1E | Class 2A/2B | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | PLC n = 30 |
TLV n = 66 |
PLC n = 187 |
TLV n = 304 |
PLC n = 152 |
TLV n = 299 |
PLC n = 78 |
TLV n = 161 |
PLC n = 17 |
TLV n = 26 |
| eGFR slope (ml/min/1.73 m2 per yr) | –1.79 | –2.10 | –3.59 | –2.32 | –3.89 | –2.99 | –4.93 | –3.46 | –1.66 | –1.34 |
| 95% CI | –3.08, –0.51 | –2.69, –1.52 | –4.18, –3.00 | –2.68, –1.95 | –4.54, –3.24 | –3.34, –2.64 | –5.87, –3.99 | –4.00, –2.92 | –3.63, 0.32 | –2.63, –0.04 |
| Treatment effect (ml/min/1.73 m2 per yr) | –0.31 | 1.27 | 0.89 | 1.47 | 0.32 | |||||
| 95% CI | –1.60, 0.98 | 0.68, 1.87 | 0.25, 1.54 | 0.53, 2.41 | –1.65, 2.30 | |||||
| P value | 0.64 | <0.001 | 0.007 | 0.002 | 0.75 | |||||
CI, confidence interval; eGFR, estimated glomerular filtration rate; PLC, placebo treatment group; TKV, total kidney volume.
Composite Secondary Endpoint: Time to Clinical Progression
Complications related to ADPKD and associated with disease progression were grouped into a composite endpoint defined as time to clinical progression. The clinical events comprising the composite endpoint were predetermined and included the following: a 25% reduction in the inverse of serum creatinine (roughly equivalent to a 30% decline in eGFR by the CKD-EPI formula), clinically significant kidney pain, worsening hypertension, and worsening albuminuria. TEMPO 3:4 previously showed fewer ADPKD-related events in tolvaptan- compared to placebo-treated patients, with a hazard ratio (HR) of 0.87 (95% confidence interval [CI] = 0.78, 0.97; P < 0.01). Analysis of the composite endpoint by class showed a significant beneficial effect in classes 1C (HR = 0.80; 95% CI = 0.67, 0.97; P = 0.02) and 1E (HR = 0.77; 95% CI = 0.61, 0.98; P = 0.03). No significant effect was observed in groups 1D (HR = 0.91; 95% CI = 0.76, 1.10; P = 0.33), 1B (HR = 1.03; 95% CI = 0.67, 1.57; P = 0.91), and 2A and 2B (HR = 1.71; 95% CI = 0.95, 3.09; P = 0.08) (Figure 2).
Figure 2.
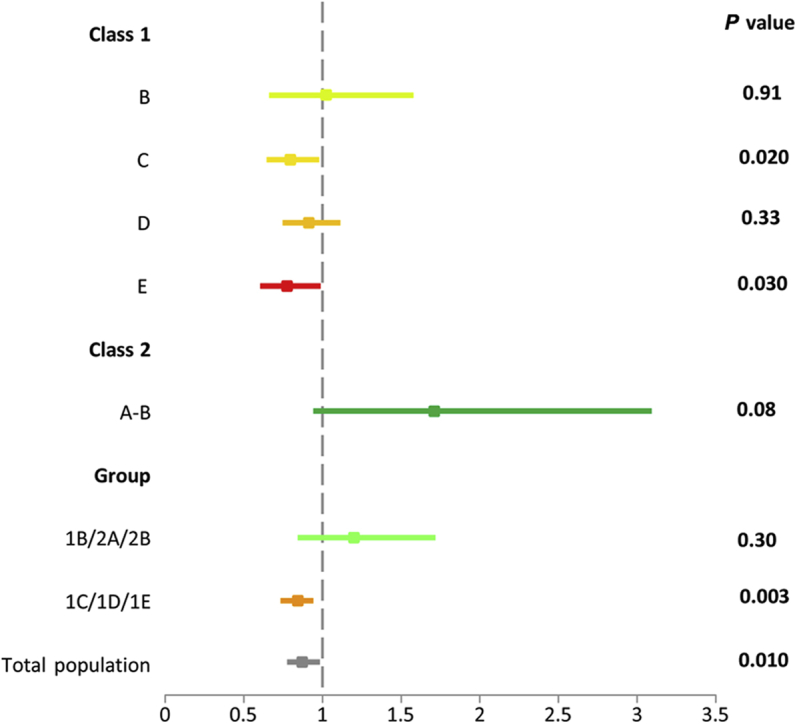
Time to multiple composite autosomal dominant polycystic kidney disease (ADPKD) events by image classification: hazard ratios (95% confidence intervals) for the secondary endpoint of ADPKD-related events by image classification and exclusion/inclusion recommended groups.
Adverse Events
Frequencies of adverse events, including elevations of liver enzymes, were not significantly different among the imaging classes.
Outcome Measures by Previous Recommendation to Exclude/Include From RCTs
To ascertain how implementation of our recommendation to exclude class 1A, 1B, and 2 patients for prognostic enrichment would have affected the results of the TEMPO 3:4, we combined classes 1B/2A/2B (n = 151), which would have been excluded, and 1C/1D/1E (n = 1285), which would have been included in the trial (n = 1436). We found that tolvaptan had a beneficial effect in the patients with moderate to severe disease (1C/1D/1E), reducing the rate of increase in TKV by −2.79% per year (P < 0.001) and the rate of eGFR decline by 1.11 ml/min/1.73 m2 per year (P < 0.001). On the other hand, tolvaptan had only a modest effect of borderline statistical significance on the rate of increase in TKV (−1.4% per year, P = 0.052) without a significant effect on the rate of change in eGFR (−0.14 ml/min/1.73 m2 per year; P = 0.79) in classes 1B/2A/2B (Table 4). Moreover, analysis of the secondary composite endpoint showed fewer ADPKD-related events per 100 person-years of follow-up with tolvaptan than with placebo only in classes 1C/1D/1E (44 vs. 52; HR = 0.84, 95% CI = 0.75, 0.94; P = 0.003) but not in classes 1B/2A/2B (41 vs. 34; HR = 1.20, 95% CI = 0.85, 1.71; P = 0.30) (Figure 2, Table 5).
Table 4.
TKV and eGFR slopes by recommendation in Irazabal et al.,17
and treatment group
| Recommendation to exclude/include | Patients to exclude 1Aa/1B/2A/2Bb |
Patients to include 1C/1D/1E |
||
|---|---|---|---|---|
| Treatment | PLC n = 46 |
TLV n = 89 |
PLC n = 412 |
TLV n = 723 |
| TKV slope (%/yr) | 2.99 | 1.53 | 5.78 | 2.91 |
| 95% CI | 1.51, 4.48 | 0.63, 2.43 | 5.18, 6.39 | 2.55, 3.28 |
| Treatment effect (%/yr) | –1.44 | –2.79 | ||
| 95% CI | –2.91, 0.01 | –3.38, –2.20 | ||
| P value | 0.052 | <0.001 | ||
| Treatment | PLC n = 47 |
TLV n = 92 |
PLC n = 417 |
TLV n = 764 |
| eGFR slope (ml/min/1.73 m2 per yr) | –1.74 | –1.88 | –3.93 | –2.82 |
| 95% CI | –2.81, –0.67 | –2.44, –1.33 | –4.33, –3.53 | –3.05, –2.59 |
| Treatment effect (ml/min/1.73 m2 per yr) | –0.14 | 1.11 | ||
| 95% CI | –1.21, 0.93 | 0.71, 1.51 | ||
| P value | 0.79 | <0.001 | ||
CI, confidence interval; eGFR, estimated glomerular filtration rate (estimated by CKD-EPI equation); PLC, placebo treatment group; TKV, total kidney volume.
Because of the entry criteria, none of the patients enrolled into TEMPO 3:4 were class 1A.
Only 1 patient classified as 2B in this study.
Table 5.
Composite secondary endpoint: time to clinical progression by recommendation in Irazabal et al.,16 and treatment group
| Recommendation to exclude/include | Patients to exclude 1Aa/1B/2A/2Bb |
Patients to include 1C/1D/1E |
||
|---|---|---|---|---|
| Treatment (no. of subjects) | PLC 48 |
TLV 103 |
PLC 433 |
TLV 851 |
| ADPKD-related composite (no. of total events) | 45 | 106 | 620 | 936 |
| Events/100 person-yr | 33.52 | 40.93 | 51.93 | 44.36 |
| HR (95% CI) | 1.20 (0.85, 1.71) | 0.84 (0.75, 0.94) | ||
| P value | 0.3028 | 0.0032 | ||
| Treatment (no. of subjects) |
PLC 47 |
TLV 97 |
PLC 427 |
TLV 813 |
| Worsening kidney function (no. of total events) | 1 | 2 | 63 | 41 |
| Events/100 person-yr | 0.75 | 0.77 | 5.3 | 1.95 |
| HR (95% CI) | 1.06 (0.10, 11.27) | 0.37 (0.25, 0.55) | ||
| P value | 0.9623 | <0.0001 | ||
| Treatment (no. of subjects) | PLC 48 |
TLV 103 |
PLC 433 |
TLV 851 |
| Clinically significant kidney pain (no. of total events) | 8 | 11 | 89 | 102 |
| Events/100 person-yr | 5.96 | 4.25 | 7.46 | 4.83 |
| HR (95% CI) | 0.71 (0.27, 1.83) | 0.64 (0.46, 0.90) | ||
| P value | 0.4745 | 0.0109 | ||
| Treatment (no. of subjects) | PLC 48 |
TLV 103 |
PLC 433 |
TLV 851 |
| Worsening hypertension (no. of total events) | 30 | 72 | 396 | 658 |
| Events/100 person-yr | 22.35 | 27.80 | 33.17 | 31.19 |
| HR (95% CI) | 1.22 (0.78, 1.93) | 0.92 (0.79, 1.08) | ||
| P value | 0.3899 | 0.3077 | ||
| Treatment (no. of subjects) | PLC 48 |
TLV 103 |
PLC 433 |
TLV 851 |
| Worsening albuminuria (no. of total events) | 7 | 23 | 96 | 170 |
| Events/100 person-yr | 5.21 | 8.88 | 8.04 | 8.06 |
| HR (95% CI) | 1.69 (0.79, 3.61) | 0.99 (0.79, 1.23) | ||
| P value | 0.1793 | 0.8965 | ||
ADPKD, autosomal dominant polycystic kidney disease; CI, confidence interval; HR, hazard ratio; PLC, placebo treatment group; TLV, tolvaptan treatment group. Significant results are highlighted in bold.
Because of the entry criteria, none of the patients enrolled into TEMPO 3:4 were class 1A.
Only 1 patient classified as 2B in this study.
Discussion
The progression of ADPKD is characterized by lifetime, unrelenting development and growth of renal cysts, renal enlargement, and destruction of renal parenchyma, while renal function remains deceptively normal for decades due to the compensatory capacity of the surviving nephrons.5 Only at advanced stages of the disease does the destruction of nephrons exceed the compensatory capacity of the kidney and an accelerated decline in kidney function ensues. This pattern of progression constitutes a challenge for the design of clinical trials because measurements of GFR are not informative at early stages of the disease, and interventions at advanced stages, when measurements of GFR are informative, may be less likely to demonstrate favorable results.5
A large body of experimental evidence supports the importance of vasopressin in the pathogenesis of ADPKD20, 21, 22, 23, 24, 25, 26, 27 and provided the rationale for TEMPO 3:4, a phase 3, multicenter, double-blind, placebo-controlled, 3-year trial to determine whether the vasopressin V2 receptor antagonist tolvaptan can slow the growth of the kidneys and the decline of kidney function and can delay a composite endpoint of events associated with progression of the disease, including kidney pain, worsening hypertension, worsening albuminuria, and 25% reduction in the inverse of serum creatinine. The design of TEMPO 3:4 relied on understanding the challenge posed by the typical course of the disease.17 Inclusion criteria were formulated to enroll patients at a relatively early stage of disease, defined by an estimated creatinine clearance of 60 ml/min or more and a high likelihood of rapid disease progression reflected by a kidney volume of at least 750 mL at a relative young age of 50 years or less. TEMPO 3:4 was the first large randomized clinical trial in ADPKD jointly including age, kidney volume, and renal function in the inclusion criteria. As a result, TEMPO 3:4 participants at baseline (39 years, 1690 ml, and 82 ml/min/1.73 m2)8 were at a later stage of the disease compared to those in the Suisse trial of sirolimus (32 years, 907 mL, and 92 ml/min/1.73 m2)13 or to the Polycystic Kidney Disease Treatment Network (HALT PKD) Study A blood pressure trial (37 years, 1210 ml, and 92 ml/min/1.73 m2),28 but at an earlier stage of the disease compared to the everolimus trial (45 years, 1970 mL, 55 ml/min/1.73 m2)14 or to the Polycystic Kidney Disease Treatment Network (HALT PKD) Study B blood pressure trial (49 years, 48 ml/min/1.73 m2).29
Although the entry criteria for TEMPO 3:4 jointly considered age, TKV, and eGFR to enroll patients likely to be informative and to increase the power to detect a treatment effect, a more precise classification of disease severity has been recently proposed with the same goal.16 The purpose of the post hoc analysis presented in this manuscript was to determine how the use of this classification in TEMPO 3:4 would have affected the results of the trial. The analysis confirmed that the entry criteria used in TEMPO 3:4 were successful in selecting a population enriched for patients with severe, rapidly progressing disease as evidenced by the low number of class 1A (0%), class 1B (106 or 7.4%), and class 2 (45 or 3.1%) patients, possibly contributing to the positive results of the trial. The analysis also showed that exclusion of these 151 patients (10.5% overall) would have resulted in numerically although not significantly higher treatment effects on TKV slopes (2.79% per year, P < 0.001, compared to 2.71% per year, P < 0.001) and on treatment eGFR slopes (1.11 ml/min/1.73 m2 per year, P < 0.001, compared to 0.98 ml/min/1.73 m2 per year, P < 0.001).
At the request of the Food and Drug Administration, the power calculation for the TEMPO 3:4 trial was based on the key secondary endpoint, using an α level of 0.01. The trial was thus powered to around 80%, with an assumption of a hazard ratio of 0.8 favorable to tolvaptan in the key secondary endpoint. When the trial was unblinded, the observed hazard ratio was 0.865, with a P value of 0.0095. Thus the post hoc power of the TEMPO 3:4 trial was 51% for an α level of 0.01. Had the trial been designed with a patient population based on Irazabal classes 1C/1D/1E, the post hoc power would have been 70% for an α level of 0.01, assuming a hazard ratio of 0.84 as observed in the analysis presented herein, and everything else remaining unchanged. For a more commonly used α value of 0.05, the post hoc power of TEMPO 3:4 would be 72%, and the post hoc power of the presumed TEMPO 3:4 trial with Irazabal classes 1C/1D/1E would have been 88%.
It is important to point out that the Irazabal classification for prognostic enrichment requires only the application of an algorithm available online, which is based on TKV, age, and height of the patient, data which were already available at the time of screening in TEMPO 3:4. It does not require additional images or expense. Therefore, it is reasonable to conclude that it would have enhanced the efficiency of the trial by providing numerically higher treatment effects on the rates of kidney growth and eGFR decline and greater power to detect a significant effect on the key secondary endpoint, with a lower number of patients and consequently lower cost. Because only patients with rapidly progressive ADPKD are likely to benefit from and be treated with novel therapies as these become available, the generalizability of the results does not become a problem when entry into clinical trials is restricted to classes 1C/1D/1E.
This study has limitations inherent to a post hoc analysis and was not powered to analyze the endpoints by class of disease severity. Furthermore, the entry criteria for TEMPO 3:4 had already been selected to enroll a cohort of patients with ADPKD and rapidly progressive disease. Nevertheless, the analysis suggests that had enrollment been restricted to class 1C, 1D, and 1E patients, similar or slightly stronger treatment effects would have been obtained with 10.5% fewer patients, and further supports the use of prognostic enrichment strategies such as the image classification in the design of RCTs for ADPKD to increase power and potentially decrease costs.
Disclosure
VET, EH, OD, ABC, RTG, RDP, JO, and FSC are members of the Steering committee of the TEMPO 3:4 study. VET, OD, ABC, RTG, RDP, and PCH have received research funding from Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ); ABC has received consultancy fees from Otsuka Pharmaceutical Development & Commercialization, Inc. EH has received research funding and consultancy fees from Otsuka Pharmaceutical Co., Ltd. JO, WZ, JDB, and FSC are employees of Otsuka Pharmaceutical Development & Commercialization, Inc.
Acknowledgments
This trial was funded by Otsuka Pharmaceuticals. Co., Ltd. Tokyo, Japan, and Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, Maryland, USA. We thank the patients involved in the TEMPO 3:4 trials for their participation and contribution; the trial investigators, sub-investigators, radiologists, study coordinators and nurses; the trial managers, trial monitors (Parexel International Corporation), data managers, programmers and statisticians; the University of Wisconsin Statistical Data Analysis Center; the members of the Independent Data Monitoring Committee and Clinical Events Committee; and the support of the Mayo Clinic Robert M. and Billie Kelley Pirnie Translational Polycystic Kidney Disease Center (DK090728).
References
- 1.Torres V.E., Harris P.C., Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Grantham J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 3.Chapman A.B., Bost J.E., Torres V.E. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris P.C., Torres V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest. 2014;124:2315–2324. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grantham J.J., Torres V.E., Chapman A.B. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 6.Chapman A.B., Torres V.E., Perrone R.D. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5:102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higashihara E., Torres V.E., Chapman A.B. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clin J Am Soc Nephrol. 2011;6:2499–2507. doi: 10.2215/CJN.03530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggenenti P., Remuzzi A., Ondei P. Safety and efficacy of long-acting somatostatin treatment in autosomal dominant polcysytic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 10.van Keimpema L., Nevens F., Vanslembrouck R. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Hogan M.C., Masyuk T.V., Page L.J. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caroli A., Perico N., Perna A. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 13.Serra A.L., Poster D., Kistler A.D. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 14.Walz G., Budde K., Mannaa M. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 15.Perico N., Antiga L., Caroli A. Sirolimus therapy to half the progression of ADPKD. J Am Soc Nephrol. 2010;21:1031–1040. doi: 10.1681/ASN.2009121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irazabal M.V., Rangel L.J., Bergstralh E.J. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres V.E., Meijer E., Bae K.T. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis. 2011;57:692–699. doi: 10.1053/j.ajkd.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Therneau T.M., Grambsch P.M. Springer; New York: 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 20.Yasuda G., Jeffries W.B. Regulation of cAMP production in initial and terminal inner medullary collecting ducts. Kidney Int. 1998;54:80–86. doi: 10.1046/j.1523-1755.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 21.Mutig K., Paliege A., Kahl T. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol. 2007;293:F1166–F1177. doi: 10.1152/ajprenal.00196.2007. [DOI] [PubMed] [Google Scholar]
- 22.Carmosino M., Brooks H.L., Cai Q. Axial heterogeneity of vasopressin-receptor subtypes along the human and mouse collecting duct. Am J Physiol Renal Physiol. 2007;292:F351–F360. doi: 10.1152/ajprenal.00049.2006. [DOI] [PubMed] [Google Scholar]
- 23.Gattone V.H., 2nd, Wang X., Harris P.C. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 24.Torres V.E., Wang X., Qian Q. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Gattone V., 2nd, Harris P.C. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–851. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Wu Y., Ward C.J. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer E., Gansevoort R.T., de Jong P.E. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant. 2011;26:2445–2453. doi: 10.1093/ndt/gfr069. [DOI] [PubMed] [Google Scholar]
- 28.Schrier R.W., Abebe K.Z., Perrone R.D. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres V.E., Abebe K.Z., Chapman A.B. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]