Abstract
Introduction
Kidney disease (KD), including acute kidney injury, is common, severe and leads to significant mortality in the developing world. However, simple tools to facilitate diagnosis and guide treatment are lacking. We studied the diagnostic performance of saliva urea nitrogen (SUN) measured by dipstick to diagnose KD in a low-resource setting.
Methods
Medical admissions to a tertiary hospital in Malawi had serum creatinine tested at presentation; SUN was measured using a dipstick. Patients with serum creatinine above normal range underwent serial measurements of SUN and blood urea nitrogen for up to 7 days. Hospital outcome was recorded in all patients.
Results
A total of 742 patients were included (age 41 ± 17·3 years, 56.1% male); 146 (19.7%) had KD, including 114 (15.4%) with acute kidney injury. SUN >14 mg/dl had a sensitivity of 0.72 and a specificity of 0.87 to diagnose KD; specificity increased to 0.97 when SUN levels were combined with self-reported urine output. The diagnostic performance of SUN was comparable with the one of blood urea nitrogen (SUN area under curve, 0.82; 95% confidence interval, 0.78–0.87; blood urea nitrogen area under curve, 0.82; 95% confidence interval, 0.59–1.0). SUN >14 mg/dl on admission was an independent predictor of all-cause mortality (hazard ratio = 2.43 [95% confidence interval, 1.63–3.62]).
Discussion
SUN measured by dipstick can be used to identify patients with KD in a low-resource setting. SUN is an independent predictor of mortality in this population.
Keywords: acute kidney injury, chronic kidney disease, hemodialysis, sepsis
Acute kidney injury (AKI) is common worldwide, causing high morbidity and mortality, particularly in the developing world.1, 2 Here, AKI primarily affects young patients with limited comorbidity, is predominantly community acquired, and is commonly caused by infective illnesses, volume depletion, and nephrotoxicity.3, 4
Many deaths resulting from AKI in low-resource settings (LRS) may be preventable.5 However, a number of major challenges exist when managing AKI in these areas: (i) a disparity in health care resources available in urban compared with rural areas; (ii) poor awareness among patients and health care workers of AKI and the need for its early detection and treatment; (iii) a scarcity of trained personnel and resources for renal replacement therapy; and (iv) a lack of reliable and cost-effective tools to diagnose AKI.5, 6
The lack of medical and laboratory infrastructure in LRS makes the development of an inexpensive, noninvasive, and reliable bedside diagnostic tool to identify patients with kidney disease (KD), including AKI, essential.5 Saliva urea nitrogen (SUN) was first described in the 1840s,7 and several studies have since evaluated the diagnostic capability of SUN to detect renal impairment.8, 9, 10, 11 SUN parallels blood urea nitrogen (BUN) and urea reaches saliva by diffusive transport from the salivary glands. Alongside bicarbonate, it is responsible for the buffering capacity of saliva.12 SUN concentration can vary according to stimulation of the salivary glands and the amount of saliva produced. SUN can be measured by laboratory techniques but also by a simple dipstick method.13
The SUN dipstick has been suggested as a potential screening tool for acute and chronic KD.14, 15 Indeed, in our previous studies in developed settings, SUN strips demonstrated good diagnostic performance to detect kidney dysfunction, especially at the higher levels of BUN.14 In the present study, we aimed to explore the diagnostic performance of this tool in a LRS, where access to laboratory measurements of renal function is often limited, and where the SUN test may be of greatest value.
Methods
Study Design, Setting, and Participants
We conducted a prospective observational study at Queen Elizabeth Central Hospital in Blantyre, Malawi, one of the poorest countries in the world. Queen Elizabeth Central Hospital acts as both a district hospital and a tertiary hospital for the southern region of Malawi, although the majority of patients admitted are from Blantyre district itself, with a population of approximately 1 million.
All patients aged 14 years or older admitted to the general medical wards between 27 April 2015 and 17 July 2015 were eligible. Patients unable to give informed consent, patients transferred to the medical ward from another ward or hospital, and patients who were unable to provide a sufficient volume of saliva (including those with significantly reduced level of consciousness) were excluded from the study.
Patients enrolled were screened for KD with serum creatinine (sCR) measurement. Concomitantly, we measured SUN levels using a dipstick (Integrated Biomedical Technology, Elkhart, IN). Those with sCR above the local laboratory reference range (>90 μmol/l in women; >104 μmol/l in men) were managed by the nephrology team and followed with serial SUN, BUN, and sCR measurements for a period of up to 7 days or until hospital discharge if sooner. Demographic and clinical data, including signs and self-reported symptoms (increased thirst and reduced urine output) of altered volume status, were also recorded. Hospital outcome was recorded in all patients.
Data Collection
Saliva and blood samples were collected simultaneously on admission (day 0) for the measurement of SUN and sCR, and then at 24 hours (day 1) and every 48 hours thereafter (days 3, 5, and 7) for the measurement of SUN, BUN, and sCR.
SUN Measurement
SUN was assessed using a dipstick (Integrated Biomedical Technology). Subjects were asked to refrain from drinking and eating for at least 15 minutes prior to saliva collection. Unstimulated saliva was collected in a plastic cup and approximately 50 μl of saliva was used to moisten the test pad of a colorimetric SUN dipstick. The dipstick test pad contains a urease enzyme in a bound form that cleaves SUN when moistened with saliva; this leads to the formation of ammonia and hydroxyl ions resulting in a change in pH which, by a pH indicator substance, consequently changes color of the test pad. After 1 minute, the color of the test pad is compared with 6 reference pads indicating increasing SUN concentrations: 5–14 mg/dl (pad 1), 15–24 mg/dl (pad 2), 25–34 mg/dl (pad 3), 35–54 mg/dl (pad 4), 55–74 mg/dl (pad 5), and ≥75 mg/dl (pad 6) (Figure S1).15
sCR and Urea Measurement
sCR was measured by the Jaffe method16 and BUN by the urease method17 (either by Flexor Junior Clinical Chemistry Analyzer [Vital Scientific, Dieren, The Netherlands] or by Mindray Chemistry Analyzer BS-120 [Shenzhen Mindray Bio-Medical Electronics Company, Shenzhen, China]) in a local laboratory.
Definitions
AKI, acute kidney disease/disorder (AKD) without AKI, and chronic kidney disease were diagnosed and staged by Kidney Disease Improving Global Outcomes criteria.1, 18 KD is used to refer to AKI, AKD without AKI, and stable chronic kidney disease. AKD incorporates both confirmed AKI and AKD without AKI (Figure S2).
Outcome Measures
The primary outcome measure was the diagnostic performance of the SUN dipstick to detect KD, alone and in combination with self-reported changes in urine output and thirst. Secondary outcome measures were the agreement of SUN with BUN at presentation and during management of KD, and the ability of SUN to predict in-hospital mortality in this population.
Statistical Analyses
For the statistical analyses, urea results were converted to BUN (mg/dl) and SUN was transformed to a continuous variable by choosing the midpoint for each range. Descriptive statistics were presented as mean ± SD and as median and interquartile range, depending on data distribution.
The agreement between SUN and BUN was tested by one-way analysis of variance with a post hoc Bonferroni correction for multiple testing. Differences between BUN and SUN (midpoint concentration of the respective test pad range) were displayed as error bars (with 95% confidence intervals [CI]) at different days and represented as a modified Bland-Altman plot using BUN as the reference method.19 Agreement between SUN and BUN over the entire period was also tested using linear mixed effects models with random intercepts for each subject and random slopes for each 2-day period.
Diagnostic performance of SUN and BUN to detect KD was analyzed using sensitivity and specificity, and by the area under the receiver operating characteristics curve at each of the observation days. Optimal diagnostic thresholds were determined based on the maximum Youden’s index (Youden’s index = sensitivity + specificity − 1). In addition, sensitivity and specificity were used to evaluate the diagnostic performance of SUN to detect KD combined with the 2 self-reported clinical parameters, namely, increased thirst and reduced urine output.20
Cox proportional hazard models were constructed to evaluate the predictors of death in this population. Age, gender, the presence of diabetes, and SUN levels were assessed. Kaplan-Meier curves were used to assess the mortality risk in patients stratified by the absence or presence of SUN levels >14 mg/dl (i.e., SUN > test pad 1). All surviving patients were censored at day 15.
A 2-sided P value less than 0.05 was considered statistically significant. Analyses were done using R 3.2.1 (codename “World-Famous Astronaut”; R Foundation for Statistical Computing, Vienna, Austria).21
Results
Cohort Description
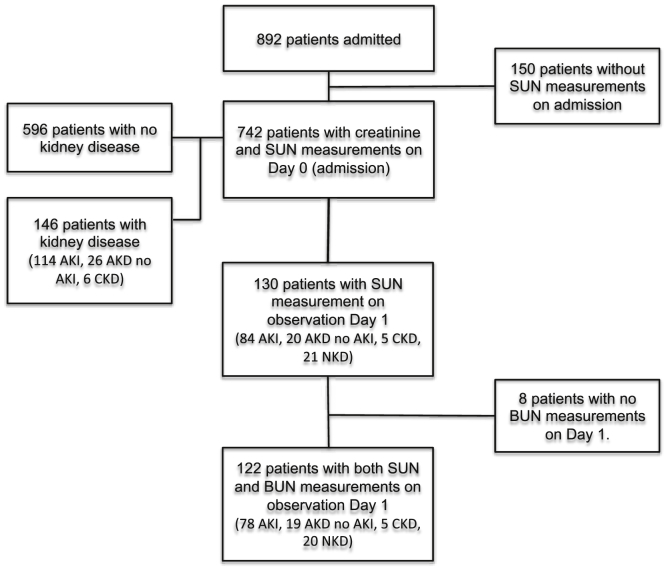
Of 892 medical patients admitted to the hospital during the study period, 742 had both sCR and SUN measurements and were thus included in the analysis (Figure 1). The mean age was 41 ± 17 years; 56% were male. A total of 146 (19.7%) patients had KD: 114 (15.4%) AKI, 26 (3.5%) AKD without AKI, and 6 (0.8%) stable chronic kidney disease. AKI was stage 3 in 67 (58.8%) patients (Table 1).
Figure 1.
Cohort description. AKD, acute kidney disease/disorder; AKI, acute kidney injury; BUN, blood urea nitrogen; CKD, chronic kidney disease; NKD, no kidney disease; SUN, saliva urea nitrogen.
Table 1.
Demographics, SUN, and serum creatinine levels on admission in the study population
| N (count) | Age (yr) | Male gender (%) | SUN (mg/dl) | Creatinine (μmol/l) | |
|---|---|---|---|---|---|
| All patients | 742 | 40.8 ± 17.3 | 56.06 | 16.8 ± 18.6 | 156 ± 335.8 |
| No kidney disease | 596 | 40.1 ± 17.2 | 55.37 | 11.1 ± 4.8 | 62.6 ± 20.5 |
| AKI stage 1 | 26 | 45.9 ± 16.2 | 38.46 | 28.2 ± 24.5 | 140.7 ± 1.7 |
| AKI stage 2 | 21 | 36.5 ± 13.9 | 61.9 | 34 ± 26.6 | 239 ± 111.9 |
| AKI stage 3 | 67 | 44.4 ± 18 | 67.16 | 53 ± 32.6 | 811.6 ± 704.2 |
| AKD with no AKI | 26 | 47 ± 17 | 53.85 | 25.3 ± 27.6 | 342.3 ± 525.9 |
| CKD | 6 | 47.7 ± 23.4 | 66.67 | 37.5 ± 28.4 | 886.5 ± 867.3 |
AKD, acute kidney disease/disorder; AKI, acute kidney injury; CKD, chronic kidney disease; SUN, saliva urea nitrogen.
A total of 130 patients had SUN measured on day 1. The characteristics of these patients are shown in Table 2. There were significantly more patients with hypovolemia as determined by the study team, self-reported reduced urine output, and increased thirst (60.5%, 29.6%, and 100%, respectively) in the group with AKI stage 3 compared with the other groups (Table S1). The most common causes of renal disease were related to sepsis and hypoperfusion: gastroenteritis (n = 24, 18.5%), heart failure (n = 21, 16.15%), pulmonary and disseminated tuberculosis (n = 13, 10%), and malaria (n = 9, 6.9%).
Table 2.
Demographics and biochemical findings (SUN, BUN, and serum creatinine) in patients who had SUN measured on day 1
| N (count) | Age (yr) | Male gender (%) | SUN (mg/dl) | BUN (mg/dl) | Creatinine (μmol/l) | |
|---|---|---|---|---|---|---|
| No kidney disease | 21 | 42 ± 20.3 | 71.43 | 15.7 ± 5.9 | 32.4 ± 35.6 | 100.1 ± 23.6 |
| AKI stage 1 | 23 | 45.5 ± 16.7 | 39.13 | 23.7 ± 22.6 | 37.7 ± 21.4 | 130.5 ± 44.4 |
| AKI stage 2 | 17 | 36.5 ± 10.8 | 58.82 | 22.7 ± 8.5 | 35.9 ± 19.1 | 193.9 ± −91.7 |
| AKI stage 3 | 44 | 41.1 ± 16.3 | 72.73 | 46.4 ± −31.7 | 92.4 ± 44.8 | 760.1 ± 761.6 |
| AKD with no AKI | 20 | 45.1 ± 16.2 | 55 | 24.8 ± 24 | 45.3 ± 34.5 | 309 ± 442.7 |
| CKD | 5 | 50.2 ± 25.3 | 60 | 25.5 ± 5.5 | 75.7 ± 58.1 | 820.2 ± 970.6 |
| All | 130 | 42.3 ± 6.8 | 61.54 | 30.2 ± 25.8 | 57.7 ± 44.2 | 403.3 ± 583.4 |
AKD, acute kidney disease/disorder; AKI, acute kidney injury; BUN, blood urea nitrogen; CKD, chronic kidney disease; SUN, saliva urea nitrogen.
SUN and BUN Measurements
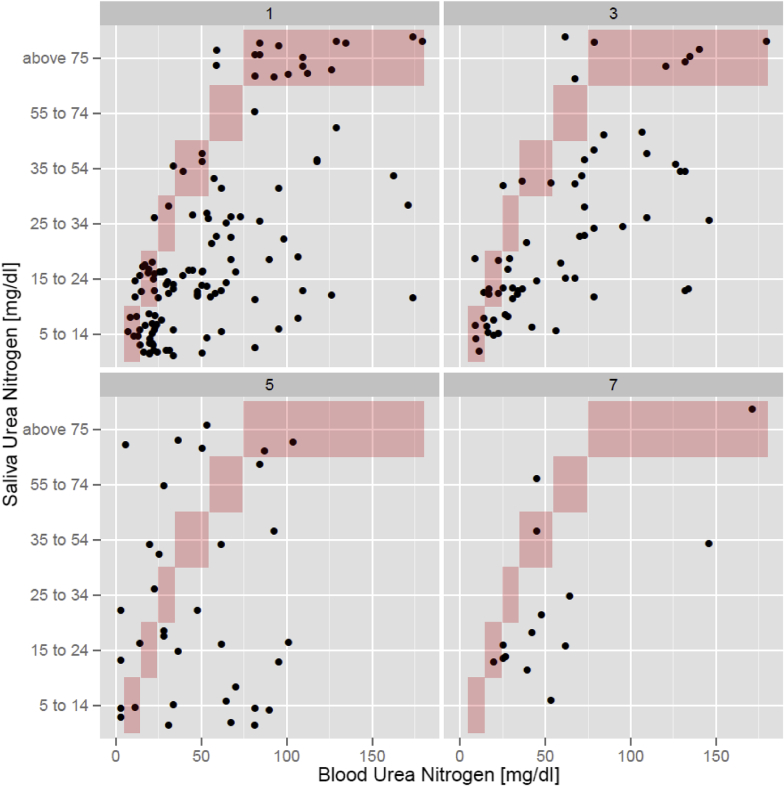
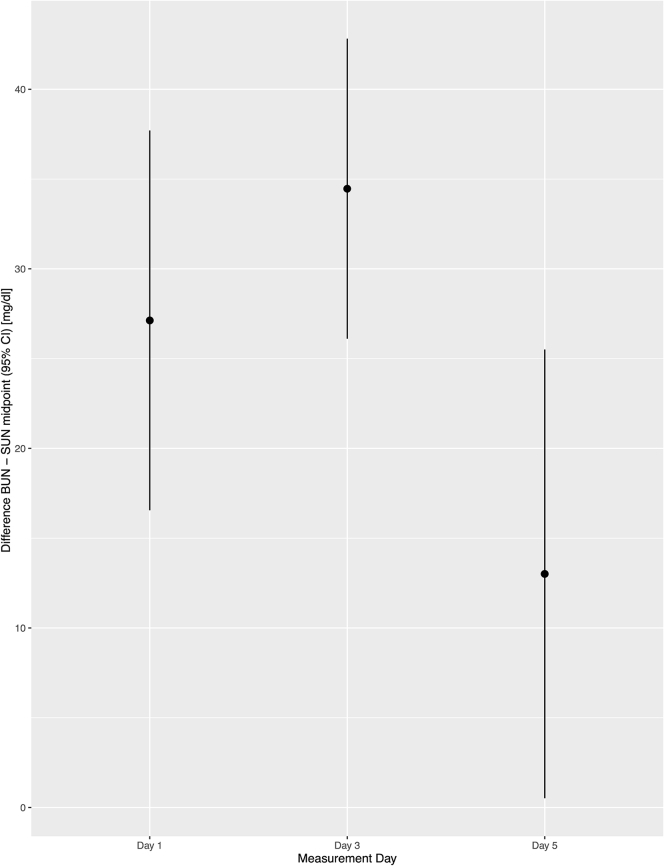
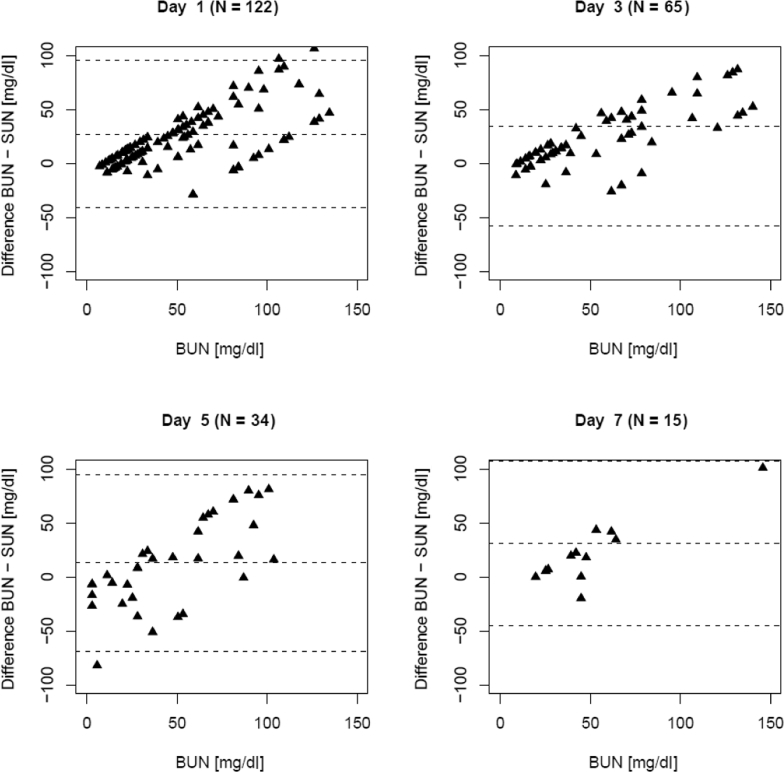
When comparing results of both parameters, there was a similar trend over time (Figure S3), with SUN consistently underestimating BUN in the follow-up period (Figure 2). However, the agreement between BUN and SUN was unaffected as patients underwent treatment (Figure 3). Plotting the differences (BUN – SUN midpoint) as a function of BUN and as a modified Bland-Altman plot showed consistent nonsignificant biases and proportional errors (Figure 4).
Figure 2.
Scatterplot demonstrating the relationship between saliva urea nitrogen and blood urea nitrogen on days 1, 3, 5, and 7.
Figure 3.
Point estimates and 95% confidence intervals of the difference between blood urea nitrogen (BUN) and saliva urea nitrogen (SUN) (quantified as the midpoint of the categories of the dipstick) on days 1, 3, and 5.
Figure 4.
Bland-Altman plot indicating the difference between blood urea nitrogen (BUN) and saliva urea nitrogen (SUN) as a function of the BUN on days 1, 3, 5, and 7. Dotted lines represent the mean difference ± SD at each observation day. The data indicate a consistent systematic and proportional bias consistent throughout the observation period.
When BUN is stratified by SUN categories, analysis of variance with Bonferroni correction did not show significant differences between the lower test pad numbers (e.g., test pads 1 and 2, or 2 and 3); however, significant differences were seen when lower test pads were compared with test pads that reflect higher levels of SUN (e.g., test pad 1 and pads 3–6, or test pads 2–4 and 6) (Table S2). These differences suggest improved performance of SUN to diagnose KD at more elevated BUN levels but lower accuracy of SUN at lower levels of BUN.
Longitudinal data analysis of BUN as a predictor of SUN using a linear mixed effects model showed BUN to significantly associate with SUN (0.26 mg/dl SUN increase per 1 mg/dl BUN increase; P < 0·001).
Diagnostic Performance of SUN and BUN
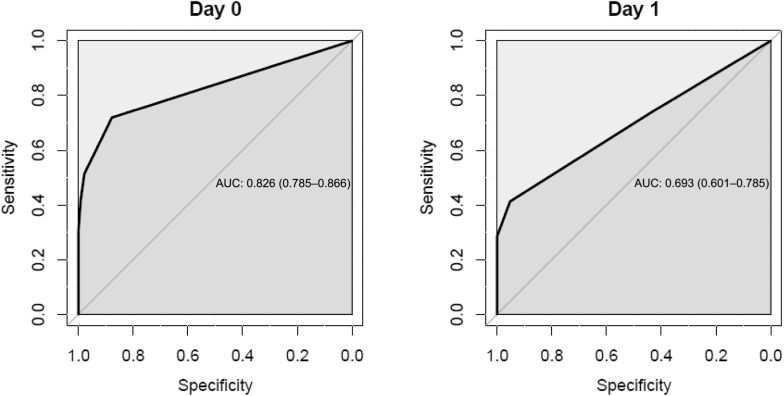
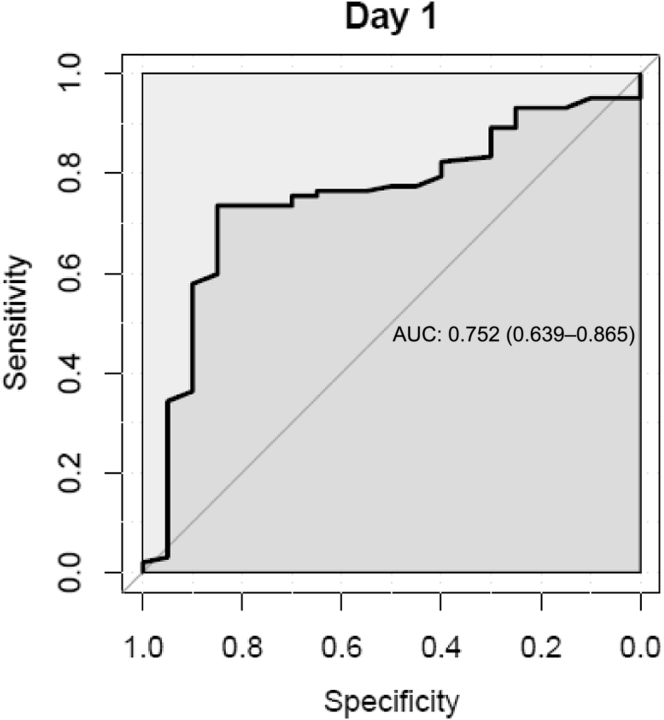
The area under the receiver operator characteristics curve for SUN to detect KD decreased from 0.82 (95% CI, 0.78–0.85) on observation day 0 (screening day) to 0.69 (95% CI, 0.50–0.78) on observation day 1 (Figure 5). Sensitivity and specificity were highest at day 0 (0.71 and 0.87, respectively). The optimal diagnostic threshold for SUN to diagnose KD was test pad 2 (based on the maximum Youden’s index according to the sensitivity and specificity of SUN at day 0). For BUN, the area under the receiver operator characteristics curve was 0.82 (95% CI, 0.59–1.0) on day 1 (Figure 6). The sensitivity and specificity of patient-reported oliguria alone to detect KD in this cohort was 0.3 and 0.79, respectively. Self-reported increased thirst analyzed combined with SUN >14 mg/dl (SUN >test pad 1) did not show significant improvement in the sensitivity and specificity to detect KD. When analyzing self-reported decreased urine output combined with SUN >14 mg/dl (SUN >test pad 1) to diagnose KD, significant differences in the sensitivity (0.22) and specificity (0.97) were demonstrated. Although sensitivity is low, the specificity increased by 0.1 up to 0.97 when self-reported information on urine output was added.
Figure 5.
Receiver operating characteristic curves of saliva urea nitrogen to detect kidney disease (acute kidney injury [AKI], acute kidney disease/disorder without AKI, and chronic kidney disease) on days 0 and 1. AUC, area under curve.
Figure 6.
Receiver operating characteristic curves of blood urea nitrogen to detect kidney disease (acute kidney injury [AKI], acute kidney disease/disorder without AKI, and chronic kidney disease) on day 1. AUC, area under curve.
Outcome Analyses
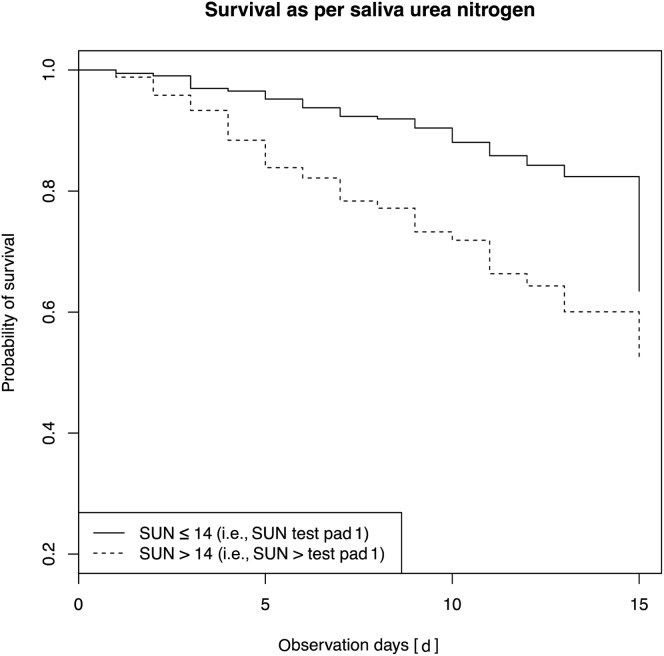
Hospital outcome was determined in 702 patients. Of these patients, 104 (14·8%) died, with 17 (2.4%) doing so after the censoring at day 15. Age, gender, the presence of diabetes, and SUN results were evaluated as predictors of time to in-hospital death; elevated SUN >14 mg/dl (SUN > test pad 1) was the only factor that was a significant independent predictor of all-cause mortality (hazard ratio = 2.23 [95% CI, 1.50–3.32]) (Figure 7).
Figure 7.
Kaplan-Meier analysis demonstrating survival probability for all patients stratified by the presence or absence of saliva urea nitrogen (SUN) >14 mg/dl (i.e., SUN > test pad 1).
Discussion
Simple and inexpensive bedside tools aiding diagnosis and guiding treatment of KD are lacking. The main finding of this study was that SUN measured by a dipstick demonstrated good diagnostic performance and longitudinal agreement with BUN in patients with predominantly AKD in an LRS in sub-Sahara Africa.
The results confirm earlier reports on the agreement between SUN and BUN.14, 15 In addition, they corroborate the notion that SUN dipsticks can be used for monitoring the evolution of renal function.22 Of note, BUN and SUN agreed well throughout all observation days, with SUN consistently reflecting BUN levels over time. Our data also corroborate previous findings of SUN levels being lower than BUN.14, 15, 22 Furthermore we confirmed good agreement between BUN and SUN at higher levels of BUN, but a lower level of accuracy of SUN to detect more subtle changes in renal function.
The diagnostic performance of SUN to detect KD decreased over the course of the study period, which may be explained by treatment initiation on admission resulting in decreased BUN and consequently SUN levels. As a consequence, sensitivity and specificity during the follow-up period was affected. In addition, loss to follow-up needs to be considered that affects not only sample size and subsequently the CI, but also the distribution of disease and diagnostic markers in the respective population analyzed at each day. For the concept of the current manuscript we deemed the results on day 0 as most important.
In this study, a level of SUN >14 mg/dl (SUN > test pad 1) was identified as the optimal threshold to diagnose KD at day 0 (screening day), with good sensitivity and specificity (0.71 and 0.87, respectively). When assessing the diagnostic performance of SUN levels combined with the information regarding urine output, we found a higher specificity to diagnose KD (0.97) at the expense of lower sensitivity (0.22). These findings demonstrate that SUN strips have a better screening performance (sensitivity) to detect KD when applied alone but a better diagnostic performance to exclude those without KD (specificity) when used combined with patient-reported urine output. This is of significant clinical relevance in an LRS. Patients at high risk for AKI (e.g., suffering from malaria or other infectious illness) are routinely evaluated at health centers and district hospitals by health care workers with only basic medical training and without access to laboratories.23 Significant numbers of patients at risk of KD are seen each day. We feel the SUN dipstick may be a useful clinical tool for these health care workers to use alongside clinical evaluation of the patient to try and identify those patients at highest risk of KD and poor outcome. According to the data in this study, a patient, for example, without a change in self-reported urine volume and a SUN less than 15 mg/dl (i.e., test pad 1) would have a 3% of chance of having AKI. We believe this is powerful information to have.
Moreover, given the SUN strip’s diagnostic performance demonstrated in this study, and its consistent agreement with BUN on all observation days, our data suggest that SUN may be a suitable tool not only to screen for AKI but also to monitor treatment progression in LRS, and should be considered for use at remote health care facilities in patients who are being treated locally for kidney injury. A positive result for SUN should immediately trigger specific diagnostic and therapeutic interventions such as volume assessment and fluid replacement as appropriate (oral or i.v.), avoidance of nephrotoxins, and management of the underlying disease (e.g., antimicrobials or antimalarial drugs). A SUN strip suggestive of severe renal injury (e.g., test pads 3–6), or progressive SUN levels over time despite treatment, especially in rural areas with limited health care resources, should trigger referral to the next level of health care with better infrastructure and possibly renal replacement therapy.
Elevated SUN was demonstrated to be an independent predictor of time to death in this population. This makes SUN an important additional tool in informing clinicians about the severity of a patient’s disease process and their prognosis. In LRS this may trigger more intensive monitoring, expedite management, and prompt earlier referral to the next tier of the health care system with the aim of increasing the probability of survival for these patients.
There are some limitations of the SUN strips to highlight. Limitations for the accuracy of the test and heterogeneity of the results may be related to differences of salivary flow rate and composition in terms of circadian rhythms of salivary glands, variable degrees of patient hydration, and the potential interference of oral bacterial flora with urea degradation in the cavity.12, 24, 25 Furthermore, collection of saliva in a patient with reduced conscious level, while possible, may be more challenging and was not attempted in this study. The dipstick quantifies salivary urea, but does not provide information on other electrolyte levels (e.g., potassium), which can be done using other more sophisticated point-of-care devices.
However, there are also major strengths to this technique. The SUN strip is a relatively cheap (approximately US$1/test strip), noninvasive, bedside tool of easy application, which allows quick evaluation of renal function. It does not require power or refrigerated storage and may therefore be applicable in even the remotest of settings. In low-middle income countries where many people, due to infrastructural, financial, and political issues, often do not receive appropriate diagnosis and treatment,2, 26 SUN strips could be of great utility to increase the identification of KD, to improve the diagnostic capability of health care facilities, to raise awareness about the need of earlier management, and, above all, to improve patient outcome from AKI.
Conclusion
This study is the first effort to apply a bedside diagnostic tool to detect and follow-up patients affected by KD in an LRS, in this case in Malawi in sub-Sahara Africa. Here, as in previous studies in developed settings, SUN demonstrated good diagnostic performance to screen, diagnose, and follow patients with acute and chronic kidney injury. Moreover, SUN was shown to be an independent predictor of all-cause mortality in this population. Semiquantitative SUN measurements by a simple dipstick method in parallel with a clinical evaluation may assist in the diagnosis of AKI, especially under circumstances of limited health care resource. Studies will now address the capability of SUN strips to detect KD in different populations and in even more rural settings, and assess the impact on patient outcome of this diagnostic tool.
Disclosure
VC-S was a fellow of the ISN and received scholarship from the Brazilian Government (CAPES) during part of the time when the study was conducted. NL and PK hold stock in Fresenius Medical Care. During this study, RP-F received a scholarship from the Brazilian Council for Research Support (CNPq). None of the other authors have declared competing interests.
Acknowledgments
We acknowledge the generous provision of SUN dipsticks free of charge by Integrated Biomedical Technology. The results presented in this paper have not been published previously. This study was funded by The International Society of Nephrology (ISN) (Research and Prevention Program), and The Royal Society of Tropical Medicine and Hygiene. The role of these organizations was provision of funding only. The study was approved by the Malawi College of Medicine Research and Ethics Committee (P.11/14/1660), and all patients provided written informed consent at enrollment.
RDRE, VC-S, JGR, HD, GD, NWL, PK, and RP-F designed the study. RDRE, UH, AC, MM, FH, and ZK collected the data and managed the study patients. JGR performed statistical analyses. RDRE and VC-S wrote the manuscript. All authors revised the manuscript and have approved the final version.
Footnotes
Figure S1. Saliva urea nitrogen (SUN) dipstick reference pads (1–6) of increasing urea nitrogen range.
Figure S2. Definitions of AKI, AKD without AKI, CKD, and NKD.
Figure S3. Line plots representing (a) BUN and (b) SUN at all observation days.
Table S1. Demographic and clinical-related parameters on day 1 in patients with kidney dysfunction.
Table S2. BUN levels of all patients on all observation days stratified as per SUN.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Saliva urea nitrogen (SUN) dipstick reference pads (1–6) of increasing urea nitrogen range.
Definitions of AKI, AKD without AKI, CKD, and NKD.
Line plots representing (a) BUN and (b) SUN at all observation days.
Demographic and clinical-related parameters on day 1 in patients with kidney dysfunction.
BUN levels of all patients on all observation days stratified as per SUN.
References
- 1.KDIGO AWG KDIGO 2012 clinical practice guideline for the evaluation and management of acute kidney injury. Kidney Int Suppl. 2012;2:19–36. [Google Scholar]
- 2.Lewington A.J., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lameire N.H., Bagga A., Cruz D. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 4.Cerda J., Bagga A., Kher V., Chakravarthi R.M. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 5.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 6.Lunyera J., Kilonzo K., Lewington A., Yeates K., Finkelstein F.O. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67:834–840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Wright S. Case of ascites in which, during spontaneous ptyalism that occurred after tapping, urea was detected in saliva. Lancet. 1841;1:753–758. [Google Scholar]
- 8.Akai T., Naka K., Yoshikawa C. Salivary urea nitrogen as an index to renal function: a test-strip method. Clin Chem. 1983;29:1825–1827. [PubMed] [Google Scholar]
- 9.Barnett G.D., Bramkamp R.G. Influence of the rate of secretion on the urea concentration of saliva. Proc Soc Exp Biol Med. 1929;27:118–120. [Google Scholar]
- 10.Forland M., Shannon I.L., Katz F.H. Parotid-fluid urea nitrogen for the monitoring of hemodialysis. N Engl J Med. 1964;271:37–38. doi: 10.1056/NEJM196407022710107. [DOI] [PubMed] [Google Scholar]
- 11.Hench P.S., Aldrich M. The concentration of urea in saliva. JAMA. 1922;79:1409–1412. [Google Scholar]
- 12.Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 13.Amerongen A.V. Saliva: properties and functions. In: Wong D.T., editor. Salivary Diagnostics. 2nd ed. Wiley-Blackwell; New Delhi, India: 2008. pp. 27–36. [Google Scholar]
- 14.Calice-Silva V., Vieira M.A., Raimann J.G. Saliva urea nitrogen dipstick—a novel bedside diagnostic tool for acute kidney injury. Clin Nephrol. 2014;82:358–366. doi: 10.5414/CN108370. [DOI] [PubMed] [Google Scholar]
- 15.Raimann J.G., Kirisits W., Gebetsroither E. Saliva urea dipstick test: application in chronic kidney disease. Clin Nephrol. 2011;76:23–28. doi: 10.5414/cn106826. [DOI] [PubMed] [Google Scholar]
- 16.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 17.Kay W.W., Sheehan H.L. Accuracy in the determination of blood-urea by the urease aeration-titration method. Biochem J. 1934;28:1784–1794. doi: 10.1042/bj0281784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KDIGO CWG KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;2:19–62. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 19.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein S., Obuchowski N.A., Lieber M.L. Clinical evaluation of diagnostic tests. AJR Am J Roentgenol. 2005;184:14–19. doi: 10.2214/ajr.184.1.01840014. [DOI] [PubMed] [Google Scholar]
- 21.Team RDC . R Foundation for Statistical Computing; Vienna: 2010. R: a language and environment for statistical computing. [Google Scholar]
- 22.Raimann J.G., Calice-Silva V., Thijssen S. Saliva urea nitrogen continuously reflects blood urea nitrogen after acute kidney injury diagnosis and management: longitudinal observational data from a collaborative, international, prospective, multicenter study. Blood Purif. 2016;42:64–72. doi: 10.1159/000445041. [DOI] [PubMed] [Google Scholar]
- 23.Olowu W.A., Niang A., Osafo C. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4:e242–e250. doi: 10.1016/S2214-109X(15)00322-8. [DOI] [PubMed] [Google Scholar]
- 24.Burne R.A., Chen Y.Y. Bacterial ureases in infectious diseases. Microbes Infect. 2000;2:533–542. doi: 10.1016/s1286-4579(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 25.Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol. 1972;220:529–545. doi: 10.1113/jphysiol.1972.sp009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand S., Cruz D.N., Finkelstein F.O. Understanding acute kidney injury in low resource settings: a step forward. BMC Nephrol. 2015;16:5. doi: 10.1186/1471-2369-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Saliva urea nitrogen (SUN) dipstick reference pads (1–6) of increasing urea nitrogen range.
Definitions of AKI, AKD without AKI, CKD, and NKD.
Line plots representing (a) BUN and (b) SUN at all observation days.
Demographic and clinical-related parameters on day 1 in patients with kidney dysfunction.
BUN levels of all patients on all observation days stratified as per SUN.