Abstract
Introduction
Atypical hemolytic uremic syndrome is a thrombotic microangiopathy, which is linked to hereditary or autoimmune defects in complement activators or regulators present in blood and on vascular endothelial cells. Acute thrombotic microangiopathy episodes are typically preceded by infections, which by themselves would not be expected to manifest HUS. Thus, it is possible that the host immune response contributes to the precipitation of aHUS. However, the mechanisms involved are not fully understood. We hypothesized that neutrophils trigger aHUS via initiating platelet aggregate formation on complement-activated endothelial cells.
Methods
We investigated neutrophil adhesion to complement-activated endothelial cells under static and flow conditions in vitro and ex vivo.
Results
Our results show that complement activation on endothelial cells promotes neutrophil adhesion, which is significantly reduced when the complement terminal pathway is blocked. When neutrophils and platelets are perfused simultaneously, neutrophils adhering to endothelial cells also induce the formation of platelet-neutrophil aggregates on these cells. Sera from patients with aHUS recapitulated these results.
Discussion
Therefore, our findings of (i) neutrophils adhering to complement-activated endothelial cells, (ii) the formation of neutrophil-platelet aggregates on endothelial cells, and (iii) the ability of aHUS serum to induce similar effects identify a possible role for neutrophils in aHUS manifestation.
Keywords: complement, endothelium, hemolytic uremic syndrome, inflammation, neutrophils, platelets
Thrombotic microangiopathy (TMA) is characterized by endothelial cell (EC) activation and/or injury and subsequent thrombus formation in the microvasculature, especially of the kidney glomeruli.1 TMA can manifest in the context of a broad spectrum of conditions involving the classical and/or alternative pathways of complement.1 Complement activation is physiologically inhibited on the surface of the vascular endothelium by plasma-borne and EC surface-bound regulators. These include the soluble complement factor H (CFH), which is dysfunctional in a significant portion of patients with atypical hemolytic uremic syndrome (aHUS), and membrane cofactor protein/CD46, decay-accelerating factor/CD55, and protectin/CD59 with the latter inhibiting the formation of the membrane attack complex (MAC; C5b-9).2, 3, 4 Loss of complement regulation on vascular ECs results in enhanced C3b deposition and MAC formation, inducing a proinflammatory phenotype and eventually clot formation.5, 6
Manifestation of aHUS is highly variable in terms of first presentation, severity of clinical presentation, and risk of recurrence.1, 7, 8 Initial TMA onset and subsequent acute episodes (flare-ups) are often preceded by infections, pointing to a critical role for inflammatory triggers and/or immune responses in manifestation of aHUS.7 Complement and neutrophils play major roles in immune defense and have the potential for mutual activation.9, 10 Although a role for neutrophils in triggering TMA in the context of complement dysregulation has not been established, neutrophil infiltration has been observed in biopsies of patients with TMA11, 12 (and personal unpublished observation). In this study, we examined whether complement activation can induce neutrophil adhesion and subsequent platelet aggregate formation on complement-activated ECs.
Concise Methods
Patients
Ethics approval was obtained from the Research Ethics Board at The Hospital for Sick Children, Toronto, Ontario. A signed consent was obtained from all patients and/or their parents, respectively.
Blood Outgrowth Endothelial Cell Culture and Preparation of Neutrophils and Platelets
Blood outgrowth endothelial cells (BOECs) were obtained from healthy controls and cultured in Endothelial Basal Medium 2 (Lonza, Walkersville, MD) supplemented with Endothelial Cell Growth Medium (EGM-2 BulletKit; Lonza Walkersville Inc., Walkersville, MD), 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), and 5% antibiotic-antimycotic mix (Sigma).13 Neutrophils were isolated from whole blood as described.14 In short, neutrophils were isolated using Polymorphprep (Axis-Shield, Dundee, Scotland), erythrocytes were lysed, and washed neutrophils were resuspended in Roswell Park Memorial Institute 1640 medium (WISENT, St Bruno, QC, Canada) containing 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Gibco, Life Technologies, Carlsbad, CA). Platelets were isolated from same donors as described.15 In short, whole blood was drawn in acid citrate dextrose (1:7) tubes and after centrifugation platelet-rich plasma was isolated and washed twice with phosphate-buffered saline/acid citrate dextrose.
Complement Activation on Cultured ECs
Complement activation on BOECs was achieved via a method previously established by us13 and others.16 Briefly, BOECs were incubated with antibodies blocking the surface complement regulators CD46, CD55, and CD59 using the monoclonal mouse anti-human antibodies GB24 (IgG1, kindly provided by John Atkinson, St. Louis, MO),17, 18 BRIC216 (IgG1), and BRIC229 (IgG2b, both from International Blood Group Reference Laboratory, NHS Blood and Transplant, Bristol, UK), respectively,19 at a concentration of 5 μg/ml. Normal human serum (NHS) prepared from whole blood of adult donors was used as a source of complement factors. Complement-inactivated serum was prepared by heating (heat-inactivated serum [HUS])20 or exhaustion with cobra venom factor (1 U/ml at 37°C for 90 minutes, Quidel, San Diego, CA). Specific inactivation of the complement cascade was achieved using C3-, C5-, and C6-depleted serum (Complement Technology, Tyler, TX). The rabbit erythrocyte lysis assay was used to confirm complement inactivation.21
Patients With aHUS
Serum from patients with aHUS due to CFH mutation (n = 2), CFH autoantibodies (n = 1), and C3 mutation (n=1) was taken from the biorepository of our KidCOM study (www.kidcom.ca) and was used without prior blockade of complement regulators. Detailed information in particular on the complement status of these patients is presented in Table 1.
Table 1.
Summary of diagnostic work-up and treatment of patients with aHUS whose serum was used for neutrophil adhesion studies
| Patient no. | Genetics | CFH Abs | Treatment |
|---|---|---|---|
| 1 | Not tested | Positive | None |
| 2 | CFH—heterozygous variant of unknown significance (c.3644G>A [p.Arg1215Gln]) |
Negative | Plasma infusion |
| 3 | CFH—heterozygous disease causing mutation (c.3148A>T [p.Asn1050Tyr]) CFHR-3/CFHR-1—homozygous deletion |
Positive | Plasma infusion |
| 4 | CFHR-3/CFHR-1—homozygous deletion | Positive | Eculizumab |
| 5 | C3—homozygous variant of unknown significance (c.304C>G [p.Arg102Gly]) | Negative | Eculizumab |
Eculizumab treatment followed a weight- and/or age-based standard protocol.
aHUS, atypical hemolytic uremic syndrome; CFH Abs, CFH autoantibodies; DEAP HUS, deficiency of complement factor H-related (CFHR) proteins and CFH autoantibody-positive hemolytic uremic syndrome.
Neutrophil Adhesion to BOECs Under Static Conditions
BOECs were grown to confluence overnight in a 96-well enzyme-linked immunosorbent assay plate (Sarstedt, Nümbrecht, Germany), exposed to anti-CD46/CD55/CD59 and 50% NHS for 1 hour or tumor necrosis factor-α (20 ng/ml) for 4 hours. Serum was washed off and 100 μl of 1 × 106/ml calcein-labeled neutrophils in Roswell Park Memorial Institute/N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid was added for 30 minutes. As previously described, nonadherent cells were removed by centrifugation of the 96-well plate upside down at 100 × g for 1 minute.22 Neutrophil adhesion was quantified using a fluorescent plate reader at excitation and emission wavelengths of 494 and 517 nm, respectively.
Neutrophil and Platelet Adhesion to BOECs Under Flow Conditions
Neutrophils and platelets were exposed to BOECs under flow conditions using a Bioflux microfluidic system (Fluxion Biosciences, South San Francisco, CA) as previously described.22 Briefly, the recording channels of 48-well (0–20 dyne/cm2) Bioflux plates were coated with 0.05 mg/ml of rat-tail collagen type I in 0.02 M acetic acid before the introduction of BOECs, which were grown to confluence, washed, and exposed to blocking antibodies for 30 minutes. Calcein-labeled (2.5 μM, Life Technologies) neutrophils were perfused simultaneously with 50% serum (NHS, heat-inactivated serum, C5-depleted serum, C6-depleted serum, patient serum) at a flow rate of 1 dyne/cm2, and images and video were obtained after 5 minutes using a ×4 objective on a Nikon Ti epifluorescence microscope using a Qimaging CCD camera and NIS software. Neutrophil adhesion was quantified via total green fluorescence intensity within the measurement channel, obtained via the Bioflux software or counted. For double adhesion experiments, platelets (600 × 106/ml) and neutrophils (12 × 106/ml) in RPMI-1640/HEPES were mixed with NHS at a ratio of 1:1:2, whereas the control channel was loaded with RPMI-1640/HEPES NHS-containing platelets but no neutrophils.
Flow Cytometry Analysis of Neutrophil Activation
Neutrophils (0.5 × 106/ml) were incubated with various types of treatment (calcein 2.5 μM; TNF-α 20 ng/ml) for 30 minutes, washed with phosphate-buffered saline, and incubated with 5 μl of Alexa 647 anti-human CD11b antibody (Biolegend, San Diego, CA) for 30 minutes and a fixable viability dye eFluor 780 (eBioscience, Affymetrix, Santa Clara, CA). At least 10,000 events were recorded using the Attune Acoustic Focusing Cytometer (Life Technologies) and analyzed using FlowJo software. Results are given as median fluorescence intensity. Cells were gated for live cells (red laser 536 nm, emission channel 2) and forward and side scatter. CD11b was recorded via the red laser 536 nm/emission channel 1, and calcein on the blue laser 488 nm/emission channel 1. To correct for spectral overlap during multicolor flow cytometry experiments, color compensation was performed each time.
Results
Complement Activation on BOECs Causes Neutrophil Adhesion
To determine whether neutrophils adhere to ECs, we isolated human neutrophils and allowed them to interact with BOECs from healthy donors under various complement-activating conditions. As recently described in detail, complement activation on BOECs was achieved by preincubation of BOECs with antibodies against CD59, or the combination of antibodies against membrane-anchored complement regulators CD46, CD55, and CD59 with 50% NHS as a source of complement.13
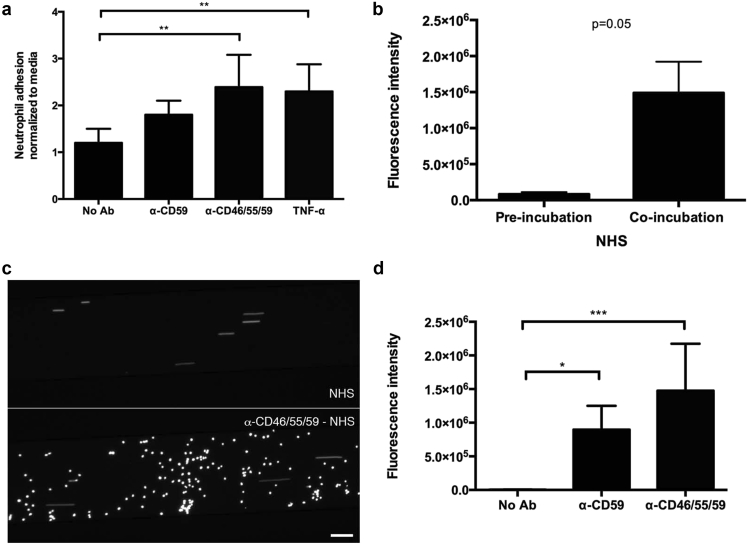
Endothelial adhesion of calcein-stained neutrophils was examined under both static and microfluidic conditions. We confirmed that calcein staining per se did not activate neutrophils (Supplementary Figure S1). Under static conditions, complement activation resulted in neutrophil adhesion to ECs subsequent to complement fixation. The number of neutrophils adhering to ECs subsequent to complement activation was comparable to that achieved in ECs stimulated with TNF-α (Figure 1a). Of note, no neutrophil adhesion occurred when non-complement fixing antibodies were used alone (e.g., anti-CD46 or anti-CD55 antibodies; data not shown). Similar results were obtained in microfluidic conditions (Figure 1b–d). When neutrophils were perfused over ECs in a microfluidic chamber at a rate of 1 dyne/cm2, pretreatment with CD59 antibody alone or—to a higher extent—with the combination of CD46, CD55, and CD59 antibodies resulted in enhanced neutrophil adhesion. Neutrophil adhesion was higher when NHS was co-perfused with neutrophils as compared with pretreatment of cells with NHS for 60 minutes (Figure 1b). Perfused neutrophils adhered to complement-activated ECs within 2 minutes and images were taken after 5 minutes (Figure 1c and d, Supplementary Videos S1–S3). For all future experiments, neutrophils were co-perfused with NHS.
Figure 1.
Neutrophil adhesion to complement-challenged BOECs under static and dynamic conditions. (a) BOECs cultured as monolayers in 96-well plates were treated with 50% NHS with or without prior incubation with anti-CD46, -CD55, and -CD59 antibodies. Cells incubated for 4 hours with TNF-α 20 ng/ml served as positive control. Calcein-labeled neutrophils were introduced for 30 minutes and then removed before wells were read for green fluorescence emission. Total fluorescence was normalized to values obtained for BOECs incubated with media only; n = 5, **P < 0.01 (1-way ANOVA with Dunnett’s multiple comparison test). (b–d) Calcein-labeled neutrophils were perfused over BOECs in a Bioflux chamber. (b) Markedly enhanced neutrophil adhesion was observed when neutrophils were co-perfused with 50% NHS for 5 minutes compared with preincubation with 50% NHS for 1 hour and then subsequent exposure to neutrophils (2-tailed t-test). (c) Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 with 50% NHS over untreated BOECs (top channel) or cells incubated with anti-CD46, -CD55, and -CD59 antibodies (bottom channel) showed marked adhesion after 5 minutes to cells with combined complement regulator blockade. (d) Quantification of neutrophil adhesion to BOECs within the measurement channel via total green fluorescence intensity shows the effect of various complement activation conditions, with combined incubation with anti-CD46, -CD55, and -CD59 antibodies showing significant neutrophil adhesion (n = 3–8, *P < 0.05, ***P < 0.001; 1-way ANOVA with Dunnett’s multiple comparison test). ANOVA, analysis of variance; BOECs, blood outgrowth endothelial cells; NHS, normal human serum; TNF-α, tumor necrosis factor-α.
Neutrophil Adhesion Is Complement-Mediated and Terminal Pathway Dependent
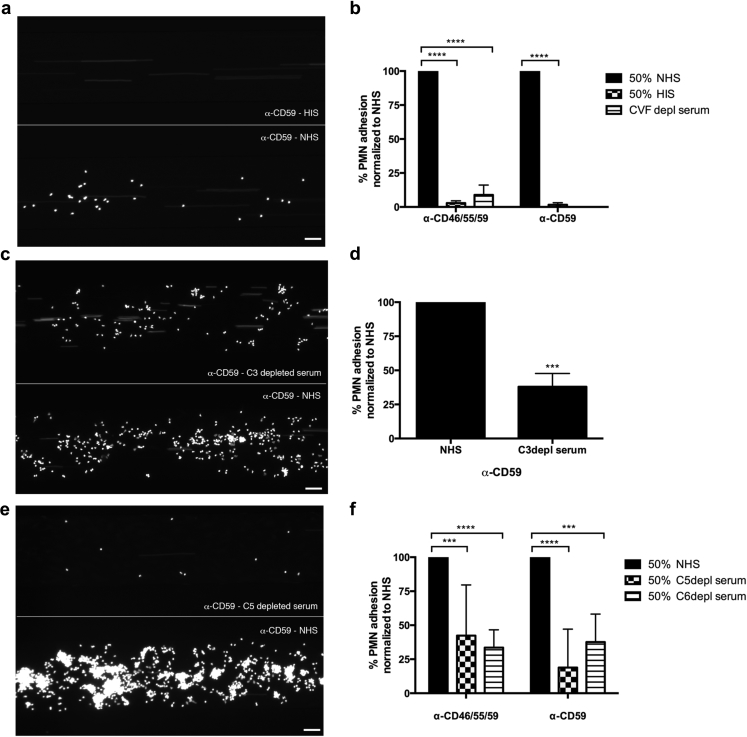
Neutrophil adhesion to BOECs was abolished when complement inactive serum (HIS) was used (Figure 2a and b, Supplementary Video S1). The same effect was achieved by the pretreatment of serum with cobra venom factor (Figure 2b, images not shown). Cobra venom factor is a potent activator of the C3 and C5 convertases and renders sera complement inactive within 90 minutes via depleting active complement components.23 Preventing terminal pathway activation by using serum depleted of C3 (Figure 2c and d), C5 (Figure 2e and f, Supplementary Video S2), or C6 (Figure 2f, images not shown), neutrophil adhesion was significantly reduced. This was seen when BOECs were pretreated with anti-CD59 antibody alone (Figure 2a, c, and e) or the combination of anti-CD46, CD55, and CD59 antibodies (Supplementary Figure S2). A hemolysis assay with rabbit erythrocytes was used to confirm that complement depletion (heat-inactivated serum, cobra venom factor, or C3-/C5-/C6-depleted sera) was successful (data not shown).24
Figure 2.
Complement blockade at the level of C3 and C5 decreases neutrophil adhesion to BOECs. (a,b) Neutrophil adhesion to BOECs with anti-CD59 antibody incubation dropped to background levels in the presence of serum depleted of complement activity by heat-inactivated serum (HIS); results are summarized in (b) including cobra venom factor (CVF). (c,d) Neutrophil adhesion was also reduced relative to NHS when C3-depleted serum was used; results are summarized in (d). (e,f) Neutrophil adhesion was decreased relative to NHS when C5-depleted serum was used; results are summarized in (f) including C6-depleted serum. Fluorescence intensity in (b), (d), and (e) is normalized to the value obtained for 50% NHS in each experiment, n = 3–6; ***P < 0.001, ****P < 0.0001 (2-way ANOVA with Dunnett’s multiple comparison test, or unpaired t-test with Welch’s correction in Figure 2d). Bar = 50 μm. ANOVA, analysis of variance; BOECs, blood outgrowth endothelial cells; NHS, normal human serum; PMNs, polymorphonuclear leukocytes.
Taken together, these results (Figures 1 and 2) indicate that neutrophil adhesion to ECs is complement-mediated and terminal pathway dependent.
Sera From Patients With aHUS Result in Neutrophil Adhesion to BOECs in a Microfluidic Chamber
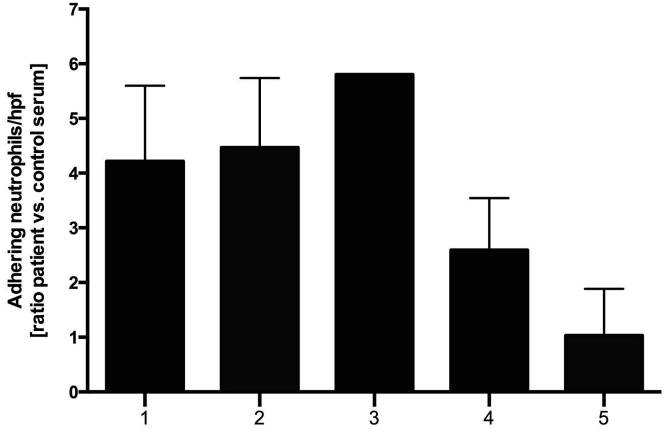
Furthermore, we were interested to examine if neutrophil adhesion to ECs can be observed when serum from patients with aHUS is used. Sera were taken from 5 patients with aHUS (patients 1–5; Table 1) in clinical remission (i.e., normal platelet count, no hemolysis, and normal kidney function). Genetic and biochemical work-up identified CFH autoantibodies in patient 1 (no genetic testing performed), a heterozygous CFH variant in patient 2 (c.3644G>A [p.Arg1215Gln]), deficiency of complement factor H-related (CFHR) proteins and CFH autoantibody-positive HUS25 with CFHR-3/CFHR-1 deletion and CFH autoantibodies in patients 3 and 4 in which patient 3 was combined with a disease causing CFH mutation (c.3148A>T [p.Asn1050Tyr]), and a homozygous C3 variant (c.304C>G [p.Arg102Gly]) in patient 5. Perfusion of serum from all patients with aHUS caused an up to 6-fold increase in neutrophil adhesion to ECs when compared with control serum (Figure 3). In keeping with a predominant role of the terminal complement pathway in neutrophil adhesion to ECs, neutrophil adhesion was reduced to a minimum when using serum of the 2 patients (patients 4 and 5) treated with the C5 antibody eculizumab, which blocks the terminal complement pathway.
Figure 3.
Serum from patients with aHUS results in neutrophil adhesion to BOECs. BOECs cultured in the BioFlux microperfusion chamber were exposed to 50% serum from patients with aHUS in clinical remission (see Table 1 for details) and calcein-labeled neutrophils at 1 dyne/cm2. Number of adhering neutrophils was counted per high power field (hpf) and ratio over adhering neutrophils incubated with control serum was calculated. n = 4 for all patients except patient 3. aHUS, atypical hemolytic uremic syndrome; BOECs, blood outgrowth endothelial cells.
Complement Fixation on ECs Causes Platelet-Neutrophil Aggregation
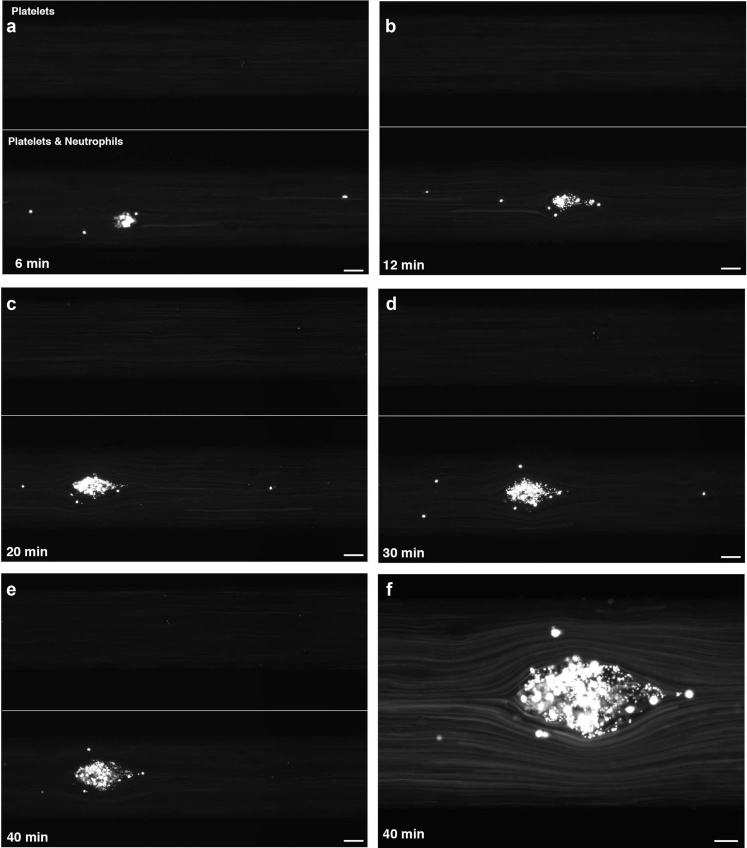
The functional consequences of neutrophil adhesion to ECs were further investigated by introducing platelets in the microfluidic chamber. Perfusion of platelets with 50% NHS after pretreatment with CD46, CD55, and CD59 antibodies resulted in adhesion of a moderate number of platelets on activated ECs without the formation of obvious platelet aggregates.13 However, simultaneous perfusion of neutrophils (3 × 106/ml) and platelets (150 × 106/ml) in 50% NHS over pretreated ECs resulted in neutrophil adhesion and the subsequent formation of neutrophil-platelet aggregates. These aggregates increased in size over the entire observation period of 40 minutes (Figure 4, Supplementary Video S3). Our results suggest that in the presence of platelets complement-mediated neutrophil adhesion to ECs induces the formation of neutrophil-platelet aggregates beginning as microaggregates and evolving into larger aggregates.
Figure 4.
BOEC-adherent neutrophils promote platelet adhesion. (a–e) BOECs with combined incubation with anti-CD46, -CD55, and -CD59 antibodies were exposed to 50% NHS and either calcein-labeled platelets alone (upper channel) or labeled neutrophils and platelets together (lower channel). In this typical experiment, bright neutrophil-platelet aggregates can be observed (original magnification ×4) to develop and increase in size over 6 (a), 12 (b), 20 (c), 30 (d), and 40 minutes (e). Bar = 50 μm. Note: Adhesion of single platelets was not detectable at the detector intensity setting used for these experiments. (f) A higher magnification (original magnification ×20) image shows that aggregates contain both platelets and neutrophils (small and large cells, respectively). Bar = 20 μm. BOECs, blood outgrowth endothelial cells; NHS, normal human serum.
Discussion
TMA is characterized by thrombus formation in the microvasculature, predominantly of the kidney glomeruli. TMA can occur as primary or secondary disease, and TMA pathogenesis is regulated by different mechanisms.1, 26 Recently, complement dysregulation on ECs—caused by genetic or autoimmune defects—has been identified as the cause for aHUS,4, 27 and the complement system has consequently gained a prominent role as treatment target for this clinical condition.28, 29 Of note, complement defects and complement-targeting treatments are also increasingly recognized as relevant to different types of TMA (reviewed in Riedl et al.1), a development highlighting the existence of a complement-based TMA spectrum. Our findings further the understanding of complement-mediated TMA pathology by reconciling the relevance of infection and the potential role of host immune components, particularly neutrophils, in aHUS pathogenesis.
The common finding of preceding infections in patients with TMA either at first presentation or during flare-ups7, 8 and studies linking complement activation with a proinflammatory phenotype (i.e., neutrophil activation)5, 6, 9, 30 highlight an important yet poorly understood role for neutrophils in TMA pathogenesis. Although previously only speculated upon,9 our results demonstrate that neutrophil adhesion is caused by complement—in particular, MAC/C5b-9—deposition on ECs, and that complement-mediated neutrophil adhesion to ECs facilitates platelet adhesion and the formation of platelet-neutrophil aggregates.
Camous et al.9 linked the complement and inflammatory systems further by showing that activation of neutrophils via cytokines results in complement activation on neutrophils, thus engaging a positive feedback loop with further activation of neutrophils. In keeping with this concept, we observed neutrophil adhesion to ECs to occur rapidly (within 2 minutes) after complement activation. Our results, however, point toward a different mechanism. Whereas the studies by Camous et al. highlight a significant role for C5a/C5a receptor interaction, our results identify a key role for the terminal complement pathway and the formation of MAC/C5b-9, as neutrophil adhesion to ECs was not different between the use of C3-, C5-, and C6-depleted serum with the first 2 conditions preventing the generation of C3a and C5a and blocking the induction of the terminal complement cascade, however, the latter still allowing for the formation of functional C5a.
Our in vitro findings of complement-induced neutrophil adhesion to ECs were confirmed in an in vitro/ex vivo study using serum from 5 patients with aHUS. Under flow conditions, neutrophil adhesion was observed in all patients. Although the effect was small in the 2 patients treated with the terminal complement blocker eculizumab (patients 4 and 5), neutrophil adhesion was significantly (up to 6-fold) increased in patients without specific treatment (patient 1) and the 2 patients with plasma infusion treatment (patients 2 and 3). As previously established, when testing serum of a patient with aHUS with a CFH mutation (c.3572C>T [p.Ser1191Leu]) who was first treated with plasma infusions and subsequently with eculizumab,21 plasma infusion achieves only partial (terminal) complement blockade and is inferior to eculizumab. This difference in treatment efficacy may account for the difference in EC neutrophil adhesion when comparing not treated (patient 1), plasma-treated (patients 2 and 3), and eculizumab-treated (patients 4 and 5) patients. Our findings not only support a key role for the terminal complement pathway in neutrophil adhesion to ECs, but also suggest that neutrophil adhesion can be used in an in vitro/ex vivo system using patient serum, neutrophils, and control ECs to monitor disease activity and treatment response, respectively, in patients with aHUS.
Our findings are in keeping with a recently published study by Noris et al.31, who investigated MAC/C5b-9 deposition on ECs using serum samples from patients with aHUS collected at different stages of disease activity and treatment. MAC/C5b-9 deposition on ECs was enhanced with serum of nontreated, but suppressed with serum of eculizumab-treated patients. In a subgroup of patients with incomplete complement control, however, MAC/C5b-9 deposition on ECs was not fully suppressed, a finding that correlated with clinical signs of aHUS flare-ups in these patients.31
As TMA is a disease characterized by thrombus formation in the microvasculature, we next investigated if complement-mediated neutrophil adhesion to ECs had a functional consequence with respect to thrombus formation. Neutrophils were reported to capture platelets causing lethal vascular occlusions, the production of reactive oxygen species, and damage to ECs in a mouse model of transfusion-related lung injury.32 Also, on activation, platelets express several surface molecules, which allow them to interact with other cells, for example, neutrophils or ECs.33 In previous studies of the role of complement and platelets in the pathogenesis of TMA, Licht et al.34 showed that normal washed platelets aggregate in the absence of serum because CFH autoantibodies induced functional CFH deficiency. Finally, in a mouse model of Shiga toxin-induced TMA, Morigi et al.35 were able to link complement activation—induced by Shiga toxin and lipopolysaccharides—to P-selectin upregulation and thrombus formation on ECs.
To study the consequences of complement-mediated neutrophil adhesion for platelet adhesion and aggregation, we perfused a microfluidic chamber simultaneously with serum and isolated neutrophils and platelets, devoid of coagulatory factors, which are known to enhance complement activation on their own.36 Interestingly, perfusion of platelets in combination with neutrophils in physiological concentrations resulted in the formation of platelet-neutrophil aggregates. These aggregates increased in size over time (40-minute observation period) by recruiting more neutrophils and platelets and may represent microthrombi in a system with an intact coagulation system.
In summary, our results show that complement activation on ECs results in neutrophil adhesion, under both static and microfluidic conditions. Neutrophil adhesion is complement-mediated and terminal pathway dependent. Neutrophil adhesion serves as nidus for the formation of neutrophil-platelet aggregates. Taken together, our findings strongly point toward a key role for neutrophils in the pathogenesis of complement-mediated TMA.
Statistical Analysis
Data are expressed as means ± SD. Data were analyzed using Graphpad Prism 7 (GraphPad Software Inc, La Jolla, CA). Statistical tests were used as stated (mainly paired t-test and 1-way analysis of variance). A P value < 0.05 was considered statistically significant.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors were supported by the following research grants: MR received a Marietta Blau-Stipend of the OeAD-GmbH, funded by the Austrian Federal Ministry for Science and Research, and a Restracomp Fellowship from the Hospital for Sick Children. DGN was funded in part by Restracomp and a Transplant Center Fellowship by The Hospital for Sick Children, Toronto, Ontario. MAK was supported by a Mitac postdoctoral fellowship. WHAK was supported by operating grants from the Canadian Institutes of Health Research (CIHR; MOP-81208 and MOP-259952). NP was funded by Cystic Fibrosis Canada (3029, 3160) and CIHR research grant (MOP-134761). CL was funded by the American Society of Nephrology (2009 Norman Siegel Research Scholar Grant), the Heart and Stroke Foundation of Ontario (NA 6716), the Kidney Foundation of Canada (KFOC120001), and SickKids intramural grants.
Footnotes
Figure S1. Calcein did not activate neutrophils. Using flowcytometry, no change in neutrophil CD11b expression was observed after exposure to 30 minutes of calcein. (A) Histogram shows the fluorescent intensity for the following conditions: unstained (gray line), positive control TNF-α (40 ng/ml for 30 minutes, gray dotted line), neutrophil in media (black line) and neutrophils pretreated with calcein (2.5 μM, black dotted line). A summary of three experiments is shown in (B). Pretreatment with TNF-α (40 ng/ml) resulted in a significant (**P < 0.01) increase of CD11b.
Figure S2. Neutrophil adhesion to BOECs was complement-mediated and terminal pathway dependent. Neutrophil adhesion to BOECs following combined anti-CD46, anti-CD55, and anti-CD59 antibody incubation dropped to background levels when using heat-inactivated serum (complement depleted) or when using C5-depleted serum (terminal-pathway inactive). A summary of the results can be found in Figures 2b, d, and f.
Video S1. Neutrophil adhesion to BOECs is complement-mediated. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 over BOECs treated with anti-CD46, anti-CD55, and anti-CD59 antibodies (upper and bottom channel) and exposed to either NHS (bottom channel) or complement inactive serum (HIS, upper channel) showed marked neutrophil adhesion in the presence of complement. Video starts 20 seconds after first neutrophils were observed in channel.
Video S2. Neutrophil adhesion to BOECs is terminal pathway dependent. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 in NHS (bottom channel) or C5 depleted serum (upper channel) were exposed over BOECs with anti-CD46, anti-CD55, and anti-CD59 antibody pretreatment (upper and bottom channel). Neutrophil adhesion was reduced when C5 depleted serum was used. Video starts 30 seconds after first neutrophils were observed in channel.
Video S3. Neutrophils adhering to complement-challenged BOECs initiate the formation of neutrophil-platelet aggregates. BOECs with combined anti-CD46, anti-CD55, and anti-CD59 antibody treatment were exposed to 50% NHS and either calcein-labeled platelets alone (upper channel) or labeled neutrophils and platelets together (lower channel). Perfusion of both resulted in formation of neutrophil-platelet-aggregates. Video sequence start 90 seconds after first neutrophils/platelets were observed in channel.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Calcein did not activate neutrophils. Using flowcytometry, no change in neutrophil CD11b expression was observed after exposure to 30 minutes of calcein. (A) Histogram shows the fluorescent intensity for the following conditions: unstained (gray line), positive control TNF-α (40 ng/ml for 30 minutes, gray dotted line), neutrophil in media (black line) and neutrophils pretreated with calcein (2.5 μM, black dotted line). A summary of three experiments is shown in (B). Pretreatment with TNF-α (40 ng/ml) resulted in a significant (**P < 0.01) increase of CD11b.
Neutrophil adhesion to BOECs was complement-mediated and terminal pathway dependent. Neutrophil adhesion to BOECs following combined anti-CD46, anti-CD55, and anti-CD59 antibody incubation dropped to background levels when using heat-inactivated serum (complement depleted) or when using C5-depleted serum (terminal-pathway inactive). A summary of the results can be found in Figures 2b, d, and f.
Neutrophil adhesion to BOECs is complement-mediated. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 over BOECs treated with anti-CD46, anti-CD55, and anti-CD59 antibodies (upper and bottom channel) and exposed to either NHS (bottom channel) or complement inactive serum (HIS, upper channel) showed marked neutrophil adhesion in the presence of complement. Video starts 20 seconds after first neutrophils were observed in channel.
Neutrophil adhesion to BOECs is terminal pathway dependent. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 in NHS (bottom channel) or C5 depleted serum (upper channel) were exposed over BOECs with anti-CD46, anti-CD55, and anti-CD59 antibody pretreatment (upper and bottom channel). Neutrophil adhesion was reduced when C5 depleted serum was used. Video starts 30 seconds after first neutrophils were observed in channel.
Neutrophils adhering to complement-challenged BOECs initiate the formation of neutrophil-platelet aggregates. BOECs with combined anti-CD46, anti-CD55, and anti-CD59 antibody treatment were exposed to 50% NHS and either calcein-labeled platelets alone (upper channel) or labeled neutrophils and platelets together (lower channel). Perfusion of both resulted in formation of neutrophil-platelet-aggregates. Video sequence start 90 seconds after first neutrophils/platelets were observed in channel.
References
- 1.Riedl M., Fakhouri F., Le Quintrec M. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 2.Merle N.S., Church S.E., Fremeaux-Bacchi V., Roumenina L.T. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merle N.S., Noe R., Halbwachs-Mecarelli L. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vriese A.S., Sethi S., Van Praet J. Kidney disease caused by dysregulation of the complement alternative pathway: an etiologic approach. J Am Soc Nephrol. 2015;26:2917–2929. doi: 10.1681/ASN.2015020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan B.P. The membrane attack complex as an inflammatory trigger. Immunobiology. 2016;221:747–751. doi: 10.1016/j.imbio.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fremeaux-Bacchi V., Fakhouri F., Garnier A. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide french series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresin E., Rurali E., Caprioli J. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camous L., Roumenina L., Bigot S. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- 10.Huber-Lang M., Younkin E.M., Sarma J.V. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crew R.J., Radhakrishnan J., Cohen D.J. De novo thrombotic microangiopathy following treatment with sirolimus: report of two cases. Nephrol Dial Transplant. 2005;20:203–209. doi: 10.1093/ndt/gfh334. [DOI] [PubMed] [Google Scholar]
- 12.Meehan S.M., Kremer J., Ali F.N. Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin J Am Soc Nephrol. 2011;6:395–403. doi: 10.2215/CJN.05870710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noone D.G., Riedl M., Pluthero F.G. Von Willebrand Factor regulates complement on endothelial cells. Kidney Int. 2016;90:123–134. doi: 10.1016/j.kint.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cote O., Clark M.E., Viel L. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One. 2014;9:e96217. doi: 10.1371/journal.pone.0096217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S., Huang Y.W., Reheman A. The cell motility modulator Slit2 is a potent inhibitor of platelet function. Circulation. 2012;126:1385–1395. doi: 10.1161/CIRCULATIONAHA.112.105452. [DOI] [PubMed] [Google Scholar]
- 16.Triantafilou K., Hughes T.R., Triantafilou M., Morgan B.P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 17.Turner N.A., Walker J.H., Ball S.G., Vaughan P.F. Phorbol ester-enhanced noradrenaline secretion correlates with the presence and activity of protein kinase C-alpha in human SH-SY5Y neuroblastoma cells. J Neurochem. 1996;66:2381–2389. doi: 10.1046/j.1471-4159.1996.66062381.x. [DOI] [PubMed] [Google Scholar]
- 18.Liszewski M.K., Leung M., Cui W. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46) J Biol Chem. 2000;275:37692–37701. doi: 10.1074/jbc.M004650200. [DOI] [PubMed] [Google Scholar]
- 19.Jurianz K., Maslak S., Garcia-Schuler H. Neutralization of complement regulatory proteins augments lysis of breast carcinoma cells targeted with rhumAb anti-HER2. Immunopharmacology. 1999;42:209–218. doi: 10.1016/s0162-3109(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 20.Soltis R.D., Hasz D., Morris M.J., Wilson I.D. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology. 1979;36:37–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Heinen S., Pluthero F.G., van Eimeren V.F. Monitoring and modeling treatment of atypical hemolytic uremic syndrome. Mol Immunol. 2013;54:84–88. doi: 10.1016/j.molimm.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi S., Yuen D.A., Bajwa A. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J Am Soc Nephrol. 2013;24:1274–1287. doi: 10.1681/ASN.2012090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane C.G., Muller-Eberhard H.J., Aikin B.S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970;105:55–69. [PubMed] [Google Scholar]
- 24.Heinen S., Jozsi M., Hartmann A. Hemolytic uremic syndrome: a factor H mutation (E1172Stop) causes defective complement control at the surface of endothelial cells. J Am Soc Nephrol. 2007;18:506–514. doi: 10.1681/ASN.2006091069. [DOI] [PubMed] [Google Scholar]
- 25.Jozsi M., Licht C., Strobel S. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111:1512–1514. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- 26.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 27.Noris M., Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 28.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 29.Loirat C., Fakhouri F., Ariceta G. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 30.Kilgore K.S., Ward P.A., Warren J.S. Neutrophil adhesion to human endothelial cells is induced by the membrane attack complex: the roles of P-selectin and platelet activating factor. Inflammation. 1998;22:583–598. doi: 10.1023/a:1022362413939. [DOI] [PubMed] [Google Scholar]
- 31.Noris M., Galbusera M., Gastoldi S. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidalgo A., Chang J., Jang J.E. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarbock A., Polanowska-Grabowska R.K., Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Licht C., Pluthero F.G., Li L. Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114:4538–4545. doi: 10.1182/blood-2009-03-205096. [DOI] [PubMed] [Google Scholar]
- 35.Morigi M., Galbusera M., Gastoldi S. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol. 2011;187:172–180. doi: 10.4049/jimmunol.1100491. [DOI] [PubMed] [Google Scholar]
- 36.Krisinger M.J., Goebeler V., Lu Z. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120:1717–1725. doi: 10.1182/blood-2012-02-412080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calcein did not activate neutrophils. Using flowcytometry, no change in neutrophil CD11b expression was observed after exposure to 30 minutes of calcein. (A) Histogram shows the fluorescent intensity for the following conditions: unstained (gray line), positive control TNF-α (40 ng/ml for 30 minutes, gray dotted line), neutrophil in media (black line) and neutrophils pretreated with calcein (2.5 μM, black dotted line). A summary of three experiments is shown in (B). Pretreatment with TNF-α (40 ng/ml) resulted in a significant (**P < 0.01) increase of CD11b.
Neutrophil adhesion to BOECs was complement-mediated and terminal pathway dependent. Neutrophil adhesion to BOECs following combined anti-CD46, anti-CD55, and anti-CD59 antibody incubation dropped to background levels when using heat-inactivated serum (complement depleted) or when using C5-depleted serum (terminal-pathway inactive). A summary of the results can be found in Figures 2b, d, and f.
Neutrophil adhesion to BOECs is complement-mediated. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 over BOECs treated with anti-CD46, anti-CD55, and anti-CD59 antibodies (upper and bottom channel) and exposed to either NHS (bottom channel) or complement inactive serum (HIS, upper channel) showed marked neutrophil adhesion in the presence of complement. Video starts 20 seconds after first neutrophils were observed in channel.
Neutrophil adhesion to BOECs is terminal pathway dependent. Calcein-labeled neutrophils perfused in a Bioflux system at 1 dyne/cm2 in NHS (bottom channel) or C5 depleted serum (upper channel) were exposed over BOECs with anti-CD46, anti-CD55, and anti-CD59 antibody pretreatment (upper and bottom channel). Neutrophil adhesion was reduced when C5 depleted serum was used. Video starts 30 seconds after first neutrophils were observed in channel.
Neutrophils adhering to complement-challenged BOECs initiate the formation of neutrophil-platelet aggregates. BOECs with combined anti-CD46, anti-CD55, and anti-CD59 antibody treatment were exposed to 50% NHS and either calcein-labeled platelets alone (upper channel) or labeled neutrophils and platelets together (lower channel). Perfusion of both resulted in formation of neutrophil-platelet-aggregates. Video sequence start 90 seconds after first neutrophils/platelets were observed in channel.