Abstract
Introduction
Limited studies have evaluated risk of stroke associated with the use of NSAIDs in patients with end-stage kidney disease. We examined the adverse effects of selective and nonselective NSAID use on the risk of stroke in dialysis patients.
Methods
A case-crossover study was conducted using medical claims data from the National Health Insurance Research Database in Taiwan. We identified patients with ischemic and hemorrhagic stroke (defined as International Classification of Diseases, 9th revision, Clinical Modification codes 433, 434, and 436 for ischemic stroke and 430 and 431 for hemorrhagic stroke) from inpatient claims during the period from 2003 to 2012. Conditional logistic regression models with adjustment for potential confounders were used to determine the effects of NSAID use on stroke.
Results
A total of 1190 dialysis patients with stroke were identified from 2003 to 2012. The results indicate a 1.31-fold increased risk of stroke related to NSAID use during the 30 days prior to a stroke (AOR = 1.31; 95% CI: 1.03–1.66); likewise, an excessive risk of ischemic stroke was observed (AOR = 1.34; 95% CI: 1.02–1.77). When classifying NSAIDs into selective and nonselective groups, nonselective NSAID use was significantly associated with an increased risk of stroke (AOR = 1.27; 95% CI: 1.00–1.61).
Discussion
In summary, the results show supportive evidence that NSAID use increased the risk of stroke in dialysis patients, which suggests the importance of closely monitoring the transient effects of initial NSAID treatment to patients on dialysis.
Keywords: dialysis, nonsteriodal anti-inflammatory drugs, stroke
During the past few decades, nonsteroidal anti-inflammatory drugs (NSAIDs) have been extensively used worldwide for managing fever, inflammation, and pain.1 However, previous studies have indicated that use of individual NSAIDs was associated with an elevated risk of cerebrovascular events.2, 3, 4 As such, substantial concern has turned to the cerebrovascular safety related to NSAID use. In particular, current clinical guidelines discourage the use of NSAIDs in patients with previous history of cerebrovascular disease as well as those at high risk for cerebrovascular harm.5
Patients on dialysis are a susceptible population for cerebrovascular disease. For example, Toyoda et al.6, 7 provided supportive evidence that stroke is common in patients with end-stage kidney disease who are undergoing hemodialysis or peritoneal dialysis). Seliger et al.8 has reported that incidence of stroke (both ischemic and hemorrhagic stroke) in patients on dialysis is particularly higher than that in the general population. However, whereas some attention has been given to the safety of NSAID use in patients with renal function issue,9, 10 whether NSAIDs use exerts an effect on elevated risk of stroke in dialysis patients remains largely unclear.
To determine the effect of NSAID use on risk of stroke in dialysis patients, we carried out a case-crossover study using nationwide medical claims data in Taiwan to evaluate the associations between NSAID use and risk of stroke (either ischemic or hemorrhagic) in dialysis patients.
Materials and Methods
Data Source
The data used in this study were derived from the Longitudinal Health Insurance Database, which comprises the reimbursement medical claims data of Taiwan’s National Health Insurance Program, which contains demographic characteristics, disease diagnoses, ambulatory care and inpatient claims data, and prescription records from National Health Insurance Program enrollees. Of note, the Longitudinal Health Insurance Database was constructed by randomly selecting 1 million enrollees from the Registry for Beneficiaries of the National Health Insurance Program in 2000, 2005, and 2010, respectively. Thus, a total of 3 million enrollees were included in this study. All medical claims data from 1 January 2000 to 31 December 2012 were included as the data source. The study protocol was approved by the Institutional Review Board of the National Health Research Institutes, Taiwan.
Study Subjects
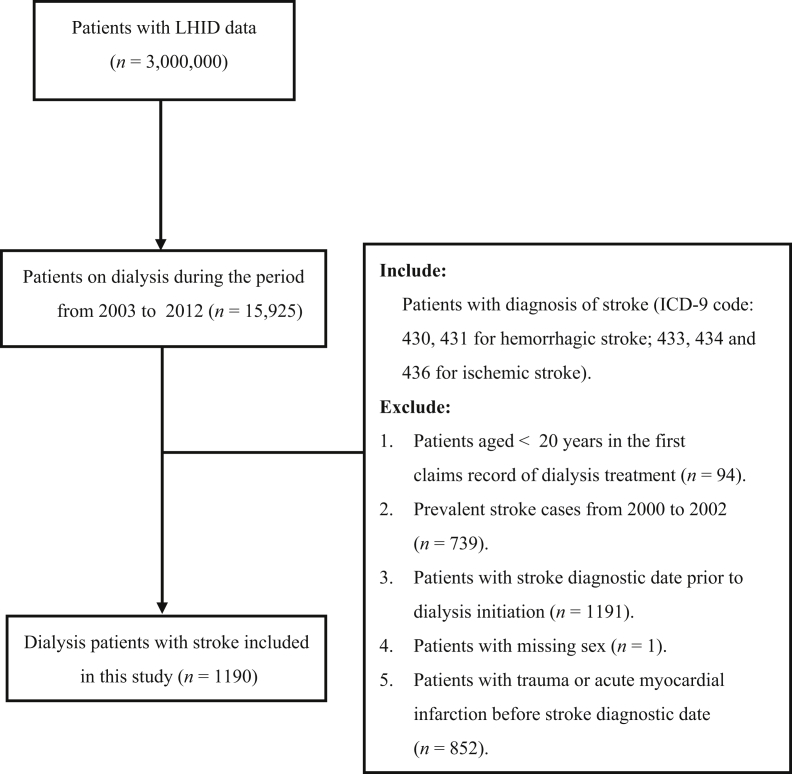
We first identified dialysis patients; that is, patients with a primary diagnosis of end-stage renal disease under International Classification of Diseases, 9th revision, Clinical Modification code 585 and who receive treatment using hemodialysis, peritoneal dialysis, or hemofiltration. Among those patients, we further identified study participants as patients with a hospitalization record for a primary diagnosis of a stroke event (International Classification of Diseases, 9th revision, Clinical Modification codes 433, 434, and 436 for ischemic stroke and 430 and 431 for hemorrhagic stroke). The index date was defined as the date that the participant was diagnosed as having a hospitalized medical record of a stroke. Patents were excluded based on the following criteria: (i) age <20 years in the first claims record of dialysis during the study period; (ii) previous inpatient admissions or outpatient visits for stroke during the period from 2000 to 2002; (iii) diagnosis of stroke prior to initiation of initiation; (iv) sex data were missing; and (v) previous diagnosis of trauma or acute myocardial infarction before the diagnosis of stroke. As a result, a total of 1190 dialysis patients, who also had incident cases of stroke from 2003 to 2012, were identified and included for further analyses in this study. Figure 1 presents the detailed flow chart regarding inclusions and exclusions of the study participants.
Figure 1.
Flow diagram of inclusion/exclusion criteria for study population. ICD-9, International Classification of Diseases, 9th revision; LHID, Longitudinal Health Insurance Database.
Exposure to NSAIDs
We obtained information on NSAID exposure according to prescription records in the National Health Insurance Research Database (NHIRD). Each prescription record in the NHIRD contains the types of prescribed drugs, time of prescription, and duration of drug supply and dosage. We identified all prescription records for NSAIDs based on the Anatomic Therapeutic Chemical code M01A, which was developed by the World Health Organization Collaborating Centre.11 In detail, the following NSAIDs were investigated in this study: (i) selective cyclooxygenase 2 (COX-2) inhibitors: celecoxib and etoricoxib; and (ii) nonselective NSAIDs: salicylates, propionic acid derivatives, acetic acid derivatives, enolic acid derivatives, and anthranilic acid derivatives. In addition, the route of administration (oral or parenteral) for NSAID exposure was also examined in this study (Supplementary Table S1).
Covariates
The following time-varying covariates were included and adjusted for in the subsequent analyses: (i) concomitant medication use related to stroke, such as antihypertensive agents (β-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, calcium-channel blockers, and loop diuretics), antidiabetic agents (insulin, sulfonylurea, thiazolidinediones, and glinides), statins, and anticoagulants (except for aspirin);12 and (ii) health care utilization, which was calculated as the number of outpatient visits during the case and control periods, respectively.13
Data Analysis
We defined the case period as 1 to 30 days prior to the index date and the control period as 91 to 120 days prior to the index date for each study participant. This same definition for the case and control periods was used in previous studies regarding pharmacological properties of NSAIDs.12, 14 In this study, we applied a population-based, case-crossover study design to determine the relationship between NSAID use and the risk of stroke, including ischemic and hemorrhagic stroke among dialysis patients. We performed conditional logistic regression models to compare the effect of NSAID use on stroke between case and control periods. Crude and adjusted odd ratios (ORs) were computed, with and without adjustment for the above-mentioned factors of time-varying medications and health care utilization. In addition to overall NSAID use, further analyses were conducted for the use of selective, nonselective, and each individual NSAID, respectively, and for the route of administration (oral or parenteral) for NSAIDs, with and without adjustment for covariates.
We also carried out subgroup analyses to examine the modifying effects of various characteristics, including age, sex, Charlson comorbidity index (CCI) score,15 heart disease, type 2 diabetes mellitus, and anticoagulant use. Moreover, sensitivity analyses were applied to test the robustness of the results. That is, we repeated the analyses using 3 alternative time windows: (i) 1 to 15 days before the index date as the case period and 31 to 45 days before the index date as the control period; (ii) 1 to 30 days before the index date as the case period and 61 to 90 days before the index date as the control period; and (iii) 1 to 30 days as the case period and 121 to 150 days as the control period. We declared statistical significance using a P value <0.05. All of the analyses were performed using SAS version 9.2 for Windows (SAS Institute, Cary, NC).
Results
We identified a total of 1190 dialysis patients who were hospitalized for incident ischemic or hemorrhagic stroke during the period from 2003 to 2012. The mean age at the onset of stroke was 62.9 ± 12.2 years, and 51.3% of the study subjects were women. Among them, 78.6% of dialysis patients were hospitalized for ischemic stroke and 21.4% were hospitalized for hemorrhagic stroke. Detailed information regarding demographic characteristics, concomitant medication use CCI score, and health care utilization are reported in Table 1.
Table 1.
Demographic and clinical characteristics of the study subjects
| Characteristics | n | % |
|---|---|---|
| Demographics | ||
| Age group (yr) | ||
| 20–64 | 613 | 51.51 |
| ≥65 | 577 | 48.49 |
| Sex | ||
| Women | 611 | 51.34 |
| Men | 579 | 48.66 |
| Concomitant medication | ||
| Antihypertensives | 216 | 18.15 |
| Statins | 293 | 24.62 |
| Insulin | 357 | 30.00 |
| Sulfonylurea | 245 | 20.59 |
| Thiazolidinediones | 76 | 6.39 |
| Glinides | 186 | 15.63 |
| β-blockers | 599 | 50.34 |
| ACE-I/ARB | 544 | 45.71 |
| Calcium-channel blockers | 776 | 65.21 |
| Loop diuretics | 370 | 31.09 |
| Vitamin K antagonists | 47 | 3.95 |
| Nonaspirin antiplatelet agents | 385 | 32.35 |
| Low-dose aspirin | 0 | 0 |
| Anticoagulants | 595 | 50.00 |
| Charlson Comorbidity Index score | ||
| 1–3 | 338 | 28.40 |
| ≥4 | 852 | 71.60 |
| Health care use during 1 year before stroke | ||
| No. of outpatient visits | ||
| 0 | 2 | 0.17 |
| 1–26 | 609 | 51.18 |
| ≥27 | 579 | 48.66 |
| No. of inpatient visits | ||
| 0 | 499 | 41.93 |
| 1 | 293 | 24.62 |
| ≥2 | 398 | 33.45 |
| Dialysis modality | ||
| HD | 1110 | 93.28 |
| PD | 80 | 6.72 |
| Dialysis duration prior to stroke | Mean ± SD | |
| HD | 1110 | 1079.15 ± 926.11 |
| PD | 80 | 1115.15 ± 879.89 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HD, hemodialysis; PD, peritoneal dialysis.
Table 2 shows the association of NSAID use with the risk of overall stroke, ischemic stroke, and hemorrhagic stroke, respectively. The results suggest that overall NSAIDs use during the 30 days prior to the stroke index date was associated with an increased risk of stroke and ischemic stroke, after controlling for confounding factors (adjusted OR [AOR]: 1.31; 95% confidence interval [CI]: 1.03–1.66 for stroke; AOR: 1.34; 95% CI: 1.02–1.77 for ischemic stroke). When classifying overall NSAIDs into selective, nonselective, and individual NSAIDs, positive associations with stroke were found for the use of nonselective NSAIDs (AOR: 1.27; 95% CI: 1.00–1.61), propionic acid (AOR: 2.14; 95% CI: 1.16–3.95), and anthranilic acid (AOR: 1.94; 95% CI: 1.08–3.49), separately. Additionally, a significant association with ischemic stroke was found for the use of propionic acid (AOR: 2.34; 95% CI: 1.14–4.82). We also observed that both oral and parenteral NSAID administration were significantly associated with stroke, but a positive association with ischemic stroke was only found for parenteral NSAID administration (Table 2). Because blood pressure data were not available in the present study, we classified study subjects into 2 groups: subjects with and without hypertension, separately; we repeated the analysis by using patients with and without hypertension, respectively. The results were comparable between patients with and without hypertension (Supplementary Table S2A and 2B).
Table 2.
Risk of stroke, ischemic stroke, and hemorrhagic stroke in relation to NSAID use among patients with renal dialysis
| Case period (1-30 days before index date) |
Control period (91-120 days before index date) |
Crude |
Adjusteda |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | OR | 95% CI | |
| Stroke (n = 1,190) | ||||||||
| NSAIDs overall | 445 | 37.39 | 375 | 31.51 | 1.57b | (1.25–1.97) | 1.31 | (1.03–1.66) |
| Selective | 47 | 3.95 | 41 | 3.45 | 1.26 | (0.73–2.18) | 1.18 | (0.67–2.09) |
| Nonselective | 419 | 35.21 | 353 | 29.66 | 1.53 | (1.22–1.91) | 1.27 | (1.00–1.61) |
| Salicylates | 207 | 17.39 | 192 | 16.13 | 1.24 | (0.89–1.74) | 1.11 | (0.78–1.59) |
| Propionic acid | 51 | 4.29 | 27 | 2.27 | 2.50 | (1.40–4.46) | 2.14 | (1.16–3.95) |
| Acetic acid | 166 | 13.95 | 126 | 10.59 | 1.49 | (1.13–1.98) | 1.16 | (0.86–1.57) |
| Enolic acid | 54 | 4.54 | 41 | 3.45 | 1.46 | (0.91–2.37) | 1.33 | (0.80–2.20) |
| Anthranilic acid | 45 | 3.78 | 21 | 1.76 | 2.33 | (1.34–4.05) | 1.94 | (1.08–3.49) |
| Oral | 414 | 34.79 | 356 | 29.92 | 1.47 | (1.17–1.85) | 1.23 | (0.96–1.56) |
| Parenteral | 76 | 6.39 | 40 | 3.36 | 2.29 | (1.47–3.56) | 1.94 | (1.22–3.06) |
| Ischemic stroke (n = 935) | ||||||||
| NSAIDs overall | 361 | 38.61 | 300 | 32.09 | 1.66 | (1.28–2.15) | 1.34 | (1.02–1.77) |
| Selective | 41 | 4.39 | 37 | 3.96 | 1.20 | (0.66–2.17) | 1.10 | (0.59–2.07) |
| Nonselective | 339 | 36.26 | 282 | 30.16 | 1.59 | (1.24–2.06) | 1.28 | (0.97–1.68) |
| Salicylates | 173 | 18.50 | 157 | 16.79 | 1.31 | (0.91–1.89) | 1.15 | (0.77–1.70) |
| Propionic acid | 40 | 4.28 | 19 | 2.03 | 2.91 | (1.47–5.77) | 2.34 | (1.14–4.82) |
| Acetic acid | 133 | 14.22 | 102 | 10.91 | 1.48 | (1.08–2.04) | 1.13 | (0.80–1.59) |
| Enolic acid | 48 | 5.13 | 35 | 3.74 | 1.57 | (0.93–2.64) | 1.38 | (0.79–2.41) |
| Anthranilic acid | 30 | 3.21 | 17 | 1.82 | 1.87 | (1.00–3.50) | 1.58 | (0.80–3.12) |
| Oral | 338 | 36.15 | 284 | 30.37 | 1.58 | (1.22–2.05) | 1.29 | (0.98–1.70) |
| Parenteral | 60 | 6.42 | 32 | 3.42 | 2.22 | (1.36–3.63) | 1.86 | (1.12–3.11) |
| Hemorrhagic stroke (n = 255) | ||||||||
| NSAIDs overall | 84 | 32.94 | 75 | 29.41 | 1.29 | (0.81–2.06) | 1.15 | (0.69–1.90) |
| Selective | 6 | 2.35 | 4 | 1.57 | 1.67 | (0.40–6.97) | 1.55 | (0.36–6.75) |
| Nonselective | 80 | 31.37 | 71 | 27.84 | 1.31 | (0.81–2.13) | 1.18 | (0.70–2.00) |
| Salicylates | 34 | 13.33 | 35 | 13.73 | 0.91 | (0.39–2.14) | 0.85 | (0.33–2.18) |
| Propionic acid | 11 | 4.31 | 8 | 3.14 | 1.60 | (0.52–4.89) | 1.54 | (0.43–5.48) |
| Acetic acid | 33 | 12.94 | 24 | 9.41 | 1.53 | (0.83–2.82) | 1.27 | (0.66–2.45) |
| Enolic acid | 6 | 2.35 | 6 | 2.35 | 1.00 | (0.29–3.45) | 1.00 | (0.28–3.65) |
| Anthranilic acid | 15 | 5.88 | 4 | 1.57 | 4.67 | (1.34–16.24) | 6.44 | (1.38–29.98) |
| Oral | 76 | 29.80 | 72 | 28.24 | 1.13 | (0.69–1.85) | 1.04 | (0.61–1.77) |
| Parenteral | 16 | 6.27 | 8 | 3.14 | 2.60 | (0.93–7.29) | 1.97 | (0.66–5.88) |
CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio.
Covariates adjusted in the conditional logistic regression models include antihypertensive (β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, and loop diuretics), antidiabetic agents (insulin, sulfonylurea, thiazolidinediones, and glinides), statins, and anticoagulants (except for aspirin) and number of outpatient visits.
P < 0.05 is in bold.
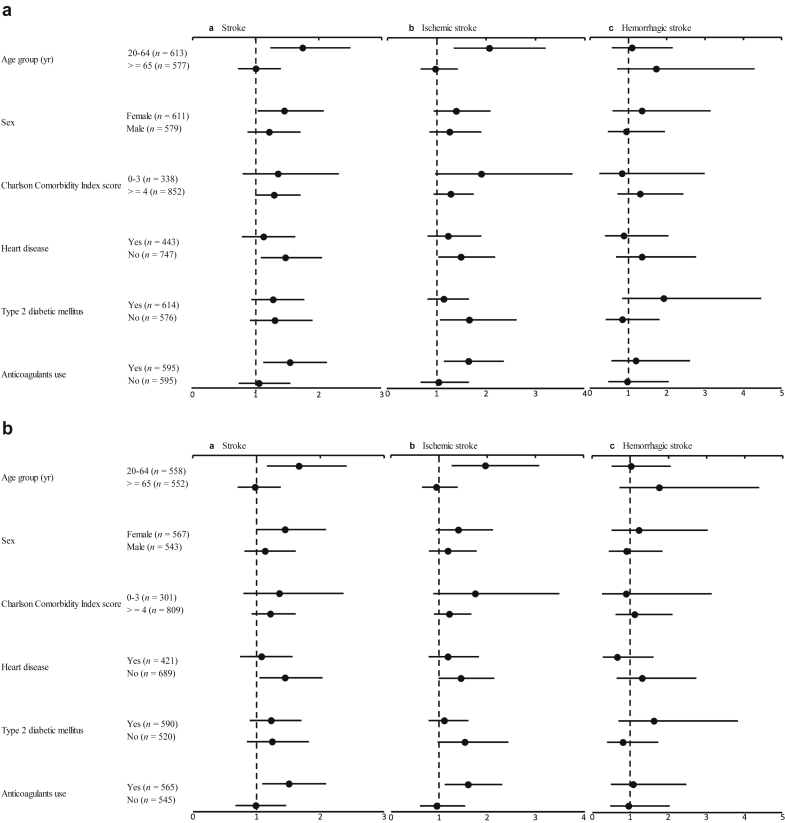
Figure 2a shows the relationships between NSAIDs use and the risks of overall stroke and ischemic and hemorrhagic stroke in dialysis patients, stratified by age, sex, and various clinical conditions. The results indicated that NSAIDs use significantly elevated the risk of stroke and ischemic stroke among patients aged 20 to 64 years, with a CCI score higher than 3, and with anticoagulants use, but no significant interaction was found between NSAIDs use and age, CCI score, or anticoagulants use, individually, was found. Similar results were observed when including patients on peritoneal dialysis (Figure 2b). In addition, when we also examined the interaction of NSAIDs use with age, sex, and various clinical conditions, separately, we did not find any significant interactions. Likely, we examined the age effect by adding an interaction term between age and NSAID use in a regression model but found no interaction.
Figure 2.
Risk of stroke in relation to nonsteroidal anti-inflammatory drug use among patients with (a) renal dialysis and (b) hemodialysis, stratified by age, sex, and various clinical characteristics.
Sensitivity analysis was carried out using different time windows, specifically: 1 to 15 days for the case period and 31 to 45 days for the control period; 1 to 30 days for case period and 61 to 90 days for control period; and 1 to 30 days for case period and 121 to 150 days for control period, separately. No overt changes were found in relation to the associations between NSAID use and increased risks of overall, ischemic, and hemorrhagic stroke across the different time windows (Table 3).
Table 3.
Risk of stroke, ischemic stroke, and hemorrhagic stroke in relation to NSAID use among patients with renal dialysis, based on different lengths for case and control periods
| Case period 1-15 days Control period 31-45 days |
Case period 1-30 days Control period 61-90 days |
Case period 1-30 days Control period 91-120 days |
Case period 1-30 days Control period 121-150 days |
|||||
|---|---|---|---|---|---|---|---|---|
| COR (95% CI) | AORa (95% CI) | COR (95% CI) | AOR (95% CI) | COR (95% CI) | AOR (95% CI) | COR (95% CI) | AOR (95% CI) | |
| Stroke | ||||||||
| NSAIDs overall | 1.65 (1.28–2.11)b | 1.36 (1.04–1.78) | 1.63 (1.29–2.05) | 1.37 (1.07–1.75) | 1.57 (1.25–1.97) | 1.31 (1.03–1.66) | 1.77 (1.42–2.20) | 1.52 (1.20–1.91) |
| Selective | 0.94 (0.46–1.90) | 0.83 (0.40–1.72) | 1.37 (0.76–2.47) | 1.18 (0.64–2.18) | 1.26 (0.73–2.18) | 1.18 (0.67–2.09) | 1.26 (0.76–2.09) | 1.19 (0.70–2.02) |
| Nonselective | 1.70 (1.31–2.19) | 1.39 (1.06–1.84) | 1.65 (1.30–2.08) | 1.37 (1.07–1.76) | 1.53 (1.22–1.91) | 1.27 (1.00–1.61) | 1.79 (1.44–2.24) | 1.52 (1.20–1.92) |
| Salicylates | 1.34 (0.93–1.93) | 1.18 (0.79–1.76) | 1.50 (1.04–2.16) | 1.39 (0.95–2.05) | 1.24 (0.89–1.74) | 1.11 (0.78–1.59) | 1.32 (0.95–1.83) | 1.18 (0.83–1.68) |
| Propionic acid | 2.00 (1.08–3.72) | 1.51 (0.79–2.87) | 1.59 (0.93–2.71) | 1.32 (0.75–2.32) | 2.50 (1.40–4.46) | 2.14 (1.16–3.95) | 1.72 (1.05–2.82) | 1.27 (0.76–2.15) |
| Acetic acid | 2.20 (1.51–3.20) | 1.74 (1.18–2.57) | 1.58 (1.18–2.13) | 1.25 (0.92–1.71) | 1.49 (1.13–1.98) | 1.16 (0.86–1.57) | 1.65 (1.25–2.18) | 1.29 (0.96–1.73) |
| Enolic acid | 1.15 (0.63–2.09) | 1.00 (0.54–1.86) | 1.71 (1.03–2.83) | 1.44 (0.85–2.43) | 1.46 (0.91–2.37) | 1.33 (0.80–2.20) | 1.74 (1.04–2.90) | 1.61 (0.94–2.76) |
| Anthranilic acid | 1.14 (0.64–2.02) | 0.90 (0.49–1.64) | 1.30 (0.81–2.09) | 0.92 (0.56–1.53) | 2.33 (1.34–4.05) | 1.94 (1.08–3.49) | 2.56 (1.44–4.57) | 2.26 (1.23–4.13) |
| Oral | 1.49 (1.15–1.92) | 1.22 (0.93–1.60) | 1.46 (1.15–1.84) | 1.23 (0.96–1.58) | 1.47 (1.17–1.85) | 1.23 (0.96–1.56) | 1.65 (1.32–2.06) | 1.41 (1.11–1.79) |
| Parenteral | 3.69 (2.00–6.81) | 2.79 (1.48–5.26) | 2.76 (1.68–4.55) | 2.22 (1.32–3.74) | 2.29 (1.47–3.56) | 1.94 (1.22–3.06) | 2.65 (1.69–4.17) | 2.28 (1.42–3.67) |
| Ischemic stroke | ||||||||
| NSAIDs overall | 1.93 (1.45–2.57) | 1.52 (1.11–2.08) | 1.71 (1.31–2.23) | 1.40 (1.06–1.86) | 1.66 (1.28–2.15) | 1.34 (1.02–1.77) | 1.79 (1.39–2.30) | 1.52 (1.16–1.98) |
| Selective | 1.17 (0.54–2.52) | 0.97 (0.43–2.19) | 1.53 (0.80–2.94) | 1.29 (0.66–2.53) | 1.20 (0.66–2.17) | 1.10 (0.59–2.07) | 1.38 (0.79–2.42) | 1.27 (0.70–2.31) |
| Nonselective | 1.95 (1.45–2.63) | 1.52 (1.10–2.10) | 1.73 (1.32–2.26) | 1.41 (1.06–1.88) | 1.59 (1.24–2.06) | 1.28 (0.97–1.68) | 1.79 (1.39–2.30) | 1.49 (1.14–1.95) |
| Salicylates | 1.44 (0.95–2.16) | 1.16 (0.74–1.82) | 1.66 (1.11–2.48) | 1.50 (0.97–2.30) | 1.31 (0.91–1.89) | 1.15 (0.77–1.70) | 1.39 (0.97–2.00) | 1.25 (0.85–1.83) |
| Propionic acid | 3.71 (1.61–8.56) | 2.78 (1.17–6.63) | 2.07 (1.09–3.92) | 1.61 (0.82–3.15) | 2.91 (1.47–5.77) | 2.34 (1.14–4.82) | 1.89 (1.07–3.34) | 1.26 (0.69–2.31) |
| Acetic acid | 2.25 (1.48–3.41) | 1.69 (1.09–2.62) | 1.51 (1.09–2.10) | 1.19 (0.84–1.68) | 1.48 (1.08–2.04) | 1.13 (0.80–1.59) | 1.55 (1.13–2.11) | 1.21 (0.87–1.69) |
| Enolic acid | 1.50 (0.76–2.95) | 1.38 (0.68–2.81) | 2.12 (1.19–3.77) | 1.71 (0.94–3.12) | 1.57 (0.93–2.64) | 1.38 (0.79–2.41) | 1.80 (1.04–3.11) | 1.67 (0.94–2.98) |
| Anthranilic acid | 1.06 (0.54–2.10) | 0.77 (0.38–1.59) | 0.96 (0.56–1.67) | 0.67 (0.38–1.21) | 1.87 (1.00–3.50) | 1.58 (0.80–3.12) | 2.45 (1.22–4.95) | 2.40 (1.15–5.04) |
| Oral | 1.73 (1.29–2.32) | 1.37 (0.99–1.88) | 1.55 (1.18–2.02) | 1.29 (0.97–1.72) | 1.58 (1.22–2.05) | 1.29 (0.98–1.70) | 1.66 (1.29–2.15) | 1.41 (1.08–1.85) |
| Parenteral | 4.22 (2.04–8.73) | 3.01 (1.41–6.44) | 2.88 (1.63–5.08) | 2.19 (1.21–3.94) | 2.22 (1.36–3.63) | 1.86 (1.12–3.11) | 2.45 (1.49–4.03) | 2.17 (1.29–3.67) |
| Hemorrhagic stroke | ||||||||
| NSAIDs overall | 0.97 (0.57–1.62) | 0.89 (0.50–1.60) | 1.38 (0.86–2.23) | 1.15 (0.68–1.96) | 1.29 (0.81–2.06) | 1.15 (0.69–1.90) | 1.69 (1.07–2.67) | 1.55 (0.92–2.61) |
| Selective | 0.25 (0.03–2.24) | 0.14 (0.01–1.48) | 0.75 (0.17–3.35) | 0.66 (0.13–3.37) | 1.67 (0.40–6.97) | 1.55 (0.36–6.75) | 0.83 (0.25–2.73) | 0.67 (0.18–2.45) |
| Nonselective | 1.04 (0.61–1.78) | 1.01 (0.55–1.87) | 1.39 (0.86–2.26) | 1.16 (0.67–1.99) | 1.31 (0.81–2.13) | 1.18 (0.70–2.00) | 1.82 (1.13–2.90) | 1.72 (1.00–2.95) |
| Salicylates | 1.00 (0.43–2.31) | 0.97 (0.36–2.61) | 0.90 (0.37–2.22) | 0.87 (0.32–2.33) | 0.91 (0.39–2.14) | 0.85 (0.33–2.18) | 1.00 (0.45–2.23) | 0.97 (0.37–2.55) |
| Propionic acid | 0.50 (0.15–1.66) | 0.47 (0.14–1.62) | 0.75 (0.26–2.16) | 0.52 (0.15–1.84) | 1.60 (0.52–4.89) | 1.54 (0.43–5.48) | 1.29 (0.48–3.45) | 1.08 (0.35–3.31) |
| Acetic acid | 2.00 (0.86–4.67) | 1.91 (0.75–4.88) | 1.92 (0.98–3.76) | 1.49 (0.73–3.07) | 1.53 (0.83–2.82) | 1.27 (0.66–2.45) | 2.14 (1.14–4.04) | 1.99 (0.96–4.09) |
| Enolic acid | 0.33 (0.07–1.65) | 0.26 (0.07–1.37) | 0.71 (0.23–2.25) | 0.59 (0.18–1.97) | 1.00 (0.29–3.45) | 1.00 (0.28–3.65) | 1.33 (0.30–5.96) | 1.39 (0.28–6.80) |
| Anthranilic acid | 1.33 (0.46–3.84) | 1.57 (0.49–5.07) | 3.50 (1.15–10.63) | 3.57 (0.93–13.66) | 4.67 (1.34–16.24) | 6.44 (1.38–29.98) | 2.80 (1.01–7.77) | 2.59 (0.84–7.93) |
| Oral | 0.85 (0.50–1.48) | 0.79 (0.43–1.45) | 1.20 (0.74–1.95) | 1.00 (0.58–1.72) | 1.13 (0.69–1.85) | 1.04 (0.61–1.77) | 1.59 (0.98–2.58) | 1.43 (0.84–2.44) |
| Parenteral | 2.50 (0.78–7.96) | 2.28 (0.67–7.69) | 2.40 (0.85–6.81) | 2.08 (0.63–6.85) | 2.60 (0.93–7.29) | 1.97 (0.66–5.88) | 3.75 (1.25–11.30) | 3.46 (0.98–12.24) |
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; NSAID, nonsteroidal anti-inflammatory drug.
Covariates adjusted in the conditional logistic regression models include antihypertensives (β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, and loop diuretics), antidiabetic agents (insulin, sulfonylurea, thiazolidinediones, and glinides), statins, and anticoagulants (except for aspirin), and number of outpatient visits.
P < 0.05 is in bold.
Discussion
The results of this study suggest that the use of NSAIDs significantly increased the risk of stroke among dialysis patients. We found overall NSAID use to be associated with a 31% greater risk of stroke, after adjusting for confounding factors. In addition to overall NSAIDs, the use of nonselective and a certain number of individual nonselective NSAIDs also showed an adverse effect on elevated risk of stroke. A greater risk of stroke was found when delivering NSAIDs parenterally compared with using NSAIDs in oral form.
Several studies have provided suggestive data that NSAID use is positively associated with the risk of stroke,12, 16, 17, 18 but limited studies have been conducted for assessing the cerebrovascular effects of NSAIDs in high-risk populations, especially Asian populations. To the best of our knowledge, the present study is among the few research investigations that has attempted to investigate the risk of stroke associated with selective and nonselective NSAIDs, individually, in dialysis patients.
Our results are in line with previous observational studies conducted in different ethnic populations. For example, in a nested case-control study using a United Kingdom cohort, Andersohn et al.4 examined the association between the use of COX-2 selective NSAIDs and ischemic stroke. Their results showed that the use of a certain kind of individual COX-2 selective NSAIDs, for example, rofecoxib and etoricoxib, increased the risk of ischemic stroke.4 In a network meta-analysis, Trelle et al.19 evaluated the cardiovascular safety of NSAIDs use based on 26 previous trials and concluded that various individual NSAIDs increased the risk of stroke. In contrast, several other studies have reported discrepant results.20, 21, 22 However, the inconsistent results reported from different studies might be likely due to different enrollment criteria, study designs, or small sample sizes.
The findings of the present study indicated that parenteral administration of NSAIDs led to a greater risk of stroke, especially ischemic stroke, than did the oral use of NSAIDs. Our results suggested that parenteral NSAIDs may cause detrimental effects on hemodynamic function in the brain, most likely due to a rapid transit of a high concentration of NSAIDs in cerebral circulation. However, to date, biologically plausible mechanisms have not been well studied. It will be of important to further investigate this issue. Despite no additional benefit from using parenteral administration compared with using oral administration,23 detrimental effects related to different routes of NSAIDs administration have barely been examined. Additionally, due to the nature of anticoagulants in use, which could have a significant effect on the risk of stroke and stroke subtypes, we have treated anticoagulant use as a covariate in the analytical models in the present study. Certainly, further investigation of the cerebrovascular safety regarding different routes of NSAIDs administration or effect of anticoagulant use or both would be merited.
In addition to potential effects of NSAID use on stroke, several previous studies have reported controlling and adjusting for potential confounding factors in their analyses including the use of concomitant medications, specifically, antihypertensive agents, antidiabetic agents, statins, and anticoagulants. For example, Tziomalos et al.24 investigated the effects of the use of various classes of antihypertensive agents on acute ischemic stroke and found a reverse association between severe ischemic stroke and diuretic use, but not for angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium-channel blockers. In addition, Yoshii et al.25 examined the effects of pioglitazone treatment on primary cardiovascular events in a Japanese population. They reported no association between 2 years of pioglitazone treatment and a reduction of primary cardiovascular events.
In addition to adjusting for concomitant medication in the models, we also performed subgroup analyses to investigate the effect of age, sex, CCI score, heart disease, type 2 diabetes, and anticoagulant use, respectively. In our subgroup analyses, we observed a higher stroke risk with NSAID use among subjects aged 20 to 64 years than in subjects aged >65 years. We further examined the age effect by adding an interaction term between age and NSAID use in a regression model but found no interaction. Whether there is an age-related modifiable effect on the association between NSAID use and risk of stroke warrants further investigation. In addition, we observed an elevated risk of stroke related to NSAID use in subjects with higher CCI scores (≧4) and with anticoagulant use, respectively. Because subjects with higher CCI scores or who are on anticoagulant treatment are vulnerable populations for stroke, caution should be taken when delivering NSAIDs to these high-risk groups. Of note, when we included patients with previous acute myocardial infarction history and repeated the analyses. Similar results were found (Supplementary Table S3).
The observed adverse effects of NSAIDs use on stroke and ischemic stroke in dialysis patients could possibly be elucidated by the following biologically plausible mechanisms. First, studies have suggested that NSAIDs play a role in elevating systemic vascular resistance and decreasing glomerular filtration rate in susceptible populations.26, 27 Hence, it is likely that NSAIDs cause cerebrovascular harm to those on dialysis treatment. Second, it has been documented that NSAIDs inhibit the activity of the COX isozymes, COX-1 and COX-2, and have influence on decreasing total renal perfusion.28 As a result, NSAIDs may cause changes in renal blood flow, worsen edema, and increase blood pressure.29, 30 Third, previous reports have demonstrated that COX-2 plays a role in modulating harmful actions of angiotensin II, consequently affecting the regulation of renal function and blood pressure.31, 32 Fourth, nonselective NSAIDs, acting as a COX-1 inhibitor, could inhibit thromboxane A2 synthesized in platelets and consequently interfere with platelet aggregation and thrombosis formation, resulting in increasing risk of bleeding tendency.33, 34, 35 On the other hand, both nonselective and selective NSAIDs, could also act on vascular endothelial cells to inhibit prostacyclin I2 production, induce vasoconstriction, and interfere with platelet aggregation.36 Therefore, NSAIDs use may also increase incidence of ischemic cerebrovascular events. Furthermore, the manifestations in diverse effects of NSAIDs depend on different pharmacokinetic characteristics of individual agents. Both hemorrhagic and ischemic strokes could thus be increased if NSAIDs are unguardedly taken by patients with dialysis, a high-risk group vulnerable to hemodynamic instability. Last but not least, several studies have provided strong evidence that NSAIDs or coxibs inhibit the homeostatic function of prostaglandins (including prostaglandin I2, or prostacyclin, and prostaglandin E2) in the kidney and have an impact on adverse renal reactions, which may lead to sodium and potassium retention, nephritis, acute renal failure, and analgesic nephropathy.37, 38, 39
In this study, several limitations should be noted. First, data on a number of potential confounding factors, for example, body mass index, lipid levels, tobacco smoking, and alcohol consumption, are not available in the NHIRD. However, because a case-crossover design was used in this study, these confounding factors were unlikely to vary during the defined case and control periods of the present study. Second, similar to most countries, some NSAIDs are available over the counter without a prescription in Taiwan. Thus, we were not able to evaluate the effects of over-the-counter NSAID use. However, this issue might be undifferentiated; subsequently, this would reduce the estimated risk and such bias would drag the observed results toward the null. Third, due to the constraints of a small sample size of individual NSAIDs examined in this study, we did not have sufficient power to examine the dose response of NSAID use. It would be important to further investigate this issue when the sample size allows. Fourth, we could only evaluate the transient effects of NSAID use due to the nature of a case-crossover study. Additional investigation will also be needed to scrutinize the long-term effects of NSAID use on stroke. Fifth, this is among the few studies to determine the adverse effects of NSAID use on stroke in dialysis patients, a population at high-risk for cerebrovascular events. Future studies will be merited to confirm the results from our study. Sixth, in the present study, blood pressure data are not available in the NHIRD. Thus, we performed analyses in patients with and without hypertension, separately. The results were comparable between patients with and without hypertension (Supplementary Table 2A and 2B). However, it is likely that the observed increased risk with NSAID use might still be partially explained by the unmeasured blood pressure, thus the results presented in this study should be interpreted with caution. Likewise, no laboratory data for C-reactive protein, albumin, hemoglobin, or erythropoiesis-stimulating agent use were available in the NHIRD, thus the effect of laboratory indices could not be evaluated in this study. Further investigation on these unmeasured factors could be important. Seventh, we applied a case-crossover design to investigate only the short-term effects of NSAID use on stroke among dialysis patients, thus, we were not able to associate long-term effects of NSAIDs and stroke in this study.
In conclusion, NSAID use was associated with an excessive risk of stroke among dialysis patients. Although it is not practical to suggest that physicians entirely avoid prescribing NSAIDs in clinical practice, even to high-risk patients, NSAIDs should be prescribed with caution and NSAID use should be based on careful clinical evaluations of benefits and risks, especially in predisposed populations.
Disclosure
All the authors declared no competing interests.
Acknowledgments
H-JT and C-CH are supported in part by grants from the National Health Research Institutes (PH-104-PP-14, PH-104-SP-05, PH-104-SP-16, PH-105-SP-05, and PH-105-SP-02 to H-JT; PH-104-SP-08 to C-CH); C-CH is also supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST 103-2314-B-400-008). We thank Tami R. Bartell at Ann and Robert H. Lurie Children's Hospital of Chicago, Stanley Manne Children’s Research Institute for English editing. This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration of the Ministry of Health and Welfare, Taiwan, and managed by the National Health Research Institutes, Taiwan (Registered numbers: 99081, 99136, 99287, 101014, NHRID-101-548, NHIRD-103-042). The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, the Ministry of Health and Welfare or National Health Research Institutes.
Footnotes
Table S1. List of different classes of selective and nonselective nonsteroidal anti-inflammatory drugs, respectively.
Table S2A. Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis and hypertension.
Table S2B. Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis, but without hypertension.
Table S3. Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs in patients with renal dialysis including patients with previous acute myocardial infarction history.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
List of different classes of selective and nonselective nonsteroidal anti-inflammatory drugs, respectively.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis and hypertension.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis, but without hypertension.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs in patients with renal dialysis including patients with previous acute myocardial infarction history.
References
- 1.Patrono C., Baigent C. Nonsteroidal anti-inflammatory drugs and the heart. Circulation. 2014;129:907–916. doi: 10.1161/CIRCULATIONAHA.113.004480. [DOI] [PubMed] [Google Scholar]
- 2.Solomon S.D., McMurray J.J., Pfeffer M.A., for the Adenoma Prevention with Celecoxib Study I Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 3.Bresalier R.S., Sandler R.S., Quan H., for the Adenomatous Polyp Prevention on Vioxx Trial Investigators Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 4.Andersohn F., Schade R., Suissa S., Garbe E. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs and the risk of ischemic stroke: a nested case-control study. Stroke. 2006;37:1725–1730. doi: 10.1161/01.STR.0000226642.55207.94. [DOI] [PubMed] [Google Scholar]
- 5.Antman E.M., Bennett J.S., Daugherty A., for the American Heart Association Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 6.Toyoda K., Fujii K., Ando T. Incidence, etiology, and outcome of stroke in patients on continuous ambulatory peritoneal dialysis. Cerebrovasc Dis. 2004;17:98–105. doi: 10.1159/000075776. [DOI] [PubMed] [Google Scholar]
- 7.Toyoda K., Fujii K., Fujimi S. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis. 2005;45:1058–1066. doi: 10.1053/j.ajkd.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Seliger S.L., Gillen D.L., Longstreth W.T., Jr. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen S.L., Fosbol E.L., Kamper A.L. Use of nonsteroidal anti-inflammatory drugs prior to chronic renal replacement therapy initiation: a nationwide study. Pharmacoepidemiol Drug Saf. 2012;21:428–434. doi: 10.1002/pds.3227. [DOI] [PubMed] [Google Scholar]
- 10.Hsu C.C., Wang H., Hsu Y.H. Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: Nationwide Longitudinal Cohort Study. Hypertension. 2015;66:524–533. doi: 10.1161/HYPERTENSIONAHA.114.05105. [DOI] [PubMed] [Google Scholar]
- 11.Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: World Health Organization, 2009.
- 12.Chang C.H., Shau W.Y., Kuo C.W. Increased risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study. Stroke. 2010;41:1884–1890. doi: 10.1161/STROKEAHA.110.585828. [DOI] [PubMed] [Google Scholar]
- 13.Chuang S.Y., Yu Y., Sheu W.H. Association of short-term use of nonsteroidal anti-inflammatory drugs with stroke in patients with hypertension. Stroke. 2015;46:996–1003. doi: 10.1161/STROKEAHA.114.007932. [DOI] [PubMed] [Google Scholar]
- 14.Gislason G.H., Jacobsen S., Rasmussen J.N. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113:2906–2913. doi: 10.1161/CIRCULATIONAHA.106.616219. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Haag M.D., Bos M.J., Hofman A. Cyclooxygenase selectivity of nonsteroidal anti-inflammatory drugs and risk of stroke. Arch Intern Med. 2008;168:1219–1224. doi: 10.1001/archinte.168.11.1219. [DOI] [PubMed] [Google Scholar]
- 17.Fosbol E.L., Olsen A.M., Olesen J.B. Use of nonsteroidal anti-inflammatory drugs among healthy people and specific cerebrovascular safety. Int J Stroke. 2014;9:943–945. doi: 10.1111/j.1747-4949.2012.00863.x. [DOI] [PubMed] [Google Scholar]
- 18.Caughey G.E., Roughead E.E., Pratt N. Stroke risk and NSAIDs: an Australian population-based study. Med J Aust. 2011;195:525–529. doi: 10.5694/mja11.10055. [DOI] [PubMed] [Google Scholar]
- 19.Trelle S., Reichenbach S., Wandel S. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bak S., Andersen M., Tsiropoulos I. Risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nested case-control study. Stroke. 2003;34:379–386. [PubMed] [Google Scholar]
- 21.Choi N.K., Park B.J., Jeong S.W. Nonaspirin nonsteroidal anti-inflammatory drugs and hemorrhagic stroke risk: the Acute Brain Bleeding Analysis study. Stroke. 2008;39:845–849. doi: 10.1161/STROKEAHA.107.497040. [DOI] [PubMed] [Google Scholar]
- 22.Mangoni A.A., Woodman R.J., Gilbert A.L., Knights K.M. Use of non-steroidal anti-inflammatory drugs and risk of ischemic and hemorrhagic stroke in the Australian veteran community. Pharmacoepidemiol Drug Saf. 2010;19:490–498. doi: 10.1002/pds.1945. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz N.A., Turturro M.A., Istvan D.J., Larkin G.L. Patients' perceptions of route of nonsteroidal anti-inflammatory drug administration and its effect on analgesia. Acad Emerg Med. 2000;7:857–861. doi: 10.1111/j.1553-2712.2000.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 24.Tziomalos K., Giampatzis V., Bouziana S.D. Effects of different classes of antihypertensive agents on the outcome of acute ischemic stroke. J Clin Hypertens (Greenwich) 2015;17:275–280. doi: 10.1111/jch.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshii H., Onuma T., Yamazaki T., for the PROFIT-J Study Group Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: the PROFIT-J study. J Atheroscler Thromb. 2014;21:563–573. [PubMed] [Google Scholar]
- 26.Whelton A., Fort J.G., Puma J.A., SUCCESS VI Study Group Cyclooxygenase-2—specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther. 2001;8:85–95. doi: 10.1097/00045391-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Amer M., Bead V.R., Bathon J. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204–212. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- 28.Brater D.C., Harris C., Redfern J.S., Gertz B.J. Renal effects of COX-2-selective inhibitors. Am J Nephrol. 2001;21:1–15. doi: 10.1159/000046212. [DOI] [PubMed] [Google Scholar]
- 29.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Whelton A., White W.B., Bello A.E., for the SUCCESS-VII Investigators Effects of celecoxib and rofecoxib on blood pressure and edema in patients > or =65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol. 2002;90:959–963. doi: 10.1016/s0002-9149(02)02661-9. [DOI] [PubMed] [Google Scholar]
- 31.Jaimes E.A., Hua P., Tian R.X., Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298:F125–F132. doi: 10.1152/ajprenal.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green T., Gonzalez A.A., Mitchell K.D., Navar L.G. The complex interplay between cyclooxygenase-2 and angiotensin II in regulating kidney function. Curr Opin Nephrol Hypertens. 2012;21:7–14. doi: 10.1097/MNH.0b013e32834d9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia Rodriguez L.A., Varas C., Patrono C. Differential effects of aspirin and non-aspirin nonsteroidal antiinflammatory drugs in the primary prevention of myocardial infarction in postmenopausal women. Epidemiology. 2000;11:382–387. doi: 10.1097/00001648-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Catella-Lawson F., Crofford L.J. Cyclooxygenase inhibition and thrombogenicity. Am J Med. 2001;110(suppl 3A):28S–32S. doi: 10.1016/s0002-9343(00)00683-5. [DOI] [PubMed] [Google Scholar]
- 36.Caughey G.E., Cleland L.G., Penglis P.S., Gamble J.R., James M.J. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 37.Garella S., Matarese R.A. Renal effects of prostaglandins and clinical adverse effects of nonsteroidal anti-inflammatory agents. Medicine. 1984;63:165–181. doi: 10.1097/00005792-198405000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Brater D.C. Effects of nonsteroidal anti-inflammatory drugs on renal function: focus on cyclooxygenase-2-selective inhibition. Am J Med. 1999;107:65S–70S. doi: 10.1016/s0002-9343(99)00369-1. discussion 70S–71S. [DOI] [PubMed] [Google Scholar]
- 39.Brater D.C. Anti-inflammatory agents and renal function. Semin Arthritis Rheum. 2002;32(suppl 1):33–42. doi: 10.1053/sarh.2002.37216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of different classes of selective and nonselective nonsteroidal anti-inflammatory drugs, respectively.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis and hypertension.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs among patients with renal dialysis, but without hypertension.
Risk of stroke, ischemic stroke, and hemorrhagic stroke associated with current use of selective, nonselective, and individual nonsteroidal anti-inflammatory drugs in patients with renal dialysis including patients with previous acute myocardial infarction history.