Abstract
Introduction
Copeptin is increasingly used in epidemiological studies as a substitute for vasopressin. The effect of renal function per se on copeptin and vasopressin concentrations as well as their ratio have, however, not been well described.
Methods
Copeptin and vasopressin levels were measured in 127 patients with various stages of chronic kidney disease, including 42 hemodialysis patients and 16 healthy participants in this observational study. Linear (segmental) regression analyses were performed to assess the association between renal function and copeptin, vasopressin and the C/V ratio. In addition, clearance of copeptin and vasopressin by hemodialysis was calculated.
Results
Both copeptin and vasopressin levels were higher when renal function was lower, and both showed associations with plasma osmolality. The C/V ratio was stable across renal function in subjects with an eGFR >28 ml/min per 1.73 m2. In contrast, the C/V ratio increased with worsening renal function in patients with eGFR ≤28 ml/min per 1.73 m2. During hemodialysis, the initial decrease in vasopressin levels was greater compared with copeptin and, consequently, the C/V ratio increased. This was, at least in part, explained by a greater dialytic clearance of vasopressin compared with copeptin.
Discussion
Our data indicate that copeptin is a reliable substitute for vasopressin in subjects with an eGFR >28 ml/min per 1.73 m2, whereas at an eGFR ≤28 ml/min per 1.73 m2, that is, CKD stages 4 and 5, a correction for renal function is required in epidemiological studies that use copeptin as a marker for vasopressin. Intradialytic copeptin levels do not adequately reflect vasopressin levels because vasopressin clearance by hemodialysis is higher than that of copeptin.
Keywords: chronic kidney disease, copeptin, hemodialysis, kidney function, vasopressin
Arginine vasopressin is essential for maintaining fluid homeostasis in the human body. Vasopressin is released from the posterior pituitary gland in response to hyperosmolality, hypotension, and hypovolemia.1 This hormone regulates water balance via V2 receptor–mediated renal water reabsorption and increases blood pressure via V1 receptor–mediated vasoconstriction. In addition to these important physiological effects, evidence is emerging that vasopressin also has deleterious effects and may play a role in the progression of renal disease.2 Experimental data showed that desmopressin, a selective V2 receptor agonist, induced transient proteinuria3, 4 and worsened progression of CKD.3
Copeptin, a fragment of the vasopressin precursor preprovasopressin, is used as a substitute for estimating vasopressin levels because it is easier to measure and allegedly more stable ex vivo.5, 6, 7, 8 When preprovasopressin is split in the pituitary gland, copeptin and vasopressin are secreted in equimolar amounts into the circulation.5, 8, 9 Several studies have investigated the reliability of copeptin as a marker for vasopressin and have demonstrated a strong correlation between plasma vasopressin and copeptin levels in healthy individuals5, 7, 10, 11 as well as in critically ill patients.5, 12, 13 In addition, it has been shown that the plasma concentration of both peptides responds similarly to changes in fluid status and plasma osmolality.6, 14, 15 Given the recent interest in the effects of vasopressin on kidney health, measurement of copeptin in epidemiological studies gains popularity.16, 17
Vasopressin as well as copeptin are small-sized molecules (1 and 5 kDa, respectively),5, 18 and both are therefore theoretically subjected to renal clearance. As yet, it is not clear whether renal function affects both analytes to the same extent and therefore whether copeptin can be used as marker for vasopressin in patients with impaired renal function. Several studies found an inverse association between copeptin and renal function.10, 19, 20, 21, 22, 23 However, concurrent measurement of vasopressin is pivotal to understand whether increased copeptin levels accurately reflect vasopressin levels in patients with chronic kidney disease (CKD).24 In case copeptin and vasopressin are cleared similarly, the copeptin-to-vasopressin ratio (C/V ratio) is expected to be stable across the full range of kidney function.
In hemodialysis patients, differential extracorporal clearance could also result in divergent plasma copeptin and vasopressin levels, because copeptin is larger than vasopressin and may therefore be cleared less by the artificial kidney. However, copeptin removal by hemodialysis has not yet been studied.
Because copeptin is used as a surrogate for vasopressin in patients with decreased renal function while it is unknown whether renal function affects copeptin and vasopressin concentrations in a similar fashion, we investigated in the present study the association of copeptin and vasopressin in subjects over a broad range of renal function as well as the kinetics of these analytes during hemodialysis.
Methods
Participants and Study Protocol
For this study, samples were used of subjects that participated in 4 studies performed at the Nephrology Department of the University Medical Center Groningen between March 1, 2010 and March 1, 2016.24 The cohort was established from patients with either autosomal dominant polycystic kidney disease or IgA nephropathy with normal and impaired renal function from whom plasma vasopressin and copeptin levels were available and in hemodialysis patients participating in a study on vasopressin. In these studies, blood samples were drawn according to a strict protocol to allow reliable measurement of plasma copeptin and vasopressin levels after completion. Blood was collected in chilled ethylenediamine tetraacetic acid tubes, immediately centrifuged in a cooled centrifuge at 4 °C, and the plasma supernatant was stored frozen at −80 °C in plastic 2 ml aliquots until measurement that took place within 1 year of storage. Samples were available of healthy subjects (n = 15), CKD patients with either autosomal dominant polycystic kidney disease (n = 54) or IgA nephropathy (n = 16), and hemodialysis patients (n = 42). All participants gave written informed consent. The studies were performed in accordance with the principles of the Declaration of Helsinki and approved by the Medical Ethical Committee of the University Medical Center Groningen.
Outcome and Measurements
The primary outcome in the present study is the C/V ratio across different stages of renal function and its association with estimated glomerular filtration rate (eGFR). Vasopressin was measured by radioimmunoassay (DRG International Inc., Springfield, NJ) with a lower limit of detection of 0.25 pmol/l and a functional assay sensitivity of 0.5 pmol/l with 2.0 ml of plasma. The interassay and intraassay coefficients of variation were both <7% in the low and high concentration range (i.e., around 4 and 20 pmol/l, respectively). Copeptin was measured by an automated sandwich immunofluorescent assay (CT-proAVP; Thermo Fisher Scientific, B.R.A.H.M.S GmbH, Hennigsdorf, Germany) with a lower limit of detection of 0.9 pmol/l and a functional assay sensitivity of 2 pmol/l. The interassay and intraassay coefficients of variation for copeptin concentrations >15 pmol/l were <5% and 4% respectively.
In healthy subjects and in patients with CKD, GFR was estimated from plasma creatinine concentration, age, and sex with the Chronic Kidney Disease Epidemiology Collaboration equation that expresses GFR indexed for 1.73 m2 body surface area.25 In hemodialysis patients, residual renal function was assumed to be present when diuresis was ≥200 ml/day and GFR was estimated as 0.5 × (24-hour urea clearance + 24-hour creatinine clearance) and similarly expressed per 1.73 m2 body surface area.26 A 24-hour urine sample was used to estimate residual renal function, this urine sample is collected every 4 months after the longest interdialytic interval preceding the first dialysis session of the week (i.e., Monday or Tuesday).
Plasma and urine creatinine was measured with the Roche enzymatic creatinine assay. Plasma and urine urea were measured with the colorimetric method on a Roche Modular analyzer. Plasma sodium was measured with the indirect method of ion-selective electrode (Roche Modular, Mannheim, Germany) and plasma osmolality was measured by freezing-point depression (Osmo Station Osmometer, Kyoto, Japan).
In hemodialysis patients, blood samples for measurement of plasma copeptin and vasopressin were collected from the arterial line at the initiation of hemodialysis and at 30 minutes on dialysis. Clearance of copeptin and vasopressin by hemodialysis was calculated as described previously27 using simultaneously collected efferent dialysate and predialyzer blood. A detailed description is provided as Supplementary Item S1. At the same time, copeptin and vasopressin were also measured in postdialyzer blood samples to evaluate the possible change in plasma concentration over the dialyzer.
Hemodialysis was conducted in a 3-times weekly 4-hour schedule with a low-flux polysulphone dialyzer (F8 or F10, Fresenius Medical Care, Bad Hamburg, Germany). The ultrafiltration volume was set to achieve dry weight at the completion of the hemodialysis session. Specifications of the dialysis treatment are provided as Supplementary Item S2. In a subgroup of 27 hemodialysis patients, blood samples were also collected during dialysis at 60, 120, 180, and 240 minutes (just before the end of the treatment) intradialysis. Intradialytic copeptin and vasopressin concentrations were corrected for hemoconcentration,28 as described in Supplementary Item S3.
Statistical Analysis
Normally distributed variables are presented as means ± SDs, and variables with a skewed distribution as medians and interquartile ranges (IQRs). Categorical data are given as numbers and percentages. For comparison of baseline characteristics, the participants were subdivided into 4 subgroups according to kidney function: group 1: eGFR ≥90 ml/min per 1.73 m2 (n = 16); group 2: eGFR between 45 and 89 ml/min per 1.73 m2 (n = 33); group 3: eGFR <45 ml/min per 1.73 m2 not on hemodialysis (n = 36); group 4: eGFR <15 ml/min per 1.73 m2 on hemodialysis (n = 42). Differences among the 4 study groups were tested for statistical significance with a chi-square test, or 1-way analysis of variance or Kruskal-Wallis test with post hoc testing for multiple comparisons. Univariate and multivariate regression analyses were used to assess associations. Nonnormally distributed variables (i.e., copeptin, vasopressin, and osmolality) were log transformed to fulfill the requirements of normal distribution of the residuals for regression analysis. In the regression analyses, eGFR is used as a continuous variable to be able to perform break-point analyses. Segmented linear regression analysis was used to determine possible breakpoints in the association of renal function with the C/V ratio. Differences in copeptin, vasopressin, and the C/V ratio between hemodialysis patients with and without residual renal function were analyzed using the Wilcoxon signed rank test. This same test was used to analyze copeptin and vasopressin during hemodialysis, with Bonferroni correction for multiple testing, and to study differences in hemodialysis clearance characteristics of copeptin and vasopressin. Spearman correlation was used to assess correlations between copeptin, vasopressin, the C/V ratio, and dialysis vintage. Analyses were performed with SPSS version 22.0 and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). P values of <0.05 (2-tailed) were considered statistically significant.
Results
Participant Characteristics
Table 1 shows the characteristics of the entire study group and the subgroups stratified according to renal function. Age, plasma osmolality, blood pressure, use of antihypertensive medication, and plasma vasopressin and copeptin levels were progressively higher in subgroups with lower renal function (Table 1).
Table 1.
Characteristics of the overall group of participants and for participants stratified according to renal function
| eGFR (ml/min per 1.73 m2) |
|||||
|---|---|---|---|---|---|
| ≥90 (n = 16) |
45-89 (n = 33) |
<45 non-HD (n = 36) |
HD (n = 42) |
P value | |
| Age (yr) | 28 ± 10 | 47 ± 10 | 51 ± 8 | 63 ± 18 | <0.001a |
| Males | 8 (50) | 17 (52) | 19 (53) | 30 (71) | 0.21 |
| eGFR (ml/min per 1.73 m2) | 103 ± 9 | 64 ± 15 | 25 ± 9 | NA | |
| RRF (ml/min per 1.73 m2) | — | — | — | 0.4 (0.0–2.2) | NA |
| Plasma vasopressin (pmol/l) | 0.7 (0.3–1.5) | 1.2 (0.8–2.6) | 2.0 (1.2–3.2) | 5.0 (3.3–6.8) | <0.001b |
| Plasma copeptin (pmol/l) | 6.1 (2.7–8.3) | 9.0 (6.6–18.2) | 25.8 (9.4–36.7) | 170.0 (102.6–280.5) | <0.001b |
| Copeptin-vasopressin ratio | 5.3 (3.5–8.2) | 7.1 (5.1–10.2) | 11.4 (6.7–17.5) | 42.6 (31.3–57.4) | <0.001b |
| Plasma osmolality (mOsm/kg) | 282 (279–284) | 287 (284–290) | 300 (294–303) | 305 (295–310) | <0.001b |
| Volume 24-hour urine (ml) | 2195 ± 936 | 2166 ± 705 | 2489 ± 942 | 336 ± 423 | <0.001a |
| SBP (mm Hg) | 128 ± 10 | 130 ±14 | 132 ± 12 | 143 ± 25 | <0.01a |
| DBP (mm Hg) | 78 ± 9 | 84 ± 19 | 82 ± 9 | 72 ± 13 | <0.01a |
| Medication | |||||
| ACEI or ARB | 4 (25) | 19 (58) | 33 (92) | 8 (19) | <0.001 |
| Beta-blocker | 0 (0) | 3 (9) | 11 (31) | 29 (69) | <0.001 |
| CBB | 0 (0) | 1 (3) | 12 (33) | 13 (31) | <0.01 |
| Diuretic | 0 (0) | 2 (6) | 12 (33) | 10 (24) | <0.01 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HD, hemodialysis; NA, not applicable; RRF, residual renal function; SBP, systolic blood pressure.
Variables are presented as mean ± SD, n (%), or median and (interquartile range).
Analysis of variance with post hoc test Bonferroni: age differed among all 4 groups (P < 0.01) except between patients with an eGFR <45 and 45 to 89 ml/min per 1.73 m2. Volume of 24-hour urine was lower in HD patients compared with the other 3 groups (P < 0.001). SBP was higher in HD patients compared with the 3 other groups (P < 0.05). DBP was lower in HD patients compared with patients with an eGFR between <45 and 45 to 89 (P < 0.05).
Kruskal-Wallis with post hoc test for multiple comparisons: osmolality differed among all 4 groups (P < 0.001), except for participants with an eGFR between 45 to 89 and ≥90 and between patients with an eGFR <45 and on HD. Vasopressin and copeptin levels were higher in HD patients compared with the 3 other groups (P < 0.01) and in the group with an eGFR <45 compared with ≥90 (P < 0.05). The copeptin-vasopressin ratio was higher in HD patients compared with the 3 other groups (P < 0.001).
Copeptin and Vasopressin
Average plasma copeptin and vasopressin concentration in the overall group of participants was 25.9 pmol/l and 2.2 pmol/l, respectively. As shown in Table 1, plasma copeptin and vasopressin levels were significantly higher in subjects with an eGFR <45 compared with subjects with eGFR ≥90 ml/min per 1.73 m2 (P < 0.05) and in patients on dialysis compared with participants not on dialysis (P < 0.001). The C/V ratio in patients on dialysis was significantly higher than for the other 3 groups (P < 0.001), whereas no significant differences were observed among the 3 nondialysis subgroups.
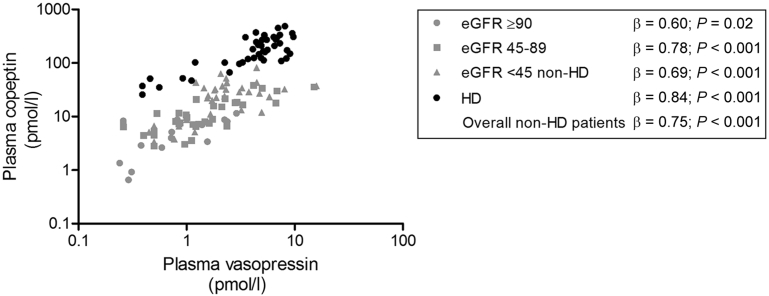
Overall, a strong association was found between copeptin and vasopressin (β = 0.77; P < 0.001). The association between copeptin and vasopressin differed between nondialysis and dialysis patients. “Dialysis treatment” was, therefore, added to the linear regression model, which had indeed a significant effect (β = 0.58; P < 0.001). Therefore, the hemodialysis patient group was analyzed separately. Copeptin and vasopressin remained strongly associated in the nondialysis subjects, including healthy individuals and CKD patients (n = 85, β = 0.75; P < 0.001), as well as in the hemodialysis patients (n = 42, β = 0.84; P < 0.001) (Figure 1).
Figure 1.
Association of plasma copeptin with vasopressin levels. Gray symbols indicate healthy participants and chronic kidney disease patients not on dialysis. Black symbols indicate hemodialysis (HD) patients. eGFR, estimated glomerular filtration rate.
Effect of Renal Function on Copeptin and Vasopressin and Their Ratio in Nondialysis Subjects
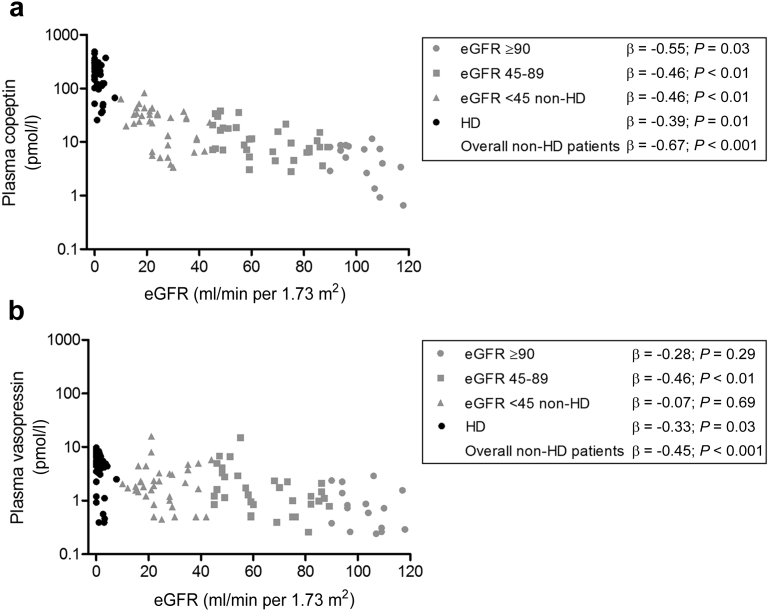
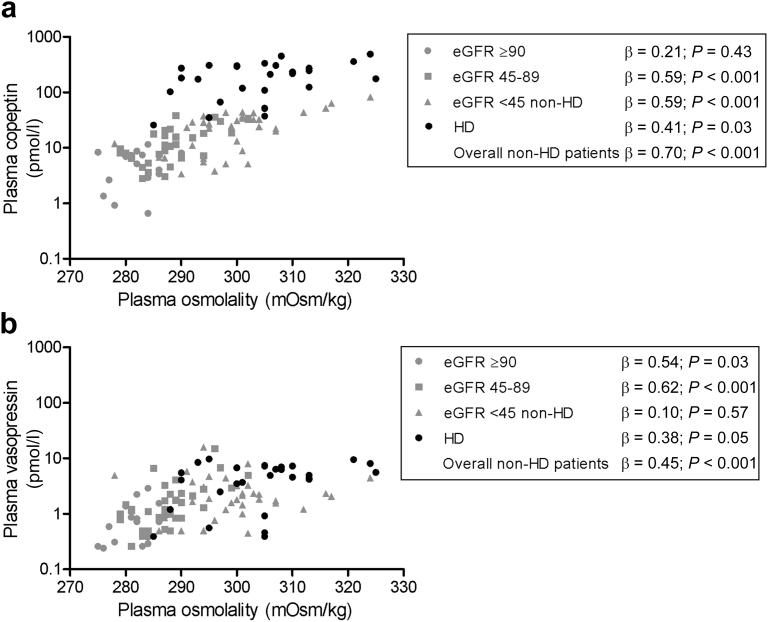
In nondialysis subjects, a significant inverse association between renal function and copeptin was found (Table 2 and Figure 2a). Associations were also observed between renal function and plasma osmolality (β = −0.78; P < 0.001), and between plasma osmolality and copeptin (β = 0.70; P < 0.001) (Figure 3a). To investigate the association of copeptin with renal function per se, this association was therefore stepwise adjusted for plasma osmolality and for other potential confounders (age, sex, and blood pressure). The final multivariate model shows that, after adjustment for these covariates, the association between copeptin and eGFR remained significant (Table 2). Besides eGFR and plasma osmolality, sex also contributed significantly to copeptin concentration (with women having significantly lower values), whereas age and blood pressure did not.
Table 2.
Associations between plasma copeptin and eGFR in healthy participants and CKD patients not on dialysis
| Univariate |
Multivariate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||
| β | P value | β | P value | β | P value | β | P value | |
| eGFR (ml/min per 1.73 m2) | −0.67 | <0.001 | −0.32 | 0.01 | −0.31 | 0.03 | −0.31 | 0.03 |
| Plasma osmolality (mOsm/kg) | 0.70 | <0.001 | 0.45 | <0.001 | 0.40 | <0.01 | 0.40 | <0.01 |
| Age (yr) | 0.48 | <0.001 | 0.04 | 0.68 | 0.04 | 0.70 | ||
| Sexa | −0.32 | <0.01 | −0.22 | <0.01 | −0.22 | <0.01 | ||
| SBP (mm Hg) | 0.13 | 0.24 | 0.02 | 0.84 | ||||
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Model 1: adjustment for plasma osmolality. Model 2: as model 1 + adjustment for age and sex. Model 3: as model 2 + adjustment for systolic blood pressure.
Men are considered the reference group.
Figure 2.
Associations of renal function with (a) copeptin and (b) vasopressin. Gray symbols indicate healthy participants and chronic kidney disease patients not on dialysis. Black symbols indicate hemodialysis (HD) patients. eGFR, estimated glomerular filtration rate.
Figure 3.
Associations of plasma osmolality with (a) copeptin and (b) vasopressin. Gray symbols indicate healthy participants and chronic kidney disease patients not on dialysis. Black symbols indicate hemodialysis (HD) patients. eGFR, estimated glomerular filtration rate.
In univariate analysis, vasopressin was also significantly inversely associated with renal function (Figure 2b) and also an association between plasma osmolality and vasopressin was found (β = 0.45; P < 0.001) (Figure 3b). In contrast to copeptin, the association between vasopressin and renal function was attenuated after adjustment for plasma osmolality, and additional adjustment for age, sex, and blood pressure (Table 3).
Table 3.
Associations between plasma vasopressin and eGFR in healthy participants and CKD patients not on dialysis
| Univariate |
Multivariate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||
| β | P value | β | P value | β | P value | β | P value | |
| eGFR (ml/min per 1.73 m2) | −0.45 | <0.001 | −0.25 | 0.11 | −0.31 | 0.09 | −0.33 | 0.08 |
| Plasma osmolality (mOsm/kg) | 0.45 | <0.001 | 0.26 | 0.10 | 0.23 | 0.15 | 0.21 | 0.18 |
| Age (yr) | 0.27 | 0.01 | −0.08 | 0.58 | −0.11 | 0.42 | ||
| Sexa | −0.16 | 0.14 | −0.10 | 0.33 | −0.10 | 0.29 | ||
| SBP (mm Hg) | 0.24 | 0.03 | 0.18 | 0.07 | ||||
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Model 1: adjustment for plasma osmolality. Model 2: as model 1 + adjustment for age and sex. Model 3: as model 2 + adjustment for systolic blood pressure.
Men are considered the reference group.
As sensitivity analyses, we also added etiology (i.e., autosomal dominant polycystic kidney disease or IgA nephropathy) to the univariate analyses, but did not find an effect of etiology on the association between eGFR and copeptin (β = −0.06; P = 0.41) or on the association between eGFR and vasopressin (β = −0.12; P = 0.24). We also added diuretic use separately to the multivariate analyses because diuretic use might affect vasopressin and copeptin levels independent of an effect on blood pressure and found that it was not significantly associated with copeptin (β = −0.15; P = 0.07) or vasopressin (β = −0.16; P = 0.13).
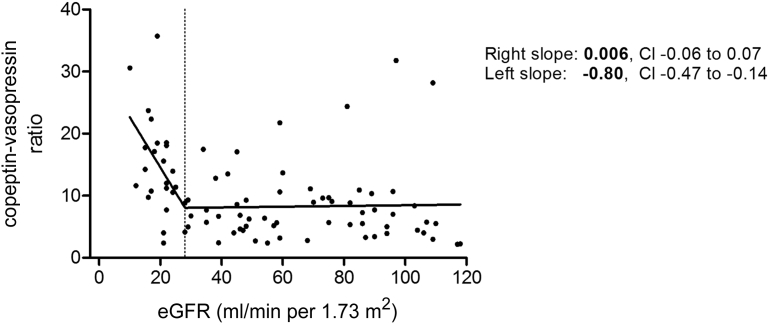
The C/V ratio was inversely associated with renal function (β = −0.36; P = 0.001), indicating that copeptin levels rose to a greater extent compared with vasopressin levels when renal function declined. Break-point analysis of the association between eGFR and the C/V ratio in nondialysis subjects indicated a change in slope at an eGFR of 28 ml/min per 1.73 m2 (Figure 4). Therefore, the association between renal function and C/V ratio was analyzed separately for subjects with an eGFR above and below this threshold. In subjects with an eGFR above 28 ml/min per 1.73 m2 (n = 60), the association between eGFR and C/V ratio was not significant (β = −0.10; P = 0.46). In contrast, in subjects with an eGFR below 28 ml/min per 1.73 m2 and not on dialysis (n = 25), eGFR was inversely associated with the C/V ratio (β = −0.46; P = 0.024). To investigate the separate associations between renal function and copeptin and vasopressin in patients with an eGFR >28 and ≤28 ml/min per 1.73 m2, similar multivariate analyses as performed for the overall group, were also performed for the 2 subgroups identified by break-point analysis (Supplementary Tables S1 and S2). Remarkably, no associations were found between vasopressin and renal function or vasopressin and plasma osmolality in patients with an eGFR ≤28 ml/min per 1.73 m2 (β = −0.14; P = 0.52, and β = 0.14; P = 0.50, respectively). In contrast, in subjects with an eGFR >28 ml/min per 1.73 m2 renal function and plasma osmolality were significantly associated with vasopressin (β = −0.46; P < 0.001, and β = 0.54; P < 0.001, respectively) and also with copeptin (β = −0.57; P < 0.001, and β = 0.57; P < 0.001, respectively). In subjects with an eGFR ≤28 ml/min per 1.73 m2, copeptin, in contrast to vasopressin, was associated with renal function and plasma osmolality (β = −0.56; P = 0.004, and β = 0.60; P < 0.001, respectively).
Figure 4.
Association of renal function with copeptin-vasopressin ratio in healthy participants and in chronic kidney disease patients not on dialysis. The dotted line indicates the break-point at an estimated glomerular filtration rate (eGFR) of 28 ml/min per 1.73 m2. CI, confidence interval.
Hemodialysis Patients
The mean total ultrafiltration volume was 2.65 ± 0.67 L. Twenty of the 42 hemodialysis dialysis patients (48%) had residual renal function. There was no difference in age, sex, plasma osmolality, or systolic blood pressure between patients with and without residual renal function, but patients without residual renal function had a significantly longer dialysis vintage compared with that of patients with residual renal function (Table 4). Patients with residual renal function had significantly lower copeptin and vasopressin levels compared with patients without residual renal function, but the pretreatment C/V ratio did not differ between both groups (Table 4). Of note, there was no significant association between dialysis vintage and plasma copeptin (β = 0.13; P = 0.40), plasma vasopressin (β = 0.07; P = 0.66) or the C/V ratio (β = 0.07; P = 0.66). There was also no correlation between the total ultrafiltration volume and the C/V ratio (r = −0.14, P = 0.38).
Table 4.
Characteristics of hemodialysis patients with and without residual renal function
| HD patients with RRF (n = 20) |
HD patients without RRF (n = 22) |
P value | |
|---|---|---|---|
| Age (yr) | 67 ± 18 | 60 ± 18 | 0.22 |
| Male | 17 (85) | 13 (59) | 0.09 |
| Dialysis vintage (mo) | 17.5 (8.3–27.3) | 37.5 (21.3–65.5) | 0.003 |
| Plasma vasopressin (pmol/l) | 4.2 (1.5–5.1) | 6.0 (4.6–8.2) | <0.01 |
| Plasma copeptin (pmol/l) | 115.9 (55.1–215.7) | 221.7 (148.6–313.8) | 0.01 |
| Copeptin-vasopressin ratio | 42.7 (30.1–59.3) | 40.4 (31.5–57.4) | 0.99 |
| Plasma osmolality (mOsm/kg) | 305 (295–307) | 307 (299–315) | 0.20 |
| SBP (mm Hg) | 145 ± 25 | 142 ± 27 | 0.64 |
HD, hemodialysis; RRF, residual renal function; SBP, systolic blood pressure.
Variables are presented as mean ± SD, n (%), or median and (interquartile range).
Effect of Hemodialysis on Copeptin, Vasopressin, and the C/V Ratio
During hemodialysis, copeptin decreased slightly from 211.0 pmol/l (IQR, 102.4–304.3) to 209.1 pmol/l (IQR, 113.0–312.4) during the first half of the dialysis session (P < 0.01) and increased again during the second half to 247.3 pmol/l (IQR, 87.2–312.0), that was not significantly different compared with predialysis (P = 0.53). Vasopressin followed a similar but more outspoken pattern, with a decrease during the first hours on dialysis (from 4.9 pmol/l [IQR, 2.5–7.3] to 2.5 pmol/l [IQR, 1.6–3.7], P < 0.01), also followed by an increase to reach a value of 3.1 pmol/l (IQR, 1.1–5.6), that was significantly different when compared with predialysis (P = 0.04). Consequently, the C/V ratio increased significantly during the first two hours on hemodialysis and remained higher during the remainder of the dialysis session.
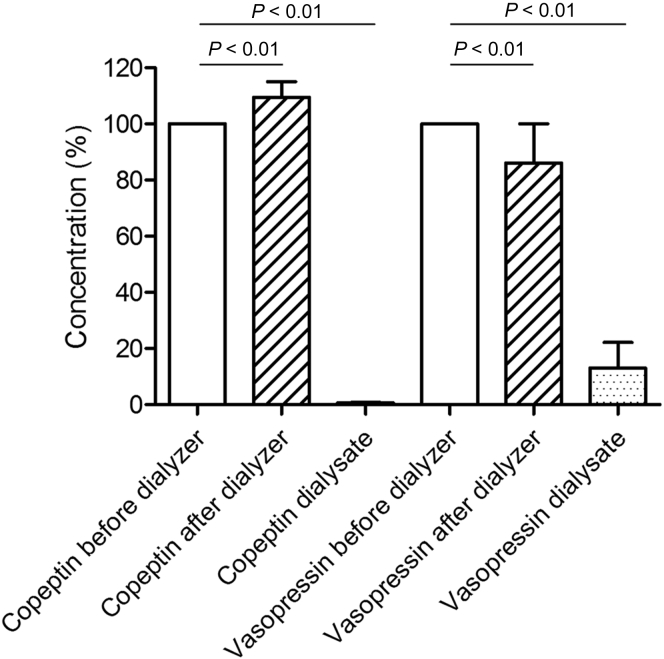
Plasma vasopressin levels decreased significantly while passing through the dialyzer from 3.9 pmol/l (IQR, 2.0–5.3) predialyzer to 3.2 pmol/l (IQR, 1.9–4.3) postdialyzer (P < 0.01), whereas copeptin levels increased significantly from 171.7 pmol/l (IQR, 112.5–296.4) predialyzer to 176.6 pmol/l (IQR, 121.7–352.8) postdialyzer (P < 0.01), presumably as a result of ultrafiltration-induced hemoconcentration. Dialysate concentrations of vasopressin and copeptin were 0.44 pmol/l (IQR, 0.33–0.59; n = 42) and 1.03 pmol/l [IQR, 0.89–1.31; n = 23], respectively. The vasopressin and copeptin clearances were 61.5 ml/min (IQR, 49.3–80.4; n = 35) and 3.5 ml/min (IQR, 2.8–6.3; n = 22), respectively. The clearance of vasopressin was significantly higher than that of copeptin (P < 0.001). Figure 5 shows the percentage change in plasma levels of vasopressin and copeptin from predialyzer to postdialyzer.
Figure 5.
Copeptin and vasopressin concentrations in blood leaving the dialyzer and in the dialysate compared to the predialyzer concentration (median and interquartile range).
Discussion
In this study we investigated the effect of renal function and hemodialysis on the association between copeptin and vasopressin levels. We showed that copeptin and vasopressin were strongly associated in the overall cohort of participants, including predialysis subjects. With declining renal function, the C/V ratio remained constant until an eGFR of 28 ml/min per 1.73 m2, after which this ratio progressively increased. In hemodialysis patients, the C/V ratio was higher than for nondialysis patients and healthy individuals, with no difference between subjects with and without residual renal function. The C/V ratio rose during a hemodialysis session, because vasopressin was cleared by dialysis to a greater extent than copeptin.
One of the 2 aims of the present study was to assess whether the C/V ratio remained stable throughout the full range of renal function to delineate whether copeptin can be used as a substitute for vasopressin in patients with impaired renal function. The ratio between copeptin and vasopressin was investigated once before in 83 CKD patients with an eGFR ranging from 7 to 61 ml/min per 1.73 m2 by Roussel et al.10 In that study, it was observed that both copeptin and vasopressin levels were higher in patients with a lower eGFR. The C/V ratio increased across quintiles of decreasing renal function and the investigators suggested a greater decrease in renal clearance rate of copeptin compared with vasopressin when renal function declines.10 In our study we investigated the association between renal function and C/V ratio more closely using break-point analysis. We add that an association between renal function and C/V ratio is only found when renal function is severely impaired (i.e., eGFR ≤28 ml/min per 1.73 m2). Furthermore, we also studied copeptin and vasopressin and their ratio in hemodialysis patients pretreatment and during hemodialysis. Another difference with our study is that samples were stored frozen during prolonged periods of time (up to 20 years vs. maximally 1 year in our study), which may have affected the concentrations of copeptin and especially vasopressin, as acknowledged by Roussel et al.10
Copeptin and vasopressin are assumed to be produced in equimolar amounts.6, 8, 9 Therefore, we expect copeptin and vasopressin and their ratio to behave similarly to changes in volume status. Still, in subjects with completely normal renal function, that is, an eGFR >90 ml/min per 1.73 m2, plasma concentration of copeptin is more than 5-fold higher than that of plasma vasopressin. This may, at least in part, be caused by a difference in apparent distribution volume. Vasopressin binds to the V1 and V2 receptors, and bound vasopressin will be sequestrated from plasma. In addition, vasopressin binds to platelets29, 30 that are removed by centrifugation during the preanalytic handling of samples for vasopressin measurement. In contrast, copeptin is presumably not bound to platelets and, to our knowledge, no copeptin receptor is known. Another explanation for lower vasopressin concentration may be that the half-life of vasopressin is shorter than that of copeptin. These 3 mechanisms may explain why even in healthy subjects with normal renal function, the C/V ratio is >1.
When renal function declines, both copeptin and vasopressin increased. This does not necessarily imply that these analytes are subject to renal clearance. It is shown that patients with lower renal function have impaired urine concentrating capacity.31 Such subjects tend to have higher plasma osmolality and, consequently, will also have higher vasopressin (and copeptin) concentration.24, 31 Indeed we found significant associations between eGFR and plasma osmolality, and between plasma osmolality and copeptin and vasopressin. In a previous study we found no association between copeptin and renal function in 134 healthy kidney donors predonation and postdonation. Copeptin levels remained unchanged after kidney donation, despite a decrease in iothalamate-measured GFR of ∼40% (from 105 to 66 ml/min per 1.73 m2).32 These data suggest that not renal clearance per se but renal disease-induced kidney damage modulates vasopressin and copeptin levels.
Importantly, when eGFR decreases progressively below 28 ml/min per 1.73 m2, copeptin levels increased to a greater extent than vasopressin levels, and consequently the C/V ratio increased. On one hand, this could be explained by a decreased clearance of copeptin compared with vasopressin. The more marked copeptin increase in severe renal insufficiency indeed suggests a renal component in the total body clearance of copeptin. Partial renal clearance of copeptin has been suggested before, as copeptin has been found in urine.7 Renal clearance, however, will presumably not be the predominant factor in the degradation of copeptin because a fast decrease in copeptin levels has been observed within minutes after the start of water loading5, 7 that is more rapid than one would expect from renal clearance alone. On the other hand, no associations between renal function and vasopressin and between plasma osmolality and vasopressin were observed in patients with an eGFR ≤28 ml/min per 1.73 m2. The relatively low vasopressin levels in this group may, therefore, be attributable to increased extrarenal clearance of vasopressin. This could also result in an increase of the C/V ratio in severely impaired renal function. Vasopressin metabolism has been studied more extensively, and it has been found that indeed only 5% to 27% of the total body clearance is accounted for by the kidneys.33, 34 Hepatic vasopressinases35 and endopeptidases and aminopeptidases36 seem to play a more important role in vasopressin clearance. One might also speculate that accumulation of uremic toxins in severe renal failure may lead to differences in copeptin and vasopressin distribution and metabolism. A study on the kinetics of copeptin and vasopressin (i.e., exact route of elimination, total body clearance and renal clearance, volume of distribution) and potential physiological effects of copeptin could clarify this. We want to emphasize that investigating the exact metabolic fate of copeptin and vasopressin is beyond the scope of the present clinical study, as this will necessitate a specific experimental design.
The second aim of the present study was to investigate the kinetics of copeptin and vasopressin during hemodialysis. The highest C/V ratio was found in hemodialysis patients. During hemodialysis, the ratio increased further, indicating that vasopressin was removed to a greater extent than was copeptin. The higher dialyzer clearance rate of vasopressin in comparison to copeptin is in line with these findings. Due to the lower clearance rate of copeptin, accumulation may occur in the interdialytic interval, provided that the half-life of copeptin that is associated with extrarenal clearance mechanisms is long enough. This could, at least in part, explain the greater increase of copeptin relative to vasopressin levels in hemodialysis patients.
This study has 2 limitations that need to be acknowledged. First, copeptin and vasopressin were measured in samples taken from different studies. However, in these studies samples were collected according to the same strict protocol to ensure optimal preanalytic sample handling, and copeptin and vasopressin measurements were performed using the same assays. Inclusion of patients from different studies explains that the number of participants differed somewhat between the study groups, that is, healthy individuals, IgA nephropathy, autosomal dominant polycystic kidney disease, and hemodialysis patients. Second, hematocrit levels in the postdialyzer bloodline was not measured. Therefore, we could not correct the postdialyzer copeptin and vasopressin levels for hemoconcentration over the dialyzer, but this does not affect the measurement of copeptin and vasopressin clearance by hemodialysis, which was the primary aim of this part of our study. Strengths of this study are that we investigated the associations among renal function, copeptin, vasopressin and their ratio in detail, and that this study is the first to investigate kinetics of copeptin in relation to vasopressin during dialysis.
In conclusion, the C/V ratio was stable across the range of renal function in patients with an eGFR above 28 ml/min per 1.73 m2. Thus, elevated copeptin levels observed in this eGFR range seem to accurately reflect vasopressin concentration. Consequently, copeptin seems a suitable marker for vasopressin in healthy subjects and patients with CKD stages 1 to 3. However, in studies that include patients with CKD stages 4 and 5, it is necessary to adjust for eGFR when studying copeptin as a marker for vasopressin. This is important for future studies in which copeptin is used as a surrogate marker for vasopressin in patients with CKD. We found no effect of etiology of renal disease on the association between eGFR and copeptin and vasopressin, which implies that our results might be generalizable for patients with CKD. In hemodialysis patients, predialysis copeptin and vasopressin showed a tight correlation. Copeptin may therefore be used as surrogate for vasopressin when assessed in blood samples drawn before dialysis. During a dialysis session, however, copeptin and vasopressin show a different kinetic profile, implying that copeptin does not adequately reflect vasopressin levels during dialysis.
Disclosure
All the authors declared no competing interests. For this research no specific grants were obtained from funding agencies in the public, commercial, or not-for-profit sector.
Footnotes
Item S1. Calculation of clearance.
Item S2. Specifications of the hemodialysis treatments.
Item S3. Hemoconcentration.
Item S4. STROBE statement.
Table S1. Associations between plasma copeptin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.
Table S2. Associations between plasma vasopressin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Calculation of clearance.
Specifications of the hemodialysis treatments.
Hemoconcentration.
STROBE statement.
Associations between plasma copeptin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.
Associations between plasma vasopressin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.
References
- 1.den Ouden D.T., Meinders A.E. Vasopressin: physiology and clinical use in patients with vasodilatory shock: a review. Neth J Med. 2005;63:4–13. [PubMed] [Google Scholar]
- 2.Bankir L., Bouby N., Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9:223–239. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Bouby N., Hassler C., Bankir L. Contribution of vasopressin to progression of chronic renal failure: study in Brattleboro rats. Life Sci. 1999;65:991–1004. doi: 10.1016/s0024-3205(99)00330-6. [DOI] [PubMed] [Google Scholar]
- 4.Bardoux P., Bichet D.G., Martin H. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant. 2003;18:497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 5.Morgenthaler N.G., Struck J., Alonso C., Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 6.Morgenthaler N.G. Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail. 2010;16(suppl 1):S37–S44. doi: 10.1111/j.1751-7133.2010.00177.x. [DOI] [PubMed] [Google Scholar]
- 7.Balanescu S., Kopp P., Gaskill M.B. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab. 2011;96:1046–1052. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- 8.Dabla P.K., Dabla V., Arora S. Co-peptin: role as a novel biomarker in clinical practice. Clin Chim Acta. 2011;412:22–28. doi: 10.1016/j.cca.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Christ-Crain M., Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12:168–176. doi: 10.1038/nrendo.2015.224. [DOI] [PubMed] [Google Scholar]
- 10.Roussel R., Fezeu L., Marre M. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab. 2014;99:4656–4663. doi: 10.1210/jc.2014-2295. [DOI] [PubMed] [Google Scholar]
- 11.Hew-Butler T., Hoffman M.D., Stuempfle K.J. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med. 2011;21:211–217. doi: 10.1097/JSM.0b013e31821a62c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jochberger S., Dorler J., Luckner G. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med. 2009;37:476–482. doi: 10.1097/CCM.0b013e3181957532. [DOI] [PubMed] [Google Scholar]
- 13.Westermann I., Dunser M.W., Haas T. Endogenous vasopressin and copeptin response in multiple trauma patients. Shock. 2007;28:644–649. doi: 10.1097/shk.0b013e3180cab33f. [DOI] [PubMed] [Google Scholar]
- 14.Morgenthaler N.G., Struck J., Jochberger S., Dunser M.W. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Bolignano D., Cabassi A., Fiaccadori E. Copeptin (CTproAVP), a new tool for understanding the role of vasopressin in pathophysiology. Clin Chem Lab Med. 2014;52:1447–1456. doi: 10.1515/cclm-2014-0379. [DOI] [PubMed] [Google Scholar]
- 16.Sontrop J.M., Huang S.H., Garg A.X. Effect of increased water intake on plasma copeptin in patients with chronic kidney disease: results from a pilot randomised controlled trial. BMJ Open. 2015;5:e008634. doi: 10.1136/bmjopen-2015-008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukaszyk E., Malyszko J. Copeptin: pathophysiology and potential clinical impact. Adv Med Sci. 2015;60:335–341. doi: 10.1016/j.advms.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Santoro A. Infusing vasopressin to prevent intradialytic hypotension. Nat Clin Pract Nephrol. 2007;3:362–363. doi: 10.1038/ncpneph0509. [DOI] [PubMed] [Google Scholar]
- 19.Ponte B., Pruijm M., Ackermann D. Copeptin is associated with kidney length, renal function, and prevalence of simple cysts in a population-based study. J Am Soc Nephrol. 2015;26:1415–1425. doi: 10.1681/ASN.2014030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer E., Bakker S.J., Halbesma N. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77:29–36. doi: 10.1038/ki.2009.397. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari S.S., Loke I., Davies J.E. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 2009;116:257–263. doi: 10.1042/CS20080140. [DOI] [PubMed] [Google Scholar]
- 22.Boertien W.E., Meijer E., Zittema D. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2012;27:4131–4137. doi: 10.1093/ndt/gfs070. [DOI] [PubMed] [Google Scholar]
- 23.Li X., Yang X.C., Sun Q.M., Chen X.D., Li Y.C. Brain natriuretic peptide and copeptin levels are associated with cardiovascular disease in patients with chronic kidney disease. Chin Med J (Engl) 2013;126:823–827. [PubMed] [Google Scholar]
- 24.Zittema D., Boertien W.E., van Beek A.P. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7:906–913. doi: 10.2215/CJN.11311111. [DOI] [PubMed] [Google Scholar]
- 25.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association Section I. measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant. 2002;17(suppl 7):7–15. doi: 10.1093/ndt/17.suppl_7.7. [DOI] [PubMed] [Google Scholar]
- 27.Franssen C.F. Oxalate clearance by haemodialysis—a comparison of seven dialysers. Nephrol Dial Transplant. 2005;20:1916–1921. doi: 10.1093/ndt/gfh971. [DOI] [PubMed] [Google Scholar]
- 28.Schneditz D., Putz-Bankuti C., Ribitsch W., Schilcher G. Correction of plasma concentrations for effects of hemoconcentration or hemodilution. ASAIO J. 2012;58:160–162. doi: 10.1097/MAT.0b013e318243660f. [DOI] [PubMed] [Google Scholar]
- 29.Preibisz J.J., Sealey J.E., Laragh J.H., Cody R.J., Weksler B.B. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension. 1983;5:I129–I138. doi: 10.1161/01.hyp.5.2_pt_2.i129. [DOI] [PubMed] [Google Scholar]
- 30.Bichet D.G., Arthus M.F., Barjon J.N., Lonergan M., Kortas C. Human platelet fraction arginine-vasopressin: potential physiological role. J Clin Invest. 1987;79:881–887. doi: 10.1172/JCI112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen E.B., Thomsen I.M., Lauridsen T.G. Abnormal function of the vasopressin-cyclic-AMP-aquaporin2 axis during urine concentrating and diluting in patients with reduced renal function: a case control study. BMC Nephrol. 2010;11:26. doi: 10.1186/1471-2369-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zittema D., van den Berg E., Meijer E. Kidney function and plasma copeptin levels in healthy kidney donors and autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol. 2014;9:1553–1562. doi: 10.2215/CJN.08690813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann G., Dingman J.F. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest. 1976;57:1109–1116. doi: 10.1172/JCI108377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses A.M., Steciak E. Urinary and metabolic clearances of arginine vasopressin in normal subjects. Am J Physiol. 1986;251:R365–R370. doi: 10.1152/ajpregu.1986.251.2.R365. [DOI] [PubMed] [Google Scholar]
- 35.Sharman A LJ Vasopressin and its role in critical care. Contin Educ Anaesth Crit Care Pain. 2008;8:134–137. [Google Scholar]
- 36.Hanoune J. The neurohypophysial system: Synthesis and metabolism of vasopressin. In: Laycock J., editor. Perspectives on Vasopressin. Imperial College Press; London, UK: 2010. pp. 39–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculation of clearance.
Specifications of the hemodialysis treatments.
Hemoconcentration.
STROBE statement.
Associations between plasma copeptin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.
Associations between plasma vasopressin and estimated glomerular filtration rate (eGFR) in healthy participants and chronic kidney disease patients not on dialysis, stratified for eGFR above or below 28 ml/min per 1.73 m2.