Abstract
Introduction
Primary focal segmental glomerulosclerosis (FSGS) is a leading cause of nephrotic syndrome and end-stage renal disease. There are no US Food and Drug Administration−approved therapies for FSGS, and treatment often fails to reduce proteinuria. Endothelin is an important factor in the pathophysiology of podocyte disorders, including FSGS. Sparsentan is a first-in-class, orally active, dual-acting angiotensin receptor blocker (ARB) and highly selective endothelin Type A receptor antagonist. This study is designed to evaluate whether sparsentan lowers proteinuria compared with an ARB alone and has a favorable safety profile in patients with FSGS.
Methods
DUET is a phase 2, randomized, active-control, dose-escalation study with an 8-week, fixed-dose, double-blind period followed by 136 weeks of open-label sparsentan treatment. Patients aged 8 to 75 years with primary FSGS will be randomized to treatment with sparsentan or irbesartan for 8 weeks.
Results
The primary efficacy objective is to test the hypothesis that sparsentan over the dose range (200 mg, 400 mg, or 800 mg daily) is superior to irbesartan (300 mg daily) in decreasing the urinary protein-to-creatinine ratio (UPC) from baseline to 8 weeks postrandomization. As secondary objectives, the trial will evaluate the proportion of patients who achieve prespecified targets of UPC reduction, changes in laboratory and quality-of-life indices, and detailed safety analysis. Analyses will be conducted at the end of the double-blind (week 8) and open-label (week 144) periods.
Discussion
This study will provide important evidence on whether dual ARB and endothelin blockade may be an effective therapeutic strategy for FSGS and may provide the rationale for next-phase trials.
Keywords: endothelin receptor antagonist, focal segmental glomerulosclerosis, irbesartan, nephrotic syndrome, proteinuria, sparsentan
Focal segmental glomerulosclerosis (FSGS) is a histopathological pattern of injury seen in some patients who present with nephrotic syndrome or isolated proteinuria.1 There is a large and heterogeneous group of known causes of FSGS, which are historically classified as primary and secondary. Primary FSGS typically has no consistently identifiable cause,1 but may occur as the result of a genetic mutation in a protein that is essential for normal podocyte structure and/or function,2 or may be caused by circulating factors that injure podocytes and increase glomerular permeability to protein.3, 4 In contrast, secondary FSGS has been linked to disorders believed to cause maladaptive injury to glomeruli following the loss of renal parenchyma or systemic metabolic changes,5 and may be caused by a variety of underlying medical conditions, such as hypertension, sickle cell anemia, or elevated body mass index.1, 6
Primary FSGS is a rare entity. However, after diabetes and hypertension, primary FSGS is one of the leading glomerular diseases to cause chronic and end-stage renal disease (ESRD), accounting for 5% of incident adult and 12% of incident pediatric ESRD cases.7, 8 Nearly 50% of affected patients with nephrotic proteinuria will require renal replacement therapy within 5 to 10 years of diagnosis.9 Primary FSGS occurs in patients of all ages and races, and the prevalence of primary FSGS has risen over the last 3 decades in both children and adults.10, 11, 12 It is uncertain whether the increased prevalence reflects an increase in the performance of kidney biopsies or an increase in predisposing risk factors such as low birth weight, use of nephrotoxic medications, or exposure to environmental chemicals and viral agents.
Primary FSGS presents as asymptomatic proteinuria or clinically evident nephrotic syndrome.1, 7 Patients may present with microscopic hematuria and elevated blood pressure (BP), and often have preserved kidney function at the time of diagnosis. The diagnosis is established by a kidney biopsy demonstrating the characteristic pathologic features of segmental sclerosis and hyalinosis of the glomerular capillary tuft.13 In the absence of kidney biopsy, particularly among children, identification of proteinuria and/or a disease-causing mutation in a podocyte protein in a patient with the appropriate history (i.e., lack of response to corticosteroids) is often sufficient to make a clinical diagnosis of FSGS.13
The first-line therapy for primary FSGS is often corticosteroids combined with renin−angiotensin system blockade with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs).6, 13, 14 In patients who do not respond to steroids, calcineurin inhibitors (CNIs), including cyclosporine and tacrolimus, are often prescribed as second-line therapy.13, 15, 16 Compared with steroid monotherapy, use of cyclosporine increases the likelihood of a complete or partial remission of proteinuria in patients.15, 17 Although tacrolimus has replaced cyclosporine as the CNI of choice at many centers, there are fewer randomized clinical trials specifically testing this agent. In patients who do not respond to therapy with a CNI, there are no established treatment options.13, 14 Agents that have been tried with marginal success include mycophenolate mofetil and rituximab.13, 14, 18, 19 Patients with resistant FSGS are at higher risk for progressing to ESRD.1, 11, 20
Attention has also focused on adjuvant, nonimmunosuppressive therapies designed to reduce proteinuria in primary FSGS.21 This basis for practice derives from evidence that reduction in proteinuria, a routinely used clinical index of treatment benefit, is nephroprotective.9, 22
Indeed, treatment-associated remission of proteinuria is 1 of the most important independent predictors of kidney survival.13, 23, 24 Studies in children and adults have indicated that there is a dose−response relationship between reduction in proteinuria and clinical outcomes, including renal survival.25, 26
Similar to ACEIs or ARBs as adjuvant therapy in primary FSGS, endothelin (ET) type A (ETA) receptor antagonists (ERAs) have demonstrated a spectrum of beneficial effects in a variety of models of glomerular diseases.27 Consequently, these agents could provide additive protective effects to ACEIs or ARBs in treating proteinuric diseases.28 Notably, combining the actions of angiotensin II and ET antagonists has demonstrated benefits in experimental models of progressive glomerulosclerosis and tubulointerstitial fibrosis.29, 30
Promising results of experimental studies have motivated clinical development of ERAs for use in patients with chronic kidney disease.31 Thus far, most of the study data have been obtained from patients with diabetic nephropathy. These studies have documented noteworthy additive benefits of ERAs on reducing proteinuria in patients who undergo optimized treatment with inhibitors of renin−angiotensin system inhibitors (RASIs).32, 33, 34 However, further development of drugs in this new class of nephroprotective agents has been hampered by reports of adverse effects. Among those, sodium and fluid retention are the most common, resulting in edema and sometimes heart failure, with the consequence of early termination of patients from some studies.27, 32
More recently, several studies identified ET as an important factor in the pathophysiology of podocyte injury. Specifically, Buelli et al35 found that ET precipitated phenotypic and functional changes in podocytes in vitro consistent with clinical observations in FSGS, and that treatment of the adriamycin-induced murine model of FSGS with type A ERAs normalized renal function and podocyte pathology in vivo. In another study, adriamycin-induced FSGS and transforming growth factor–β–induced FSGS in mice were associated with enhanced podocyte production of ET. This increased ET caused oxidative stress and injury in adjacent glomerular endothelial cells, leading to reciprocal paracrine injury to podocytes, consistent with FSGS.36 ET inhibition normalized these alterations and protected kidneys in these models of glomerular injury.
Together, these studies suggest that, in addition to their pleotropic antifibrotic, anti-inflammatory, and hemodynamic actions, ERAs may target specific molecular pathogenic steps causing FSGS. This new preclinical evidence provides a strong rationale for the use of ET receptor blockers in FSGS.
Sparsentan is a first-in-class, orally active, dual-acting angiotensin type 1 receptor blocker and highly selective ETA receptor antagonist. It is chemically similar to the AT1 receptor blocking moiety of irbesartan, an existing ARB, and was originally developed for the treatment of essential hypertension.28 This report describes, using the SPIRIT reporting criteria, a phase 2, randomized, double-blind, active-control, dose-escalation study (DUET) that will evaluate the antiproteinuric efficacy and long-term safety of sparsentan, compared with irbesartan, in patients with primary FSGS, during an 8-week double-blind study period and a 136-week open-label extension study.
Methods
Study Participants and Sites
Patients aged 8 to 75 years with biopsy-proven primary FSGS (idiopathic or due to an identified mutation) are eligible to participate in the study. Inclusion and exclusion criteria for enrollment are presented in Tables 1 and 2. Because FSGS can occur as a result of genetic mutations in structural proteins in the podocyte,2 confirmation of a disease-causing mutation in a podocyte protein will be considered to satisfy the eligibility criterion for the trial in patients who lack biopsy confirmation of the FSGS diagnosis.
Table 1.
DUET inclusion criteriaa
| 1 | Males and females who are willing and able to provide written informed consent, with consent signed by patient or legal guardian US sites: Patients aged 8−75 years EU sites: Patients aged 18−75 years |
| 2 | Biopsy-proven primary FSGS or documentation of a genetic mutation in a podocyte protein associated with the disease |
| 3 | UPC ≥ 1.0 g/g |
| 4 | eGFR > 30 ml/min/1.73 m2 |
| 5 | Mean seated BP > 100 mm Hg and < 145/96 mm Hg in patients aged ≥ 18 years Mean seated BP for patients aged < 18 years should be > 90/60 mm Hg and < 95th percentile for age, gender, and height |
| 6 | Patients must be on a stable dose of immunosuppressive medication for ≥ 1 month before randomization. The investigator should not have plans to alter the regimen during the first 8 weeks of the study except to stabilize levels. Patients who have taken rituximab or cyclophosphamide must have discontinued the medication ≥ 3 months before randomization |
BP, blood pressure; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; UPC, urine protein-to-creatinine ratio.
Patients must meet all inclusion criteria to be eligible for the study.
Table 2.
DUET exclusion criteriaa
Patients with a medical history of:
|
| Hematocrit < 27% or hemoglobin < 9 g/dl |
| Serum potassium > 5.5 mEq/l |
| Body mass index > 40 kg/m2 for adult patients or in the 99th percentile plus 5 units for pediatric patients |
| Women who are pregnant or breastfeeding or who are of child-bearing potential who are unwilling to use 2 reliable methods of contraception |
| Patients who have participated in another investigational drug study within 28 days before screening |
| Prior exposure to sparsentan |
| Patients who are unwilling to comply with the study procedures and assessments, including the ability to swallow the study drug or control capsules |
FSGS, focal segmental glomerulosclerosis.
A patient who meets any of the criteria will be excluded from the study.
The DUET study has been approved as an ancillary study of the Nephrotic Syndrome Study Network (NEPTUNE) observational study,37 and patient enrollment was offered to all sites that are members of the consortium and obtained institutional review board (IRB) approval in a timely manner. Approximately 100 patients will be enrolled at an estimated 50 sites in the United States and Europe. The sites will include academic hospitals, research centers, and community nephrology clinics. A list of study sites is available at: https://clinicaltrials.gov/ct2/show/study/NCT01613118. Approvals by local IRBs or institutional ethical committees (IECs) are required before enrollment of patients at each site.
Study Design and Treatment
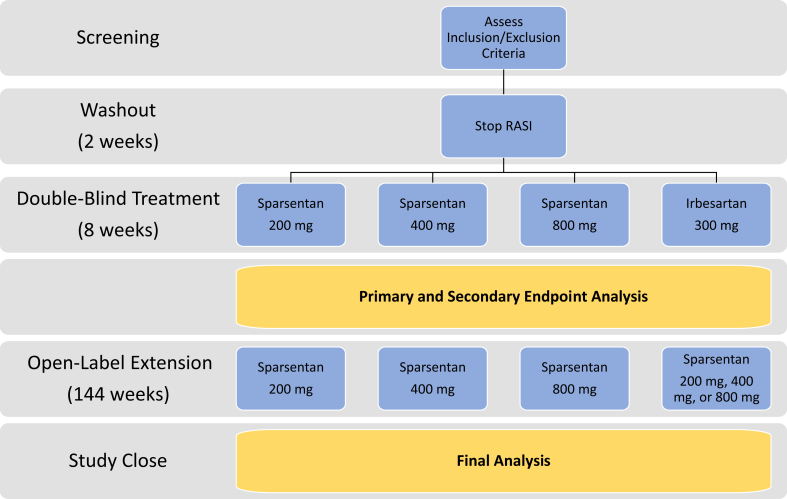
A schematic illustration of the study design is presented in Figure 1. Investigators will obtain informed consent and assent where indicated from patients or guardians. Patients will be screened to confirm eligibility and will undergo a 2-week washout period following the discontinuation of any ARB and ACEI medications. At week 0, a computer-generated randomization sequence, via an interactive Web response system, will be used to randomize patients (3:1) to receive sparsentan or irbesartan. The double-blind treatment period will last 8 weeks. Patients who agree to participate in the open-label period of the study, including those randomized to irbesartan, will receive sparsentan treatment for an additional 136 weeks. Patients in either treatment arm will receive the dose of sparsentan corresponding to their original randomization cohort. Patients will participate in the study for a total of 156 to 158 weeks, or approximately 39 months.
Figure 1.
DUET study design and analysis periods. RASI, renin−angiotensin system inhibitor.
Study participants will receive oral sparsentan 200 mg, 400 mg, or 800 mg once daily or irbesartan 300 mg once daily. Patients will be assigned into dose-escalating cohorts with incremental safety reviews by a data monitoring committee (DMC) (Figure 2). Initially, only patients aged 18 years or older will be enrolled at the lowest sparsentan dose (200 mg). After 8 patients have completed 4 weeks of treatment, the DMC will perform a safety review and will determine whether the study should continue enrollment. If the safety profile of sparsentan is acceptable, cohort 1 will begin to enroll pediatric patients (aged 8−17 years), and a second cohort comprising adult and pediatric patients (aged 8−75 years) will be randomized to receive sparsentan 400 mg or irbesartan 300 mg. This randomized, iterative enrollment process will continue until 5 dosing cohorts are fulfilled. Patients whose body weight is 50 kg or less at week 0 will receive a 50% reduction in the allocated sparsentan (i.e., 100 mg, 200 mg, or 400 mg) and irbesartan (i.e., 150 mg) doses.
Figure 2.
Progressive dosing cohorts for the DUET study. The study will progress only after safety review by the data monitoring committee (DMC).
Indications for dose reduction or patient withdrawal from the study include general medical or safety reasons, with an emphasis on safety concerns, such as low or rapidly decreasing estimated glomerular filtration rate (eGFR; < 30 ml/min), hyperkalemia, fluid retention, edema, or heart failure resistant to diuretic and other treatment; dose reduction and withdrawal may be enacted at any visit. The patients who weigh 50 kg or less and are randomized to cohort 1 (sparsentan 100 mg or irbesartan 150 mg daily during the double-blind period) and require dose reduction will be withdrawn from the study, as it is not feasible to reduce the dose below 150 mg of irbesartan or 100 mg of sparsentan. Following resolution of signs and/or symptoms requiring dose reduction, the patient may be re-escalated to the originally assigned dose and, if it is well tolerated, may be continued on that dose per protocol.
Dose increases will not be permitted during the double-blind treatment period. Dose increases for lack of efficacy will be allowed during the open-label period of the study and may be performed during any visit, including an unscheduled visit, or implemented via telephone instruction. The dose may be increased only to the highest dose level permitted by the DMC at that time.
Sparsentan will be dispensed as 100-mg capsules and irbesartan as 150-mg tablets overencapsulated in gray gelatin capsules, both provided in identical kits. Patients will be instructed to take the appropriate quantity of capsules for the assigned cohort, orally once daily before the morning meal. Adherence to study treatment will be assessed by capsule counts at each visit. Any unused medication will be returned at the end of the study.
Concomitant use of RASIs (e.g., ACEIs, ARBs, aliskiren), aldosterone blockers, potassium-sparing diuretics, interferon-β-1a, rituximab, cyclophosphamide, and long-term use of nonsteroidal anti-inflammatory drugs are not permitted during the study.
Any changes in the study protocol, such as changes in the study design, objectives or endpoints, inclusion and exclusion criteria, and/or procedures (except to eliminate an immediate hazard) will be implemented by Retrophin Inc. (San Diego, CA) or its designee. All protocol changes must be documented in protocol amendment(s). Protocol amendment(s) must be signed by Retrophin and approved by the appropriate IRB or IEC before implementation. Any changes in study conduct that result from a pending amendment will be considered protocol deviations until IRB/IEC approval is granted. Documentation of IRB/IEC approval must be provided to Retrophin or its designee.
Endpoints and Assessments
The primary efficacy endpoint for the double-blind period is the change in the urinary protein-to-creatinine ratio (UPC) from baseline to week 8. The secondary efficacy endpoint for the double- blind period is the proportion of patients in each dose group that experience a treatment-induced UPC ≤ 1.5 g/g with a >40% reduction in UPC from baseline at week 8. Tertiary efficacy endpoints include changes from baseline in 24-hour urinary protein excretion, serum albumin, serum creatinine levels, eGFR, BP, and lipid profiles. Additionally, changes from baseline in quality of life using the Pediatric Quality of Life Inventory (PedsQL v4.0, Mapi Research Trust, Lyon, France) questionnaire for patients younger than 18 years38, 39 and the 36-Item Short Form Health Survey (SF-36) questionnaire for patients aged 18 years or older will be evaluated.16, 40
Efficacy endpoints for the open-label period include the changes from week 8 to week 144 (final visit) in UPC, serum creatinine, eGFR, BP, serum albumin, plasma renin activity, serum endothelin, serum aldosterone, quality of life, and lipid profile parameters. The proportion of patients experiencing a UPC ratio ≤ 1.5 g/g with >40% reduction from baseline in UPC at each visit during open-label sparsentan treatment will also be evaluated.
The DUET trial will characterize plasma pharmacokinetics (PK) of sparsentan and irbesartan over the range of doses administered. The PK parameters will be assessed at week 0 (after the initial dose of sparsentan/irbesartan) and week 8, and will include maximum and minimum serum drug concentrations, time of the maximum and minimum drug concentrations, and area under the drug concentration−time curve for specified time intervals.
In addition, blood samples collected for biorepository will be assessed for biomarkers that may become available after the study. These biomarkers may help to elucidate the mechanisms of FSGS disease and treatment. Patients may withdraw consent for use of biorepository samples at any time.
The safety and tolerability of sparsentan will be assessed by double-blind monitoring of physical parameters, clinical laboratory tests, electrocardiograms, and echocardiographic functional parameters, as well as by monitoring of medications required to control extracellular fluid volume, edema, and BP. Patients will be evaluated by investigators for adverse events (AEs) at each study visit and with each contact outside the clinic visits (e.g., telephone calls). AEs will be assessed for severity (mild, moderate, severe) and relationship to study treatment (none, unlikely, possible, related). Medications required for controlling fluid volume, edema, and BP and any dosage changes will be documented. The safety of the study intervention will be monitored periodically by the DMC as described above. If 2 or more patients who receive study drug experience a severe AE within a system organ class that is life-threatening or results in death, and the severe AE is deemed possibly related to treatment by the investigator and/or by Retrophin, the DMC will be notified within 24 hours. The DMC may decide to immediately stop the trial, stop recruitment and dosing, or allow continued recruitment at a lower dose.
During the double-blind treatment period, clinic visits will occur at weeks 0, 1, 4, and 8. Study visits during the open-label period are scheduled in 12-week intervals ± 14 days. Assessments performed at each visit are described in Table 3. In addition, telephone calls will be used to assess safety at weeks 10, 12, 14, and 148.
Table 3.
DUET study assessment schedule
| Screening/ washout |
Double-blind treatment |
Open-label treatment |
Safety follow-up telephone call |
|||||
|---|---|---|---|---|---|---|---|---|
| week −6 to −1 |
Week 0 | Week 1 | Week 4 | Week 8 | Weeks 16−132a | Week 144 | Week 148 | |
| Interventionsb | ||||||||
| Sparsentan 200 mg | • | • | • | • | • | • | ||
| Sparsentan 400 mg | • | • | • | • | • | • | ||
| Sparsentan 800 mg | • | • | • | • | • | • | ||
| Irbesartan 300 mgc | • | • | • | |||||
| Sparsentan 200 mg, 400 mg, or 800 mgc | • | • | • | |||||
| Assessments | ||||||||
| Inclusion/exclusion criteria, complete medical history | • | |||||||
| Quality-of-life questionnaired | • | • | • | • | ||||
| Full physical examination | • | • | • | • | ||||
| Abbreviated physician examination | • | • | • | • | • | |||
| Vital sign measurements | • | • | • | • | • | • | • | |
| Prothrombin time, INR | • | • | • | • | • | • | ||
| Chemistry profile | • | • | • | • | • | • | • | |
| Complete blood cell count | • | • | • | • | • | • | • | |
| Lipid panel | • | • | •e | • | ||||
| Routine urinalysis | • | • | • | • | • | • | • | |
| Quantitative urinalysis, first void | • | • | • | • | • | • | • | |
| 24-h Quantitative urinalysis | • | • | • | • | ||||
| Renal laboratory testsf | • | • | • | •g | • | |||
| Echocardiogram | • | • | •h | • | ||||
| 12-Lead electrocardiogram | • | • | • | • | • | |||
| Pharmacokinetic samples | • | • | • | • | • | • | ||
| Study medication adherence | • | • | • | • | • | |||
| Adverse events | • | • | • | • | • | • | • | |
INR, international normalized ratio.
Clinic visits at every 12 weeks ± 14 days. Safety follow-up phone calls will be conducted at weeks 10, 12, and 14.
Study medication taken once daily.
Patients assigned to irbesartan during the double-blind treatment phase will be offered sparsentan treatment at the dose that they would have received according to the double-blind dose group in which they were enrolled.
36-Item Short Form Health Survey (SF-36) for patients aged ≥18 years; Pediatric Quality of Life Inventory (PedsQL) for patients aged <18 years.
Assessed at weeks 16, 48, and 60 through 144, not all visits.
Renin, aldosterone, endothelin, and N-terminal pro B-type natriuretic peptide for patients aged ≥18 years.
Assessed at weeks 16, 24, 48, 72, 96, and 120.
Assessed at weeks 16, 48, and 96.
Patients who discontinue from the study during either the double-blind or open-label treatment periods, for any reason, will undergo a follow-up visit, and the reason(s) for discontinuation will be documented. If a patient discontinues because of an AE, this will be noted in the case report form, and any serious AEs will be followed up until resolution or stabilization.
Data Management
The DMC will comprise 4 members, inclusive of the DMC chair, who will be chosen based on expertise in the study indication and prior indications for which the study medication was used, clinical trial methods, and/or the evaluation of laboratory/clinical results. They will be independent medical reviewers who will participate without financial, intellectual, or other competing interests. The DMC has 2 purposes through their safety review: (i) to evaluate the safety of sparsentan and to decide whether dose escalation to the next cohort should occur; and (ii) to determine the continuation, modification, or termination of the study. DMC meetings will be organized and decisions documented by the study’s contract research organization (CTI Clinical Trial & Consulting, Cincinnati, OH). The DMC charter and DMC documentation of meetings will be maintained and stored at this contract research organization.
Study data will be stored in an electronic data capture database, created and maintained by an independent data security firm (Dependable Global Solutions Inc., Falls Church, VA). Only site personnel at this facility can enter or change the data. The database will be programmed to perform edit checks to ensure complete and accurate data entry, and alerts will be initiated immediately for out-of-range values and for missing data on saving the electronic case report form. In addition, manual edit checks will be created by the monitors during source document verification and the data managers while cleaning the data. Monitoring, data management, coding, and pharmacovigilance activities will be conducted by the contract research organization, on behalf of Retrophin. Documents regarding study conduct will be maintained at the contract research organization, and their standard operating procedures are followed.
Sites will have access to their data throughout the duration of the study. After the study is completed, the database will be decommissioned, and the data, along with the queries and responses, will be provided to the sites in a per patient Portable Document Format (PDF) for only their patients. The raw data output from the clinical database will be stored at the contract research organization and transferred to Retrophin. The clinical data, including the queries and audit trail, will be provided to Retrophin. The programming for the tables, listings, and figures, along with the output, will also be transferred to Retrophin. At any time before, during, or after completion of the DUET trial, an audit may be performed by Retrophin or its designee, or a representative of a national regulatory agency may choose to inspect the site. All pertinent study data will be available for verification, audit, or inspection purposes.
The latest revision of the statistical analysis plan was finalized on 27 July 2016 before unmasking.
Statistical Analysis
The full analysis set (FAS) is defined as all randomized patients who receive at least 1 dose of study drug and have at least 1 postbaseline efficacy evaluation. The efficacy evaluable set (EES) is a subset of the FAS and is defined as all patients who have received at least 1 dose of double-blind study drug and have both baseline and week 8 UPC values. The EES will be used for the analysis of the primary efficacy endpoint. The per protocol population is a subset of the EES and excludes patients with major protocol deviations or medication compliance of ≤80% or ≥120%. Use of specific disallowed medications and other protocol deviations that exclude patients from the per protocol analysis set will be identified before unblinding the database for the primary analysis. The safety analysis set consists of all randomized patients who receive at least 1 dose of double-blind study drug and have at least 1 postbaseline safety assessment. For each analysis set, patients will be analyzed based on the treatment that they have received.
Primary Efficacy Endpoint and Analyses
The primary efficacy endpoint analyses will be based on the EES and will test the hypothesis that sparsentan is superior to irbesartan in decreasing the UPC in patients with FSGS, as measured by the change from baseline to 8 weeks’ postrandomization in 24-hour UPC ratios. To reduce skewness, the natural logarithm will be used to transform UPC data before analysis. The primary efficacy endpoint is the change from baseline to week 8 in log-transformed values of UPC. An analysis of covariance model will be the primary method of analysis for log (UPC) and other continuous data (measured as change from baseline to week 8), with treatment as a fixed effect and baseline value as a covariate. Results of the analysis will be back-transformed (via exponentiation) to obtain the geometric mean ratio of the UPC values at week 8 to the baseline value and corresponding 95% confidence intervals. The values will then be expressed as percentage change in adjusted geometric mean of the UPC for week 8 relative to baseline. Sparsentan doses or their combinations will be compared with irbesartan in the following hierarchical order:
-
•
All sparsentan doses (800 mg, 400 mg, and 200 mg) versus irbesartan
-
•
Sparsentan 800-mg and 400-mg dose groups (combined) versus irbesartan
-
•
Sparsentan 400-mg dose versus irbesartan
-
•
Sparsentan 800-mg dose versus irbesartan
Secondary and Tertiary Endpoints and Analyses
The calculation of the proportion of patients achieving UPC ≤ 1.5 g/g with 40% reduction in UPC at week 8 will be conducted using the Fisher exact test to compare sparsentan doses, or combinations of doses, with irbesartan, in a manner similar to the primary endpoint. Tertiary endpoints will be based on the EES population. Analyses of change in 24-hour urine protein excretion from baseline to week 8 will mimic those of the primary endpoint. Descriptive statistics for the change from baseline to week 8 in urinary excretion will be displayed. Analysis of covariance models will be fitted and used to compare treatments in the same manner as the primary endpoint analysis.
Analysis of quality-of-life assessments will be based on the FAS population. Summary statistics per treatment group will be tabulated for SF-36 total scores and each subdomain, and reported as overall scores and ≥5-point improvement in SF-36 physical or mental component scores. Summary statistics will be provided for PedsQL scores by visit and the change from baseline to subsequent visits.
Analyses of changes from baseline in serum albumin and changes from baseline in lipid profile (i.e., total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, very-low-density lipoprotein cholesterol) will be based on the FAS. Data will be presented as descriptive statistics with 95% confidence intervals for mean change from baseline for all sparsentan doses, mean change from baseline for all irbesartan doses, and the difference in mean changes from baseline for all sparsentan and all irbesartan groups. If confidence intervals indicate that there may be treatment differences with respect to 1 or more of these endpoints, the specific endpoint will be analyzed further using a mixed-effect model, repeated-measures analysis.
Subgroup analysis will be performed on patient subgroups of the EES, including age (patients aged >18 years vs. patients aged ≤18 years), race, baseline UPC ratio for nephrotic (UPC ≥ 3.5 g/g) versus nonnephrotic (UPC < 3.5 g/g) patients, gender, and baseline severity of kidney disease (eGFR > 90, 60–90, 45–60, and 30–45 ml/min/1.73 m2), and BP. Descriptive statistics and 95% confidence intervals will be calculated for each. Analysis of covariance will be used to assess the relationship among these prognostic factors, treatment, cohort, and the change in natural log (UPC) values.
Plan for Open-Label Analysis
All patients who receive open-label treatment and have at least 1 safety assessment after the start of the open-label phase will be included in the Safety Analysis Set. The FAS analysis set for the open-label phase will include all patients who receive open-label sparsentan and have any efficacy assessments after receiving open-label sparsentan.
In general, tables summarizing open-label efficacy and safety data will be similar to those produced for the double-blind period, with the visit schedule extended to account for long-term follow-up. Duration of total exposure, average dose, and dose modifications will be tabulated in a similar manner.
In particular, the duration of open-label exposure to sparsentan will be summarized for all patients enrolled and for groups defined by prior double-blind treatment (sparsentan or irbesartan). Duration of exposure will be calculated from the first dose (study day 1 for patients in the sparsentan group) to capture both double-blind as well as open-label exposure.
Descriptive analyses of the following endpoints will be presented separately by treatment received during the double-blind phase: change from baseline in UPC at each visit during the open-label phase, and the proportion of patients experiencing a treatment-induced reduction of UPC ratio ≤ 1.5 g/g with 40% reduction at each visit during open-label sparsentan treatment. Confidence intervals will be used to describe changes from baseline in serum albumin, lipid profile parameters, plasma renin activity, serum endothelin, serum aldosterone, and quality of life.
Safety data in the open-label phase, including physical examination parameters, laboratory tests, medication use, and incidence of AEs, will be summarized using descriptive statistics. The incidence of AEs (overall, and by severity and relationship to treatment) will be tabulated for the full cohort of subjects on open-label sparsentan. The use of concomitant medications during the open-label phase will also be summarized.
Clinical laboratory data collected during the open-label period not mentioned above will be summarized using descriptive statistics, including tabulations of mean and mean change values at each study visit, and frequency tables indicating the of number subjects with values classified as below, within, or above-normal range at each visit. Graphs of average values (± SE) will be plotted by treatment group including the group analysis based on prior double-blind irbesartan versus sparsentan treatment at all study visits, beginning from the start of open-label sparsentan until the last assessment during the open-label period.
Vital signs and electrocardiographic data measured during the open-label treatment will be presented descriptively at each study visit, with patients grouped by prior treatment (sparsentan or irbesartan). Tabulations will be similar to those produced for the double-blind period, with an extended-visit schedule.
Due to the relatively low numbers of patients per site, no site-specific analyses will be conducted, and no adjustments will be made for individual sites. All analyses will be performed using pooled data across all study sites.
Unblinding Considerations
The only planned analysis of unblinded data during the ongoing study is for the purpose of the DMC’s review of accumulating safety data. A limited number of preidentified individuals from the contract research organization responsible for the interim analysis will have access to unblinded data to prepare a safety output for the DMC review; this team will be independent of the project team and will not have access to ongoing data management activities. If a patient’s information needs to be unblinded for a medical emergency, the sites have an interactive Web response system unblinding manual that instructs them how to unblind a patient after consulting with the sponsor. If an event requires unblinding to comply with regulatory reporting requirements for a suspected, unexpected serious adverse reaction, this process is outlined in the safety management plan for the study. After database lock for the study, appropriate study team members will be unblinded, as outlined in a pre-established unblinding plan.
Sample Size and Power Calculations
The study plan is to enroll 100 patients at a 3:1 sparsentan-to-irbesartan ratio, yielding 15, 30, and 30 patients in the sparsentan 200-mg, 400-mg, and 800-mg groups, respectively, and 25 patients in the irbesartan group. A cohort may be expanded to enroll additional patients (no more than 2 times the original size), and/or additional cohorts at doses ≥ 800 mg may be added to better assess the safety and tolerability profile of sparsentan.
Based on published observations in proteinuric patients with type 2 diabetes32, 33, 34 treated with baseline RASI and add-on ET receptor antagonist, the mean reduction in UPC (expressed as the ratio of geometric means) is expected to be at least 20% greater with sparsentan compared with irbesartan (i.e., mean reduction of 40% vs. 20%). This difference represents a geometric mean ratio of 2.0. To calculate the study power, ratios were explored in the range of 1.25 to 2.5, in the original units, using a Student t test (2-sided α = 0.05) of log-transformed UPC values for sparsentan and irbesartan. Under a range of assumptions for the variability in UPC, the study has approximately 64% to 76% power to demonstrate the expected difference between sparsentan and irbesartan (e.g., 40% vs. 20% reduction in UPC). The study power will be adequate if the high doses of sparsentan have a marked effect on UPC (i.e., more than double the average percent reduction compared with irbesartan), but smaller effects may not reach statistical significance. In this scenario, analysis of combined sparsentan dose groups compared with the irbesartan group (n = 25) will provide enhanced power to detect the same difference in UPC.
Strategies for Achieving Adequate Participant Enrollment
As with all rare diseases, recruitment for clinical trials is a challenge. Recruitment for DUET will rely on the existing pool of patients at individual participating sites. To enhance enrollment, information about the trial will be disseminated via patient advocacy groups (e.g., NephCure Kidney International, King of Prussia, PA), recruitment vendors (e.g., Matthew’s Medical Group), social media, and a study website.
Discussion
Currently there is no US Food and Drug Administration−approved therapy for FSGS. This unmet clinical need has put a premium on the development of safe, well-tolerated nephroprotective agents. Initial experience with ET antagonists as derived from the results of A Study of Cardiovascular Events in Diabetes (ASCEND) trial in diabetic nephropathy32 has been disappointing because of the clinical limitations and disabling AEs, such as worsening edema and anemia, that prevented widespread application despite demonstrable reduction in proteinuria. More recent short-term studies with another ERA, atrasentan, have shown a more favorable profile at lower dose ranges together with significant add-on effect on proteinuria reduction in type 2 diabetic patients.33, 34 However, the long-term nephroprotective potential of atrasentan is not known and is currently being established in an ongoing Study of Diabetic Nephropathy with Atrasentan (SONAR) trial in type 2 diabetic patients with proteinuria.
DUET is the first study to evaluate the antiproteinuric effects and long-term safety of a dual angiotensin II and ET 1 antagonist in the nondiabetic context of primary FSGS. Evaluation of the effects on proteinuria will be specifically important in primary FSGS, a podocytopathy in which changes in proteinuria are critical in disease pathogenesis and course.
It is important to recognize the potential limitations of the DUET trial. The intended sample size is modest, and the study cohort may not reflect the diversity of patients affected by FSGS. Although participating sites are encouraged to recruit every eligible patient, which should include a representative distribution of patient demography, the study sample size will not support ancestry-stratified analysis. The protocol does not include assessment of proteinuria after discontinuation of drug treatment; therefore, it will not be possible to definitively ascertain the degree to which beneficial effects of sparsentan endure beyond the treatment period. The double-blind treatment phase is only 8 weeks in duration. However, as outlined above, the option for patients to participate in the open-label phase will enable collection of data to assess the effect of sparsentan on proteinuria for a more extended period and to clarify the safety profile. Potential unintended consequences of sparsentan use include exacerbation of edema, increased incidence of anemia and heart failure, and adverse effects on acid−base balance. Careful follow-up of the study cohort with surveillance for these and other unanticipated adverse effects will be essential before sparsentan can be considered for routine use in patients with FSGS.
Overall, this phase 2, randomized, double-blind, active control FSGS clinical trial will test the efficacy and safety of the dual ARB-ETA compared with ARB alone for the control of FSGS in children and adults. In addition to double-blind evaluation of antiproteinuric effects of sparsentan over the period of 8 weeks, the long-term, open-label treatment will provide information on sparsentan safety and clinically relevant outcomes, namely prevention of increased serum creatinine concentration and progression to ESRD. Sparsentan may be the first agent acting as an endothelin antagonist that is suitable for extended use as a nephroprotective agent. This would be especially notable in patients with a glomerular disease such as FSGS who are susceptible to edema as part of their clinical phenotype.
Disclosure
DSG, PN, SA, TS, KEM, VKD, KEM, PP, and HT are investigators for the DUET study. PN and SA have acted as consultants to Retrophin, Inc. DSG has received research funding, paid to the University of Michigan, from Bristol-Myers Squibb, NephCure Kidney International, and Retrophin, and has acted as consultant to Complexa, Dimerix, and Janssen. TS has been an investigator for studies funded by Alexion and Mallinckrodt. HT has acted as consultant to Bristol-Myers Squibb, Genzyme, Kaneka, Optherion, and Otsuka. RK, MEM, JLH, and AS are employees of Retrophin and may hold Retrophin stock and/or other options.
Acknowledgments
This study is fully funded by Retrophin, Inc. The study sponsor has been actively involved in study design, day-to-day study operations, data management, and has engaged in analysis and interpretation of data, writing of the report, and has taken an active role in the decision to submit this report for publication. The authors were involved in the decision to submit this manuscript and will take public responsibility for all aspects of the publication. Editorial support for this manuscript was provided by Shelly Asiala-Heckner, PharmD, CMPP, of JB Ashtin.
Trial registration: NCT01613118. Date of registration on 04 June 2012.
Please see the Supplementary Declarations for further information on trial status, ethics approval and consent to participate, availability of data and materials, and authors' contributions/roles and responsibilities.
Footnotes
AS was an employee of Retrophin, Inc. at the time of manuscript development.
Supplementary Material
References
- 1.D’Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 2.Sadowski C.E., Lovric S., Ashraf S. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiser J., Nast C.C., Alachkar N. Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21:417–421. doi: 10.1053/j.ackd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronbichler A., Leierer J., Oh J. Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. Biomed Res Int. 2016;2016:2150451. doi: 10.1155/2016/2150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg A.Z., Naicker S., Winkler C.A. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11:150–160. doi: 10.1038/nrneph.2015.9. [DOI] [PubMed] [Google Scholar]
- 6.Messina M., Gallo E., Mella A. Update on the treatment of focal segmental glomerulosclerosis in renal transplantation. World J Transplant. 2016;6:54–68. doi: 10.5500/wjt.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spino C., Jahnke J.S., Selewski D.T. Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr. 2016;4:25. doi: 10.3389/fped.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R., Li Y., Robinson B. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66:Svii. doi: 10.1053/j.ajkd.2015.05.001. S1−305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 10.Hanko J.B., Mullan R.N., O’Rourke D.M. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24:3050–3054. doi: 10.1093/ndt/gfp254. http://dx.doi.org/10.1093/ndt/gfp254 [DOI] [PubMed] [Google Scholar]
- 11.Kiffel J., Rahimzada Y., Trachtman H. Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis. 2011;18:332–338. doi: 10.1053/j.ackd.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava T., Simon S.D., Alon U.S. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13:13–18. doi: 10.1007/s004670050555. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:135. [Google Scholar]
- 14.Beer A., Mayer G., Kronbichler A. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. Biomed Res Int. 2016;2016:4192578. doi: 10.1155/2016/4192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattran D.C., Appel G.B., Hebert L.A. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 16.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2011;79:678–685. doi: 10.1038/ki.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matalon A., Valeri A., Appel G.B. Treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2000;20:309–317. [PubMed] [Google Scholar]
- 18.Sethna C.B., Gipson D.S. Treatment of FSGS in children. Adv Chronic Kidney Dis. 2014;21:194–199. doi: 10.1053/j.ackd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Lau E.W., Ma P.H., Wu X. Mycophenolate mofetil for primary focal segmental glomerulosclerosis: systematic review. Ren Fail. 2013;35:914–929. doi: 10.3109/0886022X.2013.794687. [DOI] [PubMed] [Google Scholar]
- 20.Kitiyakara C., Eggers P., Kopp J.B. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825. [PubMed] [Google Scholar]
- 21.Pullen N., Fornoni A. Drug discovery in focal and segmental glomerulosclerosis. Kidney Int. 2016;89:1211–1220. doi: 10.1016/j.kint.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cravedi P., Ruggenenti P., Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8:301–306. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos E., Stangou M., Papagianni A. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:1348–1356. doi: 10.1093/ndt/15.9.1348. [DOI] [PubMed] [Google Scholar]
- 24.Cattran D.C., Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 25.Gipson D.S., Chin H., Presler T.P. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 26.Troyanov S., Wall C.A., Miller J.A. Toronto Glomerulonephritis Registry G: focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 27.Kohan D.E., Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86:896–904. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komers R., Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877–R884. doi: 10.1152/ajpregu.00425.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagliardini E., Corna D., Zoja C. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297:F1448–F1456. doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- 30.Zoja C., Cattaneo S., Fiordaliso F. Distinct cardiac and renal effects of ETA receptor antagonist and ace inhibitor in experimental type 2 diabetes. Am J Physiol Renal Physiol. 2011;301:F1114–F1123. doi: 10.1152/ajprenal.00122.2011. [DOI] [PubMed] [Google Scholar]
- 31.Meyers K.E., Sethna C. Endothelin antagonists in hypertension and kidney disease. Pediatr Nephrol. 2013;28:711–720. doi: 10.1007/s00467-012-2316-4. [DOI] [PubMed] [Google Scholar]
- 32.Mann J.F., Green D., Jamerson K. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohan D.E., Pritchett Y., Molitch M. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763–772. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Zeeuw D., Coll B., Andress D. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buelli S., Rosano L., Gagliardini E. Beta-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol. 2014;25:523–533. doi: 10.1681/ASN.2013040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daehn I., Casalena G., Zhang T. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadegbeku C.A., Gipson D.S., Holzman L.B. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Varni J.W., Seid M., Rode C.A. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.