Abstract
Obesity causes various structural, hemodynamic, and metabolic alterations in the kidney. Most of these are likely to be compensatory responses to the systemic increase in metabolic demand that is seen with obesity. In some cases, however, renal injury becomes clinically apparent as a result of compensatory failure. Obesity-related glomerulopathy is the best known of such disease states. Factors that may sensitize obese individuals to renal compensatory failure and associated injury include the severity and number of obesity-associated conditions or complications, including components of metabolic syndrome, and the mismatch of body size to nephron mass, due to nephron reductions of congenital or acquired origin.
Keywords: chronic kidney disease, hyperfiltration, metabolic syndrome, nephron number, obesity, obesity-related glomerulopathy
With the worldwide epidemic of obesity, the increase in obesity-related complications is becoming a serious socioeconomic problem.1, 2, 3 The kidney may exhibit health disorders related to obesity. Obesity not only increases the risk of progression of preexisting renal diseases but is itself also an independent risk factor of renal injury.4, 5 Obesity-related glomerulopathy (ORG) is the best-known renal disease secondary to obesity. From observation of this unique disease state, significant knowledge has accumulated regarding the clinicopathological characteristics of renal injury in obesity.6, 7, 8, 9 Importantly, however, there is a large difference between the fraction of the general population that is obese and the fraction that actually develops renal impairment. In addition, the severity of obesity-related renal impairment is not necessarily related to the severity of obesity. Thus, it is conceivable that obesity is not the sole factor causing obesity-related renal injury, and that there may be additional or predisposing factors that explain the considerable differences among individuals in susceptibility to renal injury due to obesity.
In this review, we will outline (i) the renal structural, hemodynamic, and metabolic alterations in obesity and obesity-related renal impairment; (ii) the clinicopathological features of renal injury associated with obesity (primarily in ORG); and (iii) the potential additional or predisposing factors that may sensitize patients to renal structural or functional compensatory failure and subsequent injury.
Renal Alterations in Obesity or Obesity-Related Renal Impairment
Kidney Weight
Studies of adult autopsies have shown that kidney weight increases with increasing body mass index (BMI).10 The weight of kidneys from autopsies of obese individuals has been found to be significantly greater than those of normal-weight controls.11 An autopsy study of obese children who died in traumatic accidents found that the weights of all organs except the brain, including the heart, kidneys, pancreas, liver and spleen, were heavier than those of height-, age-, and sex-matched control children.12 Although the mechanism of increased kidney weight in obesity is unknown, it may be related to compensatory hypertrophy of individual nephrons, as a result of increased tubular and glomerular functions associated with obesity. In addition, intracellular or extracellular accumulation of fluid and lipid components may contribute to the increased weight of the obese kidney.
Glomerular Hypertrophy
Numerous morphometric studies of factors related to glomerular size have been performed, using autopsies or biopsies of nondiseased and diseased kidneys.13, 14, 15, 16 It has been consistently observed in many of these studies that body size, especially as defined by obesity or BMI, is one of the most important determinants of glomerular size. In obese subjects, glomerular sizes are larger, even in the absence of apparent renal disease or injury.13, 14, 15 Glomerular hypertrophy in obesity may be largely attributable to compensatory changes accompanying glomerular hyperfiltration. Glomerular hyperfiltration can be reduced in obese subjects by weight loss, but it has not been clarified whether glomerular size decreases during such “backtracking” of glomerular function. Although the increases in glomerular size found in obese subjects may be due in part to an increase in the number of glomerular capillaries, no previous study has directly tested this hypothesis in humans.
Tubular Hypertrophy
Compared with those of the glomeruli, there have been very few studies of structural alterations in renal tubules associated with obesity. Morphometric analysis may be hampered by the structural complexity of renal tubules. A study of biopsy samples from proteinuric obese patients found that the cross-sectional area of proximal tubular epithelial cells was 33% larger, and the proximal tubular lumen 54% larger, than in proteinuric nonobese patients.17
Hemodynamic Changes
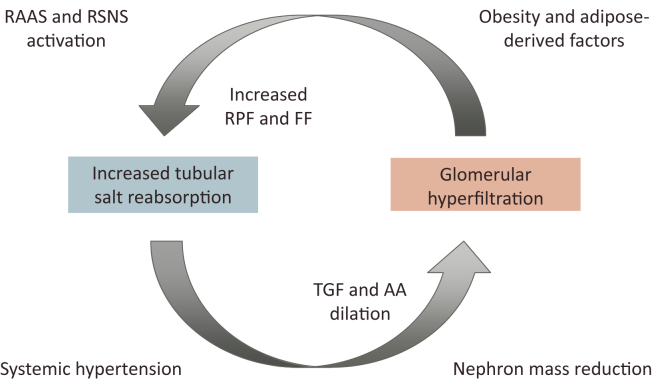
It is known that changes in intrarenal hemodynamics are characteristic of obesity. Previous animal experiments and intervention studies in obese subjects have demonstrated that renal plasma flow (RPF) and glomerular filtration rate (GFR) both increase with obesity.18, 19, 20, 21 Renal tubular overload in obesity is characterized by an increase in the filtration fraction (GFR/RPF), and may stimulate sodium and water reabsorption in the proximal tubules, resulting in decreased pre-glomerular vascular resistance via the tubuloglomerular feedback mechanism.22 A dilation of the glomerular afferent arterioles leads to a further increase in the GFR (glomerular hyperfiltration). Although the origin of such a vicious circle between increased salt reabsorption in the tubules and glomerular hyperfiltration remains unclear, such alterations in renal hemodynamics may constitute the most important pathophysiological basis for the renal abnormalities of obesity (Figure 1). Consistent with this hypothesis, cetazolamide, a carbonic anhydrase inhibitor that inhibits salt reabsorption in the proximal tubule, has been shown to reduce GFR in obese nondiabetic subjects.23
Figure 1.
Hemodynamic abnormalities and factors promoting obesity-related renal injury. Renal plasma flow (RPF) and glomerular filtration rate (GFR) increase in obesity. Such renal tubular overload in obesity is characterized by an increase in the filtration fraction (FF: GFR/RPF) and may stimulate sodium and water reabsorption in the proximal tubules, decreasing preglomerular vascular resistance via tubuloglomerular feedback (TGF). A dilation of the glomerular afferent arterioles (AAs) leads to a further increase in GFR, that is, glomerular hyperfiltration. Various factors associated with obesity, including adipose-derived factors, activation of the renin−angiotensin−aldosterone system (RAAS) and renal sympathetic nervous system (RSNS), systemic hypertension, and nephron mass reduction, constitute and promote this vicious circle.
Obesity is often accompanied by systemic hypertension, via several mechanisms.24 Systemic hypertension caused by obesity additionally increases glomerular blood flow through the dilated AAs, with reduced autoregulatory capacity, and promotes irreversible arteriolosclerotic changes that further promote glomerular hypertension and hyperfiltration.
Increased Salt Sensitivity
The changes in renal hemodynamics found in obesity are closely linked to increased salt sensitivity.25 In fact, compared to lean subjects, obese subjects are more likely to develop salt-sensitive hypertension and proteinuria from excessive salt intake.26, 27 One important mechanism by which salt sensitivity is increased with obesity is an activation of the intrarenal renin−angiotensin−aldosterone system (RAAS).28, 29 Activation of renal sympathetic nerves may also be implicated in the increased salt reabsorption seen in obesity.30, 31
RAAS Activation
Adipose tissue is known to contain all components of the RAAS system, and activation of this system occurs in obese adipose tissue.32, 33 Production of angiotensinogen, aldosterone, and aldosterone-stimulating factor is increased in obese adipocytes.34, 35, 36 Plasma aldosterone concentrations in obese subjects are high, correlated with visceral fat mass, and decreased by weight loss.37, 38
In obese individuals, the RAAS is activated in renal tissue, and increases sodium reabsorption by several mechanisms.39, 40, 41 A prospective crossover study conducted in obese patients with proteinuria showed that therapy with an aldosterone antagonist more effectively reduced proteinuria than did an angiotensin-converting enzyme inhibitor.42 In spontaneously hypertensive obese rats, renal injury and proteinuria were correlated with elevated blood aldosterone concentrations.43 Notably, that study showed that expression of aldosterone receptors in glomerular podocytes was enhanced, and that glomerular injury and proteinuria were ameliorated by the administration of an aldosterone receptor antagonist. Thus, the activation of the intrarenal RAAS system, especially involving aldosterone or its receptor, is likely to play a major role in the development of proteinuric renal injury associated with obesity.
Glucose Metabolism
The kidneys play an important role in the regulation of glucose homeostasis via glucose utilization, gluconeogenesis, and glucose reabsorption.44 Of these, renal glucose reabsorption is the main contributor to glucose homeostasis in the kidney. Reabsorption of glucose in the proximal tubules is mediated by sodium−glucose co-transporters (SGLTs), specifically SGLT-2, which controls 90% of all glucose reabsorption in the kidney.45 Hyperglycemia and angiotensin II are known to upregulate the expression of SGLT-2.46, 47 Thus, in obesity, in which both hyperglycemia and RAAS activation occur, renal tubular reabsorption of glucose may be increased via upregulation of the expression of SGLT-2.
In addition to reducing glycemia, therapy with an SGLT-2 inhibitor also slowed the progressive loss of renal function in proteinuric patients with diabetes.48 Animal models of diabetes have demonstrated that SGLT-2 inhibition affects the tubuloglomerular feedback system. A study using dapagliflozin resulted in reduced reabsorption of glucose in the proximal tubules, leading to an increase in distal delivery of glucose and sodium, and a decrease in GFR.49 It is conceivable that similar results may be observed in nondiabetic obesity-related renal disease, suggesting a potential role for SGLT2 in such patients.
Adipose-Derived Inflammation
Obesity is a chronic low-grade inflammatory condition, in which adipose tissue serves as the source of inflammatory cytokines.50 Visceral adipose tissue produces less adiponectin and more pro-inflammatory cytokines, including tumor necrosis factor−α (TNF-α) and interleukin-6 (IL-6), which can induce insulin resistance and promote endothelial dysfunction.51
Adipocytes can facilitate vasculogenesis locally and in distal organs by secreting angiogenic factors.52 In a study using obese Zucker rats, significant increases in cortical and medullary microvascular density developed in parallel with intrarenal inflammation.53 Importantly, a similar increase in microvasculature was found in a hypercholesterolemia model, and was inhibited by thalidomide, a potent anti-inflammatory and antiangiogenic agent. Inhibition was accompanied by restoration of renovascular endothelial function but decreased basal renal hemodynamics.54 These findings suggest that neovascularization in the obese or hypercholesterolemic kidney involves a compensatory mechanism to sustain basal renal vascular function.
Deposition of Lipid Components
Accumulation of fat components in cells, originally a vital function for conserving intracellular energy, is increased in obesity.55 Numerous experiments in vivo and in vitro have suggested an association between intrarenal accumulation of lipid components and renal injury.56 However, our understanding of fat accumulation in the human kidney has been very limited in comparison to what we know about this process in other organs, such as the heart, liver, and skeletal muscle. A recent study demonstrated that the degree of accumulation of triglycerides in the human renal cortex was correlated with BMI.57 Although deposition of triglycerides was observed in both glomerular and tubular cells, it was predominantly found in proximal tubular cells.57
Renal Injury Associated With Obesity
Obesity-Related Glomerulopathy
Autopsy cases in which proteinuric renal injury was associated with extreme obesity first appeared more than 40 years ago, and have been followed by additional autopsy and biopsy cases.58, 59, 60, 61, 62 In recent years, this chronic renal complication of obesity has been named obesity-related glomerulopathy, and its clinical and histopathological features have been identified and clarified in cases from several countries.6, 7, 8, 9 In general, the diagnostic criteria of ORG are defined as BMI values of 30 kg/m2 or more and the exclusion of other renal diseases, both clinically and histopathologically.6 ORG does not necessarily occur in individuals with more severe obesity. Studies have shown that, among patients with even a moderate degree of obesity (BMI values < 30 kg/m2), a subgroup exists that demonstrates the clinical and histopathological characteristics of typical ORG.8, 63
Clinical Features of ORG
Clinically, isolated proteinuria of unknown onset, with or without renal impairment, is the initial symptom in most cases. The magnitude of urinary protein excretion, and of renal functional reserve at the time of biopsy, varies among cohorts identified in different countries.6, 7, 8, 9 This may be attributed to different biopsy policies or to additional effects of already-advanced renal injury present at the time of diagnosis, independent of BMI. Although the absence of a decrease in serum protein levels, even in the presence of relatively high urinary protein excretion, is characteristic, the mechanism behind this remains unclear. The presence of full nephrotic syndrome is unusual in ORG. Therefore patients with ORG rarely display obvious symptoms such as edema. Other clinical findings commonly associated with ORG are hypertension and dyslipidemia.
Few studies have examined the long-term outcomes of patients with ORG.6, 7, 8, 9 The typical clinical course is stable or slowly progressive proteinuric renal impairment, but the long-term outcomes include progression to end-stage renal disease in 10% to 33% of patients diagnosed with ORG. Factors associated with progression include older age, renal dysfunction, and greater proteinuria at presentation, as well as greater time-averaged proteinuria during follow-up.6, 7, 9
Renal Histopathology of ORG
Typical features of renal histopathology for ORG patients include glomerulomegaly and focal segmental glomerulosclerosis (FSGS) (Figure 2)6, 64 Glomerulomegaly is very likely a result of abnormalities in renal hemodynamics associated with obesity, including increased RPF and GFR.20, 21 To date, there is no consensus on the quantitative definition of glomerulomegaly in ORG. A morphometric study found that mean glomerular volume in ORG patients with preserved renal function was about 3-fold that seen in control subjects.64 The glomerular capillaries of ORG patients appear to be increased in number, suggesting de novo formation of microvessels. The expression of capillary growth−promoting factors, such as vascular endothelial growth factor (VEGF), is increased in the glomerular tissues of ORG patients, supporting this idea.65
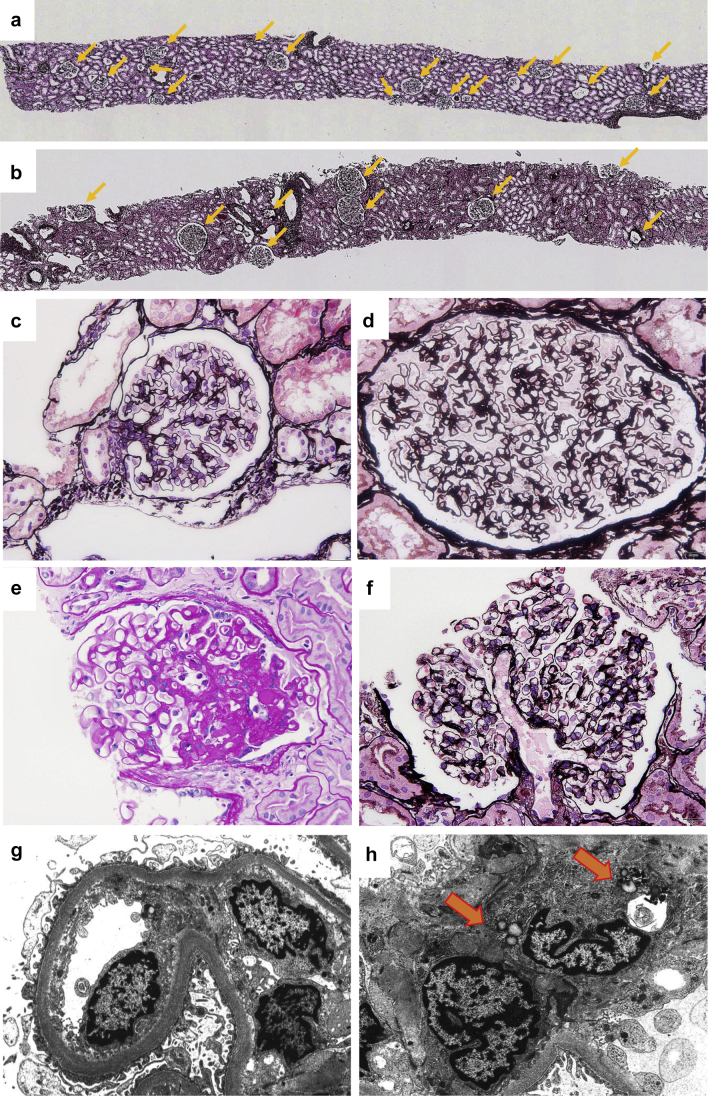
Figure 2.
Histopathology of obesity-related glomerulopathy. Representative renal biopsy findings in (a) a nonobese kidney transplantation donor showing normal glomerular density (5.0/mm2), and in (b) a patient with obesity-related glomerulopathy (ORG) showing a low glomerular density (2.0/mm2). Arrows indicate nonsclerotic glomeruli (periodic acid−methenamine silver stain, original magnification ×25). Glomerulus from (c) a nonobese patient showing minimal change nephrotic syndrome, and (d) extremely hypertrophied glomerulus (glomerulomegaly) in an ORG patient (periodic acid−methenamine silver stain, original magnification ×400). In ORG, (e) lesions of segmental glomerular sclerosis are often found in relation to the vascular pole of glomeruli (perihilar variant) (periodic acid−Schiff stain, original magnification ×400). (f) Dilated glomerular afferent arterioles in a patient with ORG (periodic acid−methenamine silver stain, original magnification ×400). Electron micrograph showing (g) mild thickening of glomerular basement membrane, mild podocyte foot process effacement, and widening of the subendothelial space in glomeruli of ORG (original magnification ×5000). Arrows indicate (h) intracytoplasmic lipid vacuoles in glomerular mesangial cells found in ORG (original magnification ×5000).
The glomerular density (number of glomeruli per unit of renal cortical area) has been found to be lower in biopsy specimens of ORG patients versus control subjects.64 Although the origin of this low glomerular density in biopsy specimens of ORG patients has not been determined, it may be due to the lower number of nephrons in these patients.
FSGS lesions are not observed in all cases of ORG, and may represent differences in the degree of obesity or renal impairment. In a report from China, however, there was no significant difference in glomerular size or number of cases showing FSGS between groups based on BMI.8 Among subtypes of FSGS, ORG exhibits a predominance of the perihilar variant.6 It is believed that this reflects an excessive pressure load on the vascular poles of glomeruli, due to the renal hemodynamic abnormalities of obesity. Obesity-induced glomerular hypertrophy and glomerulomegaly may cause glomerular podocytes to enlarge their foot processes to cover the expanded glomerular surface area. Consistent with this, a relative reduction in the coating area of glomerular podocytes on the glomerular surface is found in patients with ORG.66 This may cause changes in podocyte function and a consequent loss in protein selectivity, podocyte detachment, and replacement by matrix deposition, leading to FSGS.
Differential Diagnosis of ORG
Diagnosis of ORG is defined morphologically as glomerulomegaly with or without FSGS lesions.6, 64 Patients whose renal biopsy specimens show histopathological evidence of other primary or secondary renal diseases, including immune-complex glomerulonephritis and diabetic nephropathy, are excluded. Increased glomerular basement membrane thickness alone is not a criterion for exclusion, because obese patients can exhibit increased glomerular basement membrane thickness in the absence of diabetes.67, 68 Likewise, the presence of hypertension is not an exclusion criterion. The biopsy specimens of some obese hypertensive patients show moderate to severe vascular lesions, which are accompanied by collapsed glomeruli. Patients with such histological features are diagnosed with hypertensive nephrosclerosis rather than ORG. In electron microscopy studies, the incidence of foot process fusion among glomerular podocytes is lower in ORG (∼40%) than in idiopathic FSGS (∼75%).6 Focal lipid vacuoles are occasionally seen in the cytoplasm of glomerular mesangial cells and tubular epithelial cells.56
Factors Predisposing Obese Individuals to Renal Injury
Obesity Type
There are major functional differences among adipocytes that are related to their anatomical location in visceral or subcutaneous fat. The enhanced pathologic profile of visceral fat relative to subcutaneous fat gives it a greater potential to increase cardiovascular risks, and increases the propensity toward hypertension and risk of chronic kidney disease (CKD).69, 70, 71
Components of Metabolic Syndrome
Obesity is a risk factor for all components of metabolic syndrome: impaired glucose tolerance, hypertension, and dyslipidemia. Each component can induce kidney injury and may exacerbate pre-existing renal disease.72 Combinations of components may synergistically increase the risk of CKD, and the risk of progression of pre-existing CKD.73 It is possible that the duration and severity of each disease condition is correlated with the severity of renal impairment due to obesity. Recent studies have shown that CKD incidence in metabolically normal obese subjects is not different from that in metabolically normal nonobese subjects.74 In addition, the incidence of CKD in metabolically abnormal obese subjects is higher than that in metabolically abnormal nonobese subjects. It is therefore conceivable that obesity increases the risk of renal injury in combination with these metabolic abnormalities.
Other Obesity-Associated Complications
Sleep apnea and nocturnal hypoxemia are frequent complications of obesity, and these disease states have been associated with hypertension and loss of renal function.75 Obese patients with sleep apnea often have pulmonary hypertension, which is known to increase right ventricular overload.76 This may lead to increased renal vein pressure and congested intrarenal circulation, increasing activation of RAAS and salt reabsorption. Nonalcoholic fatty liver disease often accompanies obesity. In a meta-analysis, nonalcoholic fatty liver disease was shown to be associated with an increased risk of incidence and progression of CKD.77 These findings suggest that conditions frequently accompanying obesity may play additive roles in the progression of renal injury associated with obesity.
Nephron Mass Reduction
In a case series of ORG patients with FSGS, 8 of 15 patients exhibited an apparent reduction in the number of nephrons, due to congenital anomalies of the kidney and urinary tract, such as unilateral renal agenesis.7 Obesity has also been identified as a risk factor for proteinuria and renal dysfunction in follow-up studies of patients with unilateral nephrectomy or with congenital renal agenesis.78, 79 These results suggest that obesity may participate in the development of renal injury under conditions of severe renal mass reduction. Glomerular enlargement and scarring caused by a mismatch between body size and nephron mass may result in further reductions in nephron mass, leading to a vicious circle of glomerular compensatory failure and injury.
Nephron Number
The etiology of reduced nephron number, in the absence of any apparent renal morphological or functional abnormalities, remains incompletely understood. Nephrogenesis in humans begins at week 9 of gestation, and the final number of nephrons is determined by weeks 34 to 36; nephron number does not increase after birth. Recent autopsy studies have demonstrated considerably greater variability in total nephron number in the normal population than was previously suspected (up to a 10-fold difference).80, 81 Total nephron number is correlated with weight at birth, suggesting the importance of the intrauterine environment.82 Notably, the marked variability in glomerular number has been reported to have important implications for susceptibility to renal insufficiency and progression to end-stage renal disease, suggesting links among weight at birth, nephron number, and susceptibility to progressive renal disease.83 Interestingly, the glomerular density observed in renal biopsy specimens of ORG patients is extremely low relative to that of control subjects.64 Experimentally, rats raised on a low-calorie diet during the gestation period develop obesity, metabolic syndrome, diabetes, and hypertension when fed a high-calorie diet after birth.84 The body size−nephron mass mismatch that is potentially present in obese patients with ORG may therefore originate from the fetal environment. Studies of birthweight and the total number of nephrons, in obese subjects with and without ORG, would be required to test this hypothesis.
As with the number of nephrons, significant individual differences in the number of glomerular podocytes exist.85 Glomerular podocytes do not readily divide, and respond to glomerular enlargement by extending their foot processes. Thus, a low podocyte number may also affect the adaptive capacity in response to the glomerular enlargement seen in obesity.
Chronic Kidney Disease
A reduction in nephron mass is most frequently observed during the progression of CKD. The fraction of sclerotic glomeruli increases with the progression of renal impairment in chronic progressive renal diseases of any cause, and the remnant glomeruli exhibit compensatory hyperfiltration and enlargement.86 In the nephron mass reduction seen in chronic renal disease patients, obesity may additionally influence renal impairment. For example, BMI values of 25 kg/m2 or more increase the risk of disease progression in patients with IgA nephropathy.87 On the other hand, weight loss is effective for attenuating the progressive loss of renal function in obese patients with diabetic or nondiabetic renal disease.88 These results suggest that obesity may contribute to the further progression of already recognized CKD of any cause by inducing additional obesity-related hemodynamic and metabolic loading.
Aging
The loss of glomeruli with age occurs throughout adult life, with a mean predicted loss of ∼4500 glomeruli per kidney per year from 18 to 70 years of age.89 Thus, as with congenital differences in total nephron number among individuals, reduction during normal aging can greatly influence susceptibility to renal injury in obese individuals.
A recent, detailed analysis of nephrosclerotic specimens suggested that the progressive loss of glomerular podocytes is part of the process of normal aging.90 Thus, aging-related decreases in the number of glomerular podocytes may also contribute to a functional mismatch between reduced podocyte number and the glomerular enlargement seen in obesity.
The above-mentioned additional and predisposing factors that may sensitize obese individuals to renal compensatory failure and injury are summarized in Table 1.
Table 1.
Factors predisposing obese individuals to renal injury
| Obesity type |
| Visceral fat obesity |
| Components of metabolic syndrome |
| Impaired glucose tolerance |
| Hypertension |
| Dyslipidemia |
| Obesity-associated conditions or complications |
| Sleep apnea syndrome |
| Pulmonary hypertension and right ventricular overload |
| Nonalcoholic fatty liver disease |
| Low nephron number |
| Low birth weight |
| Intra-uterine growth retardation |
| Preterm birth |
| Nephron mass reduction |
| Congenital anomalies of the kidney and urinary tract |
| Nephrectomy |
| Progressive loss of functioning nephron |
| Chronic kidney disease of any cause |
| Aging |
Conclusion
Obesity causes various structural, hemodynamic, and metabolic alterations in the kidney. In addition to the results from experimental systems, detailed observational studies on the clinicopathological features of patients with obesity or ORG have contributed much to our knowledge of these alterations. Understanding the factors that predispose patients to the renal adaptive and maladaptive changes associated with obesity could direct the establishment of preventive and therapeutic interventions to slow the epidemic of CKD.
Disclosure
All the authors declared no competing interests.
References
- 1.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;26:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration. Whitlock G., Lewington S., Sherliker P. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;28:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Beydoun M.A., Liang L., Caballero B., Kumanyika S.K. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Chen X., Song Y. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 5.Fox C.S., Larson M.G., Leip E.P. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 6.Kambham N., Markowiz G.S., Valeri A.M. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 7.Praga M., Hernandez E., Morales E. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.M., Li S.J., Chen H.P. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi N., Koike K., Hirano K. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol. 2013;17:379–385. doi: 10.1007/s10157-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 10.Mandal R., Loeffler A.G., Salamat S., Fritsch M.K. Organ weight changes associated with body mass index determined from a medical autopsy population. Am J Forensic Med Pathol. 2012;33:382–389. doi: 10.1097/PAF.0b013e3182518e5f. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske B.L., Napier J. Glomerular sclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45–50. doi: 10.1159/000166902. [DOI] [PubMed] [Google Scholar]
- 12.Naeye R.L., Roode P. The sizes and numbers of cells in visceral organs in human obesity. Am J Clin Pathol. 1970;54:251–253. doi: 10.1093/ajcp/54.2.251. [DOI] [PubMed] [Google Scholar]
- 13.Samuel T., Hoy W.E., Douglas-Denton R. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 14.Hoy W.E., Hughson M.D., Zimanyi M. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin Nephrol. 2010;74(suppl 1):S105–S112. doi: 10.5414/cnp74s105. [DOI] [PubMed] [Google Scholar]
- 15.Puelles V.G., Zimanyi M.A., Samuel T. Estimating individual glomerular volume in the human kidney: clinical perspectives. Nephrol Dial Transplant. 2012;27:1880–1888. doi: 10.1093/ndt/gfr539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi N., Utsunomiya Y., Koike K. Factors related to the glomerular size in renal biopsies of chronic kidney disease patients. Clin Nephrol. 2013;79:277–284. doi: 10.5414/CN107817. [DOI] [PubMed] [Google Scholar]
- 17.Tobar A., Ori Y., Benchetrit S. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8:e75547. doi: 10.1371/journal.pone.0075547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henegar J.R., Bigler S.A., Henegar L.K. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 19.Reisin E., Messerli F.G., Ventura H.O., Frohlich E.D. Renal haemodynamic studies in obesity hypertension. J Hypertens. 1987;5:397–400. [PubMed] [Google Scholar]
- 20.Chagnac A., Weinstein T., Korzets A. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 21.Chagnac A., Weinstein T., Herman M. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 22.Vallon V., Richter K., Blantz R.C. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 23.Zingerman B., Herman-Edelstein M., Erman A. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS One. 2015;10:e0137163. doi: 10.1371/journal.pone.0137163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall J.E., do Carmo J.M., da Silva A.A. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;13(116):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strazzullo P., Barba G., Cappuccio F.P. Altered renal sodium handling in men with abdominal adiposity: a link to hypertension. J Hypertens. 2001;19:2157–2164. doi: 10.1097/00004872-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Rocchini A.P., Key J., Bondie D. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 27.Verhave J.C., Hillege H.L., Burgerhof J.G., PREVEND Study Group Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004;256:324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim S., Soltani-Bejnood M., Quignard-Boulange A. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J Biomed Biotechnol. 2006;2006:27012. doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C., Lin Y., Luo R. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am J Physiol Renal Physiol. 2016;310:F351–F363. doi: 10.1152/ajprenal.00223.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss N.G. Renal function and renal afferent and efferent nerve activity. Am J Physiol. 1982;243:F425–F433. doi: 10.1152/ajprenal.1982.243.5.F425. [DOI] [PubMed] [Google Scholar]
- 31.Kassab S., Kato T., Wilkins F.C. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 32.Cooper R., McFarlane-Anderson N., Bennett F.I. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens. 1997;11:107–111. doi: 10.1038/sj.jhh.1000391. [DOI] [PubMed] [Google Scholar]
- 33.Achard V., Boullu-Ciocca S., Desbriere R. Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R274–R282. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- 34.Bochud M., Nussberger J., Bovet P. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 35.Rossi G.P., Belfiore A., Bernini G. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 36.Ehrhart-Bornstein M., Arakelyan K., Krug A.W. Fat cells may be the obesity–hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30:865–870. doi: 10.1081/erc-200044122. [DOI] [PubMed] [Google Scholar]
- 37.Tuck M.L., Sowers J., Dornfeld L. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 38.Engeli S., Böhnke J., Gorzelniak K. Weight loss and the renin–angiotensin–aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 39.Ohsawa M., Tamura K., Wakui H. Deletion of the angiotensin II type 1 receptor-associated protein enhances renal sodium reabsorption and exacerbates angiotensin II-mediated hypertension. Kidney Int. 2014;86:570–581. doi: 10.1038/ki.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peti-Peterdi J., Warnock D.G., Bell P.D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 41.Rozansky D.J. The role of aldosterone in renal sodium transport. Semin Nephrol. 2006;26:173–181. doi: 10.1016/j.semnephrol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Morales E., Huerta A., Gutiérrez E. The antiproteinuric effect of the blockage of the renin-angiotensin-aldosterone system (RAAS) in obese patients. Which treatment option is the most effective? Nefrologia. 2009;29:421–429. doi: 10.3265/Nefrologia.2009.29.5.5448.en.full. [DOI] [PubMed] [Google Scholar]
- 43.Nagase M., Yoshida S., Shibata S. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 44.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeFronzo R.A., Davidson J.A., Del P.S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 46.Rahmoune H., Thompson P.W., Ward J.M. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 47.Bautista R., Manning R., Martinez F. Angiotensin II-dependent increased expression of Na+-glucose cotransporter in hypertension. Am J Physiol Renal Physiol. 2004;286:F127–F133. doi: 10.1152/ajprenal.00113.2003. [DOI] [PubMed] [Google Scholar]
- 48.Wanner C., Inzucchi S.E., Lachin J.M., EMPA-REG OUTCOME Investigators Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 49.Thomson S.C., Rieg T., Miracle C. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisse B.E. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 51.Hamdy O., Porramatikul S., Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 52.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 53.Iliescu R.I., Chade A.R. Progressive renal vascular proliferation and injury in obese zucker rats. Microcirculation. 2010;17:250–258. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chade A.R., Bentley M.D., Zhu X. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 55.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 56.de Vries A.P., Ruggenenti P., Ruan X.Z., ERA-EDTA Working Group Diabesity Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2:417–426. doi: 10.1016/S2213-8587(14)70065-8. [DOI] [PubMed] [Google Scholar]
- 57.Bobulescu I.A., Lotan Y., Zhang J. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014;9:e101285. doi: 10.1371/journal.pone.0101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisinger J.R., Kempson R.L., Eldridge F.L., Swenson R.S. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
- 59.Warnke R.A., Kempson R.L. The nephrotic syndrome in massive obesity. Arch Pathol Lab Med. 1978;102:431–438. [PubMed] [Google Scholar]
- 60.Kasiske B.L., Crosson J.T. Renal disease in patients with massive obesity. Arch Intern Med. 1986;146:1107–1109. [PubMed] [Google Scholar]
- 61.Jennette J.C., Charles L., Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep apnea syndrome. Am J Kidney Dis. 1987;10:470–472. doi: 10.1016/s0272-6386(87)80196-8. [DOI] [PubMed] [Google Scholar]
- 62.Verani R.R. Obesity-associated focal segmental glomerulosclerosis: pathological features of the lesions and relationship with cardiomegaly and hyperlipidemia. Am J Kidney Dis. 1992;20:629–634. doi: 10.1016/s0272-6386(12)70230-5. [DOI] [PubMed] [Google Scholar]
- 63.Okabayashi Y., Tsuboi N., Sasaki T. Glomerulopathy associated with moderate obesity. Kidney Int Rep. 2016;1:250–255. doi: 10.1016/j.ekir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuboi N., Utsunomiya Y., Kanzaki G. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7:735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y., Liu Z., Xiang Z. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 66.Chen H.M., Liu Z.H., Zeng C.H. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48:772–779. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Kato S., Nazneen A., Nakashima Y. Pathological influence of obesity on renal structural changes in chronic kidney disease. Clin Exp Nephrol. 2009;13:332–340. doi: 10.1007/s10157-009-0169-3. [DOI] [PubMed] [Google Scholar]
- 68.Goumenous D.S., Kawar B., El Nahas M. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24:3732–3738. doi: 10.1093/ndt/gfp329. [DOI] [PubMed] [Google Scholar]
- 69.Fox C.S., Massaro J.M., Hoffmann U. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 70.Kaess B.M., Pedley A., Massaro J.M. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young J.A., Hwang S.J., Sarnak M.J. Association of visceral and subcutaneous adiposity with kidney function. Clin J Am Soc Nephrol. 2008;3:1786–1791. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahba I.M., Mak R.H. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka H., Shiohira Y., Uezu Y. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–374. doi: 10.1038/sj.ki.5000050. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto Y., Tanaka M., Okada H. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10:578–583. doi: 10.2215/CJN.08980914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turek N.F., Ricardo A.C., Lash J.P. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–833. doi: 10.1053/j.ajkd.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolignano D., Rastelli S., Agarwal R. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61:612–622. doi: 10.1053/j.ajkd.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 77.Musso G., Gambino R., Tabibian J.H. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Praga M., Hernandez E., Herrero J.C. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111–2118. doi: 10.1111/j.1523-1755.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez E., Gutierrz E., Morales E. Factor influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005;68:263–270. doi: 10.1111/j.1523-1755.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 80.Nyengaard J.R., Bendtsen T.F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 81.Hoy W.E., Bertram J.F., Denton R.D. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 82.Hughson M., Farris A.B., 3rd, Douglas-Denton R. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;83:S32–S37. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 83.Silverwood R.J., Pierce M., Hardy R. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013;84:1262–1270. doi: 10.1038/ki.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vickers M.H., Breier B.H., Cutfield W.S. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 85.Puelles V.G., Douglas-Denton R.N., Cullen-McEwen L.A. Podocyte number in children and adults: associations with glomerular size and numbers of other glomerular resident cells. J Am Soc Nephrol. 2015;26:2277–2288. doi: 10.1681/ASN.2014070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luyckx V.A., Brenner B.M. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 87.Bonet F., Deprele C., Sassolas A. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2003;37:720–727. doi: 10.1016/s0272-6386(01)80120-7. [DOI] [PubMed] [Google Scholar]
- 88.Morales E., Valero M.A., Leon M. Benefical effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41:319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 89.Luyckx V.A., Bertram J.F., Brenner B.M. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 90.Hodgin J.B., Bitzer M., Wickman L. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol. 2015;26:3162–3178. doi: 10.1681/ASN.2014080752. [DOI] [PMC free article] [PubMed] [Google Scholar]