Introduction
It is well known that the strict control of serum inorganic phosphorus (IP) and potassium (K) concentrations is important for the prevention of various complications and improving the prognosis of patients with chronic kidney disease (CKD). Hyperkalemia is associated with sudden cardiac death in hemodialysis (HD) patients and hyperphosphatemia is associated with an increase in cardiovascular events and mortality in CKD patients.1, 2 It is a common practice to restrict IP and K intake when HD patients present with these electrolyte abnormalities.3 However, these abnormalities are not always caused by an excessive intake. We evaluated and treated a CKD case with hyperphosphatemia, hyperkalemia, and azotemia, likely caused by poor carbohydrate intake.
Case Presentation
A 54-year-old man was admitted to our hospital because of right hemiparesis and motor aphasia. He had been on peritoneal dialysis (PD) to treat kidney failure secondary to nephrosclerosis 4 years prior to this admission, and then HD had been added after a year of PD due to poor fluid removal and dialysis efficiency. His dialysis course was complicated by uncontrolled blood pressure and large interdialytic weight gain while on HD. Despite his disease, he had been a “workaholic” and had a good appetite. His blood urea nitrogen was always high, and sometimes showed high K and IP levels due to excessive intake. Two calcium channel blockers, 1 angiotensin-converting enzyme inhibitor, 1 beta-adrenergic blocker, and 1 alpha blocker were prescribed for severe hypertension. His past medical history was significant for acute coronary syndrome, which occurred 4 years prior to this admission, just before PD initiation. He did not have a history of diabetes mellitus or impaired glucose tolerance.
On admission, the patient’s blood pressure was 150/90 mm Hg, pulse rate was 72 beats/min and irregular, and respiration rate was 20 breaths/min. Physical examination of chest and abdomen showed no abnormalities except for irregular heart rhythm. Right hemiparesis was noted in the upper and the lower extremities. He showed motor aphasia and ideomotor apraxia. The magnetic resonance imaging of the brain revealed multiple infarctions of the bilateral brain cortex. Electrocardiography showed atrial fibrillation. A cardiogenic brain embolism was diagnosed. His serum chemistry on admission was as follows: K: 3.6 mmol/l, corrected calcium: 10.8 mg/dl, urea nitrogen (UN): 44.0 mg/dl, and IP: 5.1 mg/dl.
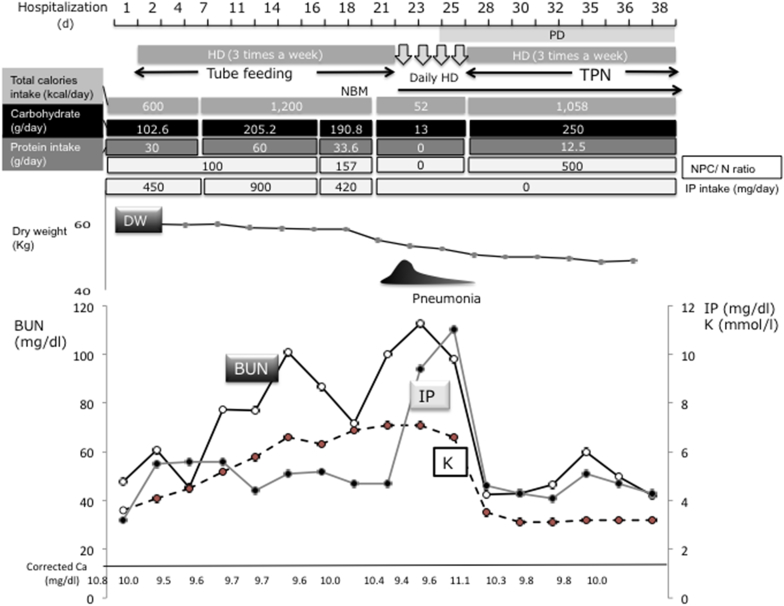
Clinical course of this patient is shown in Figure 1. The third day after admission, tube feeding and rehabilitation was initiated, and daily PD treatment was changed to HD 3 times a week. Because increases in serum K and IP concentrations were noted, the formula for tube feeding was changed to one intended for CKD patients (from 1,200 kcal, protein 60 g/d, K 1,800 mg/d, IP 900 mg/d to 1,200 mg/d protein 33.6 g/d, K 360 mg/d, IP 420 mg/d) from day 18. The patient developed congestive heart failure and bacterial pneumonia on day 21. In addition to antibiotics and fluid removal by HD, tube feeding was on hold for a few days because the team suspected aspiration pneumonia. Then his blood UN, IP, and K levels increased rapidly requiring daily HD. The peak levels were UN 112.7 mg/dl, IP 11.1 mg/dl, and K 7.3 mmol/l, respectively. It is of note that serum creatine phosphokinase was also increased. Serum albumin was 3.8 g/dl, and glucose, glycated hemoglobin, and serum lipid profiling were all within normal range. During this period, the patients had received only 52 kcal/d from peripheral i.v. fluid.
Figure 1.
Clinical course of this patient during hospitalization. BUN, blood urea nitrogen; Ca, calcium; DW, dry weight; HD, hemodialysis; IP, inorganic phosphorous; K, potassium; NBM, nothing by mouth feeding; NPC/N, nonprotein calorie–nitrogen ratio; PD, peritoneal dialysis; TPN, total parenteral nutrition.
After the initiation of total parenteral nutrition containing 250 g/d of carbohydrate, his serum UN, K, and IP rapidly improved.
Discussion
High-concentrations of serum K and IP are common among HD patients.1 Hyperkalemia in CKD is often caused by the excessive ingestion of potassium-rich foods. Hyperphosphatemia is usually caused by increased intake of IP from meats, fishes, beans, and dairy products. Elevated serum urea nitrogen may be due to excessive protein intake or insufficient dialysis. Because the patients appeared to eat liberally without sticking to the diet restriction when he was maintained on outpatient PD+HD, we assumed elevated levels of K, IP, and UN came from excessive intake.
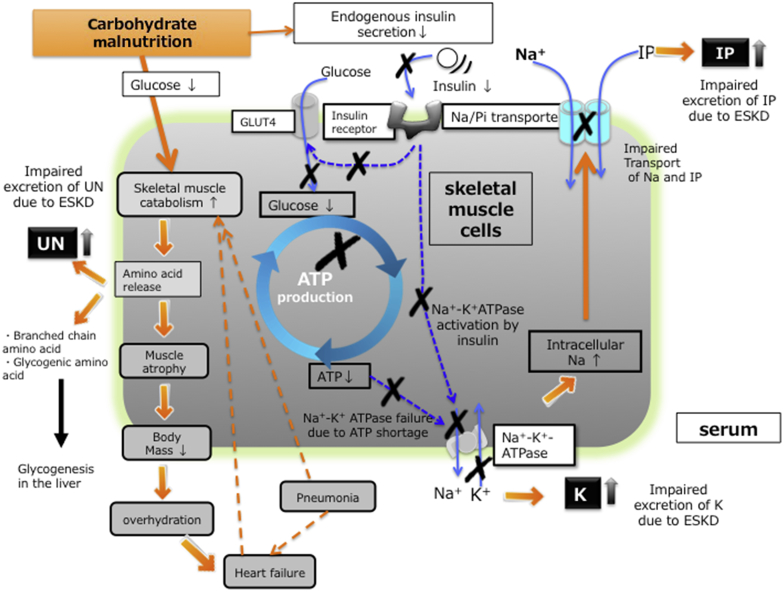
It is also known low intake or malnutrition or both can lead to hypophosphatemia. In fact, hypophosphatemia is often seen in the patients with advanced stage cancer, intensive care unit patients after major surgery or cachectic patients with old age.4, 5 On the other hand, some patients with severe malnutrition develop remarkable increase in serum IP levels.6, 7 Wada and Shinoda7 reported a patient with anorexia nervosa with the IP level of 12.8 mg/dl that was corrected by dietary modification. In addition, O’Connor et al.8 reported the temporal development of hyperphosphatemia in 11 patients with lactic acidosis, suggesting that the high amount of adenosine triphosphate (ATP) degeneration with glycolysis due to tissue hypoxia could cause hyperphosphatemia. Hyperphosphatemia was also reported in the patients with hemorrhagic shock and diabetic ketoacidosis.9, 10 The increased IP could be explained by local glycolysis and lactic acidosis due to the decreased tissue perfusion in the former cases and by the impairment of glucose metabolism due to a lack of insulin effects in the latter case. The common feature of these cases was that the patients showed increased catabolism or glycolysis and a rapid decline of IP after treatment. This kind of hyperphosphatemia is thought to be caused by a shift from the intracellular space to the extracellular space of IP. In our case, after long-term starvation, endogenous carbohydrate and ATP are depleted in many organs and skeletal muscle. The reduction of ATP decreases activity of Na+-K+-ATPase in these cells and reduces the sodium concentration gradient across the plasma membrane.11 As a result, the sodium-dependent IP transporter NaPi-III, in skeletal muscle cells cannot efficiently move IP from extracellular fluid into the cells. The increases in serum K seen in this case can also be explained by carbohydrate malnutrition, and a reduction in Na-K-ATPase activity. The low carbohydrate reduces the endogenous insulin secretion, which leads to a decreased insulin-dependent K transport through glucose transporter 4 (GLUT4). Carbohydrate malnutrition is also well known as a cause of a high UN levels. Carbohydrate shortage leads to the consumption of glycogen in the initial phase. If it persists, then glycogenic amino acids are utilized for glyconeogenesis in the liver, during which process, ammonium is produced, and then converted to urea in the kidney via the ornithine cycle.
Nutritional management in critically ill patients is often inadequate.12, 13 In general, protein intake <0.6 to 0.8 g/kg/d and energy intake >33 to 35 kcal/kg/d (in the actual survey weight) and about 300 to 500 of the nonprotein calorie–nitrogen ratio are recommended for the prevention of catabolism in CKD patients.14, 15 In addition, > 70% of energy should come from carbohydrates in critically ill patients. Therefore our patient should have received > 1,750 kcal/d of carbohydrate with 300 to 500 of nonprotein calorie–nitrogen ratio.
However, not all critically ill HD patients present with the degree of hyperkalemia, hyperphosphatemia, and high UN levels seen in our patient even when nutrition is inadequate. We speculate that a liberal diet and physical activity before admission could preserve this patient’s muscle mass (Figure 2). Unlike most CKD patients on chronic HD, where sarcopenia is prevalent, this patient appeared to have larger intracellular store of K, IP, and protein in his muscle, which was leaked when carbohydrate malnutrition occurred. During his hospital course, he developed pneumonia complicated with heart failure that may be caused by relative overhydration due to weight loss after admission, both of which might have partly accelerated hypercatabolism.
Figure 2.
The mechanisms of releasing inorganic phosphorous (IP), potassium (K), and urea nitrogen (UN) under the carbohydrate malnutrition in present case. ATP, adenosine triphosphate; ESKD, end-stage kidney disease; GLUT4, glucose transporter 4.
Conclusion
We report a case of a dialysis patient who developed remarkably high serum K, IP, and UN concentration after hospitalization due to stroke. These laboratory results were likely to be due to carbohydrate malnutrition in the face of preserved muscle mass.
Disclosure
All the authors declared no competing interests.
References
- 1.Pun P.H. The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis. 2014;21:480–488. doi: 10.1053/j.ackd.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Gallieni M., De Luca N., Santoro D. Management of CKD-MBD in non-dialysis patients under regular nephrology care: a prospective multicenter study. J Nephrol. 2016;29:71–78. doi: 10.1007/s40620-015-0202-4. [DOI] [PubMed] [Google Scholar]
- 4.Taylor B.E., Huey W.Y., Buchman T.G. Treatment of hypophosphatemia using a protocol based on patient weight and serum phosphorus level in a surgical intensive care unit. J Am Coll Surg. 2004;198:198–204. doi: 10.1016/j.jamcollsurg.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Zazzo J.F., Troche G., Ruel P., Maintenant J. High incidence of hypophosphatemia in surgical intensive care patients: efficacy of phosphorus therapy on myocardial function. Intensive Care Med. 1995;21:826–831. doi: 10.1007/BF01700966. [DOI] [PubMed] [Google Scholar]
- 6.Bonne O.B., Gur E., Berry E.M. Hyperphosphatemia: an objective marker for bulimia nervosa? Compr Psychiatry. 1995;36:236–240. doi: 10.1016/0010-440x(95)90088-d. [DOI] [PubMed] [Google Scholar]
- 7.Wada K., Shinoda T. A case report of an anorexia nervosa patient with end-stage renal disease due to pseudo Bartter's syndrome and Chinese herb nephropathy requiring maintenance hemodialysis. Ther Apher Dial. 2008;12:417–420. doi: 10.1111/j.1744-9987.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor L.R., Klein K.L., Bethune J.E. Hyperphosphatemia in lactic acidosis. N Engl J Med. 1977;297:707–709. doi: 10.1056/NEJM197709292971307. [DOI] [PubMed] [Google Scholar]
- 9.Sternbach G.L., Varon J. Severe hyperphosphatemia associated with hemorrhagic shock. Am J Emerg Med. 1992;10:331–332. doi: 10.1016/0735-6757(92)90013-n. [DOI] [PubMed] [Google Scholar]
- 10.Kebler R., McDonald F.D., Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79:571–576. doi: 10.1016/0002-9343(85)90053-1. [DOI] [PubMed] [Google Scholar]
- 11.Marinella M.A. The refeeding syndrome and hypophosphatemia. Nutr Rev. 2003;61:320–323. doi: 10.1301/nr.2003.sept.320-323. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom Rehal M., Fiskaare E., Tjader I., Norberg A., Rooyackers O., Wernerman J. Erratum to: “Measuring energy expenditure in the intensive care unit: a comparison of indirect calorimetry by E-sCOVX and Quark RMR with Deltatrac II in mechanically ventilated critically ill patients.”. Crit Care. 2016;20:104. doi: 10.1186/s13054-016-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer P., Hiesmayr M., Biolo G. Pragmatic approach to nutrition in the ICU: expert opinion regarding which calorie protein target. Clin Nutr. 2014;33:246–251. doi: 10.1016/j.clnu.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Singer P., Berger M.M., Van den Berghe G. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 15.McClave S.A., Taylor B.E., Martindale R.G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N. JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]