Abstract
Introduction
The risk of major adverse events associated with chronic kidney disease (CKD) could potentially be reduced with effective medical interventions. The impact of multifaceted interventions as compared with usual care in patients with nondiabetic CKD is unclear. We performed a systematic review to analyze the impact of multifaceted interventions on reducing the risk of major adverse events in this population.
Methods
Systematic review and meta-analysis. We searched MEDLINE, EMBASE, CINAHL and the Cochrane Library databases for medical literature published up to November 2016. Published original studies and abstracts were reviewed that reported on adult patients in a community or specialty care setting, with 2 or more CKD risk factors, treated with a combination of more than 2 interventions. We included randomized controlled trials, observational studies, and systematic reviews. Studies focused on diabetic patients were excluded. The intervention was defined as a treatment with a combination of 2 or more interventions compared with the usual care. The outcomes were defined as a reduction in the risk of adverse clinical outcomes (renal replacement therapy, all-cause hospitalizations, all-cause and cardiovascular mortality, cardiovascular events) as primary outcomes. Secondary outcomes were optimal risk factor control (attaining guideline concordant blood pressure, reduction of proteinuria, smoking cessation).
Results
Five of the 5846 unique citations from our initial literature search met our study criteria. All identified studies reported on patients with CKD and their management. In comparison with usual care, multifaceted interventions tended to reduce all-cause mortality (risk ratio: 0.81, 95% confidence interval: 0.63–1.03) and were associated with a lower risk of progression to kidney failure requiring dialysis (risk ratio: 0.57, 95% confidence interval: 0.35–0.94). Multifaceted interventions were not associated with reducing risk of all-cause hospitalizations (risk ratio: 0.93, 95% confidence interval: 0.71–1.23) or improved blood pressure control (mean difference: −0.48, range: −2.5 to 1.55 mm Hg).
Discussion
Multifaceted interventions targeting multiple risk factors tended to reduce the risk of all-cause mortality and reduced the risk to progress to end-stage kidney failure in patients with CKD. There is a need for high-quality studies that can rigorously evaluate a set of interventions targeting multiple domains of CKD management in the population with nondiabetic CKD due to paucity of data in the current published literature.
Keywords: multifaceted care, nondiabetic CKD, outcomes, systematic review
Chronic kidney disease (CKD) is a significant burden on patients and the health care system, as it leads to end-stage kidney disease requiring dialysis or transplantation and accelerates the development of cardiovascular disease.1, 2, 3 Many strategies have been used to delay progression of CKD to end-stage kidney disease and to prevent cardiovascular events.3, 4 These include pharmaceutical interventions (e.g., control of blood pressure, reduction of proteinuria, reduction of other risk factors for CKD, and cardiovascular disease), behavioral and/or lifestyle changes (e.g., counseling for weight loss, smoking cessation, dietary restrictions, and exercise), and organizational changes (case management, multidisciplinary care, and use of an ancillary health care workforce).1, 5, 6
There has been no clear consensus on the most effective combination of these approaches (pharmacologic, behavioral/lifestyle, and organizational changes) in reducing risk of adverse clinical outcomes for patients with CKD.7, 8 A number of studies in the past have focused on individual risk factors, a single domain of intervention, and/or a specific population with CKD.9, 10, 11 Despite the calls for a paradigm shift from a single risk factor care approach to multiple risk management, data on the effectiveness of multifaceted interventions (a combination of at least 2 of pharmacologic, behavioral/lifestyle, and/or organizational changes) compared with the usual care in reducing the risk of adverse events in nondiabetic CKD are sparse.6, 10, 12, 13
We conducted a systematic review to summarize the evidence on the benefits of multifaceted interventions compared with the usual care. The overarching aim was first to synthesize the evidence for benefits through prevention of adverse clinical outcomes (myocardial infarction, strokes, end-stage kidney disease, and amputations from peripheral vascular diseases), and second, to synthesize the evidence of benefit on surrogate markers of CKD care (e.g., control of hypertension, reduction in proteinuria, smoking cessation).
Methods
We followed standard guidelines for the conduct and reporting of systematic reviews14 using a standard protocol registered in PROSPERO International Prospective Register of Systematic Reviews (Registration # CRD42013003597).
Data Sources, Search, and Review Strategy
We utilized the services of a medical librarian (TC) to do a comprehensive literature search using a combination of controlled vocabulary terms (MeSH, Emtree where applicable) and keywords for identification of relevant published studies. Searches were conducted in Ovid Medline (from 1946), Ovid EMBASE (from 1974), Ovid Cochrane, and EBSCO CINAHL (from 1937). Original searches were run to December 2014, and subsequent updated searches were completed up to 13 November 2016. The searches were limited to humans, adults, English language, and to study designs being systematic reviews, randomized controlled trials (RCTs) (using the Cochrane Highly Sensitive Search strategy for RCTs available at http://handbook.cochrane.org/), and cohort studies. The full search strategy is provided in Supplementary Appendix S1.
To ensure a quality and consistent review, content experts in the research group (AKB, JO) screened titles and abstracts of each retrieved citation for relevance, and any study considered potentially relevant was extracted for an in-depth review and evaluation. The full text of each retrieved publication was evaluated by 2 independent assessors (AS, BQ) for inclusion in the systematic review using eligibility criteria defined a priori. Studies were eligible if they compared the impact of multifaceted interventions (defined as treating ≥2 CKD risk factors) versus the usual care and related that to the outcomes of mortality (all-cause and cardiovascular), end-stage kidney disease, major adverse cardiovascular events such as fatal and nonfatal myocardial infarction, strokes, and peripheral vascular diseases. In a secondary analysis, we reviewed studies that investigated the relationship of multifaceted care versus the usual care and surrogate outcome parameters such as control of hypertension, reduction in proteinuria, and smoking cessation. The references of included studies were reviewed for additional studies, but no attempt was made to review the gray literature (technical reports, memoranda, and government reports) because of difficulty in acquiring this kind of data in a systematic way, and thus the review was restricted to published literature only.
Data Abstraction
We abstracted data for the studies that met all criteria for full-text review based on the population, intervention, comparator, outcomes criteria. The population was adult patients (>18) in a community or specialty care with 2 or more CKD risk factors. The intervention was defined as any intervention specifically designed to address 2 or more of the risk factors. The intervention could be pharmacological; behavioral/lifestyle including education, self-management, and education for adults; and/or organizational including implementation of policy changes or health care system redesign (health system reorganization). The comparator was usual care and outcomes were adverse clinical events and surrogate parameters as were enumerated previously. Two reviewers (AS, BQ) extracted data into an electronic spreadsheet, and disagreements were resolved by mutual discussions between the reviewers and the investigators (AKB, JO).
Data Synthesis and Analysis
The initial analysis was qualitative, summarizing the key study parameters such as design, population type and demographics, mode of intervention, setting, and the specific outcomes. We summarized study characteristics and quality based on standard criteria. Quantitative analyses were used to pool data on the key study outcomes to allow for a reasonable comparison with effect sizes. Risk ratios were computed for dichotomous outcomes, and mean differences computed for continuous outcome variables. Studies were pooled using a DerSimonian-Laird random-effects method. Statistical heterogeneity was assessed with the I2-statistic. The risk of bias in included studies was assessed using a well-validated Quality Checklist for Health Care Intervention Studies.15 This checklist enhanced the assessment and synthesis of published studies and reports on the quality of study design, statistical analysis, and reporting of results. We also utilized the criteria defined by Higgins et al.16 (performance bias, detection bias, attrition bias, reporting bias, and other bias) for evaluation of RCTs to minimize bias.
Results
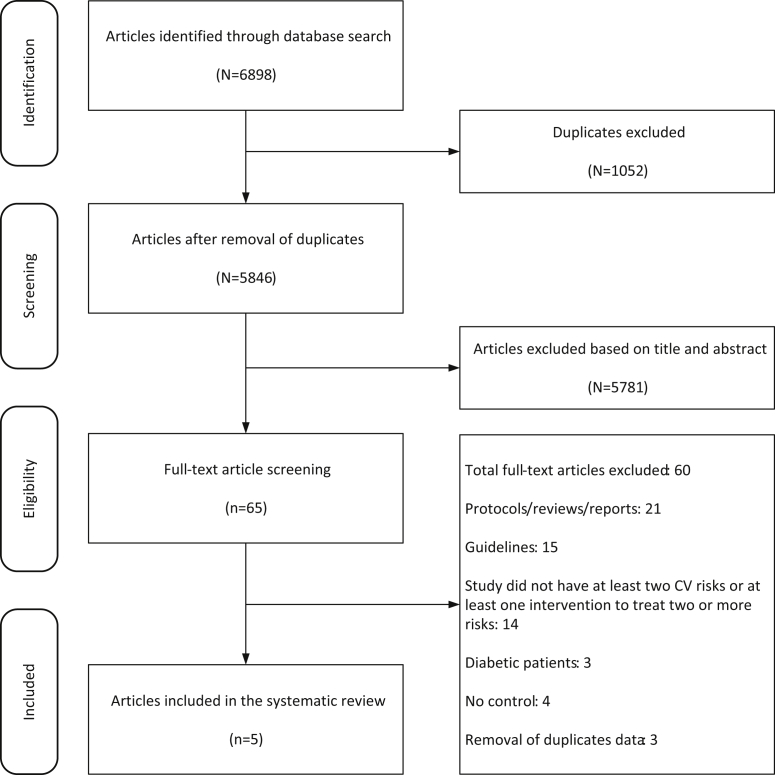
Our search originally yielded 6898 citations that were reduced to 5846 citations after removal of duplicate titles. These were screened by title and abstract against our a priori inclusion criteria and resulted in the selection of 65 articles, the full text of which was then retrieved and reviewed independently by 2 primary reviewers (AS, BQ), and this was further verified by the investigators (AKB, JO). Of the selected full articles, 36 were excluded based on study designs (protocols, guidelines, narrative reviews), 14 based on interventions, and 7 because of population of interest; 3 others were excluded because they were duplicate data (multiple publications from the same study) (Figure 1).
Figure 1.
Study selection criteria. CV, cardiovascular.
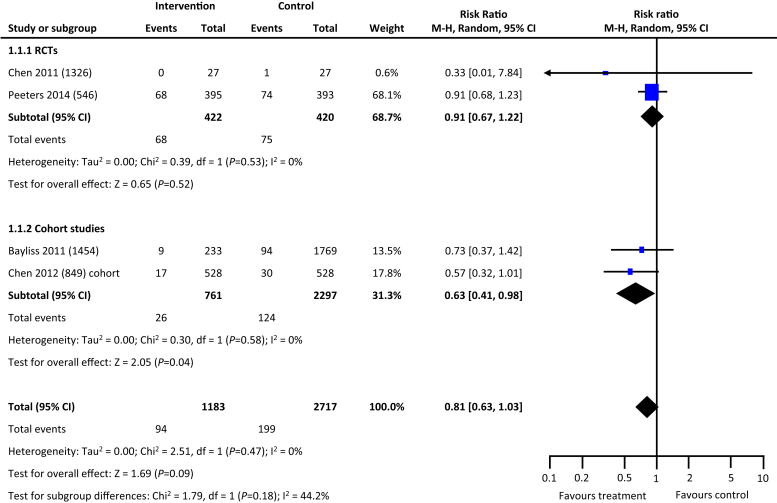
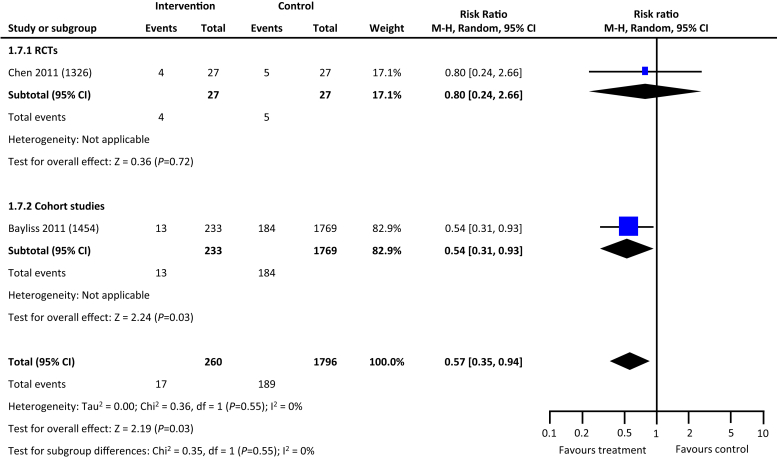
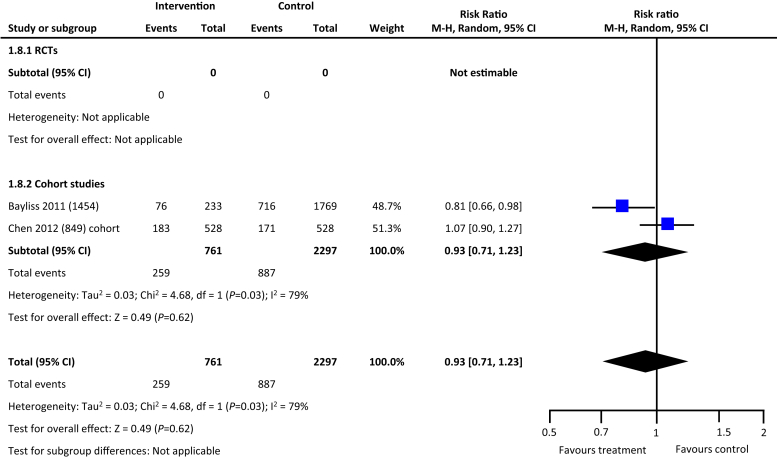
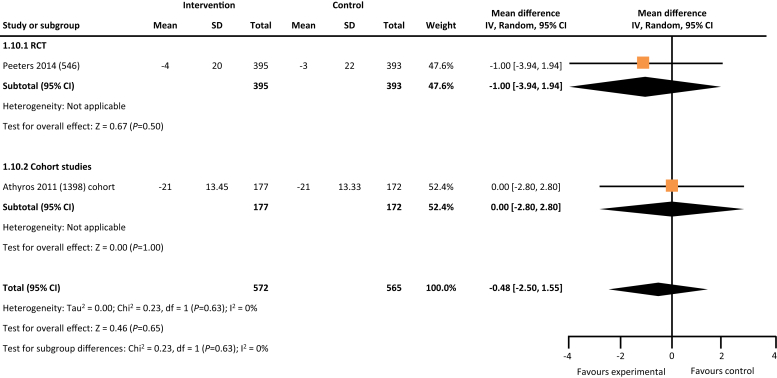
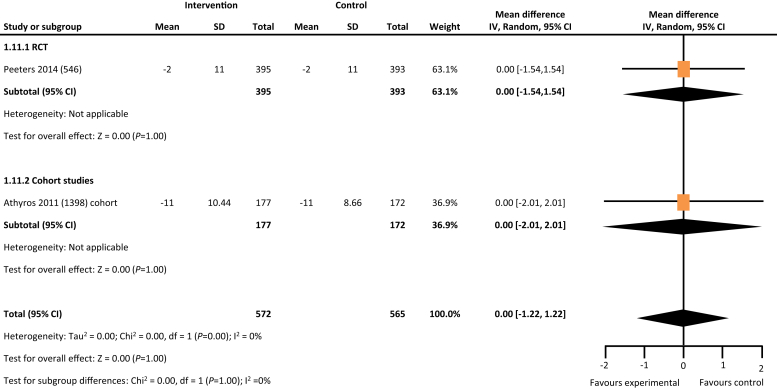
From the 5846 citations reviewed, we identified 5 studies across 4 countries that met our study criteria.17, 18, 19, 20, 21 As depicted in Table 1, 2 studies were RCTs, and the remaining 3 were cohort studies. Four of the 5 studies reported data on hard outcomes of all-cause mortality and dialysis. Two of the 3 cohort studies reported on incident all-cause hospitalization. One RCT and 1 cohort study of the 5 studies reported on blood pressure control (Table 1). In comparison with the usual care, multifaceted interventions were associated with a tendency for lower risk of all-cause mortality (risk ratio: 0.81, 95% confidence interval: 0.63–1.03, P = 0.09) (Figure 2) and reduced risk for progression to kidney failure requiring dialysis (risk ratio: 0.57, 95% confidence interval: 0.35–0.94, P = 0.03) (Figure 3). Multifaceted interventions were not associated with reducing risk of all-cause hospitalizations (risk ratio: 0.93, 95% confidence interval: 0.71–1.23, P = 0.62) (Figure 4) or improved blood pressure control (mean difference: −0.48 [−2.5 to 1.55]) (Figures 5 and 6).
Table 1.
Study characteristics
| Study | Country | Type of study | Data source, period, patient sample | Intervention | Control | Outcome studied | Summary of findings |
|---|---|---|---|---|---|---|---|
| Chen et al.20 (2011) | Taiwan | Randomized controlled trial | Outpatient clinic, Chang Gung Memorial Hospital Jan. 2008–Dec. 2008 27 SMS 27 non-SMS |
SMS: provision of health information, patient education, telephone-based support, and support group. Support came from nurses, dieticians, peers, and volunteers Lectures given on renal health, nutrition, lifestyle, and pharmacological management |
Non-SMS: customary care from the same physicians. No extensive health information was provided. Patients transferred to the SMS group after 12-mo follow-up | Mortality Reaching dialysis |
SMS group had a significant improvement in CKD knowledge Higher absolute eGFR in SMS Hospitalizations were lower in SMS |
| Peeters et al.21 (2014) | Netherlands | Randomized controlled trial | Hospital Patient enrollment April 2004–Dec. 2005; study conclusion August 2011 395 NPC+PC 393 PC |
NPC+ PC: NPC coached to improve patient self-management in addition to physician care to reduce decline of kidney function Lifestyle management, use of mandatory medication, and following CKD guidelines |
PC: only physician care according to CKD guidelines | Mortality Systolic blood pressure changes Diastolic blood pressure changes |
Improved renal outcomes and reduced rate of renal decline apparent after >2-yr follow-up |
| Bayliss et al.18 (2011) | United States | Retrospective cohort | Patient enrollment March 2005–June 2009 233 MDT care 1769 usual care |
MDT: consisted of a nephrologist, renal clinical pharmacy specialist, diabetes nurse educator, renal dietician, social worker, and nephrology nurse Weekly team meetings to review patient charts and progression of care. Patients asked to keep log of home blood pressure and blood sugar | Usual care: primary care physician managed chronic conditions and collaborated with a nephrologist outside the integrated care plan | Mortality Reaching dialysis Hospitalizations |
MDT care on average had fewer chronic conditions and lower rate of physician visits Fewer patients requiring dialysis and lower rates of hospitalization for MDT care |
| Chen et al.19 (2013) | Taiwan | Prospective cohort | Five hospitals 2008–2010 528 MDC 528 usual care |
MDC: care under nephrologist, nephrology nurse educator, renal dietitian, social worker, pharmacy specialist, and surgeon | Usual care: care under primary care physicians, and a number of specialists | Mortality Hospitalizations |
Risk of mortality was lowered in MDC |
| Athyros et al.17 (2011) | United Kingdom | Prospective cohort | Patient enrollment 2005–2008; 3.5-yr study 172 group A1 (target LDL-C <100 mg/dl) 177 group B1 (target LDL-C <130 mg/dl) |
Group A1: Original group A consisted of 566 patients; those with stage 3 CKD were characterized into group A1 | Group B1: original group B consisted of 557 patients; those with stage 3 CKD were characterized into group B1 | Systolic blood pressure changes Diastolic blood pressure changes |
Estimated 10-yr CVD risk was reduced by 52% for both groups compared with baseline 6 CVD nonfatal events occurred in group B1, whereas none occurred in group A1 |
CKD, chronic kidney disease; CVD, cardiovascular disease; LDL-C, low-density lipoprotein-cholesterol; MDC, multidisciplinary care; MDT, multidisciplinary team; NPC, nurse practitioner care; PC, physician care; SMS, self-management support.
Figure 2.
Multifaceted interventions and risk of mortality. CI, confidence interval; RCT, randomized controlled trial.
Figure 3.
Multifaceted interventions and risk of kidney failure (initiation of dialysis). CI, confidence interval; RCT, randomized controlled trial.
Figure 4.
Multifaceted interventions and risk of all-cause hospitalization. CI, confidence interval; RCT, randomized controlled trial.
Figure 5.
Multifaceted interventions and changes in systolic blood pressure. CI, confidence interval; RCT, randomized controlled trial.
Figure 6.
Multifaceted interventions and changes in diastolic blood pressure. CI, confidence interval; RCT, randomized controlled trial.
Risk of Bias Assessment
Only 2 of the 5 reviewed studies were rated as high quality; the remaining 3 were classified as low quality or unclear risk of bias based on the criteria of sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective outcome reporting (reporting bias), as well as small sizes and insufficient follow-up data for the cohort studies (Supplementary Table S1).22, 23
Discussion
Given the high morbidity and mortality, and rising health care costs associated with CKD and its complications globally, it is imperative to put in place effective strategies to stem the tide of CKD and related adverse clinical outcomes in our communities. Attempts have been made to reduce the population impact of CKD by risk factor modification in primary and secondary prevention strategies.7, 9, 10 Approaches vary, with some strategies targeting individual risk factors, whilst others are multifaceted in nature, encompassing nonpharmacological interventions such as lifestyle modification regarding diet, physical activity, smoking and alcohol use, counseling and education, and pharmacological interventions using evidence-based therapies. Although there is evidence of a clear benefit of multifaceted interventions in diabetes-related CKD,6, 10, 12 this evidence is less abundant for targeted therapies in patients with nondiabetic CKD.
In this review, we have examined the evidence on the effectiveness of multifaceted interventions in CKD care and relationships to clinical outcomes in the population with nondiabetic CKD. We have found that multiple interventions targeted to multiple risk factors were associated with nonsignificant reduction in the risks of all-cause mortality and with reduced risk of kidney failure requiring dialysis. There were no significant associations between multifaceted interventions and the risk of all-cause hospitalization and blood pressure control.
There have been calls for a paradigm shift from a single risk factor care approach to multifaceted strategies to reduce adverse clinical outcomes in CKD. However, data on the effectiveness of these interventions on nondiabetic CKD at the population level are still sparse. Several trials and observational studies reported conflicting outcomes.12, 13, 19, 20 Furthermore, care delivery practices for CKD have not changed much in the last few decades. The current model of care is not sustainable due to an exponential rise in the number of patients, an aging population, as well as conflicting demands on the time of primary care practitioners.9 Moreover, emerging data point to the possibility of leveraging a combination of risk factors and/or markers that affect disease progression and outcome.10, 11, 24, 25 Therefore, there is a need to move from the traditional single risk control approach to a strategy that encompasses all facets of care inclusive of patient, provider, and systems-level factors.
What are the implications of our findings? Our data have identified a key knowledge gap, that is, the absence of high-quality evidence to guide multifaceted chronic disease management programs in CKD care that are comprehensive enough to change patient outcomes. This work demonstrated a dearth of information on the most effective strategy for CKD management to enhance optimal patient care toward mitigating the risk for adverse clinical outcomes. This is the first analysis to synthesize the available evidence comparing multifaceted interventions versus usual care, providing new information for the design or update of policies and guidelines for CKD care. Furthermore, our findings will link and broker the essential pillars of evidence translation and implementation, and care delivery innovation and refining of policies targeted for CKD care delivery. Specifically, this work has provided synthesized evidence that demonstrated the gross lack of data on the role of multiple interventions for optimal CKD care delivery that could positively impact clinically relevant outcomes.
The major limitation is the small number of studies found to meet the criteria despite a broad scope of questions underpinning the review.23 This is an important finding in itself as it defines areas where studies are needed to close the identified knowledge gaps. Second, the heterogeneity, small sample sizes, and suboptimal study quality in the studies reviewed have hampered the internal validity and generalizability of our findings. A third limitation is the lack of a standard taxonomy to define a multifaceted care that hampered our ability to select and include studies based on a type of care delivered in a consistent manner.
In conclusion, multifaceted CKD care interventions targeting multiple risk factors appeared to reduce the risk for adverse clinical outcomes in the population with nondiabetic CKD. There is a need for high-quality studies that can rigorously study and evaluate a set of interventions targeting multiple domains of CKD management that encompasses not only pharmacologic, but behavioral/lifestyle, and organizational changes. Many efforts are being geared in this direction such as the Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of Nurse Practitioners (MASTERPLAN), and similar initiatives would enhance the quality of information vital for the development of concrete strategies to mitigate adverse consequences associated with nondiabetic CKD. A set of combined strategies may prove to be most effective in reducing the risk of adverse clinical outcomes in the population with nondiabetic CKD when tested in large-scale and high-quality studies, and this will overcome the current challenges due to paucity of data in the current literature.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work supported by an internal grant by the Faculty of Medicine and Dentistry, University of Alberta.
Footnotes
Appendix S1. Full search strategy.
Table S1. Risk of bias assessment.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Author Contributions
AKB and BB created the research idea and designed the study. AKB, BB, BQ, AS, JO, and TC acquired the data. AKB, BB, BQ, AS, JO, TC, and BV analyzed and interpreted the data. BV performed the statistical analysis. Each author contributed important intellectual content during manuscript drafting and accepts accountability for the overall work. AKB and BB take responsibility that this study has been reported honestly, accurately, and transparently, and that no important aspects of the study have been omitted.
Supplementary Material
Full search strategy.
Risk of bias assessment.
References
- 1.Meguid El, Nahas A., Bello A.K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 2.James M.T., Hemmelgarn B.R., Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–1309. doi: 10.1016/S0140-6736(09)62004-3. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt K.U., Coresh J., Devuyst O. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 4.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 6.Strand H., Parker D. Effects of multidisciplinary models of care for adult pre-dialysis patients with chronic kidney disease: a systematic review. Int J Evid Based Healthc. 2012;10:53–59. doi: 10.1111/j.1744-1609.2012.00253.x. [DOI] [PubMed] [Google Scholar]
- 7.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 8.Stevens P.E., Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 9.Remuzzi G., Benigni A., Finkelstein F.O. Kidney failure: aims for the next 10 years and barriers to success. Lancet. 2013;382:353–362. doi: 10.1016/S0140-6736(13)60438-9. [DOI] [PubMed] [Google Scholar]
- 10.Tricco A.C., Ivers N.M., Grimshaw J.M. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379:2252–2261. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 11.Shojania K.G., Ranji S.R., McDonald K.M. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 12.Lau C., Vistisen D., Toft U. The effects of adding group-based lifestyle counselling to individual counselling on changes in plasma glucose levels in a randomized controlled trial: the Inter99 study. Diabetes Metab. 2011;37:546–552. doi: 10.1016/j.diabet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen O. Intensified multifactorial treatment of patients with type 2 diabetes: what can be achieved and does it pay off? J Diabetes. 2009;1:83–89. doi: 10.1111/j.1753-0407.2009.00014.x. [DOI] [PubMed] [Google Scholar]
- 14.Swartz M.K. The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care. 2011;25:1–2. doi: 10.1016/j.pedhc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athyros V.G., Karagiannis A., Ganotakis E.S. Association between the changes in renal function and serum uric acid levels during multifactorial intervention and clinical outcome in patients with metabolic syndrome. A post hoc analysis of the ATTEMPT study. Curr Med Res Opin. 2011;27:1659–1668. doi: 10.1185/03007995.2011.595782. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss E.A., Bhardwaja B., Ross C. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6:704–710. doi: 10.2215/CJN.06610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.R., Yang Y., Wang S.C. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3-year prospective cohort study. Nephrol Dial Transplant. 2013;28:671–682. doi: 10.1093/ndt/gfs469. [DOI] [PubMed] [Google Scholar]
- 20.Chen S.H., Tsai Y.F., Sun C.Y. The impact of self-management support on the progression of chronic kidney disease—a prospective randomized controlled trial. Nephrol Dial Transplant. 2011;26:3560–3566. doi: 10.1093/ndt/gfr047. [DOI] [PubMed] [Google Scholar]
- 21.Peeters M.J., van Zuilen A.D., van den Brand J.A. Nurse practitioner care improves renal outcome in patients with CKD. J Am Soc Nephrol. 2014;25:390–398. doi: 10.1681/ASN.2012121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan M, Berkman ND, Dryden DM, et al. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. Methods Research Report. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed]
- 23.Zeng X., Zhang Y., Kwong J.S. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 24.Wang S.M., Hsiao L.C., Ting I.W. Multidisciplinary care in patients with chronic kidney disease: a systematic review and meta-analysis. Eur J Intern Med. 2015;26:640–645. doi: 10.1016/j.ejim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Tangri N., Grams M.E., Levey A.S. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full search strategy.
Risk of bias assessment.