Abstract
Introduction
Chronic kidney disease (CKD) is associated with high cardiovascular morbidity and mortality. Recent evidence suggests that increases in both serum and intracellular magnesium (Mg) can slow or even prevent the development of vascular calcification seen in CKD. Serum calcification propensity (T50) is a novel functional test, which is associated with all-cause mortality in CKD and measures the ability of serum to delay the formation of crystalline nanoparticles. Theoretically, increasing serum Mg should improve T50 and thereby reduce the propensity towards ectopic calcification.
Methods
We conducted a randomized placebo-controlled double-blinded clinical trial to investigate the safety of 2 different doses of oral Mg supplementation in subjects with CKD stages 3 and 4 as well as their effects on intracellular Mg and T50. Thirty-six subjects with CKD stages 3 and 4 were randomized to one of 3 groups (placebo, elemental Mg 15 mmol/d or elemental Mg 30 mmol/d) given as slow-release Mg hydroxide and followed for 8 weeks.
Results
Thirty-four subjects completed the trial. Intracellular Mg remained stable throughout the trial despite significant increases in both serum and urine Mg. T50 increased significantly by 40 min from 256 ± 60 (mean ± SD) to 296 ± 64 minutes (95% confidence interval, 11–70, P < 0.05) in the Mg 30 mmol/d group after 8 weeks. No serious adverse events related to the study medication were reported during the study.
Discussion
Oral Mg supplementation was safe and well tolerated in CKD stages 3 and 4 and improved T50, but did not increase intracellular Mg. Further studies are needed to investigate the long-term effects of Mg supplementation in CKD stage 3 and 4 and whether improvement in calcification propensity is related to clinical endpoints.
Keywords: calcification propensity, chronic kidney disease, magnesium
Chronic kidney disease (CKD) affects approximately 13% of the general population1 and is associated with increased risk of cardiovascular disease (CVD) because of both traditional and nontraditional CVD risk factors.2 Among the nontraditional CVD risk factors, disturbances in mineral and bone disease are associated with vascular calcification,3 which is associated with increased cardiovascular mortality in end-stage renal disease.4 Key factors in this regard are phosphate (PO4) and calcium (Ca), which can precipitate and induce an osteogenic transformation in the vascular smooth muscle cells of the arteries causing them to calcify and stiffen.3
Epidemiological studies have found associations between higher levels of serum magnesium (Mg) and improved survival among patients suffering from CKD5 and end-stage renal disease,6, 7, 8, 9, 10, 11, 12, 13 and higher levels of serum Mg (sMg) are associated with reduced progression of CKD.5, 14 These associations are thought to be mediated by an antagonistic effect of Mg on the procalcifying milieu in CKD.15 In vitro calcifications induced by Ca and high concentrations of PO4 can be prevented or reversed by adding or increasing Mg, which appears to be mediated by both upregulation of factors that inhibit calcification and downregulation of factors that promote calcification.16, 17, 18, 19, 20 Also, 2 small clinical trials of Mg supplementation in end-stage renal disease have shown reduced progression of vascular calcification.21, 22 Thus, Mg supplementation could potentially be a therapeutic option to attenuate the progression of vascular calcification in CKD. Because studies have shown that influx of Mg into the vascular smooth muscle cell is important for prevention of vascular calcification,16, 18, 19 measurement of any effect of Mg therapy on intracellular Mg (iMg) would be of interest. However, because of the renal excretion of Mg, there is a risk that Mg therapy might result in toxic hypermagnesemia in patients with reduced kidney function. So far, no studies have examined the effect of oral Mg supplementation on iMg in subjects with CKD stages 3 and 4, or the safety of this intervention.
Serum calcification propensity (T50) is a novel functional test, which determines the ability of serum to resist Ca/PO4 precipitation by measuring the time-point of conversion from primary to secondary calciprotein particle.23 Previous studies have shown that low T50 predicts all-cause mortality in CKD stages 3 and 424 and kidney transplant recipients,25, 26 as well as graft failure in kidney transplant recipients.25 T50 is believed to reflect the propensity towards ectopic calcification, although so far low T50 has not been directly associated with progression of vascular calcification. In vitro data indicate that Mg improves T50,23 but so far this has not been examined in a clinical trial.
We conducted a randomized placebo-controlled double-blinded clinical trial to investigate the efficacy and safety of 2 different doses of oral Mg supplementation on iMg in subjects with CKD stages 3 and 4, as well as any effects on T50. We hypothesized that greater doses of Mg supplementation would result in a greater increase in iMg compared with placebo as well as improving T50 in subjects with CKD stages 3 and 4 and low or low-normal sMg.
Methods
Subjects
Subjects were recruited between October 2014 and February 2015 from the outpatient clinic at the Division of Nephrology, Roskilde County Hospital, Denmark. All patients were screened before planned visits to the clinic and those matching trial criteria were offered participation in the trial. Inclusion criteria were age > 18 years, estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2, total sMg < 0.82 mmol/l, safe contraceptive treatment in women of childbearing age, and written informed consent. Exclusion criteria were current treatment with hemodialysis or peritoneal dialysis, kidney transplant recipient, treatment with Mg-containing medication or supplements, parathyroid hormone (PTH) > 66 ρmol/l, cancer, other medical condition that in the opinion of the investigators would prohibit participation in the trial, pregnancy or breastfeeding, allergies to any contents of the study medication, and participation in other interventional trials.
Design
The trial was an investigator-initiated double-blinded placebo-controlled clinical trial in which subjects were randomized in a ratio of 1:1:1 to 8 weeks of oral treatment with either placebo twice daily, slow-release Mg hydroxide 360 mg (equivalent to 15 mmol of elemental Mg) (Mablet, Gunnar Kjems ApS, Copenhagen, Denmark) once daily and placebo once daily, or slow-release Mg hydroxide 360 mg twice daily. Mg hydroxide and placebo tablets were identical in appearance, constituents, and containers, and did not contain Ca. Study medication was packed in consecutively numbered containers according to a computer generated block-randomized randomization list, and was administered to subjects consecutively as they entered the trial. Subjects and investigators were blinded to the study medication during the course of the trial.
At weeks 0, 4, and 8, sublingual epithelial cells were sampled for iMg and nonfasting blood samples, 24-hour urine samples were collected, and an electrocardiogram was performed. Subjects were instructed to maintain their usual diets and not to begin treatment with Mg-containing medication or supplements for the duration of the trial. Adherence was assessed by pill count at week 8.
The primary endpoint was a change in iMg as assessed by energy dispersive X-ray microanalysis of sublingual epithelial cells,27 as this has been shown previously to correlate with iMg in human atrial cardiomyocytes.28 Based on previous studies,29, 30, 31, 32, 33 an increase in iMg of 2.0 mEq/l with a SD of 2.0 mEq/l was considered clinically relevant. With a probability of type 1 error (α) of 5% and power of 80% (1 − β) a sample size of 10 subjects per group would be necessary to detect a difference of 2.0 mEq/l. A dropout rate of 15% was anticipated, and therefore 36 subjects in total were included in the trial (12 subjects per group). Dropouts were not replaced.
Secondary endpoints were blood levels of total Mg, PO4, ionized Ca, PTH, 25-hydroxy vitamin D3 (25-OH-D3), T50, fetuin-A, albumin, bicarbonate, intact fibroblast growth factor 23 (FGF23), eGFR, 24-hour urinary Mg (uMg) and PO4 (uPO4), and electrocardiogram-measured QTc interval.
Ethics
The trial is in compliance with the Helsinki Declaration II of 1975, revised 1983, and was approved 5 May 2014 by the Danish National Committee on Biomedical Research Ethics (SJ-398) and the Danish Data Protection Agency. The trial was registered at ClinicalTrials.gov (NCT02216877). All subjects gave written informed consent before initiating the trial.
Laboratory Analysis
iMg was measured in sublingual epithelial cells scraped from the mucosa adjacent to the frenulum and immediately fixed on a carbon slide with cytology fixative. The slides were examined with a scanning electron microscope (FEI; Thermo Fisher Scientific, Waltham, Massachusetts), and suitable cells were identified. iMg was measured with radiographic analysis of individual epithelial cells (EXA; Intracellular Diagnostics, Medford, Oregon) (normal range 34.0–42.0 mEq/l). Reported values are the mean of 5 to 10 cells per subject; a specimen was rejected if variance exceeded 2%. This method is used to assess total cellular magnesium and cannot differentiate free Mg from bound species.
Blood samples were drawn from subjects in nonfasting states. Samples for measurement of T50, fetuin-A, and FGF23 were collected and immediately stored at –80°C. The analyses were performed in bulk in the same analytical run so as to eliminate interassay variation. Blood levels of total Mg, ionized Ca, PO4, PTH, 25-OH-D3, creatinine, hemoglobin, bicarbonate, and albumin were measured on a routine basis by the same standardized methods at the local department of clinical biochemistry. Urine samples were collected over 24 hours in 2.5-liter containers, which contained 100 ml of 2.5% hydrochloric acid. uMg and uPO4 were analyzed on a routine basis by the same standardized analysis at the local laboratory.
T50 was measured using a standardized method previously described.23 FGF23 was measured using the human intact FGF23 ELISA kit 60-6500 (Immutopics, San Clemente, CA), and fetuin-A was measured using the human intact Quantikine human Fetuin A ELISA kit DFTA00 (R&D Systems, Minneapolis, MN). PTH was measured using the second-generation intact PTH immunoassay (reference values 1.5–7.6 ρmol/l) (ADVIA Centaur, Siemens, Erlangen, Germany). eGFR was calculated using the CKD-EPI formula.
Statistical Analysis
The biostatistical evaluation was performed blinded using SPSS version 22.0.0.0 (IBM Corporation, Armonk, NY). Continuous data were described as mean ± SD for data of a normal distribution, and as median and interquartile range (25th–75th percentile) for data of a non-normal distribution. For assessment of within-group changes, a one-way analysis of variance with repeated measures or a Friedman test (both with post hoc Bonferroni correction for multiple measures) was applied for data with Normal or non-Normal distributions, respectively. For assessment of between-group changes of iMg, sMg, uMg, and T50, a mixed 2-way analysis of variance with repeated measures was applied. All tests were 2-sided tests and a P < 0.05 was considered statistically significant.
Results
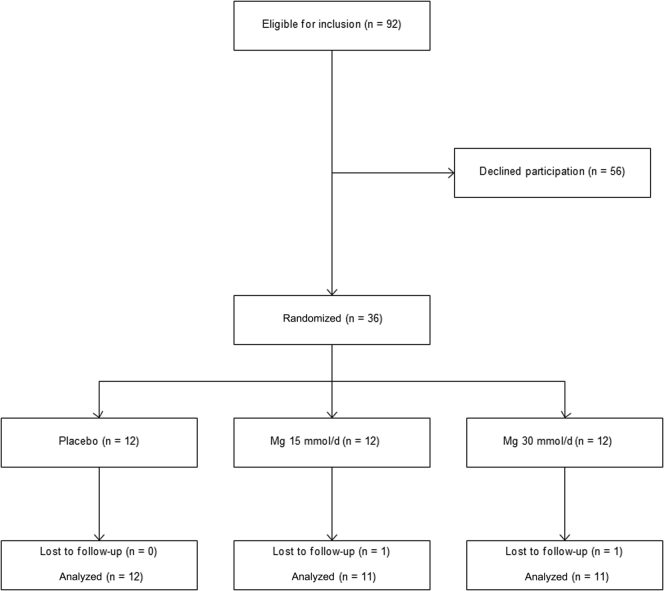
Ninety-two subjects met inclusion criteria for the trial, and of these, 36 agreed to participate (Figure 1). Demographic characteristics of trial subjects are displayed in Table 1. According to tablet count at final follow-up visit compliance >95% was achieved in all subjects.
Figure 1.
CONSORT diagram. Mg, magnesium.
Table 1.
Demographic information
| Characteristics | Total (n = 34) | Placebo (n = 12) | Mg 15 mmol/d (n = 11) | Mg 30 mmol/d (n = 11) |
|---|---|---|---|---|
| Male sex, no. (%) | 28 (82) | 12 (100) | 9 (82) | 7 (64) |
| White race, no. (%) | 33 (97) | 11 (92) | 11 (100) | 11 (100) |
| Age (yr) | 65.9 ± 8.7 | 70.4 ± 7.7 | 61.4 ± 10.2 | 65.4 ± 5.8 |
| Body mass index (kg/m2) | 27.9 ± 4.5 | 27.5 ± 3.4 | 27.8 ± 3.0 | 28.4 ± 6.6 |
| Smoker | ||||
| Active, no. (%) | 9 (26) | 2 (16) | 4 (36) | 3 (27) |
| Previous, no. (%) | 15 (44) | 8 (67) | 4 (36) | 3 (27) |
| Never, no. (%) | 10 (29) | 2 (17) | 3 (27) | 5 (46) |
| Comorbidities | ||||
| Diabetes mellitus | ||||
| Type 1, no. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type 2, no. (%) | 11 (32) | 3 (25) | 4 (36) | 4 (36) |
| Coronary artery disease, no. (%) | 6 (18) | 4 (33) | 1 (9) | 1 (9) |
| Heart failure, no. (%) | 1 (3) | 1 (8) | 0 (0) | 0 (0) |
| Hypertension, no. (%) | 32 (94) | 10 (83) | 11 (100) | 11 (100) |
| Dyslipidemia, no. (%) | 23 (68) | 10 (83) | 6 (55) | 7 (64) |
| Cerebrovascular disease, no. (%) | 5 (15) | 2 (17) | 1 (9) | 2 (18) |
| Peripheral artery disease, no. (%) | 3 (9) | 0 (0) | 2 (18) | 1 (9) |
| Cause of CKD | ||||
| Diabetes mellitus type 2, no. (%) | 6 (18) | 2 (17) | 1 (9) | 3 (27) |
| Hypertension, no. (%) | 8 (24) | 4 (33) | 3 (27) | 1 (9) |
| Chronic glomerulonephritis, no. (%) | 9 (27) | 5 (42) | 4 (36) | 0 (0) |
| Polycystic kidney disease, no. (%) | 2 (6) | 0 (0) | 0 (0) | 2 (18) |
| Interstitial nephropathy, no. (%) | 1 (3) | 0 (0) | 0 (0) | 1 (9) |
| Other, no. (%) | 8 (24) | 1 (8) | 3 (27) | 4 (36) |
| Medical therapy | ||||
| ACE inhibitor, no. (%) | 12 (35) | 5 (42) | 3 (27) | 4 (36) |
| ARB, no. (%) | 13 (38) | 3 (25) | 6 (55) | 4 (36) |
| Beta-blocker, no. (%) | 16 (47) | 5 (42) | 5 (46) | 6 (55) |
| Calcium-channel blocker, no. (%) | 21 (62) | 7 (58) | 6 (55) | 8 (73) |
| Loop diuretic, no. (%) | 10 (29) | 5 (42) | 2 (18) | 3 (27) |
| Thiazide-like diuretic, no. (%) | 7 (21) | 1 (8) | 4 (36) | 2 (18) |
| Statin, no. (%) | 19 (56) | 8 (67) | 5 (46) | 6 (55) |
| Phosphate binder, no. (%) | 3 (9) | 2 (17) | 1 (9) | 0 (0) |
| 25-Hydroxy vitamin D, no. (%) | 18 (53) | 10 (83) | 5 (46) | 3 (27) |
| 1,25-Dihydroxy vitamin D, no. (%) | 2 (6) | 1 (8) | 1 (9) | 0 (0) |
| Sodium bicarbonate, no. (%) | 3 (9) | 2 (17) | 1 (9) | 0 (0) |
| PPI, no. (%) | 15 (44) | 4 (33) | 7 (64) | 3 (27) |
| Amiloride, no. (%) | 2 (6) | 0 (0) | 1 (9) | 1 (9) |
| Spironolactone, no. (%) | 2 (6) | 1 (8) | 0 (0) | 1 (9) |
| CNI, no. (%) | 1 (3) | 1 (8) | 0 (0) | 0 (0) |
| Systolic blood pressure (mm Hg) | 138.4 ± 14.7 | 138.8 ± 14.7 | 139.1 ± 16.5 | 137.5 ± 14.1 |
| Diastolic blood pressure (mm Hg) | 82.3 ± 9.7 | 82.3 ± 9.3 | 83.3 ± 11.4 | 81.3 ± 9.2 |
| Heart rate beats/min | 75.6 ± 14.3 | 73.9 ± 10.5 | 78.5 ± 19.4 | 74.6 ± 12.94 |
| eGFRCKD-EPI (ml/min per 1.73 m2) | 32.6 ± 12.1 | 29.6 ± 14.4 | 36.2 ± 12.8 | 32.3 ± 8.4 |
| Proteinuria (g/d) | 1.63 ± 1.38 | 1.87 ± 1.33 | 1.76 ± 1.54 | 1.20 ± 1.30 |
| Hemoglobin (mmol/l) | 8.09 ± 0.86 | 8.15 ± 0.92 | 8.01 ± 0.96 | 8.09 ± 0.75 |
| Albumin (g/l) | 37.5 ± 3.1 | 36.2 ± 3.1 | 38.2 ± 3.2 | 38.4 ± 2.7 |
ACE, angiotensin converting enzyme; ARB, angiotensin 2 receptor blocker; CKD, chronic kidney disease; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; Mg, magnesium; PPI, proton pump inhibitor.
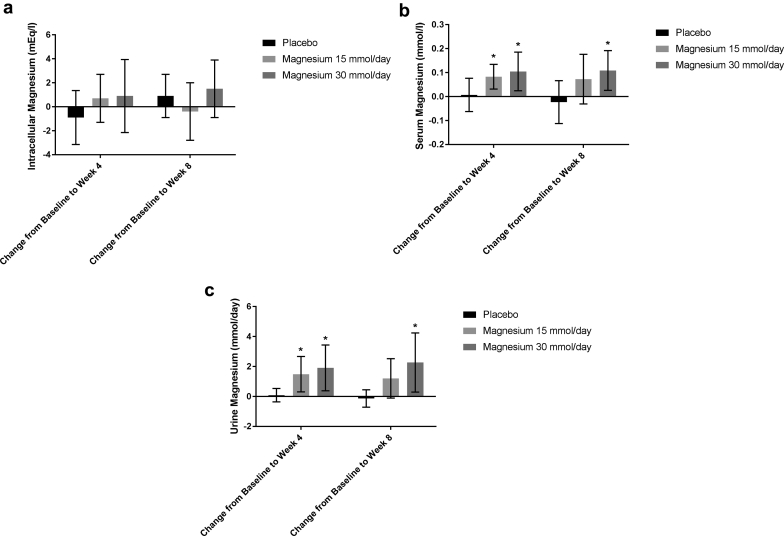
The primary study endpoint, iMg, did not change significantly compared with week 0 in any of the groups at either week 4 or week 8 (Table 2 and Figure 2a). sMg and uMg increased significantly from week 0 to week 4 in both Mg treatment groups, whereas there was no change in the placebo group (Table 2 and Figure 2b and c). At week 8, both sMg and uMg increased further compared with week 0 in the Mg 30 mmol/d group, whereas in the Mg 15 mmol/d group, only uMg was increased compared with week 0 and the change in sMg was no longer significant. There were no significant changes in sMg or uMg in the placebo group from week 0 to weeks 4 and 8. The effect of time and intervention on iMg was not statistically significant (Table 3), but there was a significant effect of time and intervention on both sMg and uMg (Table 3).
Table 2.
Treatment effect on magnesium parameters, one-way ANOVA with repeated measures, and Bonferroni-corrected post hoc tests
| Treatment group | Week 0 | Week 4 | Week 8 | P value for time effect | Difference Week 0 versus week 4 |
Difference Week 0 versus week 8 |
|---|---|---|---|---|---|---|
| Placebo | ||||||
| iMg (mEq/l) | 35.3 ± 1.9 | 34.4 ± 2.5 | 36.2 ± 2.7 | 0.11 | −0.9 (−3.1 to 1.4) | 0.9 (−0.9 to 2.7) |
| sMg (mmol/l) | 0.830 ± 0.084 | 0.837 ± 0.100 | 0.808 ± 0.100 | 0.50 | 0.007 (−0.063 to 0.076) | −0.023 (−0.112 to 0.067) |
| uMg (mmol/d) | 3.83 ± 1.62 | 3.92 ± 1.60 | 3.70 ± 2.82 | 0.78 | 0.09 (−0.70 to 0.87) | −0.13 (−1.41 to 1.14) |
| Mg 15 mmol/d | ||||||
| iMg (mEq/l) | 35.3 ± 2.7 | 36.0 ± 2.3 | 34.9 ± 2.9 | 0.40 | 0.7 (−1.3 to 2.7) | −0.4 (−2.8 to 2.0) |
| sMg (mmol/l) | 0.790 ± 0.119 | 0.873 ± 0.086 | 0.863 ± 0.110 | 0.04∗ | 0.083 (0.041 to 0.144)∗∗ | 0.073 (−0.031 to 0.176) |
| uMg (mmol/d) | 3.51 ± 1.33 | 5.00 ± 1.67 | 4.72 ± 1.75 | 0.003∗ | 1.49 (0.68 to 2.31)∗∗ | 1.21 (−0.15 to 2.58) |
| Mg 30 mmol/d | ||||||
| iMg (mEq/l) | 35.4 ± 2.7 | 36.2 ± 2.8 | 36.8 ± 2.5 | 0.30 | 0.9 (−2.2 to 3.9) | 1.5 (−0.9 to 3.9) |
| sMg (mmol/l) | 0.739 ± 0.101 | 0.844 ± 0.103 | 0.848 ± 0.099 | 0.001∗ | 0.105 (0.024 to 0.185)∗∗ | 0.109 (0.026 to 0.192)∗∗ |
| uMg (mmol/d) | 3.16 ± 1.23 | 5.08 ± 1.68 | 5.43 ± 2.08 | 0.001∗ | 1.91 (0.84 to 2.99)∗∗ | 2.27 (0.67 to 3.87)∗∗ |
Reported as mean ± SD for weeks 0, 4, and 8, and mean change with 95% confidence interval for differences between time points.
ANOVA, analysis of variance; CI, confidence interval; Mg, magnesium, iMg, intracellular magnesium; ionMg, ionized magnesium; sMg, serum magnesium; uMg, urine magnesium.
P < 0.05.
P < 0.05 after Bonferroni adjustment for multiple comparisons.
Figure 2.
(a) Mean change in intracellular magnesium compared with baseline. Error bars = 95% confidence interval. (b) Mean change in serum magnesium compared with baseline. Error bars = 95% confidence interval. *P < 0.05 after Bonferroni adjustment for multiple comparisons. (c) Mean change in 24-hour urine magnesium compared with baseline. Error bars = 95% confidence interval. *P < 0.05 after Bonferroni adjustment for multiple comparisons.
Table 3.
Change compared with baseline for magnesium parameters and serum calcification propensity, 2-way mixed ANOVA with repeated measures
| Change at week 4 Mean (CI; P value) |
Change at week 8 Mean (CI; P value) |
|
|---|---|---|
| Mg 15 mmol/d versus placebo | ||
| iMg (mEq/l) | 1.59 (−4.60 to 1.42; P = 0.41) | −1.32 (−3.97 to 1.34; P = 0.45) |
| sMg (mmol/l) | 0.076 (−0.010 to 0.162; P = 0.09) | 0.095 (−0.017 to 0.207; P = 0.11) |
| uMg (mmol/d) | 1.55 (0.45 to 2.65; P = 0.005)∗ | 1.59 (−0.09 to 3.27; P = 0.07) |
| T50 (min) | 47.7 (1.3 to 94.1; P = 0.43) | 29.1 (−17.6 to 75.9; P = 0.29) |
| Mg 30 mmol/d versus placebo | ||
| iMg (mEq/l) | 1.74 (−1.27 to 4.75; P = 0.34) | 0.53 (−2.12 to 3.18; P = 0.88) |
| sMg (mmol/l) | 0.098 (0.012 to 0.184; P = 0.02)∗ | 0.132 (0.020 to 0.243; P = 0.02)∗ |
| uMg (mmol/d) | 1.97 (0.90 to 3.04; P < 0.001)∗ | 2.85 (1.21 to 4.49; P = 0.001)∗ |
| T50 (min) | 59.6 (13.2 to 106.1; P = 0.01)∗ | 56.2 (9.5 to 102.9; P = 0.02)∗ |
| Mg 30 mmol/d versus Mg 15 mmol/d | ||
| iMg (mEq/l) | 0.15 (−2.93 to 3.22; P = 0.99) | 1.85 (−0.86 to 4.55; P = 0.23) |
| sMg (mmol/l) | 0.022 (−0.067 to 0.110; P = 0.82) | 0.036 (−0.078 to 0.151; P = 0.72) |
| uMg (mmol/d) | 0.42 (−0.68 to 1.52; P = 0.62) | 1.26 (−0.39 to 2.90; P = 0.16) |
| T50 (min) | 11.9 (−35.5 to 59.3; P = 0.81) | 27.1 (−20.7 to 74.8; P = 0.36) |
P values for the effect of time and intervention: iMg, P = 0.133; sMg, P = 0.016; uMg, P = 0.009; T50, P = 0.011.
ANOVA, analysis of variance; CI, confidence interval; Mg, magnesium; iMg, intracellular magnesium; sMg, serum magnesium; uMg, urine magnesium; T50, serum calcification propensity.
P < 0.05.
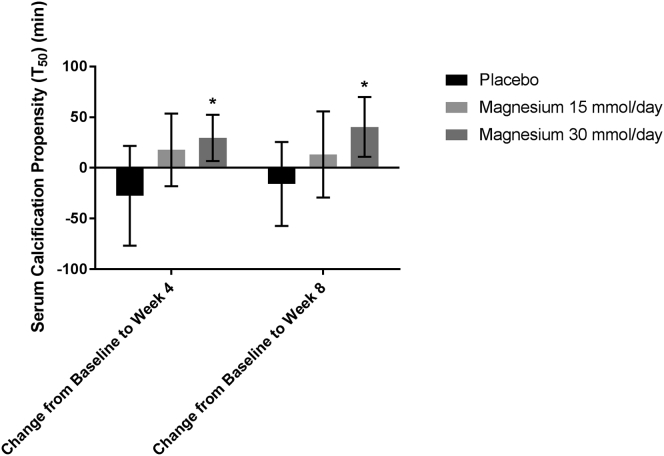
T50 increased significantly in the Mg 30 mmol/d group compared with baseline at both weeks 4 and 8, while only increasing significantly within the Mg 15 mmol/d group compared with baseline at week 4, and with no significant changes within the placebo group (Table 4 and Figure 3). There was also a significant effect of time, and intervention on T50 and post hoc tests revealed that Mg 30 mmol/d produced significantly greater changes to T50 over time compared with placebo or Mg 15 mmol/d (Table 3). Factors other than Mg known to affect T50 (fetuin-A, PO4, ionized Ca, albumin, bicarbonate) did not change significantly in either group throughout the trial (Table 4).
Table 4.
Treatment effect on serum calcification propensity and related factors, one-way ANOVA with repeated measures or Friedman test, both with Bonferroni-adjusted post hoc tests
| Treatment group | Week 0 | Week 4 | Week 8 | P value for time effect | Difference Week 0 versus week 4 |
Difference Week 0 versus week 8 |
|---|---|---|---|---|---|---|
| Placebo | ||||||
| T50 (min) | 298.4 ± 80.8 | 270.9 ± 75.4 | 282.6 ± 70.0 | 0.23 | −27.5 (−76.7 to 21.7) | −15.8 (−57.2 to 25.6) |
| ionCa (mmol/l) | 1.210 ± 0.054 | 1.206 ± 0.017 | 1.230 ± 0.041 | 0.84 | −0.005 (−0.046 to 0.036) | −0.007 (−0.044 to 0.029) |
| sPO4 (mmol/l) | 1.040 ± 0.202 | 1.118 ± 0.246 | 1.050 ± 0.258 | 0.33 | 0.078 (−0.054 to 0.210) | 0.010 (−0.115 to 0.135) |
| Fetuin-A (g/l) | 0.21 (0.16; 0.26) | 0.24 (0.13; 0.49) | 0.23 (0.13; 0.49) | 0.92 | 0.03 | 0.02 |
| Albumin (g/l) | 36.2 ± 3.1 | 35.2 ± 4.2 | 36.1 ± 3.2 | 0.39 | −1.0 (−3.5 to 1.5) | −0.1 (−2.0 to 1.8) |
| HCO3 (mmol/l) | 24.4 ± 3.4 | 24.1 ± 2.5 | 25.2 ± 2.6 | 0.42 | −0.3 (−3.2 to 2.5) | 0.8 (−1.4 to 3.1) |
| Mg 15 mmol/d | ||||||
| T50 (min) | 263.5 ± 59.1 | 281.2 ± 39.0 | 276.7 ± 35.0 | 0.40 | 17.7 (−18.1 to 53.6) | 13.3 (−29.2 to 55.8) |
| ionCa (mmol/l) | 1.193 ± 0.048 | 1.191 ± 0.041 | 1.179 ± 0.050 | 0.45 | −0.002 (−0.030 to 0.026) | −0.014 (−0.055 to 0.027) |
| sPO4 (mmol/l) | 1.082 ± 0.239 | 1.085 ± 0.234 | 1.085 ± 0.188 | 0.99 | 0.003 (−0.197 to 0.202) | 0.003 (−0.232 to 0.238) |
| Fetuin-A (g/l) | 0.22 (0.11; 0.31) | 0.28 (0.11; 0.42) | 0.27 (0.15; 0.55) | 0.53 | 0.06 | 0.05 |
| Albumin (g/l) | 38.2 ± 3.2 | 36.8 ± 3.0 | 37.5 ± 2.7 | 0.13 | −1.4 (−3.3 to 0.6) | −0.7 (−2.6 to 1.2) |
| HCO3 (mmol/l) | 25.0 ± 3.7 | 25.1 ± 3.5 | 25.7 ± 3.1 | 0.42 | 0.1 (−1.4 to 1.5) | 0.7 (−0.8 to 2.3) |
| Mg 30 mmol/d | ||||||
| T50 (min) | 256.0 ± 60.4 | 285.6 ± 69.1 | 296.4 ± 63.9 | 0.004∗ | 29.6 (6.8 to 52.5)∗∗ | 40.4 (10.9 to 69.9)∗∗ |
| ionCa (mmol/l) | 1.20 ± 0.04 | 1.21 ± 0.04 | 1.22 ± 0.06 | 0.18 | −0.01 (−0.04 to 0.02) | −0.03 (−0.08 to 0.03) |
| sPO4 (mmol/l) | 1.09 ± 0.18 | 1.09 ± 0.19 | 1.05 ± 0.20 | 0.62 | −0.00 (−0.08 to 0.08) | −0.04 (−0.18 to 0.10) |
| Fetuin-A (g/l) | 0.33 (0.11; 0.50) | 0.20 (0.13; 0.38) | 0.36 (0.22; 0.47) | 0.72 | −0.13 | 0.03 |
| Albumin (g/l) | 38.4 ± 2.7 | 38.0 ± 2.1 | 38.3 ± 1.9 | 0.77 | 0.4 (−1.5 to 2.2) | 0.1 (−1.5 to 1.7) |
| HCO3 (mmol/l) | 25.3 ± 1.8 | 26.2 ± 2.7 | 26.7 ± 2.0 | 0.08 | 0.9 (0.8 to 2.6) | 1.4 (−0.3 to 3.2) |
Reported as mean ± SD or median and interquartile range for weeks 0, 4, and 8 (as relevant), and change with 95% confidence interval or absolute change for differences between time points (as relevant).
ANOVA, analysis of variance; CI, confidence interval; ionCa, ionized calcium; HCO3, bicarbonate; Mg, magnesium; sPO4, serum phosphate; T50, serum calcification propensity.
P < 0.05.
P < 0.05 after Bonferroni adjustment for multiple comparisons.
Figure 3.
Mean change in serum calcification propensity compared with baseline. Error bars = 95% confidence interval. *P < 0.05 after Bonferroni adjustment for multiple comparisons.
eGFR did not change significantly throughout the trial, and there were no changes in blood analyses of mineral metabolism or uPO4 in any of the treatment groups (Table 5). There was a significant effect of time on change in QTc on electrocardiogram in the Mg 30 mmol/d group, but this change lost significance in post hoc tests (Table 5).
Table 5.
Treatment effect on mineral metabolism and kidney function, one-way ANOVA with repeated measures or Friedman test
| Treatment group | Week 0 | Week 4 | Week 8 | P value for time effect | Difference Week 0 versus week 4 |
Difference Week 0 versus week 8 |
|---|---|---|---|---|---|---|
| Placebo | ||||||
| eGFR (ml/min) | 29.6 ± 14.4 | 28.1 ± 13.6 | 29.6 ± 12.6 | 0.38 | −1.5 (−5.1 to 2.1) | 0.0 (−3.7 to 3.7) |
| ionCa (mmol/l) | 1.210 ± 0.054 | 1.206 ± 0.017 | 1.230 ± 0.041 | 0.84 | −0.005 (−0.046 to 0.036) | −0.007 (−0.044 to 0.029) |
| sPO4 (mmol/l) | 1.040 ± 0.202 | 1.118 ± 0.246 | 1.050 ± 0.258 | 0.33 | 0.078 (−0.054 to 0.210) | 0.010 (−0.115 to 0.135) |
| uPO4 (mg/d) | 2428 ± 751 | 2588 ± 860 | 2458 ± 365 | 0.82 | 159 (−854 to 1173) | 30 (−651 to 711) |
| PTH (pmol/l) | 10.4 (6.5:16.8) | 8.3 (7.0:16.9) | 11.6 (7.1:23.3) | 0.26 | −2.1 | 1.2 |
| 25-D3 (nmol/l) | 61.0 (48.5; 78.5) | 66.5 (53.3; 78.0) | 60.5 (56.3; 72.8) | 0.34 | 5.5 | −0.5 |
| FGF-23 (IU) | 98.0 (54.3; 173.8) | 153.0 (68.3; 277.0) | 112.0 (60.5; 357.3) | 0.56 | 55 | 14 |
| QTc (ms) | 408.3 ± 25.7 | 413.7 ± 19.7 | 414.8 ± 14.9 | 0.74 | 5.4 (−23.5 to 34.3) | 6.5 (−17.7 to 30.7) |
| Mg 15 mmol/d | ||||||
| eGFR (ml/min) | 36.2 ± 12.8 | 38.6 ± 15.1 | 36.6 ± 14.0 | 0.17 | 2.5 (−1.8 to 6.7) | 0.4 (−3.2 to 3.9) |
| ionCa (mmol/l) | 1.193 ± 0.048 | 1.191 ± 0.041 | 1.179 ± 0.050 | 0.45 | −0.002 (−0.030 to 0.026) | −0.014 (−0.055 to 0.027) |
| sPO4 (mmol/l) | 1.082 ± 0.239 | 1.085 ± 0.234 | 1.085 ± 0.188 | 0.99 | 0.003 (−0.197 to 0.202) | 0.003 (−0.232 to 0.238) |
| uPO4 (mg/d) | 2281 ± 880 | 2381 ± 647 | 2450 ± 1045 | 0.64 | 100 (−414 to 614) | 169 (−353 to 691) |
| PTH (pmol/l) | 9.3 (4.5; 18.3) | 10.1 (5.3; 13.9) | 13.8 (5.7; 17.3) | 0.34 | 0.8 | 4.5 |
| 25-D3 (nmol/l) | 46.5 (35.5; 65.3) | 49.0 (35.8; 54.8) | 40.5 (33.8; 63.3) | 0.67 | 3.5 | −6 |
| FGF-23 (IU) | 62 (53; 112) | 58 (42; 105) | 59 (56; 111) | 0.61 | −4 | −3 |
| QTc (ms) | 421.2 ± 30.3 | 418.5 ± 23.1 | 415.9 ± 30.9 | 0.73 | −2.7 (−17.2 to 11.8) | −5.3 (−27.8 to 17.2) |
| Mg 30 mmol/d | ||||||
| eGFR (ml/min) | 32.3 ± 8.4 | 32.6 ± 9.6 | 31.8 ± 9.6 | 0.86 | −0.3 (−3.7 to 3.1) | 0.5 (−3.6 to 4.5) |
| ionCa (mmol/l) | 1.20 ± 0.04 | 1.21 ± 0.04 | 1.22 ± 0.06 | 0.18 | −0.01 (−0.04 to 0.02) | −0.03 (−0.08 to 0.03) |
| sPO4 (mmol/l) | 1.09 ± 0.18 | 1.09 ± 0.19 | 1.05 ± 0.20 | 0.62 | −0.00 (−0.08 to 0.08) | 0.04 (−0.10 to 0.18) |
| uPO4 (mg/d) | 2126 ± 537 | 2027 ± 741 | 2071 ± 726 | 0.86 | 99 (−577 to 774) | 55 (−366 to 476) |
| PTH (pmol/l) | 13.2 (3.3; 17.9) | 15.1 (3.4; 19.9) | 13.0 (5.8; 15.5) | 0.81 | 1.9 | −0.2 |
| 25-D3 (nmol/l) | 63.5 (44.3; 81.0) | 64.0 (39.3; 79.3) | 58.0 (40.8; 73.0) | 0.38 | 0.5 | −5.5 |
| FGF-23 (IU) | 75 (62; 110) | 69 (57; 93) | 72 (47; 96) | 0.40 | −6 | −3 |
| QTc (ms) | 427.6 ± 21.2 | 430.3 ± 22.3 | 418.3 ± 26.0 | 0.03∗ | −2.8 (−16.4 to 10.9) | −9.2 (−4.8 to 23.3) |
Reported as mean ± SD or median and interquartile range for weeks 0, 4, and 8 (as relevant), and change with 95% confidence interval or absolute change for differences between time points (as relevant).
ANOVA, analysis of variance; 25-D3, 25-hydroxy vitamin D3; CI, confidence interval; ionCa, ionized calcium; sCa, total serum calcium; eGFR, estimated glomerular filtration rate; FGF-23, intact fibroblast growth factor 23; Mg, magnesium; sPO4, serum phosphate; PTH, parathyroid hormone; uPO4, urine phosphate; QTc, corrected QT interval.
P < 0.05.
During the course of the trial, one subject in the Mg 15 mmol/d group suffered a transitory ischemic attack and withdrew from the trial, and one subject in the Mg 30 mmol/d group withdrew because of difficulties swallowing the study medication. Further, one subject in the Mg 30 mmol/d group had side effects from the medication similar to previously experience from other medications (abdominal cramps, flushing, and palpitations), and intolerance to one of the tablet constituents was suspected (most likely talcum). There were 2 cases of community-acquired pneumonia in the placebo group that required hospital admissions, and one case of asthma exacerbation in the Mg 15 mmol/d group that required treatment with corticosteroids. None of the adverse events were considered to be due to the effects of Mg. Incidence of loosening of stool was identical (2 in each treatment group), but none of the study participants experienced frank diarrhea. None of the adverse events were attributed to Mg supplementation.
Discussion
The main finding of this clinical trial is that oral Mg supplementation using slow-release Mg hydroxide 30 mmol/d does not affect iMg as assessed by energy dispersive X-ray microanalysis after 8 weeks of treatment, despite significant increases in sMg and uMg. Furthermore, this trial has shown for the first time that calcification propensity T50 can be improved by oral Mg supplementation in CKD stages 3 and 4.
Study participants were included based on low to low-normal levels of sMg, and it is plausible that subjects may have been Mg-depleted based on the low iMg and uMg in all groups at baseline. Thus, Mg supplementation would first have to replenish total body Mg stores before “spilling over” into the serum, which could explain the relatively modest increase in sMg and uMg. We speculate that iMg stores increased in other compartments of the body (e.g., bone or muscle), and only later in epithelial cells from the sublingual mucosa, which would account for the lack of a treatment effect on iMg.
Despite 8 weeks of Mg supplementation with 15 mmol/d or 30 mmol/d on top of dietary Mg intake, uMg was only 4.72 mmol/d and 5.43 mmol/d in the 2 groups at week 8, respectively. Assuming that approximately one third of ingested Mg is available for absorption, uMg would be expected to be at least 5 mmol/d and 10 mmol/d for the 2 groups, if Mg intake was balanced by urinary excretion. The relatively small increases in uMg in the active treatment groups suggest either reduced Mg absorption in the gut, Mg retention due to total body Mg depletion, or Mg retention due to compromised renal Mg excretion. Proton pump inhibitors reduce intestinal Mg absorption, and were used by 64% and 27% of subjects in the 15 mmol/d and 30 mmol/d groups, respectively. Thus, reduced absorption of Mg might have blunted the effect of Mg supplementation. Faecal Mg was not measured in this trial, so it was not possible to assess Mg absorption in the gut.
This is the first clinical trial to show that T50 is improved by Mg supplementation in CKD stages 3 and 4. Notably, there were no significant changes to any of the other factors known to affect T50, suggesting that the change in T50 was caused by the increase in sMg alone. Indeed, adding Mg to serum ex vivo is known to increase T50.23 The final measurement of T50 in the group treated with Mg 30 mmol/d was similar to that of healthy adults without CKD,34 and the intervention shifted the mean T50 about half a SD for patients with predialysis CKD, which according to previous data might lead to a 20% risk reduction in all-cause mortality.24
Mg delays the formation of secondary calciprotein particles in the T50 test.23 A recent in vitro study convincingly demonstrated that secondary (but not primary) calciprotein particles induce calcification of vascular smooth muscle cells,35 suggesting that delaying the time until formation of secondary calciprotein particles (i.e., increasing T50) might improve calcification propensity via this mechanism. Thus, T50 appears to be a modifiable risk factor and might be useful not only for risk assessment, but also for monitoring and guiding therapy directed at inhibition of Ca/PO4 crystal formation. Whether the improvement of T50 observed in this trial is sustained over a longer treatment period, and whether this translates to a reduction in clinically relevant outcomes (e.g., cardiovascular events or all-cause mortality) will have to be studied in a future randomized clinical trial, which may use individualized and combined interventions aimed at improving T50.
Mg supplementation might be expected to lower sPO4 in the same manner as Mg-containing PO4 binders. We found no significant differences in sPO4, uPO4 or intact FGF23 in any of the groups, suggesting that Mg supplementation with the formulation used in this trial does not affect PO4 homeostasis in any clinically meaningful way. However, the sample size and increases in sMg were probably not powered to definitively rule out any interaction and larger study populations would be needed to answer this question.
Mg supplementation has previously been a concern in CKD and end-stage renal disease because of the risk of hypermagnesemia due to impaired renal excretion of Mg. There is general consensus that hypermagnesemia is likely not symptomatic at sMg < 2.0 mmol/l36 and that serious complications only occur at sMg > 3.0 mmol/l. Based on this trial, 8 weeks of Mg supplementation with slow-release Mg hydroxide is safe at doses up to 30 mmol/d in subjects with low or low-normal sMg and eGFR down to 15 ml/min per 1.73 m2 (the lowest eGFR of any subject in this trial). However, whether there are risks associated with longer treatment duration or in subjects with higher levels of sMg was not assessed in this trial.
There are limitations and strengths to the current trial. Limitations include that energy dispersive X-ray microanalysis of sublingual epithelial cells was used as a proxy for iMg, and not bone, vascular, or muscle biopsy, which are the gold standards for measurement of Mg levels in these tissues. Also, the method has not previously been applied to patients with CKD, which might have confounded the results. Furthermore, the sample size was small and the trial was of a short duration. Lastly, the formulation of slow-release Mg hydroxide used in this trial was chosen because it is widely used and readily available in Denmark. It is possible that other Mg formulations with greater bioavailability would have produced different effects on measures of Mg and on T50. Strengths of the trial include its single-center, randomized controlled and blinded study design.
In conclusion, 8 weeks of Mg supplementation using slow-release Mg hydroxide 30 mmol/d did not affect iMg in sublingual epithelial cells. Mg supplementation improved T50, which might aid in treating systemic calcification propensity. Mg supplementation was safe and well tolerated with no adverse events related to Mg treatment and no incidences of symptomatic hypermagnesemia. Slow-release Mg hydroxide 30 mmol/d might therefore be useful for increasing sMg in future clinical trials addressing vascular calcification or calcification propensity in CKD stages 3 and 4.
Disclosure
BS is the Research Director and President of IntraCellular Diagnostics, Inc., Medford, OR, USA. AP is one of the co-inventors of the T50 test and is the CEO and co-founder of Calciscon AG, Bern, Switzerland, which specializes in performing the T50 test. Gunnar Kjems ApS provided the study medication free of charge, but had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the other authors declared no competing interests.
References
- 1.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Moe S.M., Chen N.X. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 4.London G.M., Guerin A.P., Marchais S.J. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 5.Van Laecke S., Nagler E.V., Verbeke F. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med. 2013;126:825–831. doi: 10.1016/j.amjmed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi Y., Fujii N., Shoji T. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181. doi: 10.1038/ki.2013.327. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi Y., Fujii N., Shoji T. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PloS One. 2014;9:e116273. doi: 10.1371/journal.pone.0116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanbay M., Yilmaz M.I., Apetrii M. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36:228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 9.Ishimura E., Okuno S., Yamakawa T. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 10.Joao Matias P., Azevedo A., Laranjinha I. Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif. 2014;38:244–252. doi: 10.1159/000366124. [DOI] [PubMed] [Google Scholar]
- 11.Fein P., Weiss S., Ramos F. Serum magnesium concentration is a significant predictor of mortality in peritoneal dialysis patients. Adv Perit Dial. 2014;30:90–93. [PubMed] [Google Scholar]
- 12.Kurita N., Akizawa T., Fukagawa M. Contribution of dysregulated serum magnesium to mortality in hemodialysis patients with secondary hyperparathyroidism: a 3-year cohort study. Clin Kidney J. 2015;8:744–752. doi: 10.1093/ckj/sfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Roij van Zuijdewijn C.L., Grooteman M.P., Bots M.L. Serum magnesium and sudden death in European hemodialysis patients. PloS One. 2015;10:e0143104. doi: 10.1371/journal.pone.0143104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi Y., Iwatani H., Hamano T. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 2015;88:833–842. doi: 10.1038/ki.2015.165. [DOI] [PubMed] [Google Scholar]
- 15.Massy Z.A., Drueke T.B. Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol. 2015;11:432–442. doi: 10.1038/nrneph.2015.74. [DOI] [PubMed] [Google Scholar]
- 16.Montezano A.C., Zimmerman D., Yusuf H. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 17.Kircelli F., Peter M.E., Sevinc Ok E. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louvet L., Buchel J., Steppan S. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montes de Oca A., Guerrero F., Martinez-Moreno J.M. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PloS One. 2014;9:e89525. doi: 10.1371/journal.pone.0089525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelt J.G., McCabe K.M., Svajger B. Magnesium modifies the impact of calcitriol treatment on vascular calcification in experimental chronic kidney disease. J Pharmacol Exp Ther. 2015;355:451–462. doi: 10.1124/jpet.115.228106. [DOI] [PubMed] [Google Scholar]
- 21.Turgut F., Kanbay M., Metin M.R. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 22.Mortazavi M., Moeinzadeh F., Saadatnia M. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol. 2013;69:309–316. doi: 10.1159/000346427. [DOI] [PubMed] [Google Scholar]
- 23.Pasch A., Farese S., Graber S. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol. 2012;23:1744–1752. doi: 10.1681/ASN.2012030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith E.R., Ford M.L., Tomlinson L.A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol. 2014;25:339–348. doi: 10.1681/ASN.2013060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keyzer C.A., de Borst M.H., van den Berg E. Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol. 2016;27:239–248. doi: 10.1681/ASN.2014070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahle D.O., Asberg A., Hartmann A. Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients. Am J Transplant. 2016;16:204–212. doi: 10.1111/ajt.13443. [DOI] [PubMed] [Google Scholar]
- 27.Silver B.B. Development of cellular magnesium nano-analysis in treatment of clinical magnesium deficiency. J Am Coll Nutr. 2004;23:732S–737S. doi: 10.1080/07315724.2004.10719417. [DOI] [PubMed] [Google Scholar]
- 28.Haigney M.C., Silver B., Tanglao E. Noninvasive measurement of tissue magnesium and correlation with cardiac levels. Circulation. 1995;92:2190–2197. doi: 10.1161/01.cir.92.8.2190. [DOI] [PubMed] [Google Scholar]
- 29.Shechter M., Sharir M., Labrador M.J. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102:2353–2358. doi: 10.1161/01.cir.102.19.2353. [DOI] [PubMed] [Google Scholar]
- 30.Shechter M., Bairey Merz C.N., Stuehlinger H.G. Effects of oral magnesium therapy on exercise tolerance, exercise-induced chest pain, and quality of life in patients with coronary artery disease. Am J Cardiol. 2003;91:517–521. doi: 10.1016/s0002-9149(02)03297-6. [DOI] [PubMed] [Google Scholar]
- 31.McBride B.F., Min B., Kluger J. An evaluation of the impact of oral magnesium lactate on the corrected QT interval of patients receiving sotalol or dofetilide to prevent atrial or ventricular tachyarrhythmia recurrence. Ann Noninvasive Electrocardiol. 2006;11:163–169. doi: 10.1111/j.1542-474X.2006.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pokan R., Hofmann P., von Duvillard S.P. Oral magnesium therapy, exercise heart rate, exercise tolerance, and myocardial function in coronary artery disease patients. Br J Sports Med. 2006;40:773–778. doi: 10.1136/bjsm.2006.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shechter M., Saad T., Shechter A. Comparison of magnesium status using X-ray dispersion analysis following magnesium oxide and magnesium citrate treatment of healthy subjects. Magnes Res. 2012;25:28–39. doi: 10.1684/mrh.2012.0305. [DOI] [PubMed] [Google Scholar]
- 34.de Seigneux S., Ponte B., Berchtold L. Living kidney donation does not adversely affect serum calcification propensity and markers of vascular stiffness. Transpl Int. 2015;28:1074–1080. doi: 10.1111/tri.12595. [DOI] [PubMed] [Google Scholar]
- 35.Aghagolzadeh P., Bachtler M., Bijarnia R. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis. 2016;251:404–414. doi: 10.1016/j.atherosclerosis.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 36.Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]