Abstract
Introduction
In hemodialysis patients on ferric citrate hydrate, the increase in ferritin level is mainly due to the administration of the compound. We investigated possible other factors associated with ferritin level and how erythropoietin resistance index and erythropoiesis in those patients were affected. We looked at ferritin-elevating factors using data from a Japanese phase III long-term clinical trial of ferric citrate hydrate.
Methods
The factors with a strong association with ferritin levels at week 28 were selected by the process of variable selection. In addition, selected factors were analyzed by Mixed Model for Repeated Measurement. Subjects were divided into 3 groups by quantiles (<Q1, Q1–Q3, Q3<) of the most strongly correlated factors. Then the least-squares means of change of ferritin at each time point for each group were calculated. Finally, the differences of the least-squares means were examined. Changes of both erythropoiesis-stimulating agent dose and erythropoietin resistance index for each group were investigated. The differences in mean erythropoietin resistance index between groups at baseline, week 28, and week 52 were analyzed using t tests.
Results
Dose of ferric citrate hydrate showed the strongest correlation with change of ferritin and the second strongest was the reduction of erythropoiesis-stimulating agents. The mean erythropoietin resistance index was lowered in group <Q1. Group <Q1 showed significantly lower levels of ferritin at baseline.
Discussion
It is suggested that not only iron load but also the erythropoiesis-stimulating agent dose reduction may be involved in ferritin elevation during ferric citrate hydrate treatment, resulting in a decrease of erythropoietin resistance index.
Keywords: CKD, ERI, ESA, ferric citrate hydrate, ferritin, hyperphosphatemia
Hyperphosphatemia is associated with vascular calcification and mortality in patients on chronic hemodialysis.1 In patients with chronic kidney disease (CKD), control of hyperphosphatemia with phosphate binders is expected to improve prognosis.2, 3 There are 2 types of phosphate binders currently available: those containing calcium and those that are calcium free. Calcium-free phosphate binders such as sevelamer hydrochloride (or carbonate) and lanthanum carbonate may be beneficial for avoiding calcium loading.
Ferric citrate hydrate was developed as a calcium-free phosphate binder.4, 5 It was launched in 2014 in Japan ahead of other countries, and later in the USA and Taiwan. Because patients with CKD have high levels of hepcidin that downregulates intestinal iron absorption, oral administration of iron is supposedly less likely to cause elevation of serum ferritin levels than i.v. administration.6 Orally administered trivalent iron is reduced to bivalent iron in the digestive tract and is then absorbed via the divalent metal transporter 1 in the intestinal epithelium.7 Thus, ferric citrate hydrate, a trivalent iron, might be considered not to have a significant effect on serum ferritin levels. In addition, it was reported that phosphorus in the intestine attenuates iron reduction and inhibits iron absorption in the intestine.4, 7
However, both phase III long-term clinical trials of ferric citrate hydrate in hemodialysis patients that were conducted in Japan and the USA showed increases in serum ferritin, as well as dose reduction of erythropoiesis-stimulating agent (ESA) and i.v. iron preparations by administration of ferric citrate hydrate.5, 8 It has been confirmed in the clinical trials that iron from administered ferric citrate hydrate is partially absorbed and increases serum ferritin and hemoglobin levels.5, 8
There are factors other than simply iron absorption that can cause serum ferritin concentrations to rise during iron therapy. Because ferric citrate hydrate’s primary role is as a phosphate binder, it would be important to better understand factors that lead to the increase in serum ferritin found with its use. This would also aid further understanding of the pathology of iron-deficiency anemia in CKD. In this study, we investigated serum ferritin-elevating factors using data from the Japanese phase III long-term trial conducted in hemodialysis patients with hyperphosphatemia.5 Because ferric citrate hydrate administration enables the dose reduction of ESA and elevates serum hemoglobin levels, we also examined the erythropoietin resistance index (ERI) and investigated erythropoiesis.
Methods
Data from the Japanese long-term trial were used to identify the factors causing the elevation of serum ferritin and to investigate changes in ERI and the association between administration of ferric citrate hydrate and erythrocyte indices (mean corpuscular volume [MCV] and mean corpuscular hemoglobin [MCH]). The trial recruits were Japanese patients with hyperphosphatemia, and who were undergoing hemodialysis. The methods used in this study were as follows.
Identification of Serum Ferritin-Elevating Factors in Patients on Ferric Citrate Hydrate
In the Japanese long-term trial, serum ferritin levels had risen for 28 weeks after the start of ferric citrate hydrate administration (baseline; 0 wk). After week 28, the levels stabilized until week 52. Therefore, the factors with a strong association with serum ferritin levels (change of ferritin level from baseline) at week 28 were selected from the obtained data by the process of variable selection (stepwise method). The selected candidate variables were factors for which it is considered clinically plausible for there to be an association with a change of ferritin: age, gender, body weight, and serum phosphorus level (P[0 wk]), serum ferritin level (Ferritin [0 wk]), serum hemoglobin level (Hb [0 wk]), MCV [0 wk], MCH [0 wk], and transferrin saturation (TSAT [0 wk]), plus change in ESA dose at week 28 from baseline (change of ESA), average dose of ferric citrate hydrate over 28 weeks (dose of ferric citrate hydrate), and change of ERI at week 28 from baseline (change of ERI).
To avoid multicollinearity problems at variable selection by inclusion of factors that are strongly related to each other in the model, one factor was excluded using correlation analysis and single regression analysis. In addition, to investigate the correlation between the most strongly related factors and change of ferritin at each time point, selected factors were analyzed by Mixed Model for Repeated Measurement using the model described here. The exception to this was the dose of ferric citrate hydrate. We divided subjects based on their ESA dose administered by using quantiles. The calculated quantiles were that Q1 (lower 25% points) was −2500 IU/wk, median was 0 IU/wk, and Q3 (upper 25% points) was also 0 IU/wk. It was shown that the median equaled Q3 by chance. Therefore we combined a group (Q1 through to less than median) and another group (median through to less than Q3), and accordingly subjects (N = 146) were divided into 3 groups (group <Q1: n = 36, group Q1–Q3: n = 74, group Q3<: n = 36) by quantiles (<Q1, Q1–Q3, Q3<) of the most strongly correlated factors based on the change of ESA dose administered. Then the least-squares means of change of ferritin at each time point for each group were calculated, and the differences of the least-squares means were examined.
Model
The response variable was the change of ferritin level. The fixed effect was the factor with the lowest P value among selected factors by variable selection (except dose of ferric citrate hydrate). The covariates were selected factors as chosen by variable selection, time point, and time point × fixed effect except fixed effect.
Investigation of the Correlation Between Administration of Ferric Citrate Hydrate and Erythrocyte Indices
Subjects were divided into 3 groups by using quantiles of fixed effect. The dose of ESA and change of ERI for each group were investigated. The dose of ESA was calculated using the following equation:
The differences in mean ERI between groups at baseline, week 28, and week 52 were analyzed using t tests. Multiplicity was not considered. ERI was calculated using the following equation:
In addition, the change of serum hemoglobin, MCV, and MCH of each group were examined.
Results
Analysis of Serum Ferritin-Elevating Factors in Patients on Ferric Citrate Hydrate
The correlation of Pearson analysis for factors that were clinically considered to be potentially associated with a change of ferritin showed that MCV and MCH (ρ = 0.84), and also the change of ESA dose and change of ERI (ρ = 0.96) were strongly correlated. Gender and body weight (ρ = −0.47) were also correlated (Table 1). Single regression analysis showed that body weight, MCH, and change of ESA were more strongly associated with change of ferritin than gender, MCV, and change of ERI, respectively. Therefore, gender, MCV, and change of ERI were removed from candidate factors (Table 2). Secondly, age, Ferritin (0 wk), change of ESA, and dose of ferric citrate hydrate were selected as factors strongly correlated to change of ferritin from the candidate factors (age, body weight, serum P [0 wk], Ferritin [0 wk], Hb [0 wk], MCH [0 wk], TSAT [0 wk], change of ESA dose and dose of ferric citrate hydrate) by variable selection (Table 3). Change of ESA showed the second strongest correlation with change of ferritin, following dose of ferric citrate hydrate. Thus, subjects were divided into 3 groups by using quantiles of change of ESA dose (Q1 = −2500 IU/wk [lower 25% points], Q3 = 0 IU/wk [upper 25% points]), and Mixed Model for Repeated Measurement analysis was performed. As a result, the factor of the increase in ferritin was associated with not only ferric citrate hydrate dose but also the degree of decrease in ESA dose (Figure 1). The serum ferritin level tended to increase more in group <Q1 than other groups. The analysis of the difference of least mean square values revealed that the change of ferritin level had significantly increased from week 16 to 52 in the group <Q1, than in other groups (P < 0.05).
Table 1.
Correlation analysis for candidate factors that may relate to a change of ferritin
| Ferritin (0 wk) | Hb (0 wk) | MCH (0 wk) | Gender | ESA change (28–0 wk) | Ferric citrate hydrate dose (28-wk mean) | TSAT (0 wk) | Body weight | MCV (0 wk) | P (0 wk) | ERI change (28–0 wk) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.03 | 0.04 | 0.16 | −0.04 | −0.06 | −0.06 | −0.07 | −0.23a | 0.25a | −0.09 | −0.05 |
| Ferritin (0 wk) | −0.10 | 0.05 | 0.05 | 0.15 | 0.02 | 0.16 | −0.08 | 0.05 | −0.02 | 0.17 | |
| Hb (0 wk) | 0.07 | −0.04 | −0.02 | 0.10 | 0.05 | 0.08 | 0.10 | 0.08 | 0.03 | ||
| MCH (0 wk) | −0.09 | 0.21a | 0.08 | 0.23a | −0.06 | 0.84b | 0.00 | 0.22a | |||
| Gender | −0.01 | −0.05 | −0.08 | −0.47c | 0.09 | −0.02 | −0.06 | ||||
| ESA change (28–0 wk) | −0.22a | 0.28a | −0.10 | 0.13 | 0.09 | 0.96b | |||||
| Ferric citrate hydrate dose (28-wk mean) | 0.00 | 0.07 | 0.12 | 0.33a | −0.20a | ||||||
| TSAT (0 wk) | −0.13 | 0.19 | 0.10 | 0.26a | |||||||
| Body weight | −0.22a | 0.10 | −0.02 | ||||||||
| MCV (0 wk) | 0.01 | 0.14 | |||||||||
| P (0 wk) | 0.06 |
The correlation analysis for factors that were clinically considered to be potentially associated with a change of ferritin was performed.
ERI, erythropoietin resistance index; ESA, erythropoietin-stimulating agents; Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; P, phosphate; TSAT, serum ferritin and transferrin saturation.
Correlation coefficient (r): |r| ≥ 0.6.
Correlation coefficient (r): 0.2 ≤ |r| < 0.4.
Correlation coefficient (r): 0.4 ≤ |r| < 0.6.
Table 2.
Single regression analysis for factors with a correlation coefficient ≥0.4
| Variable | Estimate | P value | Lower 95% confidence interval | Upper 95% confidence interval |
|---|---|---|---|---|
| MCH (0 wk) | −4.84 | 0.49 | −18.79 | 9.11 |
| Gender | 6.91 | 0.77 | −38.83 | 52.64 |
| Body weight | 0.57 | 0.51 | −1.12 | 2.26 |
| MCV (0 wk) | 0.58 | 0.80 | −3.99 | 5.15 |
| ESA change (28–0 wk) | −0.02 | <0.0001 | −0.03 | −0.01 |
| ERI change (28–0 wk) | −9.18 | 0.0003 | −14.11 | −4.25 |
Single regression analysis showed that body weight, MCH, and change of ESA were more strongly associated with change of ferritin than gender, MCV, and change of ERI respectively. Therefore, gender, MCV, and change of ERI were removed from candidate factors.
ERI, erythropoietin resistance index; ESA, erythropoietin-stimulating agents; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume.
Table 3.
Selected variables by using variable selection
| Variable | Estimate | P value | Lower 95% confidence interval | Upper 95% confidence interval |
|---|---|---|---|---|
| Ferric citrate hydrate dose (28-wk mean) | 60.58 | <0.001 | 45.37 | 75.79 |
| ESA change (28–0 wk) | −0.01 | 0.001 | −0.02 | −0.01 |
| Age | 2.08 | 0.009 | 0.54 | 3.62 |
| Ferritin (0 wk) | 0.16 | 0.15 | −0.06 | 0.37 |
ESA, erythropoietin-stimulating agent.
Figure 1.
Adjusted mean of change of ferritin levels of 3 groups divided by using quantiles of change of erythropoiesis-stimulating agent (ESA) dose. Subjects were divided into 3 groups (group <Q1: n = 36, group Q1–Q3: n = 74, group Q3<: n = 36) by using quantiles of change of ESA dose (Q1 = −2500 IU/wk, Q3 = 0 IU/wk) and Mixed Model for Repeated Measurement analysis was performed. Then the least-squares means of change of ferritin at each time point for each group were calculated, and the differences of the least-squares means were examined. #P < 0.05: group <Q1 versus group Q1–Q3, *P < 0.05: group <Q1 versus group Q3<.
Investigation of the Correlation Between Administration of Ferric Citrate Hydrate and Erythrocyte Indices
The identification of serum ferritin-increasing factors showed that the change of ESA dose was the factor second most strongly related to the change of ferritin level, following dose of ferric citrate hydrate. The subjects were divided into groups by quantiles on the basis of the change of ESA dose, and this factor was investigated by time point for each group. As a result, we found that the baseline dose of ESA was significantly greater in group <Q1 than in the other groups. However, later in the trial the ESA dose of each group became more similar (Figure 2).
Figure 2.
Change of erythropoiesis-stimulating agent (ESA) dose in groups divided by using quantiles of change of ESA dose. The vertical axis shows dose of ESA. The subjects were divided into groups (group <Q1: n = 36, group Q1–Q3: n = 74, group Q3<: n = 36) by quantiles on the basis of change of ESA dose (Q1 = −2500 IU/wk, Q3 = 0 IU/wk), and this factor was investigated by time point for each group.
At the same time, the ERI values at baseline, week 28, and week 52 of each group were investigated. The ERI at baseline was higher in group <Q1, whereas ERI values at weeks 28 and 52 were not different between groups (Figure 3). Mean ERI at baseline was significantly different between groups by a t test, although there was no significant difference between groups at week 52 (Table 4).
Figure 3.
Box plot of erythropoietin resistance index (ERI) in groups divided by using quantiles of the change of the ESA dose. The subjects were divided into 3 groups (group <Q1: n = 36, group Q1–Q3: n = 74, group Q3<: n = 36) by quantiles on the basis of change of ESA dose (Q1 = −2500 IU/wk, Q3 = 0 IU/wk), and the ERI values were investigated at baseline, week 28, and week 52 of each group.
Table 4.
t test for mean of ERI in groups divided by using quantiles of change of ESA dose
|
P value |
|||
|---|---|---|---|
| Week 0 | Week 28 | Week 52 | |
| <Q1 versus Q1–Q3 | <0.001 | 0.05 | 0.68 |
| <Q1 versus Q3< | <0.001 | <0.001 | 0.40 |
| Q1–Q3 versus Q3< | 0.03 | 0.01 | 0.19 |
Quantiles of change of ESA dose: Q1 = −2500 IU/wk, Q3 = 0 IU/wk.
ESA, erythropoiesis-stimulating agent; ERI, erythropoietin resistance index.
Serum hemoglobin levels were almost similar in each group at baseline, although they fluctuated after week 2 in all groups. MCV in group <Q1 was lower than that in group Q3< at baseline, but increased within the reference range (80–98 fl) later in the trial. MCH in group <Q1 was lower than that in other groups at baseline, but increased within the reference range (28–32 pg) later in the trial (data not shown).
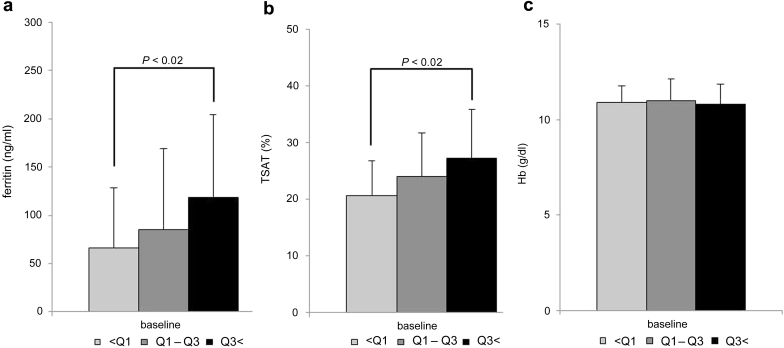
The serum ferritin level, TSAT, and hemoglobin level of each group were compared at baseline. There was no difference in hemoglobin, whereas group <Q1 showed significantly lower levels of serum ferritin and TSAT than group Q3< (Figure 4).
Figure 4.
(a) Serum ferritin, (b) TSAT, and (c) serum Hb in groups divided by using quantiles of change of ESA dose. The subjects were divided into 3 groups (group <Q1: n = 36, group Q1–Q3: n = 74, group Q3<: n = 36) by quantiles on the basis of the change of the ESA dose (Q1 = −2500 IU/wk, Q3 = 0 IU/wk). The serum ferritin level, TSAT, and hemoglobin level of each group were compared at baseline. P < 0.02: multiplicity of comparisons was taken into consideration using the Bonferroni method. ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; TSAT, serum ferritin and transferrin saturation.
Discussion
Oral administration of iron preparations has been considered less likely to cause oxidative stress than i.v. administration.9, 10 Accordingly, ferric citrate hydrate could reduce oxidative stress by reducing the necessary dose of i.v. iron preparations. However, there is still some concern that ferric citrate hydrate may affect iron metabolism even when orally administered. We investigated serum ferritin-elevating factors using data from the Japanese phase III long-term clinical trial of ferric citrate hydrate. The factors with a strong association with serum ferritin levels at week 28 were selected by the process of variable selection. In addition, selected factors were analyzed by Mixed Model for Repeated Measurement. Dose of i.v. iron preparations was not included into the factor because an increase in serum ferritin level was found despite that the dose was remarkably reduced in course of the trial.5 This study showed that change of ESA dose, age, and baseline serum ferritin levels, as well as the dose of ferric citrate hydrate, were factors strongly associated with the change of serum ferritin level during ferric citrate hydrate therapy. Also, the strongest serum ferritin-elevating factor, second only to the dose of ferric citrate hydrate, was shown to be the dose reduction of ESA. This is supported by a report of Karaboyas et al.11 In the USA, serum ferritin levels have increased despite the fact that usage of ESA preparations has decreased and usage of i.v. iron preparations has not increased since changes in the medical system in 2011. Thus, it is suggested that less use of ESA preparations might be associated with an increase in serum ferritin levels.12
Analysis of correlations between administration of ferric citrate hydrate and erythrocyte indices (MCV and MCH) was performed by dividing subjects into groups by quantiles on the basis of the change of ESA dose. Whereas hemoglobin levels were similar in all groups at baseline, MCV and MCH increased within reference ranges in accordance with a decrease of ERI in the group that showed a greater reduction of ESA dose. In addition, despite serum ferritin levels at baseline in group <Q1 being significantly lower than in other groups, they had markedly increased by ferric citrate hydrate administration. With regard to the dose of i.v. iron preparations administered, it was dramatically reduced similarly in each group in the course of the Japanese long-term trial (data not shown). This result suggests that ferric citrate hydrate can improve iron deficiency by the iron component being partly absorbed.
Orally administered iron is reduced from the trivalent to bivalent form in the digestive tract and then absorbed via divalent metal transporter 1 in the intestinal epithelium.7 However, intestinal iron absorption is suggested to be suppressed and serum ferritin levels tend not to be increased in patients with CKD, whose levels of hepcidin—which regulates iron absorption in the intestine—are high.6 Therefore, ferric citrate hydrate had been thought not to have much effect on serum ferritin levels.
On the contrary, it was observed that serum ferritin levels rise during ferric citrate hydrate treatment. In previous studies, serum ferritin levels showed an elevation up to week 24 to week 28, after which they increased only slowly or plateaued by week 52.5, 8 The stabilization of serum ferritin elevation in the later stages was observed in both phase III long-term clinical trials conducted in Japan and the USA, respectively, but the mechanism for this still remains to be studied. The results of this study suggest that patients with CKD may have subclinical iron deficiency. A change in the behavior of duodenal cytochrome B, which is involved with the reduction of iron, may be involved in the mechanism. In addition, one study has reported that the tight junctions of the intestinal epithelium were damaged in CKD model rats.13 Therefore, there may be other routes of intestinal iron absorption that do not involve divalent metal transporter 1. Sucroferric oxyhydroxide, which also contains trivalent iron, has been reported to elevate serum ferritin levels to a smaller extent than ferric citrate hydrate.14 Thus, intestinal iron absorption in patients with CKD is not fully understood.
In the Japanese long-term trial, hemoglobin levels and serum ferritin levels were elevated by ferric citrate hydrate and then plateaued according to the dose reduction of ESA.5 The results suggest that the iron supplied from ferric citrate hydrate was employed for hematopoiesis. Shoji et al.15 reported that serum ferritin levels were lowered without a change in hemoglobin levels by swapping recombinant erythropoietin for darbepoetin, a long-acting ESA preparation.15 They postulated that serum ferritin levels may have decreased because the stored iron was used as a result of the ESA preparation being administered. Long-acting ESA preparations had also been used in the Japanese long-term trial of ferric citrate hydrate. Therefore, it is considered that subclinical iron deficiency exists in hemodialysis patients as shown by low levels of serum ferritin (Figure 4a). The effect on iron usage and storage in the body by different types of ESA should be clarified in the future.
As a mechanism for the elevation of serum ferritin levels, it has been reported that acute loss of erythropoietin caused apoptosis of immature erythrocytes and that iron contained in the apoptotic cells was stored as serum ferritin.16 Thus, erythrocyte breakdown might exceed its production because of the ESA dose reduction caused by ferric citrate hydrate administration; in addition, the iron from the erythrocytes that was stored as serum ferritin might have contributed to the elevation of serum ferritin levels.
In this study, serum ferritin-elevating factors were investigated using data from the Japanese long-term trial. It is suggested that not only iron load but also the ESA dose reduction may be involved in serum ferritin elevation, resulting in a decrease of ERI. Iron supplements are suggested to improve erythropoiesis.
Limitations
This study was a retrospective study. The original primary endpoint of the Japanese long-term trial was the effect of ferric citrate hydrate on serum phosphate levels, not on serum ferritin levels. In addition, although the serum ferritin level is used to evaluate iron metabolism, it also could be affected by various factors including inflammation and ESA preparation, not just by iron administration.11, 17, 18 Further evaluation comparing serum ferritin concentration among each type of ESA preparation would be needed to determine if the ferritin elevation is different depending on individual ESA preparation. Therefore, the clinical significance of serum ferritin is not yet fully understood. Because postdialysis body weight was not obtained in the Japanese long-term trial, we used predialysis body weight to calculate ERI.
Disclosure
The study was conducted by Japan Tobacco Inc. Torii Pharmaceutical Co., Ltd. and Japan Tobacco Inc. were involved in the additional analyses and investigation of the results in this study. HH, TA, MN, K Yokoyama, and SF received consulting fees from Torii Pharmaceutical Co., Ltd. TO and K Yamada are employees of Japan Tobacco Inc. YN is employed by Torii Pharmaceutical Co., Ltd. The other author declared no competing interests.
Acknowledgment
We acknowledge the editorial support of Emedits Global Ltd. in the preparation of this manuscript.
References
- 1.Block G.A., Hulbert-Shearon T.E., Levin N.W., Port F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 2.Kestenbaum B., Sampson J.N., Rudser K.D. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R., Peralta C.A., Chen S.C., Kidney Early Evaluation Program (KEEP) Investigators No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Kidney Int. 2013;84:989–997. doi: 10.1038/ki.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nastou D., Fernández-Fernández B., Elewa U. Next-generation phosphate binders: focus on iron-based binders. Drugs. 2014;74:863–877. doi: 10.1007/s40265-014-0224-6. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama K., Akiba T., Fukagawa M. Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr. 2014;24:261–267. doi: 10.1053/j.jrn.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Garrido P., Ribeiro S., Fernandes J. Iron-hepcidin dysmetabolism, anemia and renal hypoxia, inflammation and fibrosis in the remnant kidney rat model. PLoS One. 2015;10:e0124048. doi: 10.1371/journal.pone.0124048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przybyszewska J., Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208–213. doi: 10.5114/pg.2014.45102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis J.B., Sika M., Koury M.J., Collaborative Study Group Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26:493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim P.S., Wei Y.H., Yu Y.L., Kho B. Enhanced oxidative stress in haemodialysis patients receiving intravenous iron therapy. Nephrol Dial Transpl. 1999;14:2680–2687. doi: 10.1093/ndt/14.11.2680. [DOI] [PubMed] [Google Scholar]
- 10.Akarsu S., Demir H., Selek S., Oguzoncul F. Iron deficiency anemia and levels of oxidative stress induced by treatment modality. Pediatr Int. 2013;55:289–295. doi: 10.1111/ped.12054. [DOI] [PubMed] [Google Scholar]
- 11.Karaboyas A., Zee J., Morgenstern H. Understanding the recent increase in ferritin levels in United States dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol. 2015;10:1814–1821. doi: 10.2215/CJN.02600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block G.A., Fishbane S., Rodriguez M. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis. 2015;65:728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri N.D., Yuan J., Nazertehrani S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol. 2013;38:99–103. doi: 10.1159/000353764. [DOI] [PubMed] [Google Scholar]
- 14.Floege J., Covic A.C., Ketteler M. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037–1046. doi: 10.1093/ndt/gfv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoji S., Inaba M., Tomosugi N. Greater potency of darbepoetin-α than erythropoietin in suppression of serum hepcidin-25 and utilization of iron for erythropoiesis in hemodialysis patients. Eur J Haematol. 2013;90:237–244. doi: 10.1111/ejh.12067. [DOI] [PubMed] [Google Scholar]
- 16.Rice L., Alfrey C.P., Driscoll T. Neocytolysis contributes to the anemia of renal disease. Am J Kidney Dis. 1999;33:59–62. doi: 10.1016/s0272-6386(99)70258-1. [DOI] [PubMed] [Google Scholar]
- 17.Charytan D.M., Pai A.B., Chan C.T. Dialysis Advisory Group of the American Society of Nephrology. Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol. 2015;26:1238–1247. doi: 10.1681/ASN.2014090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasuike Y., Nonoguchi H., Tokuyama M. Serum ferritin predicts prognosis in hemodialysis patients: the Nishinomiya study. Clin Exp Nephrol. 2010;14:349–355. doi: 10.1007/s10157-010-0288-x. [DOI] [PubMed] [Google Scholar]