Abstract
Introduction
We developed the Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST) to permit hemodialysis (HD) patients with central venous catheters (catheters) to shower without additional infection risk. Our primary objective was to determine the feasibility of conducting a parallel randomized controlled trial (RCT) to evaluate the impact of HIPPO-ST on catheter-related bacteremia (CRB) in adult HD patients.
Methods
Adult HD patients using catheters were recruited from 11 HD units. Patients were randomized to receive HIPPO-ST or standard care and were followed up for 6 months. Only CRB-outcome assessors were blinded. For the study to be considered feasible, 4 of 5 feasibility outcomes, each with its own statistical threshold for success, must have been achieved.
Results
A total of 68 patients were randomized (33 HIPPO-ST and 35 control) and were followed up to 6 months. Of 5 measures of feasibility, 4 were achieved: (1) accurate CRB rate documented (threshold: κ level >0.80); (2) 97.8% (279/285) of satellite HD patients with catheters were screened (threshold: >95%); (3) 88% (23/26) in the HIPPO-ST arm were successfully educated by 6 months (threshold: >80%); and (4) 0% (0/29) patients in the control arm were “contaminated,” that is, using HIPPO-ST (threshold: <5%). However, only 44.2% (72/163) of eligible patients consented to participate (threshold: >80%). The rate of CRB was similarly low in HIPPO-ST and control groups (0.68 vs. 0.88/1000 catheter days).
Discussion
This HIPPO-ST pilot study demonstrated the feasibility of the larger HIPPO-ST study, especially given the high levels of education success with the HIPPO-ST arm and the low levels of contamination in the control arm.
Keywords: catheter, hemodialysis, pilot study, randomized controlled trial, vascular access
Arteriovenous fistulas (fistula) are associated with the lowest morbidity and mortality of the 3 vascular access types, if they mature to be used to deliver adequate dialysis.1, 2 However, hemodialysis (HD) central venous catheters (catheters) are the predominant choice of vascular access for patients requiring immediate HD until either a synthetic arteriovenous graft (graft) or a fistula can be placed. Currently, despite efforts to promote increased arteriovenous-access creation and use,3 up to 80% of incident and 50% of prevalent patients in North America dialyze via a catheter.4, 5 Catheter use represents a burden on health care resources; it is associated with both the highest financial costs and the highest associated morbidity and mortality of all vascular access types.6, 7, 8, 9, 10, 11, 12, 13
Catheter-related infections drive much of this increased cost,14 with estimates of $17,000 USD to $32,000 USD for the total direct and indirect costs associated with hospitalizations due to catheter-related infection.7, 15, 16, 17 Catheter-related infections encompass catheter entry site infections, tunnel infections, and bacteremia; however, HD catheter-related bacteremia (CRB) are considered the most clinically important, as they have the potential to progress to sepsis and death.6 Thus, it is critically important to have effective prophylactic strategies as part of routine catheter care to limit catheter-related infection overall, and CRB specifically, with minimal complication risk, inconvenience, and discomfort to the patient, and at minimal cost.18
Currently, in most guideline recommendations on routine catheter care, wet submersion of the catheter or catheter entry site is not advised, including swimming, submerged baths, and showering, as it is not possible to ensure full protective coverage of the catheter entry site with dressings, ointments, or other protective barriers during these activities.19, 20, 21, 22, 23 Exposure to nonsterile, dirty, and/or damp environments may facilitate microrganism colonization and entry at the catheter entry site, potentially leading to subsequent catheter-related infection, especially if the catheter entry site is not fully healed.20, 21, 22, 23 Yet despite the potential infection risk associated with showering, patient compliance with the recommendation not to shower with their catheter is poor. Up to 77% of patients shower despite being advised not to by their health care team.24
To address the patient’s desire to shower for hygiene and quality of life reasons and to simultaneously adhere to infection prophylactic measures, several “shower techniques” have been developed as an alternative method of catheter care24, 25, 26; however, “shower techniques” have not yet been formally evaluated to ensure that they do not increase catheter-related infection risk. We developed the Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST) to permit HD patients with catheters to shower but not increase infection risk. The primary objective of this pilot study is to determine whether it is feasible to conduct a large randomized controlled trial (RCT) comparing the rate of CRB in adult satellite HD patients using HIPPO-ST versus standard catheter care over 6 months. Our secondary objectives include comparing the rate and proportions of CRB, entry site and tunnel infections, and vascular access−related satisfaction in adult HD patients using HIPPO-ST versus standard catheter care over 6 months.
Materials and Methods
Details of this pilot parallel randomized controlled trial protocol are published and registered at ClinicalTrials.gov (NCT02002169), with key study conduct information consistent with that described below.27 One major change from the published protocol was expanding recruitment into some in-center HD units due to recruitment challenges (see Discussion) as we originally planned to recruit only in satellite HD units. The rationale for this initial recruitment decision is that historically, satellite patients were more “independent”; thus we envisioned them to have a greater ability to learn and to shoulder the responsibility of performing the shower technique with minimal assistance if they were randomized to it. However, as we found that some in-center patients had characteristics similar to those of satellite patients, and as we had difficulties with recruitment, we expanded our recruitment criteria and the number of sites (different from published protocol). To fully reflect the contributions of all the centers across Ontario that developed the HIPPO-ST, the study title was also altered. The design, conduct, and reporting of this study adheres to Consolidated Standards of Reporting Trials (CONSORT) guidelines (Supplementary Material 1).28 This study took place in 3 in-center and 8 satellite HD units affiliated with 2 academic centers, University Health Network−Toronto General Hospital (UHN-TGH) and London Health Sciences Centre and 3 community centers: the Scarborough Hospital, Trillium Health Centre−The Credit Valley Hospital, and Mackenzie Health Hospital in South Central Ontario, Canada. Research ethics board approval was obtained before study initiation at all participating sites.
Eligibility Criteria
Individuals were screened by the study coordinator using predefined inclusion and exclusion criteria (Table 1). The main inclusion criteria included use of a tunneled HD catheter for >6 weeks and the ability to take a shower (with or without assistance). The main exclusion criteria included use of antibiotics at the time of enrollment and limited life expectancy.
Table 1.
Criteria for participating in the Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST): pilot randomized trial
| Inclusion criteria |
| 1. English speaking |
| 2. Age ≥18 years |
| 3. Required a catheter for vascular access: a) end-stage kidney disease (ESKD) without a functioning surgically created access; b) ESKD patient whose peritoneal dialysis problems required transfer to HD for an anticipated prolonged period |
| 4. Was willing and able to take a shower as the standard form of body cleansing if randomized to HIPPO-ST |
| 5. Trisodium citrate (4%) as standard catheter locking solution |
| 6. Catheter has been in situ for >6 weeks |
| Exclusion criteria |
| 1. Acute kidney failure, likely to be reversible with recovery of renal function |
| 2. Nontunneled catheter |
| 3. Antibiotic use by any route in the week prior to enrolling in the study, including intranasal mupirocin |
| 4. On immunosuppressant therapy |
| 5. Use of the catheter for purposes other than access for HD (e.g., TPN) |
| 6. Involvement in another interventional study related to their vascular access |
| 7. Catheter or patient life expectancy <6 months (e.g., active malignancy; serious comorbidity such as hepatic failure) |
| 8. Routine use of TPA or antibiotic as a locking solution |
| 9. Catheter insertion in location other than the neck/chest region (internal jugular or subclavian acceptable) |
ESKD, end-stage kidney disease; HD, hemodialysis; TPA, tissue plasminogen activator; TPN, total parenteral nutrition.
Interventions
Participants with healed catheter entry sites were randomized to either the Control group, which involved standard catheter care provided by HD nurses at the HD center, or to the HIPPO–ST Intervention group, which involved training and use of the HIPPO-ST when the participant showered plus standard catheter care provided by HD nurses at the HD center. In both the control and HIPPO-ST arms, participants whose HD center used a prophylactic barrier, polysporin triple ointment (PTO), at the catheter entry site as part of their standard catheter care protocol, continued to have it applied according to guideline recommendations23 or as per HD unit policy and procedures for HD patient and catheter care, a practice supported by high-level RCT evidence.29 The duration of the interventions was 6 months from the time of randomization.
Control Group
In the control group,27, 29 standard catheter care was performed by trained HD nurses and consisted of cleansing with chlorhexidine 2% or povidone (if allergic to chlorhexidine) at the catheter entry site, followed by placement of a dry gauze dressing once per week or when clinically indicated.
HIPPO-ST Group
In the HIPPO-ST group,27 participants randomized to the HIPPO-ST received a 30-minute personalized HIPPO-ST training session with the study coordinator, using the HIPPO-ST training tools (see below) in which they were taught how to do the following: (i) prior to showering, prepare all the supplies required to change their catheter entry site dressing; (ii) carefully shower to clean their body and to avoid the catheter entry site; no coverage of the catheter dressing was required; (iii) after showering, dry their body, again avoiding the catheter entry site; (iv) wash their hands with soap thoroughly; (v) carefully and gently remove and discard existing catheter entry site dressing; (vi) remove the chlorhexidine-soaked cotton swab applicator (supplied by the study) from its packaging and then cleanse the skin around the catheter entry site and catheter tube; (vii) if using PTO, apply the PTO (this PTO had the participant’s study number affixed, and participants were taught that this PTO is strictly not to be used for other reasons or to be shared by other household members); and (viii) apply new dry dressing at the catheter entry site. At the end of the training session, the participant had to successfully demonstrate the HIPPO-ST on a demonstration mannequin, evaluated against a test checklist by the study coordinator (HIPPO-ST Test), before proceeding to independent showering.
If the participant passed the HIPPO-ST Test, they were provided an educational pamphlet on the HIPPO-ST, not to be shared with other participants, to be kept as a reference and placed in their bathroom/household. They were also given the necessary supplies for the HIPPO-ST, itemized in individual sequentially numbered kits, to take home. Twelve kits were given to each patient at a time, enough for 3 showers per week for 1 month. New kits were distributed monthly. Study personnel were available to answer any questions during HD or by telephone for both study arms any time throughout the study.
Shower Technique Protocol Training Tools
The HIPPO-ST protocol was developed at an in-person meeting of nephrologist leaders of the participating centers in which several pre-existing catheter care protocols were carefully examined and revised until consensus was reached. The HIPPO-ST training tools, including an educational pamphlet, video, and demonstration mannequin, were then developed specifically for use in the HIPPO-ST pilot study by a panel of nephrologists, vascular access and HD patient education experts, and HD patients. The pamphlet and video use lay term language (education level, grade 5) with clear visual aids to explain the HIPPO-ST, as well as signs and symptoms of infection of which participants should be aware.
Recruitment
Each site had 6 months to recruit patients, from which a recruitment rate was determined. All satellite HD patients with a catheter in situ for at least 6 weeks were approached. A screening log was maintained and evaluated weekly. Participants could rescind consent from the study at any time. The target sample size was 78 participants and was calculated based on 50% eligibility, 30% refusal, 10% noncompliance, <1% loss to follow-up, and previous trials in the HD setting.24, 27, 29
Allocation Process
Once written informed consent was obtained, the participant underwent formal testing for catheter entry site healing.27 As no tests of catheter entry site healing for HD patients were found in the literature, the catheter entry site healing tests were developed for the HIPPO-ST pilot study by a panel of vascular access experts, including an expert nephrologist on HD vascular access,30, 31, 32, 33, 34, 35 a vascular access coordinator (a co-leader experienced as the first vascular access coordinator in North America),33, 36, 37, 38, 39, 40, 41, 42, 43 and an experienced HD nurse. The panel established 3 components to evaluating the entry site for healing: (i) stability of the catheter ingrowth, (ii) appearance of the catheter entry site, and (iii) integrity of the seal.27
Stability of the catheter was quantified by measuring the distance from the hub of the catheter to the entry site with a small disposable paper ruler before and after the patient took a deep breath. The catheter is generally inserted into the internal jugular vein, and protrudes from the chest just below the collar bone. If the catheter is not endothelialized in situ, the catheter may move as the patient takes a deep breath due to the muscles in the chest wall contracting. The measurement was taken during the catheter dressing change routinely conducted once weekly by the nurses. A difference between full exhalation and full inspiration of >0.23 cm indicated a failed test. The appearance of the catheter entry site was measured using a visual assessment of the skin around the point of catheter entry into the chest for signs of irritation and infection (e.g., redness, discharge, or swelling) by the HD nurse with the assistance of the study coordinator. The presence of any 2 of the following present constituted a failure of this test: redness, discharge, or swelling. The integrity of the skin seal was measured using a visual assessment of how tightly the skin is sealed around the catheter tube by the HD nurses with the assistance of the study coordinator. The integrity of the skin seal around the catheter is rated in the test as good, fair, or poor, and a rating of poor constituted a failure of this test. If two-thirds or more of the above catheter entry site healing tests were failed, standard care was applied and dressings changed once per week on dialysis as per protocol, and the catheter entry site–healing tests were repeated once weekly until two-thirds of the tests were passed, and the patient could proceed to randomization, or the study ended. As with other criteria, such as for exit site infection, the above tests have not been validated; however it was an attempt to standardize and objectively determine entry site healing, rather than have no criteria aside from the current subjective evaluation by nurses.
Upon passing two-thirds of the catheter entry site healing tests, participants were randomized via a 24-hour, telephone-accessed independent central randomization facility. Randomization involved a computer-generated randomization sequence using random block sizes with stratification by study site. Allocation of participants to the intervention was concealed to the randomization desk; however, participants and the study coordinator could not be blinded to allocation status due to the nature of the intervention. As participants were stratified by site, the number of patients from each site allocated to each arm was balanced, and potential differences in outcomes owing to site-specific practices (e.g., brand of dressings, polysporin triple ointment use) should consequently also be balanced. We therefore did not control for use of prior regimens in the analysis (regimens are determined by individual site policies).
Outcomes
Feasibility objectives and their corresponding outcome measures are listed in Table 2. The primary clinical objective (exploratory) was to compare the rate of CRB in patients with healed catheter entry sites using HIPPO-ST in addition to standard care versus standard catheter care alone over 6 months (hypothesized to be non-inferior). Catheter-related infections were adjudicated by a blinded outcomes committee, the HD Infection Control Subcommittee (HICS),36 at UHN-TGH, for confirmation and classification of the diagnosis of CRB according to the Health Canada definitions.44 The secondary clinical outcome, determining patient satisfaction with their vascular access, was measured using the Short Form Vascular Access Questionnaire (SF-VAQ), which contains 13 items, 1 item relating to patient satisfaction with the vascular access overall as measured using a 7-point Likert scale. The other 12 items involve the patient indicating their level of agreement with individual statements about having problems with physical symptoms (including pain, bleeding, swelling, bruising, social functioning, which includes daily activities, appearance, sleep, and bathing), and complications, including problems on dialysis, vascular access care, hospitalization, and concerns about vascular access longevity (Supplementary Material 2).16 For those 12 items, when the 7-point Likert scale results in low scores, this indicates satisfaction, a score of 4 indicates neutrality, and high scores indicate dissatisfaction. For example, in a previous study of hemodialysis patients,16 the item associated with the highest level of dissatisfaction overall in the social functioning domain was bathing, with values of 3.27, 1.60, and 1.29 for catheters, fistulas, and grafts, respectively, suggesting that hemodialysis patients were satisfied with their current bathing protocol overall; however, dissatisfaction was markedly higher for patients with catheters.
Table 2.
Feasibility of Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST): pilot randomized trial
| Objective | Outcome measure | Criteria for success | Results |
|---|---|---|---|
| Primary objective is to determine the feasibility of the HIPPO-ST study design defined by 5 outcomes below: | |||
| 1. To assess the accuracy of capturing the CRB rate within the satellite HD setting | The level of agreement between the date the nurse contacts the coordinator to inform them of a suspected infectiona and when the culture was sent to the laboratory | κ > 0.80 | 1.0 |
| 2. To determine the percentage of satellite HD patients with catheters who are screenedb | The percentage of HD patients with catheters who are screened for eligibility among all HD patients | >95% | 97.8% |
| 3. To determine the percentage of eligible HD patients who consent | The percentage of consented eligible patients among all eligible patients | >80% | 44.2% |
| 4. To measure the success of HIPPO-ST teaching | The percentage of patients in the intervention arm passing the Shower Technique Test at 3 and 6 months | ≥80% of patients randomized to HIPPO-ST | 88.4% |
| 5. To determine the percentage of participants in the control arm who are using aspects of the intervention | The percentage of controls who are using aspects of the HIPPO-ST that they were not using at baseline | <5% of participants in the control arm | 0% |
HD, hemodialysis.
Catheter-related infection defined by the Health Canada guidelines and determined by the independent event adjudication committee (see previously published protocol for full details27).
Screening was challenging in remotely located satellite units (compared to in-center HD).
Data Collection
Study visits took place at baseline and 3 and 6 months postrandomization. Baseline clinical, demographic, and vascular access information was obtained from the chart and/or a short interview with the participant. The catheter care survey, a measure of participant compliance and contamination with their catheter care protocol, and the SF-VAQ were administered to all participants at each study visit.45, 46 At the once-monthly monitoring visits, the study coordinator tracked use of all HIPPO-ST patients’ supplies and checked dialysis treatment sheets (resource use data will be reported separately). Feasibility outcomes were evaluated at each phase of the study (e.g., screening, recruitment, education, event determination, and documentation), with successes defined in Table 2.
All participants were clinically evaluated 3 times per week on HD by their HD nurses, who were all experienced at recognizing and managing patients with a suspected catheter-related infection, especially a CRB.27 When an infection was suspected, swabs were sent for organism identification and growth and antibiotic sensitivities. A provider (nurse practitioner or physician) and the study coordinator were notified and subsequently completed a data collection form and submitted it to the HICS36 for outcome adjudication (above).44
All baseline and outcome data were collected by study coordinators and entered into the computerized HIPPO-ST database. Only the principal investigator, study coordinator, and monitors from the research ethics boards had access to the final dataset.
Data Analysis
Descriptive statistics were calculated for all feasibility outcome measures, with the corresponding hypotheses and thresholds of success/statistical test listed in Table 2. For all clinical outcomes (deemed exploratory in nature), estimates of effect are presented as mean values for HIPPO-ST and control groups with confidence intervals only, in accordance with a checklist for the conduct of pilot studies, as this pilot study is designed to assess feasibility and not statistical significance.48 Data from the SF-VAQ are presented as means due to the ability of Likert scales to approximate interval level measurements.47, 49All analyses were based on an intention-to-treat approach and conducted using SPSS 22 software (IBM, Armonk, NY).
Results
Recruitment and Baseline Characteristics
A total of 72 patients consented to participate, and 68 participants met the criteria for healed tunneled catheter entry site. Of the participants, 35 were randomized to control and 33 to HIPPO-ST (Figure 1). The recruitment period was from November 2012 until December 2014. Recruitment was stopped before the target recruitment number of 78 was reached due to non−study-related practical limitations (i.e., funding was depleted). The participants in the HIPPO-ST and control groups were similar in their baseline characteristics (Table 3).
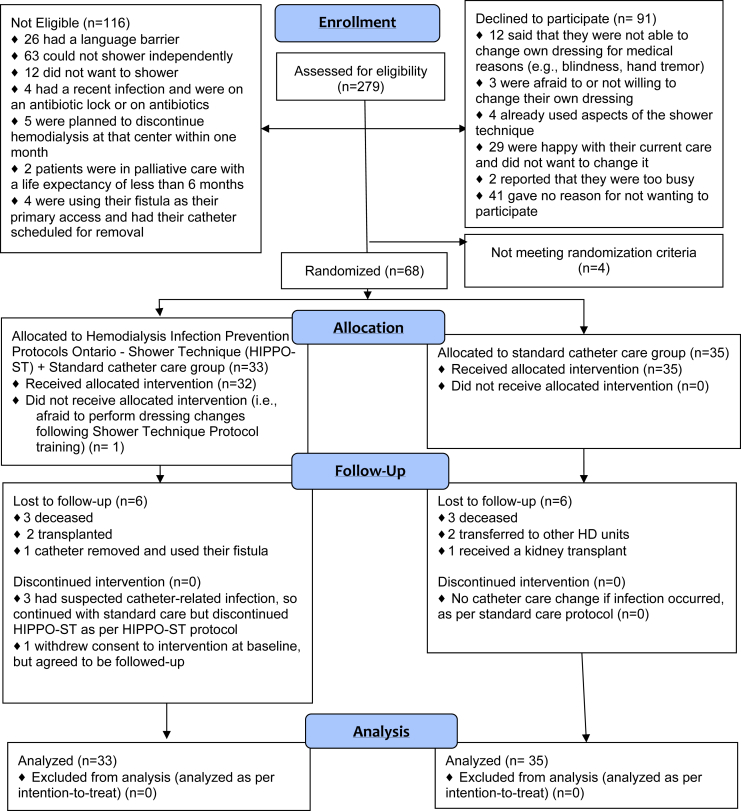
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. HD, hemodialysis; HIPPO-ST, Hemodialysis Infection Prevention Protocols Ontario—Shower Technique.
Table 3.
Baseline Characteristics of Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST) pilot study participants
| Characteristic at baseline | Control % (n) |
HIPPO-ST % (n) |
|---|---|---|
| Sociodemographics | ||
| Mean age (yr) | 53.00 | 58.41 |
| Sex | ||
| Male | 58.8 (20) | 64.5 (20) |
| Female | 41.2 (14) | 35.5 (11) |
| Ethnicity | ||
| White | 63.6 (21) | 61.3 (19) |
| Black | 6.1 (2) | 12.9 (4) |
| East Asian | 18.2 (6) | 12.9 (4) |
| South Asian | 12.1 (4) | 12.9 (4) |
| Comorbidities | ||
| Hypertension | 64.7 (22) | 80.6 (25) |
| Diabetes | 55.9 (19) | 61.3 (19) |
| Peripheral vascular disease | 8.8 (3) | 6.5 (2) |
| Coronary artery disease | 32.4 (11) | 38.7 (12) |
| Congestive heart failure | 26.5 (9) | 22.6 (7) |
| Stroke | 8.8 (3) | 9.7 (3) |
| Gastric bleeding | 15.2 (5) | 0.0 (0) |
| Chronic obstructive pulmonary disease | 0.0 (0) | 3.3 (1) |
| Malignancy | 26.9 (7) | 16.0 (4) |
| Chronic skin condition | 11.8 (4) | 6.5 (2) |
| Other conditionsa | 54.5 (18) | 48.4 (15) |
| Catheter characteristics | ||
| Mean length of follow up (catheter days) | 167 | 172 |
| Previous catheter use | 67.9 (19) | 52.2 (12) |
Some other comorbidities included psychiatric disorders, rheumatologic conditions, gastrointestinal disorders, head injury, intracranial hemorrhage, and hematologic conditions.
Primary Outcome: Feasibility of a Larger Trial
Four of the 5 objectives of feasibility were achieved at 6 months, as reported in Table 2 and detailed by each objective below.
Feasibility Objective 1: To Accurately Capture the CRB Rate Within the Satellite HD Setting
The level of agreement between the dates of (1) suspected CRB and notifying the study coordinator within 72 hours and (2) the date the catheter entry site was swabbed and sent to the microbiology laboratory was excellent (κ = 1.0; success threshold: κ > 0.80). The study coordinator was notified within 72 hours regarding all 11 cases of suspected catheter-related infection.
Feasibility Objective 2: To Determine the Percentage of Eligible HD Patients Who Were Screened
There were 285 patients with catheters during the screening period, of whom 279 patients (97.8%) were screened for eligibility to participate in the study (success threshold, >95%). Some patients were not screened due to the logistical difficulties of research staff traveling to the satellite units. Of the 279 patients screened, 163 were deemed eligible to participate (Figure 1).
Feasibility Objective 3: To Determine the Percentage of Eligible Satellite HD Patients Who Consented
Of the 163 eligible patients, 72 (44.2%) consented to participate in this study (success threshold, >80%).
Feasibility Objective 4: To Determine the Percentage of Patients Who Passed the HIPPO-ST Test
Of the patients randomized to HIPPO-ST, 100% (33/33), 100% (31/31), and 88.4% (23/26) in the study at baseline, 3, and 6 months passed the HIPPO-ST test (success threshold, ≥80%). Figure 1 provides reasons for loss to follow-up in the intervention arm. Over the study period, 3 patients in the HIPPO-ST arm who had suspected infection stopped using the HIPPO-ST during the time that the infection was suspected; however, they were still administered the HIPPO-ST test and included in the descriptive statistics above, as per intention-to-treat. All 3 patients who had a suspected infection were given the option to resume using the HIPPO-ST; however, all 3 patients declined to continue using the HIPPO-ST.
Feasibility Objective 5: To Determine the Percentage of Participants in the Control Arm Who Used Aspects of the Intervention (HIPPO-ST)
At both the 3-month (n = 32) and 6-month (n = 29) study visits, no participants in the control arm (0%) were using any aspect of the HIPPO-ST that they were not using at baseline (n = 35), as determined by the catheter care survey (success threshold, <5%). Figure 1 presents reasons for loss to follow-up in the control arm.
Catheter-Related Infection
The proportions and rates of patients with different types of catheter-related infections are reported in Table 4. The proportion of patients with a CRB in the HIPPO-ST group was 12.1% (4/33) and in the control group was 11.4% (4/35), with a mean difference between groups of 0.007 (95% confidence interval [CI] = −0.16 to 0.17). The rate of CRB in HIPPO-ST group was 0.88/1000 catheter days and in the control group was 0.68/1000 catheter days, with a mean difference between groups of 0.16 (95% CI = −2.25 to 2.65). No unexpected harm using HIPPO-ST was observed.
Table 4.
Rates and proportions of catheter-related infection in hemodialysis patients with healed catheter entry sites using Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST) versus standard catheter care over 6 months
| Catheter-related infection type | HIPPO-ST (n = 33) | Standard Care (n = 35) | Mean difference (95% CI) |
|---|---|---|---|
| Bacteremia | |||
| Rate/1000 catheter days | 0.88 | 0.68 | 0.16 (−2.25 to 2.65) |
| Proportion % (SD) | 12.1 (0.33) | 11.4 (0.318) | 0.7 (−16 to 17) |
| Entry site | |||
| Rate/1000 catheter days | 0.88 | 0.68 | 0.16 (−2.25 to 2.65) |
| Proportion % (SD) | 12.1 (0.33) | 11.4 (0.318) | 0.7 (−16 to 17) |
| Tunnel | |||
| Rate/1000 catheter days | 0.35 | 0 | 0.35 (−0.81 to 1.51) |
| Proportion % (SD) | 6.1 (0.24) | 0 (0) | 6.1 (−2.1 to 14) |
Rates and proportions compared with Poisson distribution. CI, confidence interval.
Patient Satisfaction With Vascular Access
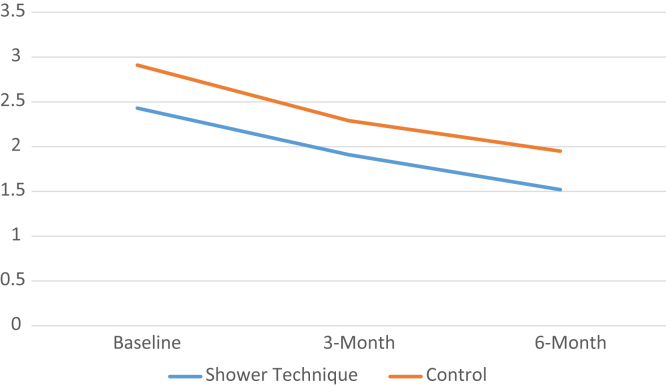
The change in patient satisfaction as measured by the Short Form Vascular Access Questionnaire (SF-VAQ) scores45, 46 are shown in Supplementary Material 2. Mean scores for both the HIPPO-ST and the control group indicated high levels of satisfaction with their vascular access overall over the course of the study (5.73 vs. 5.94, 6.24 vs. 6.25, and 5.73 vs. 6.68 at baseline, 3 months, and 6 months, respectively). The HIPPO-ST group had a similar improvement in SF-VAQ scores compared with the control group over 6 months for the item “During the past 4 weeks my vascular access caused me problems when I was bathing or showering” (Figure 2).
Figure 2.
Patient-reported levels of problems associated with vascular access when bathing or showering in hemodialysis patients with healed catheter entry sites using Hemodialysis Infection Prevention Protocols Ontario—Shower Technique (HIPPO-ST) versus standard catheter care over 6 months. The y-axis represents the level of agreement with the item “During the past 4 weeks my vascular access caused me problems when I was bathing or showering,” which was rated by using a 7-point Likert scale from 1 (strongly disagree) to 7 (strongly agree).
Discussion
The key finding in this study is that criteria for feasibility success (4/5 criterion) were met and the full HIPPO-ST study is feasible to conduct. The results from this pilot study’s exploratory analysis of the primary clinical outcome (CRB rate) indicated similar CRB rates between the intervention and control arms, suggesting that a noninferiority design for the full study would be appropriate. The percentage of patients with a CRB was similar in the HIPPO-ST group (12%) compared to the control arm (11%); the rate of CRB was slightly higher in the HIPPO-ST Group (0.88/1000 catheter days) compared to the control arm (0.68/1000 catheter days), although both were under the threshold of an excellent CRB rate (1.0/1000 catheter days).50 In addition, analysis of a secondary clinical outcome, SF-VAQ score, showed an improvement within the HIPPO-ST group in terms of their satisfaction with their catheter care relating to problems when bathing or showering; however, satisfaction was not a relatively greater improvement than the control group condition. We found that compliance with the catheter care protocol was high, and contamination of patients in the control arm (i.e., using aspects of the HIPPO-ST) was not detected. The results of the HIPPO-ST pilot study indicate that on HIPPO-ST, patients are satisfied and compliant with the HIPPO-ST, which was designed specifically to meet their needs and minimize infection risk.27
Our findings are consistent with other studies of HD catheter care with a shower component.51, 52 Both of these previous studies used a “no-dressing” shower technique in which the patients shower freely and do not have a dressing placed over the catheter entry site as part of their catheter care.51, 52 However, the HIPPO-ST in the current study differs in that patients were trained, using the HIPPO-ST tools, on how to correctly cleanse the entry site and apply a dressing following a shower up to 3 times per week. Although these studies are not directly comparable, the investigators have consistently found that a patient showering with an HD catheter in situ did not demonstrate an increase in infection risk, and patients’ quality of life was improved by not restricting their ability to shower.51, 52, 53
Another unique difference is that both prior studies did not test catheter entry site healing before introducing a shower component into catheter care, but instead used various time thresholds from the time of catheter insertion to determine shower technique eligibility.51, 52 The use of the catheter entry site healing tests prior to randomization are a critical feature of the HIPPO-ST design. For unhealed catheter entry sites, there may be a potential increased risk of infection using any shower technique, including HIPPO-ST, by extraluminal exposure to microorganisms compared with standard catheter care due to the higher number of risk exposures from showering with or without dressing changes conducted by patients. As 4 patients failed to meet entry site healing randomization criteria despite using a catheter for >6 weeks, our results suggest that duration of catheter use itself is not a reliable factor to ensure entry site healing.
A prior study in HD patients dialyzing via catheters demonstrated that patients were 3.8 times more likely to be compliant with their catheter care protocol (i.e., not to shower with a catheter in situ) if they recalled a health care provider educating them not to, compared with having no such recollection (95% CI = 1.2−4.5).24 This is consistent with the high compliance with the catheter care protocol found in our study after patient education and regular reinforcement of proper catheter dressing care and infection prevention.
From both clinical and research perspectives, it is critical to understand the infection rates and patient satisfaction associated with the HIPPO-ST in HD patients with healed catheter entry sites as a prophylactic strategy. The Centers for Disease Control and Prevention (CDC) guidelines state that the optimal method of catheter infection prophylaxis in patients with healed entry sites is an unresolved issue.54 This is reflected in a Canadian survey of 68 dialysis centers across Canada in which practice was found to be very inconsistent surrounding the recommendations made to patients for personal hygiene: 75% of centers recommended that patients clean themselves by bathing (nonsubmerged) or sponge bath, 38% recommended showering, and 5% made no recommendation at all (categories non−mutually exclusive).55 Therefore, HD patients across Canada receive conflicting, inconsistent, or no recommendations about personal hygiene care techniques and whether or not they should preserve the dryness of their dressings to prevent catheter-related infection.
Lessons Learned About How to Design the Main Trial
Given the burden of catheter-related infection and the reduced quality of life of patients from restricting their ability to shower, the study question of whether the CRB rate for HD using HIPPO-ST is noninferior to that of patients using standard catheter care remains a pressing issue that the full HIPPO-ST trial will ultimately address. Although the outcome of the CRB rate addresses the morbidity and mortality associated with the intervention being studied, we submit that equally important is patients’ satisfaction with their vascular access and its care. Thus, the full potential benefit of the HIPPO-ST is best captured by including the SF-VAQ. This insight will be helpful in designing the full HIPPO-ST study, in which both hard and surrogate, clinical and patient-focused outcomes will be crucial to fully understanding the impact of the HIPPO-ST.45 When designing the full HIPPO-ST trial we will not aim to show a large difference in a single outcome; rather, we will be evaluating several dimensions of the patient experience to incrementally improve care. In addition, we originally planned to conduct the pilot study only in satellite units due to the perception that the satellite population may be more eligible (i.e., tend to be younger and have fewer co-morbidities) than in-center patients. However, this was not so, and recruitment rates were similar in both satellite and in-center units. Therefore, in the full trial, in-center units should be considered for inclusion.
Study Limitations
There are several limitations to this study. First, this study was a pilot study, and any examination of clinical outcomes is only exploratory. The full HIPPO-ST trial is needed to answer these clinical questions. In addition, there are no fully validated vascular access−specific questionnaires, including the SF-VAQ; however the full HIPPO-ST may provide an opportunity for construct validation of this measure.56, 57 Moreover this study may be limited by selection bias, as many eligible patients declined to participate. Although we collected data on their reasons for declining to participate (Figure 1), constraints placed by the study REB prohibited collection of any additional sociodemographics or clinical data on these patients. Therefore we cannot examine whether these patients were systematically different from those who decided to participate. It is possible that patients at highest risk for infection, for example, were self-selecting out of participating in the trial. Recruitment is challenging when conducting any clinical research study but is particularly difficult in RCTs. Indeed, we failed to meet our feasibility criteria on recruitment, despite using multiple strategies to facilitate recruitment, including identification of a nurse leader (such as a vascular access coordinator at each center), extensive in-servicing with all unit nurses, and expanding recruitment into some in-center units (as we originally planned to recruit only in satellites). However, the availability of the pilot study data to patients may help alleviate patient concerns, and facilitate recruitment in the future full HIPPO-ST study.
Conclusion
Overall, we found that the full HIPPO-ST study is feasible to conduct, with a high level of compliance with the HIPPO-ST, and low levels of contamination in the control arm. The conduct of the full HIPPO-ST study will address the current paucity of evidence surrounding showering aspects of catheter care allowing patients, HD personnel, and nephrologists to make informed choices about HD catheter care.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding was provided by the Kidney Foundation of Canada. The authors thank the student volunteers, Gabrielle Ene, Elgene Yranon, Lara Pazek, and Maryum Yousefi, for their assistance with this project; all the vascular access coordinators and hemodialysis nurses who facilitated the recruitment and conduct of this study; Cynthia Bhola and Cathy Forrester for their role in developing the catheter entry site healing tests; and the hemodialysis patients who volunteered their time to participate. Clinical Trial Registration Number: NCT02002169.
Footnotes
Supplementary Material 1. CONSORT 2010 checklist of information to include when reporting a randomized trial.
Supplementary Material 2. Self-reported satisfaction with vascular access measured on Short-form Vascular Access Questionnaire (SF-VAQ) in hemodialysis patients with healed catheter entry sites using Hemodialysis Infection Prevention Protocols Ontario - Shower Technique (HIPPO-ST) versus standard catheter care over 6 months.
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
CONSORT 2010 checklist of information to include when reporting a randomized trial.
Self-reported satisfaction with vascular access measured on Short-form Vascular Access Questionnaire (SF-VAQ) in hemodialysis patients with healed catheter entry sites using Hemodialysis Infection Prevention Protocols Ontario - Shower Technique (HIPPO-ST) versus standard catheter care over 6 months.
References
- 1.Lopes A.A., Bragg-Gresham J.L., Goodkin D.A. Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res. 2007;16:545–557. doi: 10.1007/s11136-006-9143-7. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury B.D., Fissell R.B., Albert J.M. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 3.Tonnessen B.H., Money S.R. Embracing the fistula first national vascular access improvement initiative. J Vasc Surg. 2005;42:585–586. doi: 10.1016/j.jvs.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 4.USRDS. U.S. Renal Data System. USRDS 2010 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health. Costs of end-stage renal disease. Vol 2. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2010.
- 5.CORR. 2010 CORR Report—Treatment of end-stage organ failure in Canada, 1999 to 2008. Ottawa, Ontario. Available at: https://secure.cihi.ca/free_products/corr_annual_report_2010_e.pdf. Accessed December 19, 2016.
- 6.Ishani A., Collins A., Herzog C., Foley R. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int. 2005;68:311–318. doi: 10.1111/j.1523-1755.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 7.Engemann J.J., Friedman J.Y., Reed S.D. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005;26:534–539. doi: 10.1086/502580. [DOI] [PubMed] [Google Scholar]
- 8.Marr K.A., Kong L., Fowler V.G. Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int. 1998;54:1684–1689. doi: 10.1046/j.1523-1755.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- 9.Maraj S., Jacobs L.E., Kung S.C. Epidemiology and outcome of infective endocarditis in hemodialysis patients. Am J Med Sciences. 2002;324:254–260. doi: 10.1097/00000441-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 10.USRDS. U.S. Renal Data System. USRDS 2009 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
- 11.Ramanathan V., Chiu E.J., Thomas J.T. Healthcare costs associated with hemodialysis catheter-related infections: a single-center experience. Infect Control Hosp Epidemiol. 2007;28:606–609. doi: 10.1086/513617. [DOI] [PubMed] [Google Scholar]
- 12.Mokrzycki M.H., Zhang M., Cohen H. Tunnelled haemodialysis catheter bacteraemia: risk factors for bacteraemia recurrence, infectious complications and mortality. Nephrol Dial Transplant. 2006;21:1024–1031. doi: 10.1093/ndt/gfi104. [DOI] [PubMed] [Google Scholar]
- 13.Tanriover B., Carlton D., Saddekni S. Bacteremia associated with tunneled dialysis catheters: comparison of two treatment strategies. Kidney Int. 2000;57:2151–2155. doi: 10.1046/j.1523-1755.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee H., Manns B., Taub K. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–622. doi: 10.1053/ajkd.2002.34924. [DOI] [PubMed] [Google Scholar]
- 15.Reed S.D., Friedman J.Y., Engemann J.J. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2005;26:175–183. doi: 10.1086/502523. [DOI] [PubMed] [Google Scholar]
- 16.Nissenson A.R., Dylan M.L., Griffiths R.I. Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am J Kidney Dis. 2005;46:301–308. doi: 10.1053/j.ajkd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan V., Chiu E., Thomas J.T. Healthcare costs associated with hemodialysis catheter–related infections: a single-center experience. Infect Control Hosp Epidemiol. 2007;28:606–609. doi: 10.1086/513617. [DOI] [PubMed] [Google Scholar]
- 18.Kosa D., Lok C. The economics of hemodialysis catheter-related infection prophylaxis. Semin Dial. 2013;26:482–493. doi: 10.1111/sdi.12115. [DOI] [PubMed] [Google Scholar]
- 19.Kidney Foundation of Canada. Kidney Foundation Patient Fact Sheet. 2011. Available at: https://www.kidney.ca/document.doc?id=764. Accessed August 18, 2011.
- 20.Besarab A., Work J., Brouwer D. National Kidney Foundation clinical practice guidlines for vascular access: guideline 7. Prevention and treatment of catheter port complications. Am J Kidney Dis. 2006;48:S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Vanholder R., Canaud B., Fluck R. Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): a position statement of European Renal Best Practice (ERBP) NDT Plus. 2010;25:1753–1756. doi: 10.1093/ndtplus/sfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manian F.A. IDSA guidelines for the diagnosis and management of intravascular catheter-related bloodstream infection. Clin Infect Dis. 2009;49:1770–1771. doi: 10.1086/648113. author reply 1771–1772. [DOI] [PubMed] [Google Scholar]
- 23.Jindal K., Chan C.T., Deziel C. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol. 2006;17(3 Suppl 1):S1–S27. doi: 10.1681/ASN.2005121372. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence-Murphy J.A., Seiler S., Hooton A. Shower and no-dressing technique for tunneled central venous catheters: an update one year later. CANNT J. 2010;20:18. [Google Scholar]
- 25.Wu G. Trends in hemodialysis catheter bacteremia rates at Credit Valley Hospital. Paper presented at: Bloodstream Infections in Hemodialysis Patients: Local Symposium 2011; Mississauga, ON, Canada.
- 26.Kosa S. Hemodialysis Infection Prevention with Polysporin Ointment (PTO) Versus Taking a Wet Shower (HIPPO-TWO) pilot study design. Am J Kidney Dis. 2012;59:B48. [abstract]. [Google Scholar]
- 27.Kosa S.D., Gafni A., House A. Hemodialysis Infection Prevention Using Polysporin Ointment With Shower Technique in satellite Units (HIPPOSAT) pilot study design. Nephrol Open J. 2015;1:1–12. [Google Scholar]
- 28.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 29.Lok C.E., Stanley K.E., Hux J.E. Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol. 2003;14:169–179. doi: 10.1097/01.asn.0000038688.76195.a4. [DOI] [PubMed] [Google Scholar]
- 30.Lee T., Lok C., Vazquez M. Minimizing hemodialysis catheter dysfunction: an ounce of prevention. Int J Nephrol. 2012;2012:170857. doi: 10.1155/2012/170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lok C.E. Avoiding trouble down the line: the management and prevention of hemodialysis catheter-related infections. Adv Chronic Kidney Dis. 2006;13:225–244. doi: 10.1053/j.ackd.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Lok C., Thumma J., McCullough K. Catheter-related infection and septicemia: impact of seasonality and modifiable practices from the DOPPS. Semin Dial. 2013;27:72–77. doi: 10.1111/sdi.12141. [DOI] [PubMed] [Google Scholar]
- 33.Lok C.E., Appleton D., Bhola C. Trisodium citrate 4%–an alternative to heparin capping of haemodialysis catheters. Nephrol Dial transplant. 2006;22:477–483. doi: 10.1093/ndt/gfl570. [DOI] [PubMed] [Google Scholar]
- 34.Lok C.E., Mokrzycki M.H. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79:587–598. doi: 10.1038/ki.2010.471. [DOI] [PubMed] [Google Scholar]
- 35.Lok C.E., Thomas A., Vercaigne L. A patient-focused approach to thrombolytic use in the management of catheter malfunction. Semin Dial. 2006;19:381–390. doi: 10.1111/j.1525-139X.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 36.Battistella M., Bhola C., Lok C.E. Long-term follow-up of the Hemodialysis Infection Prevention with Polysporin Ointment (HIPPO) study: a quality improvement report. Am J Kidney Dis. 2011;57:432–441. doi: 10.1053/j.ajkd.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Kalman P.G., Pope M., Bhola C. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999;30:727–733. doi: 10.1016/s0741-5214(99)70112-6. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhry M., Bhola C., Joarder M. Seeing eye to eye: the key to reducing catheter use. J Vasc Access. 2011;12:120–126. doi: 10.5301/jva.2011.6390. [DOI] [PubMed] [Google Scholar]
- 39.Kalman P.G., Pope M., Bhola C. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999;30:727–733. doi: 10.1016/s0741-5214(99)70112-6. [DOI] [PubMed] [Google Scholar]
- 40.Lok C., Bhola C., Davidson I. Planning for vascular access surgery–a patient-centered approach. Vasc Access Gen Nephrologist. 2012:363–384. [Google Scholar]
- 41.Lok C.E., Bhola C., Croxford R., Richardson R.M. Reducing vascular access morbidity: a comparative trial of two vascular access monitoring strategies. Nephrol Dial Transplant. 2003;18:1174–1180. doi: 10.1093/ndt/gfg122. [DOI] [PubMed] [Google Scholar]
- 42.Lok C.E., Oliver M.J., Su J. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67:2462–2469. doi: 10.1111/j.1523-1755.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J., Rajan D., Bhola C. Reasons for hemodialysis catheter use & its complications: patient and coordinator perspectives. Am J Kidney Dis. 2007;30:B85. [abstract]. [Google Scholar]
- 44.Division of Nosocomial and Occupational Infectious Diseases, Bureau of Infectious Diseases, Laboratory Centre for Disease Control, Health Canada. Preventing infections associated with indwelling intravascular access devices. Can Commun Dis Rep. 1997;23(Suppl 8) i–iii, 1–32; i–iv, 31–16. [PubMed] [Google Scholar]
- 45.Kosa S, Al Jaishi A, Joarder B, et al. Hemodialysis patient views on their catheter care protocols. Presented at: Canadian Society of Nephrology Annual General Meeting. St John's, Newfoundland: Canadian Society of Nephrology. 2012.
- 46.Quinn R.R., Lamping D.L., Lok C.E. The Vascular Access Questionnaire: assessing patient-reported views of vascular access. J Vasc Access. 2008;9:122–128. [PubMed] [Google Scholar]
- 47.Kosa S.D., Bhola C., Lok C.E. Measuring patient satisfaction with vascular access: Vascular Access Questionnaire development and reliability testing. J Vascul Access. 2015;16:200–205. doi: 10.5301/jva.5000339. [DOI] [PubMed] [Google Scholar]
- 48.Thabane L., Ma J., Chu R. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1–10. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan G.M., Artino A.R., Jr. Analyzing and interpreting data from Likert-type scales. J Grad Med Educ. 2013;5:541–542. doi: 10.4300/JGME-5-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beathard G.A., Urbanes A. Infection associated with tunneled hemodialysis catheters. Semin Dial. 2008;21:528–538. doi: 10.1111/j.1525-139X.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence J.A., Seiler S., Wilson B., Harwood L. Shower and no-dressing technique for tunneled central venous hemodialysis catheters: a quality improvement initiative. Nephrol Nurs J. 2014;41:67. [PubMed] [Google Scholar]
- 52.Evans E.C., Hain D., Kear T.M. Hemodialysis catheter outcomes pilot study: no dressing coverage with prescribed showering. Nephrol Nurs J. 2014;41:53. [PubMed] [Google Scholar]
- 53.Grapsa E, Pantelias K. Vascular access and new trends. Available at: http://www.intechopen.com/books/updates-in-hemodialysis/vascular-access-and-new-trends. Accessed December 19, 2016.
- 54.O'Grady NP, Alexander M , Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Accessed December 19, 2016. [PubMed]
- 55.Kosa S., Lok C. A survey of hemodialysis practice patterns in Canada for hemodialysis central venous catheters. Am J Kidney Dis. 2013;61:B56. [abstract]. [Google Scholar]
- 56.Lynn M.R. Determination and quantification of content validity. Nurs Res. 1986;35:382–385. [PubMed] [Google Scholar]
- 57.Norman G, Streiner DL. Health measurement scales: a practical guide to their development and use. New York: Oxford University Press; 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT 2010 checklist of information to include when reporting a randomized trial.
Self-reported satisfaction with vascular access measured on Short-form Vascular Access Questionnaire (SF-VAQ) in hemodialysis patients with healed catheter entry sites using Hemodialysis Infection Prevention Protocols Ontario - Shower Technique (HIPPO-ST) versus standard catheter care over 6 months.