Abstract
Introduction
Although several studies suggest that the prognosis of hypertensive dialysis patients can be improved by using antihypertensive drug therapy, it is unknown whether the prescription of a particular class or combination of antihypertensive drugs is beneficial during hemodialysis.
Methods
We performed a propensity score matching study to compare the effectiveness of various classes of antihypertensive drugs on cardiovascular (CV) mortality in 2518 incident hemodialysis patients in Spain. The patients had initially received antihypertensive therapy with a renin-angiotensin system (RAS) blocker (728 patients), a ß-blocker (679 patients), antihypertensive drugs other than a RAS blocker or a ß-blocker (787 patients), or the combination of a ß-blocker and a RAS inhibitor (324 patients). These patients were followed for a maximum of 5 years (median: 2.21 yr; range: 1.04–3.34 yr).
Results
After adjustment for baseline CV risk covariates, no significant differences were observed in the risk of CV mortality between patients taking a RAS blocker and patients treated with ß-blocker–based antihypertensive therapy. The combination of a RAS blocker with a ß-blocker was associated with better CV survival than either agent alone (RAS blocker: hazard ratio [HR]: 1.68; 95% confidence interval [CI] 1.05–2.69; ß-blocker: HR: 1.59; 95% CI: 1.01–2.50; antihypertensive medication other than a RAS blocker or ß-blocker: HR: 1.67; 95% CI: 1.08–2.58).
Discussion
Our data suggested that the combination of a RAS blocker and a ß-blocker could improve survival in hemodialysis patients. Further prospective randomized controlled trials are necessary to confirm the beneficial effects of this combination of antihypertensive drugs in patients undergoing hemodialysis.
Keywords: antihypertensive drug, β-blocker, cardiovascular risk, hemodialysis, renin-angiotensin system blocker
Mortality among patients undergoing hemodialysis has improved in recent years, yet mortality rates remain unacceptably high, mostly due to cardiovascular (CV) diseases.1 Hypertension is 1 of the main factors that contributes to the hugely increased rate of CV mortality in end-stage renal disease (ESRD).2, 3, 4
Hypertension is common in patients undergoing hemodialysis. Extracellular volume overload is the main pathogenic mechanism underlying hemodialysis-related hypertension,5 although other mechanisms, such as the renin-angiotensin system (RAS) and sympathetic system hyperactivity, also contribute to maintaining hypertension in hemodialysis patients.6, 7 Isolated systolic hypertension and increased pulse pressure resulting from stiff large arteries due to arteriosclerosis are a common finding in patients undergoing hemodialysis. One large-scale epidemiological study based on United States Renal Data System data showed that pulse pressure was the most significant prognostic predictor of mortality.8
Antihypertensive drugs are generally prescribed to patients during hemodialysis, when hypertension persists despite the achievement of adequate dry weight.9 Although several studies suggest that the prognosis of hypertensive dialysis patients may be improved by using antihypertensive drug therapy,10, 11 it is unknown whether the prescription of a particular class or combination of antihypertensive drugs is beneficial during hemodialysis. In addition, because of the excessive CV mortality of these patients, such knowledge would be of enormous clinical usefulness.
In the general population, RAS blockers (i.e., angiotensin-converting enzyme inhibitors [ACEI] and angiotensin receptor blockers [ARB]) and ß-blockers have been shown to exert beneficial effects on CV events, particularly left ventricular hypertrophy, coronary heart disease (CHD), and heart failure.12, 13, 14, 15, 16 Studies comparing the efficacy of various antihypertensive treatments in reducing CV risk in ESRD patients have yielded controversial results.8, 17, 18, 19, 20, 21 Some have suggested that RAS blockers are superior to other antihypertensive drugs in reducing left ventricular hypertrophy and preventing CV risk.20 The CV benefits of ß-blockers have also been reported. A large epidemiological study performed in the United States concluded that ß-blockers improved survival and may even have induced CV protection in dialysis patients.8 A randomized controlled trial that involved patients with left ventricular hypertrophy who underwent hemodialysis reported that the ß-blocker atenolol was superior to the RAS blocker lisinopril in preventing CV morbidity and all-cause hospitalizations.22
To compare the effects of different classes of antihypertensive drugs on CV mortality in patients undergoing maintenance hemodialysis, we performed an observational study (propensity-matched analysis) of a large cohort of patients who initially received antihypertensive therapy with a RAS blocker (ACEI or ARB), a ß-blocker, other antihypertensive drugs, or the combination of a RAS blocker and a ß-blocker.
Materials and Methods
Dialysis Facilities Operated by Fresenius Medical Care in Spain
The Spanish National Health System covers the treatment of ESRD (dialysis and kidney transplantation) for the entire population and is financed through the budget of the 17 Autonomous Communities into which the Spanish state is divided. Patients with ESRD are referred to public hospitals or to dialysis facilities owned by the main dialysis companies, with which the National Health System has agreements. Patients treated at the 63 Fresenius Medical Care dialysis facilities in Spain represent approximately one-quarter of the whole hemodialysis population in Spain. These patients are similar in age, sex, etiology of kidney disease, and baseline comorbidities to the entire hemodialysis population in Spain, as reflected in a previous publication.23
Patients
Patients admitted to the 63 Fresenius Medical Care dialysis facilities in Spain who initiated hemodialysis between January 1, 2009 and December 31, 2012 were screened for inclusion in the study. The inclusion criteria were as follows: age older than 18 years; incident hemodialysis (<90 days on renal replacement therapy before starting hemodialysis in Fresenius Medical Care facilities and no previous kidney transplantation or peritoneal dialysis); registration in the EuCliD database and the Fresenius Medical Care clinical data system23; prescription of hemodialysis based on a regimen of 3 sessions per week (4 h each); and receiving a stable dose of antihypertensive medication (the same type and dose of antihypertensive drugs for at least 2 months before inclusion in the study). Exposure to antihypertensive medications was obtained from electronic prescription data, which is included in the EuCliD database. All patients gave their written informed consent for data evaluation on admission to the Fresenius Medical Care center.
Study Design
Baseline was set at 3 months after the date of renal replacement therapy. At baseline, we collected the following variables and comorbidities that, according to the existing literature, seem to be associated with antihypertensive medication and outcome: age, blood pressure (systolic and diastolic), pulse pressure, heart rate, ultrafiltration per session (all calculated as the average of the measurements taken during the baseline period, i.e., before the hemodialysis session), sex, vascular access, and potassium levels. The comorbidities recorded at baseline were diabetes mellitus, heart failure, CHD, arrhythmia, and stroke. We recorded antihypertensive medication classified according to the corresponding codes of the Anatomical Therapeutic Chemical (ATC) classification system. Patients were classified into 4 groups according to their antihypertensive medication, as follows: (i) RAS blockers (ATC group C09); (ii) ß-blockers (ATC group C07); (iii) any other antihypertensive medication not including RAS blockers or ß-blockers, peripheral vasodilators (ATC group C04), calcium-channel blockers (ATC group C08), and other drugs (ATC group C02); and (iv) combined RAS blockers and ß-blockers (ATC group C07 + ATC group C09). See Supplementary Table S1 for further details on prescriptions.
Patients were followed for a maximum of 5 years until December 31, 2014 (median: 2.21 yr; range; 1.04–3.34 yr). Patients who discontinued hemodialysis because of recovery of renal function, those who were transferred to hemodialysis facilities other than NephroCare, and those who received a successful kidney graft were censored.
All analyses were performed on an intention-to-treat basis, although it was found that most patients (84.3%) received the same type of antihypertensive medications 1 year after baseline.
Outcome
Death dates and causes were provided by the attending nephrologist using the appropriate International Classification of Diseases-10th Revision (ICD-10) codes and registered in the EuCliD database. The primary outcome measure was CV mortality, defined as death from CHD (myocardial infarction, cardiac arrest, and ventricular fibrillation), sudden death, heart failure, and cerebrovascular or vascular disease (disease of the aorta or peripheral vasculature). The secondary outcome measure was all-cause mortality, including deaths from CV diseases, infections, malignancies, and deaths from unknown causes.
Statistical Analysis
Continuous variables are reported as mean ± SD; categorical variables are reported as percentages. Bivariate comparisons between cohorts were performed using the χ2 test and the t test as appropriate. The survival analysis to evaluate all-cause mortality was performed by combining the Kaplan-Meier survival curves (compared using the log-rank test) with the univariate and multivariate Cox regression analyses. The hazard ratios and the corresponding 95% confidence intervals were calculated. Moreover, to determine which medication improved survival profiles, we constructed several adjusted competing risks regression models to calculate the corresponding subdistribution hazard ratios following the approach proposed by Fine and Gray.24 These models are used to assess the effect of the covariates on the subdistribution of a specific outcome (as previously described) in a competing risks scenario. In the present study, this scenario was kidney transplantation, change in dialysis unit, or other reasons.
The linear effect of the continuous variables was explored in several univariate models. The corresponding cutoffs were chosen in a clinically relevant way to yield balanced groups. For age, these cutoffs were 50 years or younger, 51 to 60, 61 to 70, 71 to 80, and 81 years or older. For systolic blood pressure, the cutoffs were ≤130, 131 to 140, 141 to 150, 151 to 160, and ≥161 mm Hg. For the ultrafiltration per session, these cutoffs were ≤3.00, 3.01 to 3.50, and >3.50 L. For potassium, these cutoffs were ≤4.20, 4.21 to 4.70, 4.71 to 5.20, and >5.20 mEq/l. The other covariates included in the corresponding risk models were sex (reference: female), diabetes (reference: no), heart failure (reference: no), CHD (reference: no), arrhythmia (reference: no), stroke (reference: no), and vascular access (reference: arteriovenous fistula). We also investigated the possibility of collinearity in the continuous variables throughout the examination of the variance inflation factors and the proportional hazards assumption based on the log (−log [survival]) versus log of survival time graph.
We performed a propensity score matching (PSM) analysis for the main outcome measure because patients were not randomly allocated to the 4 study groups. We then used PSM to minimize any potential confounding and selection biases in pair-by-pair comparisons. We calculated the propensity score for each patient by modeling the probability of receiving 1 of the 4 regimens of antihypertensive medication using multivariate logistic regression models based on the following covariates: age, systolic blood pressure, sex, diabetes, heart failure, CHD, arrhythmia, stroke, ultrafiltration volume per session, potassium, and concomitant antihypertensive medication. The propensity scores derived were used to match the groups at a 1:1 ratio based on a caliper matching algorithm. We applied this matching procedure by fixing a caliper parameter equivalent to 0.2 of the pooled SD of the logit of the propensity scores.25 To evaluate the quality of the different PSM models, we assessed the balance in covariates based on the absolute difference before and after matching between the groups, and after making the appropriate bivariate comparisons. Statistical analyses were performed using SPSS Statistics (version 23.0; IBM, Armonk, New York). The Fine and Gray competing risks regression models were run using the SPSS extension command COMPRISK, which uses the R “cmprsk” package.26 PSM was performed using an SPSS R-Menu27 and R3.1.1. Statistical significance was set at P < 0.05.
Results
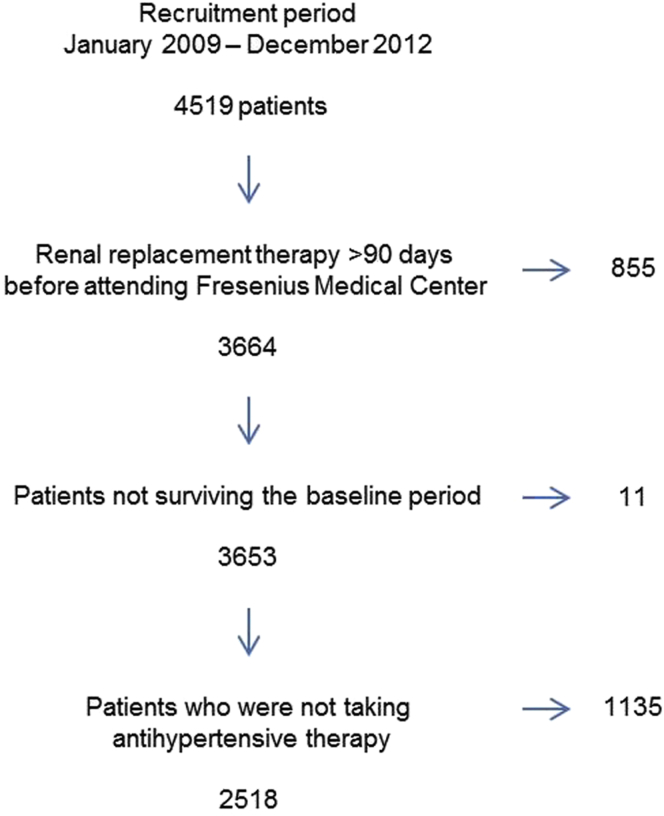
The design of the project is shown in Figure 1. We screened 4519 patients treated in the NephroCare facilities of Fresenius Medical Care centers in Spain between 2009 and 2012 for inclusion. Only 2518 patients from 63 Fresenius Medical Care clinics fulfilled the inclusion criteria (see Supplementary Table S2 for statistical comparisons between excluded and included patients). These patients were classified at baseline according to their initial main antihypertensive medication into 4 groups: (i) agents acting on the RAS if the main antihypertensive prescription was a RAS blocker (ACEIs or ARBs) (n =728); (ii) ß-blockers if the main antihypertensive medication was a ß-blocker, (n = 679); (iii) combination of RAS blockers and ß-blockers if the antihypertensive medication included a RAS blocker and a ß-blocker (n = 324); and (iv) other antihypertensive medication if the initial antihypertensive medication was any other drug not included in any of the previously defined groups (n = 787).
Figure 1.
Flowchart according to the exclusion criteria.
Descriptive Analysis
Table 1 shows the baseline demographic characteristics for systolic blood pressure, diastolic blood pressure, pulse pressure, and heart rate for the 4 groups of patients. There were no significant differences among the groups with respect to sex, percentage of patients with diabetes mellitus, or use of a catheter as vascular access. In contrast, significant differences were found for age, namely, the mean age of the patients included in the combination of RAS blockers and ß-blockers group was significantly lower. In addition, significant differences were found for both systolic and diastolic blood pressures, mean liters ultrafiltrated per session, and mean serum potassium levels, which were lower for patients treated with ß-blockers at baseline; however, the difference in pulse pressure among the groups was not significant. The heart rate was lower in the ß-blocker and the combination of the RAS blocker plus ß-blocker groups at baseline. Finally, significant differences were found for cardiac comorbidities; patients who took antihypertensive medication with ß-blockers had more episodes of heart failure, CHD, and arrhythmia at baseline than the other groups.
Table 1.
Patient characteristics at baseline according to the main antihypertensive medication
| Baseline | RAS blockers | ß-blockers | Combination RAS blockers + ß-blockers | Other | P value |
|---|---|---|---|---|---|
| N | 728 | 679 | 324 | 787 | |
| Age (yr) | 64.40 ± 15.08 | 65.84 ± 13.82 | 61.44 ± 14.88 | 67.86 ± 13.86 | <0.001 |
| Systolic blood pressure (mm Hg) | 144.00 ± 18.02 | 140.75 ± 21.02 | 146.00 ± 20.58 | 142.36 ± 17.80 | <0.001 |
| Diastolic blood pressure (mm Hg) | 72.72 ± 11.57 | 69.13 ± 13.01 | 73.14 ± 12.79 | 69.95 ± 11.29 | <0.001 |
| Pulse pressure (mm Hg) | 71.28 ± 17.12 | 71.62 ± 19.44 | 72.86 ± 18.78 | 72.41 ± 16.90 | 0.460 |
| Heart rate (beats/min) | 77.04 ± 10.41 | 70.97 ± 11.63 | 69.79 ± 10.56 | 76.74 ± 10.46 | <0.001 |
| Sex (female) | 33.93% | 34.90% | 37.35% | 33.04% | 0.564 |
| Diabetes mellitus | 41.90% | 41.09% | 46.30% | 39.52% | 0.218 |
| Heart failure (ICD-10 codes: I50) | 7.55% | 13.84% | 11.73% | 7.88% | <0.001 |
| Coronary heart disease (ICD-10 codes: I25) | 8.24% | 25.18% | 21.91% | 11.18% | <0.001 |
| Arrhythmia (ICD-10 codes: I44−I49) | 17.03% | 22.97% | 15.43% | 19.19% | 0.010 |
| Stroke (ICD-10 codes: I60−I69, G45−G46) | 12.23% | 13.40% | 11.42% | 13.21% | 0.775 |
| Ultrafiltration per session (l) | 3.13 ± 0.69 | 3.11 ± 0.71 | 3.21 ± 0.67 | 3.21 ± 0.7 | 0.007 |
| Potassium (mEq/l) | 4.82 ± 0.82 | 4.68 ± 0.79 | 4.88 ± 0.82 | 4.74 ± 0.78 | 0.001 |
| Vascular access (% catheter) | 46.57% | 51.84% | 45.68% | 50.44% | 0.112 |
ICD-10, International Classification of Diseases-10th Revision; RAS, renin-angiotensin system.
To compare the variables between the groups we used the Chi-square test for categorical factors and ANOVA for continuous variables. Statistical significance of P < 0.05 is in bold.
Survival Analysis
The participants included in this study were followed for a maximum of 5 years, until death or until withdrawal from the study (any reason). Therefore, during the observation period, 995 patients finished the study because of kidney transplantation (n = 545), change in dialysis unit (n = 366), or other reasons (n = 84). There were 575 deaths during the follow-up period. The main causes of death were CV disease (62.9%), infectious disease (14.8%), gastrointestinal disease (6.3%), cancer (6.1%), and other (9.9%).
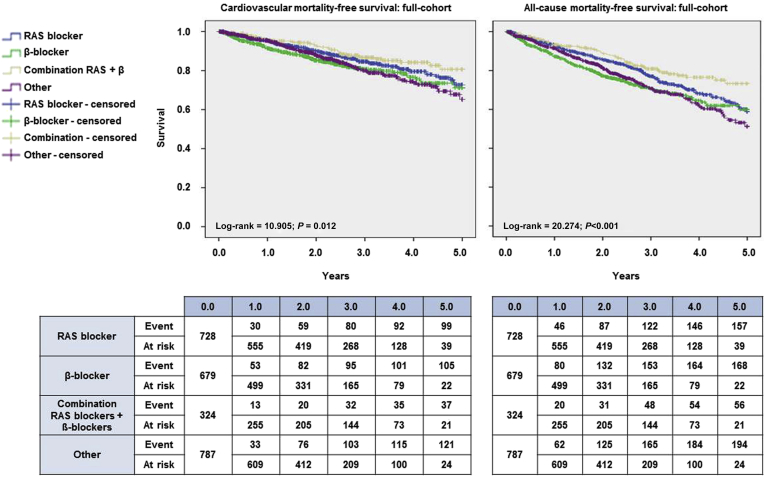
Figure 2 shows the Kaplan-Meier curves for CV and all-cause mortality before allocation based on PSM. Several Cox regression models were built to explore the association between antihypertensive medication and the outcomes studied. Because this was a multicenter study throughout the different Fresenius Medical Care dialysis facilities in Spain, the possible center effect on the outcome was tested by the corresponding univariate Cox regression. This analysis in relation to the main outcome was nonsignificant (Supplementary Table S3). Patients who received the combination of RAS blockers and ß-blockers had the best survival profile in the cohort in both the unadjusted and the adjusted models. Mortality was significantly higher in patients who took ß-blockers or other antihypertensive medication than in patients who took a combination of RAS blockers and ß-blockers (Table 2). Moreover, the adjusted competing risks regression models revealed the same results as the adjusted Cox models (Table 2).
Figure 2.
Kaplan-Meier survival plots for cardiovascular mortality (left) and all cause mortality (right). The survival curves for each medication group—renin-angiotensin system (RAS) blockers (blue), β-blockers (green), combination RAS blockers + β-blockers (gold), and other (purple)—are accompanied by their corresponding survival tables.
Table 2.
Mortality HRs and SHRs in hypertensive patients with chronic kidney disease according to antihypertensive medication
| Unadjusted HR |
Adjusted model HRa |
Competing risks model SHRb |
Matched adjusted models HRc |
|||||
|---|---|---|---|---|---|---|---|---|
| All-cause | Cardiovascular | All-cause | Cardiovascular | All-cause | Cardiovascular | All-cause | Cardiovascular | |
| Combination RAS blockers + ß-blockers | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| RAS blockers | 1.36 (1.01–1.85)d | 1.30 (0.89–1.90) | 1.16 (0.85–1.59) | 1.25 (0.84–1.87) | 1.12 (0.96–1.28) | 1.11 (0.92–1.30) | 1.55 (1.05–2.28)d | 1.68 (1.05–2.69)d |
| ß-blockers | 1.82 (1.34–2.46)e | 1.72 (1.18–2.50)f | 1.51 (1.09–2.08)f | 1.50 (1.01–2.24)d | 1.44 (1.29–1.59)d | 1.31 (1.11-1.50)d | 1.45 (1.01–2.09)d | 1.59 (1.01–2.50)d |
| Other | 1.74 (1.29–2.34)e | 1.64 (1.13–2.37)f | 1.37 (1.01–1.89)d | 1.61 (1.08–2.39)f | 1.29 (1.13–1.43)d | 1.23 (1.05-1.43)d | 1.46 (1.02–2.10)d | 1.67 (1.08–2.58)d |
HR, hazard ratio; RAS, renin-angiotensin system; SHR, subdistribution hazard ratio.
The HRs and SHR for all-cause mortality or cardiovascular mortality and their corresponding 95% confidence intervals (in parentheses) were calculated.
Adjusted Cox models were built including the following covariates: age (years); sex; diabetes mellitus; systolic blood pressure (mm Hg); heart failure (International Classification of Diseases-10th Revision [ICD-10] code: I50); coronary heart disease (ICD-10 code: I25); arrhythmia (ICD-10 codes: I44−I49); stroke (ICD-10 codes: I60−I69, G45−G46); ultrafiltration per session (l), potassium (mEq/l), and vascular access (catheter).
Adjusted Fine and Gray competing risks regression models were built including the following covariates: age (years); sex; diabetes mellitus; systolic blood pressure (mm Hg); heart failure (ICD-10 code: I50); coronary heart disease (ICD-10 code: I25); arrhythmia (ICD-10 codes: I44−I49); stroke (ICD-10 codes: I60−I69, G45−G46); ultrafiltration per session (l), potassium (mEq/l), and vascular access (catheter).
Patients on treatment with both RAS blockers and ß-blockers or any other treatment were matched 1:1 based on different propensity score matching models including the vascular access in the regression analyses.
P < 0.05; eP < 0.001; fP < 0.01; no symbol means no significant differences.
Propensity Score Matching
Because the patients were not randomly assigned to the 4 study groups and significant differences were found for the independent predictors of CV risk at baseline, we used the PSM to eliminate baseline differences among the treatment groups. Because the resulting populations were balanced in each case for all the CV risk-related covariates recorded and the class of drugs prescribed, vascular access was excluded from the matching procedure because of the null association with the decisions of the attending physicians on antihypertensive drug prescription.
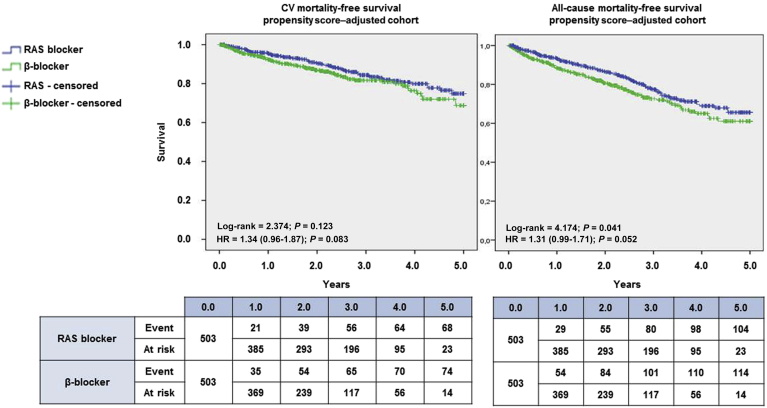
In a matched propensity score cohort, 503 patients treated with RAS blockers (ACEIs or ARBs) were compared with 503 patients treated with ß-blockers. After allocation based on PSM, baseline covariates were similar in the matched cohort, except for diastolic blood pressure and heart rate, which were not included in the PSM and were lower in the ß-blocker group (Supplementary Table S4). This adjustment revealed a nonsignificant trend toward a better CV survival profile in patients who took RAS blockers (ACEI or ARB) compared with patients who started therapy with a ß-blocker at the end of follow-up (Figure 3).
Figure 3.
Kaplan-Meier survival plots. Cardiovascular (CV) (left) and all-cause (right) survival after propensity score matching–based adjustment for β-blockers (green) and renin-angiotensin system (RAS) blockers (blue) are compared using the log-rank test. The corresponding hazard ratio (HR) is accompanied by the corresponding 95% confidence interval.
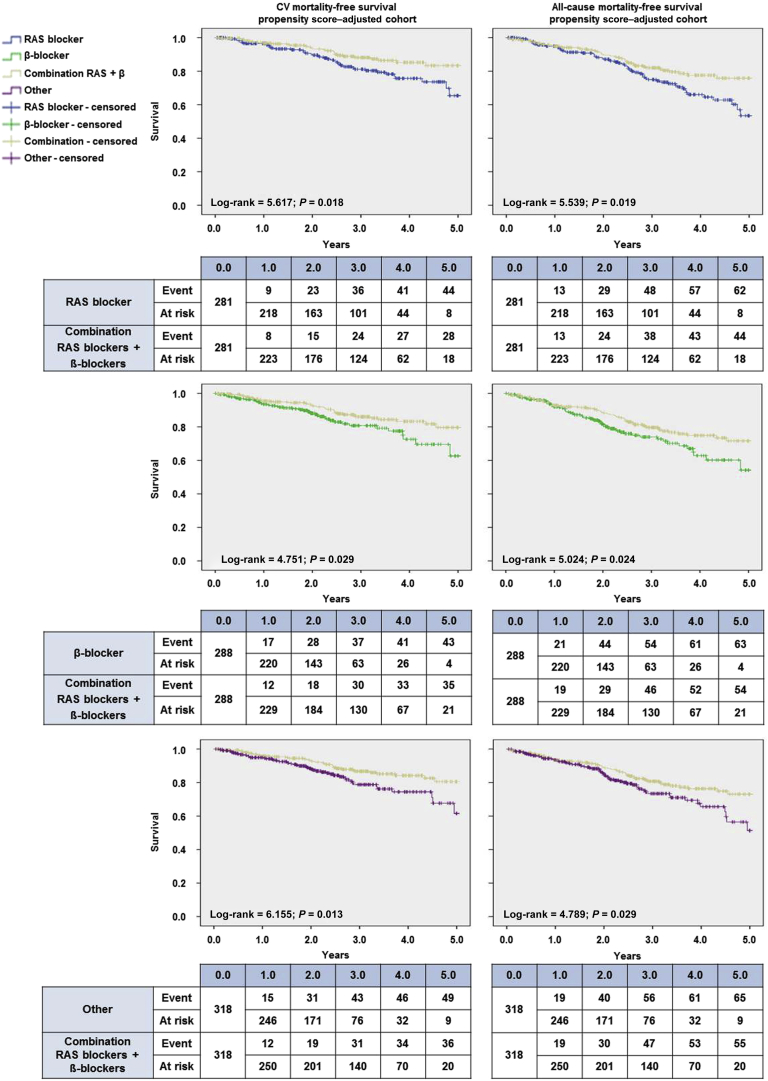
We performed a pair-by-pair comparison between the groups taking a combination of RAS blockers and ß-blockers and the other 3 cohorts. We compared 281 patients treated with the combination with 281 patients treated with RAS blockers only. Baseline characteristics were similar in the matched cohorts, except for heart rate, which was lower in the combination group (Supplementary Table S5). Furthermore, we compared 288 patients treated with the combination with 288 patients treated with ß-blockers only. Baseline characteristics were similar in the matched cohort (Supplementary Table S6). Finally, we compared 318 patients treated with the combination with 318 patients treated with other drugs and found that the baseline characteristics were similar in the matched cohort, except for heart rate, which was lower in the combination group (Supplementary Table S7).
The corresponding survival curve analyses are shown in Figure 4. In patients taking the combination of RAS blockers and ß-blockers, the survival profile was better in every model plotted and in all pairwise comparisons for CV-associated mortality and for all-cause mortality. The Cox models for the adjusted cohorts including vascular access (Table 2) indicated that taking a RAS blocker, a ß-blocker, or other antihypertensive medication was associated with a significantly increased risk of CV and all-cause mortality than taking the combination of a RAS blocker plus a ß-blocker.
Figure 4.
Kaplan-Meier survival plots after propensity score matching–based adjustment for cardiovascular (left) and all-cause mortality (right). The survival curves for each medication group—renin-angiotensin system (RAS) blockers (blue), β-blockers (green), combination RAS blockers + β-blockers (gold), and other (purple)—are accompanied by their corresponding survival tables.
Discussion
We performed an observational study using PSM to analyze the association between CV mortality and the use of different classes of antihypertensive medications in 2518 incident hemodialysis patients followed for up to 5 years. After adjustment using PSM, no significant differences in CV risk were observed with the use of either RAS blockers or ß-blockers. Our main finding was that CV survival was better with the combination of a RAS blocker with a ß-blocker than with antihypertensive therapy with either agent alone or with antihypertensive drugs other than RAS blockers or ß-blockers.
Although several studies with short observation periods failed to identify hypertension as having a major influence on CV mortality, long-term observational data showed that mortality was lower in patients with normal blood pressure than in those with high blood pressure.2, 3, 28 Epidemiological evidence from a large cohort of dialysis patients in the United States clearly showed that pre- and postdialysis blood pressure values were independently associated with mortality.8 In their prospective follow-up based on clinical and echocardiographic assessments in dialysis patients, Foley et al.29 demonstrated that each 10-mm Hg rise in mean arterial blood pressure was independently associated with concentric left ventricular hypertrophy, de novo heart failure, and de novo CHD.
The prevalence of hypertension is high in hemodialysis patients. In this study, 68% of hemodialysis patients received antihypertensive medication. Although the indication of antihypertensive medication might not be indicative of hypertension in all cases, particularly in patients with heart disease, most hemodialysis patients treated with antihypertensive drugs in the present study had high blood pressure (any degree). The prevalence of antihypertensive drug use in hemodialysis patients was similar to that reported in other studies (60%−80%).30, 31, 32, 33
Volume excess is the main cause of hypertension in dialysis patients, and a high percentage remain hypertensive mainly due to excessive sodium intake and the difficulty in eliminating the interdialytic volume excess. Other nonvolume-related mechanisms that sustain hypertension include activation of the RAS or sympathetic nervous system. The CV risk in patients with high blood pressure after intensive volume management can be reduced using antihypertensive drug therapy.10, 11 Several antihypertensive drugs have been shown to reduce blood pressure effectively and to improve prognosis in hemodialysis patients. Despite the lack of clear evidence to date of the superiority of one class of antihypertensive drug over the others, RAS blockers20 and ß-blockers have been shown to be superior to other drugs for the management of CV risk.22
The RAS not only mediates the immediate physiological effects of vasoconstriction and regulation of blood pressure, but is also implicated in inflammation, endothelial dysfunction, atherosclerosis, and congestive heart failure.34 Activation of the RAS appears to be implicated in the pathogenesis of hypertension in dialysis patients, and RAS blockers such as ACEIs and ARBs are useful, effective, and well tolerated, and are particularly indicated in patients with left ventricular hypertrophy, cardiomyopathy, or both.17, 19, 20 In this study, we included patients treated with both RAS blockers (ACEIs or ARBs) in the same group, because there was no conclusive difference between ACEIs and ARBs in terms of their CV protective effect in patients with chronic kidney disease (CKD). In addition, most influential clinical guidelines recommend that ACEIs and ARBs be used indistinctly to improve CV outcomes in CKD patients.
However, evidence to support a beneficial effect of ACEIs and ARBs in reducing CV mortality in ESRD patients is insufficient. In a randomized trial, the ACEI fosinopril was unable to significantly reduce CV risk in 397 patients who underwent hemodialysis.18 In a double-blind, randomized, placebo-controlled, 1-year intervention trial in hemodialysis patients with a predefined systolic blood pressure target of 140 mm Hg, treatment with irbesartan did not significantly affect intermediate CV endpoints such as central aortic blood pressure, carotid–femoral pulsewave velocity, left ventricular mass index, N-terminal brain natriuretic prohormone, heart rate variability, and plasma catecholamines.35 However, a significant 49% decrease in CV events was observed in a trial that included 360 patients randomized to ARBs versus no ARBs.36 In another larger multicenter, randomized trial, no significant differences in the risks of major CV events or death were recorded in 469 patients with high blood pressure on long-term hemodialysis who were assigned to receive the ARB olmesartan or another antihypertensive treatment.37
The sympathetic nervous system is also activated in CKD,7 and ß-blockers may provide multiple benefits other than control of blood pressure in coronary artery disease and heart failure, which are the main causes of mortality in ESRD patients. Therapy with ß-blockers improves coronary blood flow, protects the heart against overstimulation by catecholamines, and reduces the risk of sudden cardiac death.38
Epidemiological studies have shown a clear CV benefit of ß-blockers in patients undergoing hemodialysis,8 and 1 randomized controlled study in ESRD patients with left ventricular hypertrophy showed that the ß-blocker atenolol was clearly superior to the ACEI lisinopril in preventing CV morbidity and all-cause hospitalizations.22 In another retrospective study of 2500 dialysis patients enrolled in the United States Renal Data System with no documented history of heart failure, ß-blockers were associated with a lower risk of new heart failure, CV death, and death from any cause.39 In contrast, we found no significant CV benefit after adjustment for baseline CV risk factors in patients who received ß-blockers compared with patients who received RAS blockers.
To further study the clinical impact of combining RAS blockers with other drugs with positive CV effects, we compared the effectiveness of the combination of RAS blockers and ß-blockers (in terms of CV benefit and long-term survival) with either agent alone or with agents other than RAS or ß-blockers. We found that the combination of RAS blockers with ß-blockers carried the lowest risk of CV and all-cause death.
The CV effects of combining RAS blockers and ß-blockers have not been extensively studied in patients who receive hemodialysis, and the mechanism of this potential benefit needs to be elucidated. It seems reasonable to assume that the CV benefits of the combination in CKD patients could be potentiated by the association of the benefits of each type of drug. One study reported a beneficial effect of combining the ß-blocker carvedilol with RAS blockers in 114 hemodialysis patients with symptomatic heart failure. All the patients received either ACEIs or ARBs at baseline and were randomly assigned to placebo or carvedilol after 24 months of follow-up. Fatal myocardial infarctions, fatal strokes, and hospital admissions for worsening heart failure were lower in the carvedilol group than in the placebo group.40
Our study was subject to a series of limitations. First, because it was an observational study, we could not rule out confounding biases such as the indication of medication and other residual confounders. Second, several differences were recorded at baseline. Patients who took ß-blockers had more CV events (heart failure, CHD, and arrhythmia) and lower blood pressure than the other groups. Third, although the propensity score adjustment eliminated most baseline differences among the treatment groups and controlled for age, sex, and comorbidities, it was not possible to rule out other potential influential factors that were not adjusted. Fourth, clinical data were recorded from the medical histories, with the result that any differences in the definition of baseline comorbidities by the attending physician were not controlled. Fifth, medications taken during the pre-hemodialysis period were not recorded, and the analyses did not take into account changes in medication use over time or changes in dose. Finally, 15.7% of the study patients discontinued the initial antihypertensive medication after a year of follow-up.
Despite these limitations, this was a novel study. To our knowledge, no previous reports have analyzed the combination of RAS blockers and ß-blockers on mortality in stable ESRD patients undergoing hemodialysis.
In conclusion, we showed that in a cohort of ESRD patients who underwent long-term hemodialysis treated with antihypertensive medication, treatment with RAS blockers or ß-blockers did not lead to significant differences in the risk of CV mortality. The combination of a RAS blocker with a ß-blocker was associated with better CV survival than antihypertensive therapy with either agent alone or antihypertensive medication other than RAS blockers or ß-blockers. Further prospective randomized controlled trials are necessary to confirm the beneficial effects of this combination in patients undergoing hemodialysis.
Disclosure
The Optimizing Results in Dialysis group (ORD) is a scientific advisory board funded by Fresenius Medical Care, Spain. JV, RR, and JIM are employees of Fresenius Medical Care. All the other authors declared no competing interests.
Acknowledgments
The authors are grateful for the contribution of the doctors of the Spanish Fresenius Medical Care clinics, whose efforts in the implementation of the EuClid database made this study possible, and to Thomas O’Boyle for writing assistance.
The Optimizing Results in Dialysis group (ORD) members are Pedro Aljama, Bernard Canaud, Angel Luis Martin De Francisco, Adelheid Gauly, José Luño, Francisco Maduell, Alejandro Martin-Malo, José Ignacio Merello, Julio Pascual, Rafael Pérez-Garcia, Manuel Praga, Rosa Ramos, Stefano Stuard, Javier Varas, and Adam Zawada.
Footnotes
Table S1. Drugs description by patients groups.
Table S2. Patient characteristics at baseline among patients who were included versus excluded in the study.
Table S3. Cox analysis to analyze the plausible center effect affecting the mortality in the study.
Table S4. Baseline characteristics before and after propensity-score matching for the subgroups with renin-angiotensin system blockers and ß-blockers.
Table S5. Baseline characteristics before and after propensity-score matching for the subgroups renin-angiotensin system (RAS) blockers and combination RAS + ß-blockers.
Table S6. Baseline characteristics before and after propensity-score matching for the subgroups ß-blocker and combination renin-angiotensin system blockers + ß-blockers.
Table S7. Baseline characteristics before and after propensity-score matching for the subgroups other antihypertensive drugs and combination renin-angiotenin system blockers + ß-blockers.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Contributor Information
José Luño, Email: jose.luno@salud.madrid.org.
ORD Group:
Pedro Aljama, Bernard Canaud, Angel Luis Martin De Francisco, Adelheid Gauly, José Luño, Francisco Maduell, Alejandro Martin-Malo, José Ignacio Merello, Julio Pascual, Rafael Pérez-Garcia, Manuel Praga, Rosa Ramos, Stefano Stuard, Javier Varas, and Adam Zawada
Supplementary Material
Drugs description by patients groups.
Patient characteristics at baseline among patients who were included versus excluded in the study.
Cox analysis to analyze the plausible center effect affecting the mortality in the study.
Baseline characteristics before and after propensity-score matching for the subgroups with renin-angiotensin system blockers and ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups renin-angiotensin system (RAS) blockers and combination RAS + ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups ß-blocker and combination renin-angiotensin system blockers + ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups other antihypertensive drugs and combination renin-angiotenin system blockers + ß-blockers.
References
- 1.Sociedad Española de Nefrología. Registros de Enfermos Renales 2014. Available at: http://www.senefro.org/modules.php?name=webstructure&idwebstructure=29. Accessed October 1, 2016.
- 2.Charra B., Calemard E., Ruffet M. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41:1286–1291. doi: 10.1038/ki.1992.191. [DOI] [PubMed] [Google Scholar]
- 3.Mazzuchi N., Carbonell E., Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58:2147–2154. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas M.F., Quereda C., Teruel J.L. Effect of hypertension before beginning dialysis on survival of hemodialysis patients. Am J Kidney Dis. 2003;41:814–823. doi: 10.1016/s0272-6386(03)00029-5. [DOI] [PubMed] [Google Scholar]
- 5.Charra B., Laurent G., Chazot C. Clinical assessment of dry weight. Nephrol Dial Transplant. 1996;11:16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F., Covic A., Chazot C. Hypertension and cardiovascular risk assessment in dialysis patients. Nephrol Dial Transplant. 2004;19:1058–1068. doi: 10.1093/ndt/gfh103. [DOI] [PubMed] [Google Scholar]
- 7.Converse R.L., Jr., Jacobsen T.N., Toto R.D. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 8.Foley R.N., Herzog C.A., Collins A.J. Blood pressure and longterm mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62:1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 9.Scribner B.H. Can antihypertensive medications control BP in haemodialysis patients: yes or no? Nephrol Dial Transplant. 1999;14:2599–2601. doi: 10.1093/ndt/14.11.2599. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R., Sinha A.D. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heerspink H.J., Ninomiya T., Zoungas S. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ONTARGET Investigators. Yusuf S., Teo K.K. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S., Sleight P., Pogue J. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 14.Cohn J.N., Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 15.McMurray J.J., Ostergren J., Swedberg K. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 16.Dahlöf B., Devereux R.B., Kjeldsen S.E., LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 17.Tai D.J., Lim T.W., James M.T. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol. 2010;5:623–630. doi: 10.2215/CJN.07831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zannad F., Kessler M., Lehert P. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 19.Xie X., Liu Y., Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Wang R., Li M.X. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on left ventricular mass index and ejection fraction in hemodialysis patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Int J Cardiol. 2016;219:350–357. doi: 10.1016/j.ijcard.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Chan K.E., Ikizler T.A., Gamboa J.L. Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int. 2011;80:978–985. doi: 10.1038/ki.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal R., Sinha A.D., Pappas M.K. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29:672–681. doi: 10.1093/ndt/gft515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-García R., Palomares I., Merello J.I., (ORD group) Epidemiological study of 7316 patients on haemodialysis treated in FME clinics in Spain, using data from the EuCliD® database: results from years 2009-2010. Nefrología. 2012;32:743–753. doi: 10.3265/Nefrologia.pre2012.Jul.11549. [DOI] [PubMed] [Google Scholar]
- 24.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Austin P.C. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometr J. 2009;51:171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M.-J., Zhang X., Scheike T.H. Modeling cumulative incidence function for competing risks data. Expert Rev Clin Pharmacol. 2008;1:391–400. doi: 10.1586/17512433.1.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoemmes F. Propensity score matching in SPSS. Available at: http://arxiv.org/abs/1201.6385. Accessed December 2016.
- 28.Port F.K., Hulbert-Shearon T.E., Wolfe R.A. Pre-dialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 29.Foley R.N., Parfrey P.S., Harnett J.D. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49:1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 30.Salem M.M. Hypertension in the hemodialysis population: a survey of 649 patients. Am J Kidney Dis. 1995;26:461–468. doi: 10.1016/0272-6386(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 31.Rocco M.V., Yan G., Heyka R.J. HEMO Study Group: Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. Am J Nephrol. 2001:280–288. doi: 10.1159/000046262. [DOI] [PubMed] [Google Scholar]
- 32.Raine A.E., Margreiter R., Brunner F.P. Report on management of renal failure in Europe, XXII, 1991. Nephrol Dial Transplant. 1992;7(Suppl 2):s7–s35. [PubMed] [Google Scholar]
- 33.Rahman M., Fu P., Sehgal A.R. Interdialytic weight gain, compliance with dialysis regimen, and age are independent predictors of blood pressure in hemodialysis patients. Am J Kidney Dis. 2000;35:257–265. doi: 10.1016/s0272-6386(00)70335-0. [DOI] [PubMed] [Google Scholar]
- 34.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 35.Peters C.D., Kjaergaard K.D., Jensen J.D. No significant effect of angiotensin II receptor blockade on intermediate cardiovascular end points in hemodialysis patients. Kidney Int. 2014;86:625–637. doi: 10.1038/ki.2014.69. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H., Kanno Y., Sugahara S. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Iseki K., Arima H., Kohagura K., Olmesartan Clinical Trial in Okinawan Patients Under OKIDS (OCTOPUS) Group Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28:1579–1589. doi: 10.1093/ndt/gfs590. [DOI] [PubMed] [Google Scholar]
- 38.Lymperopoulos A., Rengo G., Koch W.J. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;30(113):739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott K.C., Trespalacios F.C., Agodoa L.Y. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164:2465–2471. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 40.Cice G., Ferrara L., D’Andrea A. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drugs description by patients groups.
Patient characteristics at baseline among patients who were included versus excluded in the study.
Cox analysis to analyze the plausible center effect affecting the mortality in the study.
Baseline characteristics before and after propensity-score matching for the subgroups with renin-angiotensin system blockers and ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups renin-angiotensin system (RAS) blockers and combination RAS + ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups ß-blocker and combination renin-angiotensin system blockers + ß-blockers.
Baseline characteristics before and after propensity-score matching for the subgroups other antihypertensive drugs and combination renin-angiotenin system blockers + ß-blockers.