To the Editor:
Immune response quality is considered as a major factor in chronic viral infections.1 Cytomegalovirus (CMV) remains one of most frequent and deleterious viral infections in kidney transplantation. For the prevention of CMV disease, prophylactic or preemptive treatments are commonly proposed. Because of side effects, new methods for tailoring of anti-CMV treatments are needed to adapt to an individual’s recipient risk.2, 3, 4 Assessment of the host immune system capacity to control CMV replication might be useful to predict viral reactivation control, but is not yet widely recommended.5, 6, 7, 8, 9 In this study, we characterized the specific memory T-cell responses against immediate early protein 1 (IE-1) and phosphoprotein 65 (pp65), 2 immunodominant proteins from the CMV virion, to better understand the immune mechanisms of viral control in CMV-seropositive patients receiving kidney transplantation.
Patients, Material, and Methods
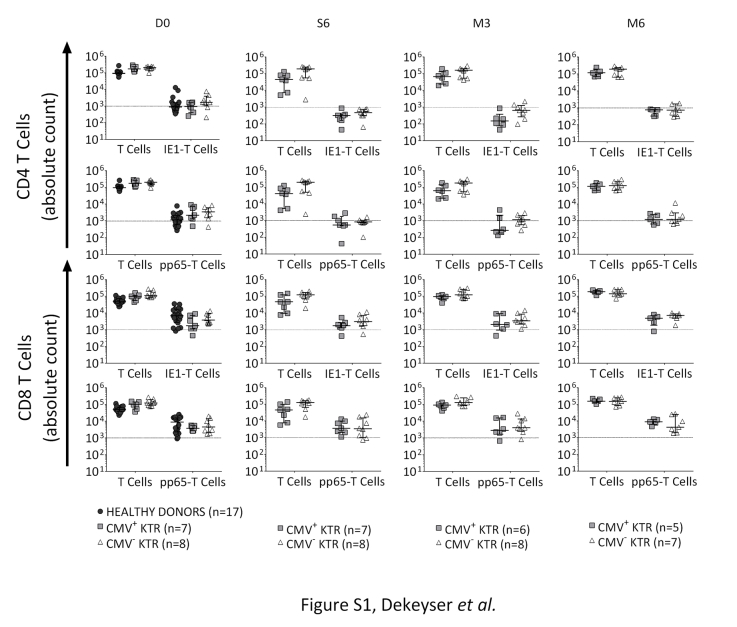
According to France’s bioethics specifications, we realized a prospective monocentric observational study. Consecutive CMV-seropositive patients receiving de novo kidney transplant (KTR) and CMV-seropositive healthy donors (French blood bank donors) were included from January to November 2013. In the KTR group, CMV-DNAemia was monitored weekly in whole blood during the 6 months’ follow-up. CMV infection or disease was defined according to International Guidelines and American Society of Transplantation recommendations for use in clinical trials.3, 4, 10 Antiviral therapy was initiated in case of CMV infection (defined by an increase of CMV-DNAemia kinetic more than 10 times between 2 consecutive samples, without symptoms, treated by preemptive valganciclovir) or CMV disease (curative ganciclovir treatment, followed by preemptive therapy). On renal transplant day (D0), 6 weeks, 3 months, and 6 months after transplantation, peripheral blood mononuclear cells were stimulated overnight with CMV peptide pools (IE-1 or pp65). After stimulation, cells were stained for memory T-cell subpopulation identification11, 12, 13 (stem-cell memory [TSCM], central-memory [TCM], effector-memory [TEM], and terminally differentiated effector T cells [TD]), and then for intracellular cytokine secretions (Figure 1, Supplementary Material and Methods online). Lymphocyte polyfunctionality was evaluated by the capacity of CMV-specific T cells to perform single, double, and triple cytokine secretions. To ensure the robust analysis of CMV-specific T-cell subpopulations, only samples with CMV-specific T-cell responses more than twice the background (no antigen) and a minimum of 40 cytokine-secreting cells were considered positives and included (Supplementary Figure S1).
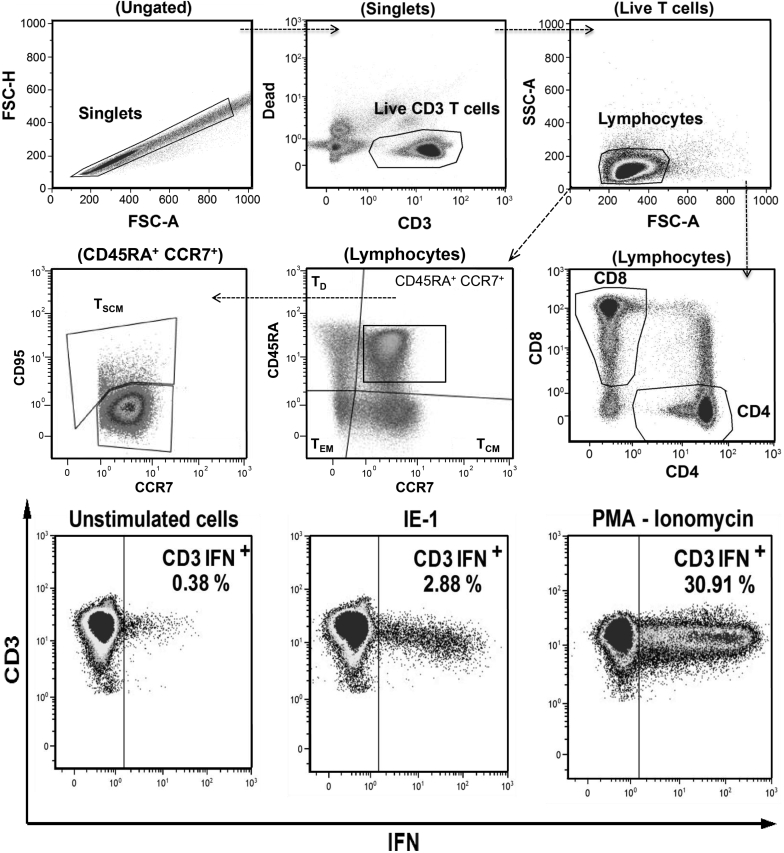
Figure 1.
Gating strategy for the identification of IE-1 and pp65-specific memory T cells. One million fresh peripheral blood mononuclear cells were incubated overnight in AIM-V medium alone (unstimulated cells) or with CMV peptides (IE-1 or pp65 peptide pools) or with positive control (PMA-ionomycin). After activation, cells were stained by surface markers (antibodies anti-CD3, -CD4, -CD8, -CCR7, -CD45RA, -CD95) and then fixed and permeabilized for intracellular cytokine staining (antibodies anti-IL2, -IFNγ, and -TNFα). Upper and middle dot-plots: singlet cell population was identified by applying an FSC-H versus FSC-A gate. Then, live CD3 T cells were identified by a gate on high CD3 and low Fixable Dye staining. T lymphocytes were identified as low SSC-A/FSC-A cells, and CD4 versus CD8 gate was applied. Memory T-cell subpopulations were identified based on expression of CCR7, CD45RA: central-memory (TCM), effector-memory (TEM) and terminally differentiated effector (TD) T cells. Stem-cell memory T cells (TSCM) were identified as CD45RA+ CCR7+ CD95+ cells. Down dot-plots: a gate was set in the unstimulated cells for the background expression of each cytokine (IL2+, TNFα+, or IFNγ+) on CD3+, CD4+, and CD8+ T lymphocytes. In IE-1, pp65, and PMA/ionomycin stimulation, the same gating strategy was used to define the cytokine-secreting cells. The percentage of cytokine-producing CD4 or CD8 T cells was determined after subtracting the background in unstimulated controls. Shown are examples on a representative healthy donor of IFNγ-secreting CD3+ T cells (percentage of CD3+ T cells expressing IFN-γ) in unstimulated cells, after IE-1 and PMA/ionomycin stimulation. Similar results were found for IL2 and TNFα secretions, and after pp65 stimulation (data not shown). Lymphocyte polyfunctionality was evaluated by the capacity of the CMV-specific T cells to perform single, double, and triple cytokine secretions. Mono-, bi-, and trifunctional T cells were identified by Boolean gates. Total frequency of polyfunctional cells was determined by summing the total number of cytokine-secreting cells. CD3 IFN+, interferonγ-producing CD3 T cells; IE-1, immediate early protein 1; IFNγ, interferonγ; pp65, phosphoprotein 65; TCM, central-memory T cells; TD, terminally differentiated effector T cells; TEM, effector-memory T cells; TSCM, stem-cell memory T cells.
Results
Clinical Data
Fifteen CMV-seropositive KTR were followed for 6 months after transplantation. As the control group, 17 CMV-seropositive healthy donors were included. Controls were comparable to KTR for age and gender (P > 0.05). Two groups of KTR were identified per the occurrence of CMV infection/disease: 7 KTR presented CMV infection/disease requiring antiviral treatment (CMV+ KTR), whereas 8 KTR did not (CMV− KTR). Median CMV-viral load peak was 55,800 copies/ml (1070–801,000) with a median time of 6 weeks after transplantation. During the follow-up period, the CMV+ KTR group presented more viral infectious complications, without a statistical difference concerning the immunosuppressive regiment, nor in graft function (Table 1).
Table 1.
Characteristics and clinical evolution of CMV-seropositive patients with de novo kidney transplantation, according to the occurrence of CMV infection/disease, during the 6 months of follow-up
| Time | CMV+ KTR (n = 7) | CMV− KTR (n = 8) | P value | |
|---|---|---|---|---|
| D0 | Male (n) | 4 | 7 | 0.282 |
| Age (yr) | 70 (49–77) | 50 (31–74) | 0.088 | |
| Diabetic and/or vascular nephropathy (n) | 4 | 4 | 1.000 | |
| Malformative uropathy (n) | 1 | 1 | 1.000 | |
| Glomerulopathy (n) | 2 | 3 | 1.000 | |
| Dialysis (mo) | 45 (23–104) | 23 (0–159) | 0.219 | |
| Hemodialysis (n) | 6 | 6 | 1.000 | |
| First kidney transplant (n) | 6 | 6 | 1.000 | |
| I and II class HLA mismatches (n) | 4 (2–8) | 5.5 (2–8) | 0.788 | |
| Thymoglobulin (n) | 3 | 3 | 1.000 | |
| Tacrolimus (n) | 3 | 6 | 0.315 | |
| Mycophenolate mofetil (n) | 6 | 8 | 0.467 | |
| Corticosteroids (n) | 6 | 8 | 0.467 | |
| CMV-specific IgG antibody (UA/ml) | 222 (101–250) | 250 (125–250) | 0.689 | |
| W6 | CMV viral load peak (copies/ml) | 55,800 (1070–801,000) | 0 (0–2867a) | **0.002 |
| Delay between CMV infection/disease and transplant day (wk) | 6.3 (2.3–11) | 0 | *0.016 | |
| Valganciclovir (n) | 5 | ‒ | ‒ | |
| Ganciclovir or valaciclovir (n) | 2 | ‒ | ‒ | |
| Creatininemia (μmol/l) | 204 (98–412) | 149 (93–210) | 0.456 | |
| M6 | Creatininemia (μmol/l) | 183 (96–542) | 140 (78–210) | 0.149 |
| Graft rejection (n) | 2 | 1 | 0.569 | |
| Graft lost (n) | 2 | 0 | 0.200 | |
| Viral infectious complications other than CMV infection/disease (n) | 6 | 2 | *0.041 |
Median (Minimum–Maximum), Mann-Whitney. *P < 0.05, **P < 0.005.
Seven KTR presented CMV infection/disease requiring treatment (CMV+ KTR), whereas 8 KTR did not (CMV− KTR). There was no statistical difference concerning the immunosuppressive regiment, nor in graft function from the transplant day to the sixth month. During the follow-up period, patients from the CMV+ KTR group had more viral infectious complications such as EBV or HSV reactivation, compared with the CMV− KTR group.
CMV, cytomegalovirus; CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV-infection/disease; CMV− KTR, CMV-seropositive kidney transplant recipients without CMV-infection/disease; D0, transplantation day; M6, 6th month after kidney transplantation; n, number; W6: 6th week after kidney transplantation.
Two patients from the CMV− KTR group presented a latent CMV, without antiviral treatment requirement (asymptomatic lower CMV viremia with spontaneous normalization between 2 consecutive samples).
At D0, CMV-Specific Memory T Cells Were Less Frequent, With Lower CD8 TSCM and TCM Counts in Patients Awaiting Renal Transplantation
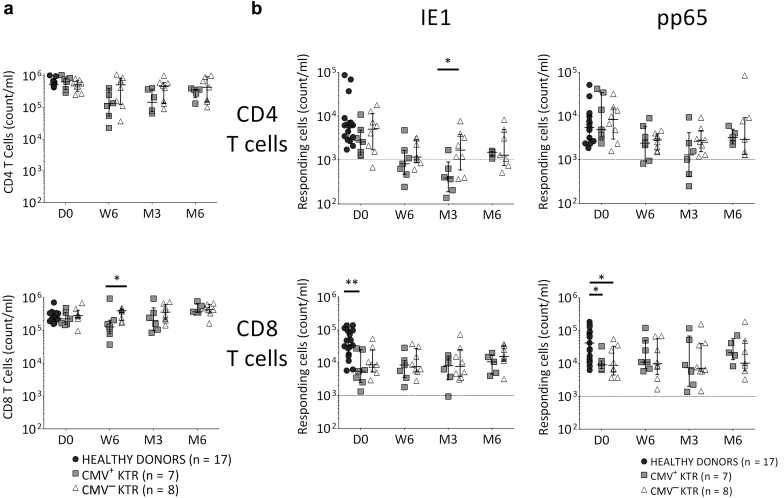
In pretransplantation, CD4 and CD8 T-cell total counts were similar in KTR groups compared with healthy donors and, on the sixth week after transplantation, CMV+ KTR presented CD8 lymphopenia compared with CMV− KTR (P = 0.04; Figure 2a). On D0, CMV-specific CD8 T cells were lower in KTR groups compared with healthy donors (IE-1: P = 0.004, pp65: P = 0.04; Figure 2b) and, on the third month after transplantation, a lower IE-1–specific CD4 T-cell count was found in the CMV+ KTR compared with the CMV− KTR group (P = 0.04; Figure 2b).
Figure 2.
Evolution of CD4 and CD8 T-cell counts and CMV-specific CD4 and CD8 T-cell counts in healthy donors and CMV-seropositive KTR, from transplant day to 6 months after transplantation. (a) On the transplant day, there were no differences in absolute cell numbers of CD4 and CD8 T cells between healthy donors and KTR groups. From the sixth week after transplantation, CMV+ KTR presented CD8 T-cell lymphopenia, compared with the CMV− KTR group. (b) On the transplant day, absolute cell numbers of CMV-specific CD8 T cells were lower in CMV-seropositive KTR, compared with healthy donors. From the third month after transplantation, CMV+ KTR presented lower absolute cell numbers of IE-1–specific CD4 T cells, compared with the CMV− KTR group. Median and 25th and 75th percentiles, Mann-Whitney or Kruskal-Wallis tests for comparison of 2 or 3 groups. *P < 0.05, **P < 0.005. The dotted line represents a strong CMV-specific response. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV infection/disease; CMV− KTR, CMV-seropositive kidney transplant recipients without CMV infection/disease; D0, transplantation day; IE-1, immediate early protein 1; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; pp65, phosphoprotein 65; W6, sixth week after kidney transplantation.
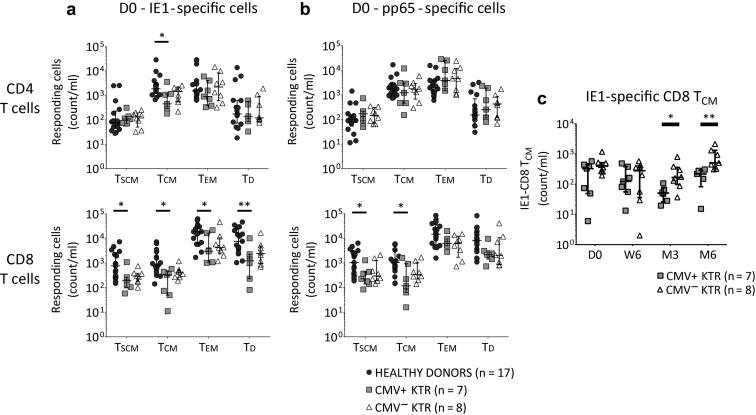
In pretransplantation, compared with healthy donors, CMV-specific TSCM and TCM were lower in CMV+ KTR (IE-1-CD4 and CD8 TCM, IE-1-CD8 TSCM, pp65-CD8 TSCM and TCM, all P < 0.05; Figure 3a and b). In healthy donors, compared with CMV+ KTR, IE-1–specific CD8 TEM and TD were also higher (P = 0.04 and P = 0.009, respectively; Figure 3a). Although the CMV-specific T-cell differentiation states were similar between the 2 KTR groups on the transplant day, on the third and sixth months after transplantation, IE-1–specific CD8 TCM counts were lower in CMV+ KTR (P = 0.01 and P = 0.003, respectively; Figure 3c).
Figure 3.
CMV-specific memory CD8 TSCM and TCM are lower in kidney transplant recipients with CMV infection/disease. (a) On the transplant day, absolute numbers of IE-1–specific CD4 TCM were lower in CMV+ KTR, compared with healthy donors. The distribution of IE-1–specific CD8 T-cell differentiation was more important in healthy donors, with an expansion of TSCM, TCM, TEM, and TD. (b) On the transplant day, without a difference in pp65-specific CD4 T-cell differentiation, absolute cell numbers of pp65-specific CD8 TSCM and TCM were lower in CMV+ KTR, compared with healthy donors. (c) On the third and sixth months after transplantation, CMV+ KTR presented a lower absolute cell numbers of IE-1–specific CD8 TCM compared with the CMV− KTR group. Median and 25th and 75th percentiles, Mann-Whitney or Kruskal-Wallis tests for comparison of 2 or 3 groups. *P < 0.05, **P < 0.005. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV infection/disease; CMV− KTR, CMV-seropositive kidney transplant recipients without CMV infection/disease; IE-1, immediate early protein 1; n, number; pp65, phosphoprotein 65; TCM, central-memory T cells; TD, terminally differentiated effector T cells; TEM, effector-memory T cells; TSCM, stem-cell memory T cells.
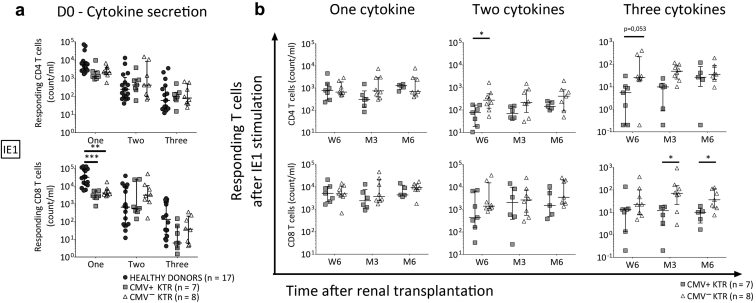
IE-1–Specific Lymphocyte Polyfunctionality Increased Early in KTR Without CMV Infection/Disease
Concerning the IE-1–specific lymphocyte polyfunctionality, in pretransplantation, IE-1–specific one-cytokine-secreting CD8 T cells were more frequent in healthy donors compared with KTR (P = 0.0001, P = 0.005, respectively; Figure 4a), without differences between the 2 KTR groups (Figure 4a). However, we observed, on the sixth week, higher double- and triple-cytokine-secretion counts of IE-1-CD4 T cells (P = 0.01, P = 0.05, respectively; Figure 4b) in CMV− KTR compared with CMV+ KTR. On the third and sixth months after transplant, triple-cytokine-secreting CD8 T-cell counts were higher in CMV− KTR (P = 0.04, P = 0.03, respectively; Figure 4b).
Figure 4.
IE-1–specific lymphocyte polyfunctionality is precociously more important in KTR without CMV infection/disease. An early increase of IE-1–specific lymphocyte polyfunctionality in kidney transplant recipients without CMV infection/disease was observed. (a) On the transplant day, the absolute number of IE-1–specific CD8 T cells producing 1 cytokine was higher in healthy donors, compared with KTR groups. No differences were found in T cells secreting double and triple cytokines on D0. (b) From the sixth week after transplantation, double-cytokine-producing CD4 T cells were higher in KTR without CMV infection/disease. On the third and sixth months after transplant, concomitant secretion of 3 cytokines by IE-1–specific CD8 T cells was higher in CMV− KTR. Median and 25th and 75th percentiles, Mann-Whitney or Kruskal-Wallis tests for comparison of 2 or 3 groups. *P < 0.05, **P < 0.005, ***P < 0.0005. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV infection/disease; CMV− KTR, CMV-seropositive kidney transplant recipients without CMV infection/disease; D0, transplantation day; IE-1, immediate early protein 1; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; W6, sixth week after kidney transplantation.
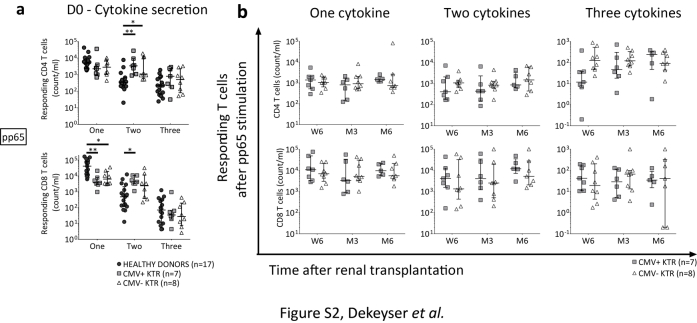
Concerning the pp65-specific lymphocyte polyfunctionality, there was no difference between KTR with or without CMV infection/disease during the follow-up period (Supplementary Figure S2b).
Discussion
Our study aimed to better understand the immune mechanisms of viral control in CMV-seropositive patients receiving kidney transplantation. Immunological memory is the key feature of the adaptive immune system to provide life-long protection against pathogens.11, 14 Here, CMV-specific memory T cells were numerically lower in patients awaiting kidney transplantation. In KTR with CMV infection/disease, we found a decrease of CMV-specific T-cell subsets with lower TSCM and TCM counts in pretransplantation, compared with healthy donors. A premature immunological aging in patients with end-stage renal failure was observed in previous studies, showing a selective loss of naive and TCM T cells.15, 16, 17, 18 In healthy aged donors, CMV-specific response may represent over 10% of the CD8 T-cell pool.19 Here, CMV-specific CD8 T cells mainly produced one cytokine in healthy donors. This relative monofunctionality in healthy donors could be explained by the expansion of IE-specific TEM and TD CD8 T cells. Interestingly, the higher TSCM and TCM counts in healthy donors can suggest increased self-renewal and multipotency capacities, providing a potential reservoir for T-cell memory.13 Lymphocyte phenotype and functionality are intrinsically linked and effector T cells may exercise effector functions in situations of viral reactivation.20 CMV-specific T-cell differentiation state was not an early predictive factor allowing identifying recipients at CMV infection/disease risk. Although immunosuppressive regimens were similar in our study, quantitative and qualitative changes have been described to occur in CMV-specific CD8 T cells in response to immunosuppressive treatment, with predominantly effector phenotype and cytotoxic capacities.21 In liver or lung transplantation, polyfunctional CMV-specific T cells have been found to be associated with a lower risk of CMV reactivation.22, 23, 24 Also, precedent studies found that higher frequencies of IE-1 but not pp65-specific CD8 T cells correlated with protection against CMV disease.25, 26, 27 Here, we found a higher IE-1–specific lymphocyte polyfunctionality in KTR without CMV infection/disease compared with those with CMV infection/disease, with a superior double-functionality of CD4 T cells at an early period, that is, 6 weeks after transplantation and a superior trifunctionality of CD8 T cells at 3 and 6 months after transplantation. Importantly, polyfunctionality of CMV-specific T cells in pretransplantation was not different between KTR with or without CMV infection/disease. Despite the small number of patients and a relatively limited lymphocyte polyfunctionality evaluation, our pilot study was prospective and assessed the CMV-specific cellular immunity in the absence of systematic prophylactic treatment. In conclusion, our study suggests that an early posttransplantation emergence of polyfunctional IE-1–specific T cells is associated with a decreased risk of CMV infection/disease, and therefore, the identification of such T cells might possibly be used as a predictive biomarker of viral replication control in the renal transplant setting. Further multicenter studies with higher numbers of patients are needed to confirm this hypothesis.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplemental Material and Methods.
Figure S1. Absolute number of T cells (CD4, CD8, CMV-specific CD4 and CD8 T cells) included in CMV-specific memory T-cell analyses during the study follow-up. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV-infection/disease; CMV- KTR, CMV-seropositive kidney transplant recipients without CMV-infection/disease; D0, day of transplantation; IE-1, Immediate Early protein 1; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; pp65: Phosphoprotein 65; W6, sixth week after kidney transplantation. Median and 25th and 75th percentiles. The dotted line represents a strong CMV-specific response.
Figure S2. Evaluation of pp65-specific T-cell polyfunctionality in KTR with or without CMV infection/disease, from the transplant day to 6 months. On the transplant day, the absolute number of pp65-specific T cells producing one cytokine was higher in healthy donors, while double-cytokine secreting pp65-specific T-cells were higher in KTR group. There were no differences between KTR with or without CMV-infection/disease during the follow-up period. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV-infection/disease; CMV- KTR, CMV-seropositive kidney transplant recipients without CMV-infection/disease; D0, transplantation day; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; pp65, Phosphoprotein 65; W6, sixth week after kidney transplantation. Median and 25th and 75th percentiles, Mann-Whithney or Kruskal-Wallis tests for comparison of 2 or 3 groups. *P<0.05, **P<0.005, ***P<0.0005.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
Absolute number of T cells (CD4, CD8, CMV-specific CD4 and CD8 T cells) included in CMV-specific memory T-cell analyses during the study follow-up. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV-infection/disease; CMV- KTR, CMV-seropositive kidney transplant recipients without CMV-infection/disease; D0, day of transplantation; IE-1, Immediate Early protein 1; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; pp65: Phosphoprotein 65; W6, sixth week after kidney transplantation. Median and 25th and 75th percentiles. The dotted line represents a strong CMV-specific response.
Figure S2.
Evaluation of pp65-specific T-cell polyfunctionality in KTR with or without CMV infection/disease, from the transplant day to 6 months. On the transplant day, the absolute number of pp65-specific T cells producing one cytokine was higher in healthy donors, while double-cytokine secreting pp65-specific T-cells were higher in KTR group. There were no differences between KTR with or without CMV-infection/disease during the follow-up period. CMV+ KTR, CMV-seropositive kidney transplant recipients with CMV-infection/disease; CMV- KTR, CMV-seropositive kidney transplant recipients without CMV-infection/disease; D0, transplantation day; M3, third month after kidney transplantation; M6, sixth month after kidney transplantation; n, number; pp65, Phosphoprotein 65; W6, sixth week after kidney transplantation. Median and 25th and 75th percentiles, Mann-Whithney or Kruskal-Wallis tests for comparison of 2 or 3 groups. *P<0.05, **P<0.005, ***P<0.0005.
References
- 1.Harari A., Dutoit V., Cellerai C. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 2.Fishman J.A. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 3.Kotton C.N., Kumar D., Caliendo A.M. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 4.Kotton C.N., Kumar D., Caliendo A.M. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 5.Lúcia M., Crespo E., Cruzado J.M. Human CMV-specific T-cell responses in kidney transplantation; toward changing current risk-stratification paradigm. Transpl Int. 2014;27:643–656. doi: 10.1111/tri.12318. [DOI] [PubMed] [Google Scholar]
- 6.Rittà M., Costa C., Sidoti F. Pre-transplant assessment of CMV-specific immune response by Elispot assay in kidney transplant recipients. New Microbiol. 2015;38:329–335. [PubMed] [Google Scholar]
- 7.Costa C., Balloco C., Sidoti F. Evaluation of CMV-specific cellular immune response by EliSPOT assay in kidney transplant patients. J Clin Virol. 2014;61:523–528. doi: 10.1016/j.jcv.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Egli A., Humar A., Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55:1678–1689. doi: 10.1093/cid/cis818. [DOI] [PubMed] [Google Scholar]
- 9.Sester M., Leboeuf C., Schmidt T. The “ABC” of Virus-Specific T Cell Immunity in Solid Organ Transplantation. Am J Transplant. 2016;16:1697–1706. doi: 10.1111/ajt.13684. [DOI] [PubMed] [Google Scholar]
- 10.Humar A., Michaels M., AST ID Working Group on Infectious Disease Monitoring American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 11.Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L., Lugli E., Ji Y. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lugli E., Dominguez M.H., Gattinoni L. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Berg P.J.E.J., van Stijn A., Ten Berge I.J.M. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J Clin Virol. 2008;41:213–217. doi: 10.1016/j.jcv.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Betjes M.G.H., Huisman M., Weimar W. Expansion of cytolytic CD4+CD28- T cells in end-stage renal disease. Kidney Int. 2008;74:760–767. doi: 10.1038/ki.2008.301. [DOI] [PubMed] [Google Scholar]
- 16.Betjes M.G.H., Langerak A.W., van der Spek A. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80:208–217. doi: 10.1038/ki.2011.110. [DOI] [PubMed] [Google Scholar]
- 17.Litjens N.H.R., van Druningen C.J., Betjes M.G.H. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol. 2006;118:83–91. doi: 10.1016/j.clim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Betjes M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 19.Khan N., Hislop A., Gudgeon N. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen E.M., Gamadia L.E., Baars P.A. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 21.Gamadia L.E., Rentenaar R.J., Baars P.A. Differentiation of cytomegalovirus-specific CD8(+) T cells in healthy and immunosuppressed virus carriers. Blood. 2001;98:754–761. doi: 10.1182/blood.v98.3.754. [DOI] [PubMed] [Google Scholar]
- 22.Nebbia G., Mattes F.M., Smith C. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant. 2008;8:2590–2599. doi: 10.1111/j.1600-6143.2008.02425.x. [DOI] [PubMed] [Google Scholar]
- 23.Snyder L.D., Medinas R., Chan C. Polyfunctional cytomegalovirus-specific immunity in lung transplant recipients receiving valganciclovir prophylaxis. Am J Transplant. 2011;11:553–560. doi: 10.1111/j.1600-6143.2010.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder L.D., Chan C., Kwon D. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016;193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunde T., Kirchner A., Hoffmeister B. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel P., Bold G., Presber F. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol. 2009;20:238–242. doi: 10.1016/j.trim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Bestard O., Lucia M., Crespo E. Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant. 2013;13:1793–1805. doi: 10.1111/ajt.12256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.