ABSTRACT
Background: Long non-coding RNA MALAT1 (Metastasis-associated lung Adenocarcinoma transcript-1) has been demonstrated to play a critical role in the regulation of cancer progression and metastasis. However, little is known about MALAT1 in nasopharyngeal carcinoma (NPC) pathogenesis and progression.
Methods: Quantitative real-time PCR (qRT-PCR) was conducted to measure the expression of MALAT1, miR-124 and Capn4 mRNA in NPC cell lines. The protein level of Capn4 was examined by western blot analysis. Cell proliferation was detected by MTT assay, trypan blue exclusion method and colony formation analysis. Cell invasion was determined by transwell chamber assay. Expression of EMT-related proteins was detected by western blot. The potential targets of MALAT1 and miR-124 were verified by target prediction and luciferase reporter assay.
Results: MALAT1 and Capn4 were upregulated while miR-124 expression was downregulated in NPC cell lines. MALAT1 knockdown inhibited proliferation, invasion and EMT of NPC cells. Moreover, MALAT1 improved Capn4 expression by sponging miR-124. MALAT1 upregulation abated miR-124-induced repression on NPC cell proliferation, invasion and EMT. Furthermore, Capn4 overexpression reversed the inhibitory effect of MALAT1 silencing on proliferation, invasion and EMT of NPC cells.
Conclusion: MALAT1 promoted proliferation, invasion and EMT of NPC cells through de-repressing Capn4 by sponging miR-124. The present study revealed a novel MALAT1/miR-124/Capn4 regulatory axis in NPC, contributing to a better understanding of the NPC pathogenesis and providing a promising therapeutic target for NPC therapy.
KEYWORDS: MALAT1, miR-124, Capn4, proliferation, invasion, NPC
Introduction
Nasopharyngeal carcinoma (NPC) is a highly metastatic and invasive malignant tumor that derived from epithelial cells lining on the nasopharynx.1 The long-term survival of NPC patients has improved a lot with the advance of diagnosis and treatment, but for locally advanced and metastatic NPC, the outcome remains unsatisfactory due to local relapse and distant metastasis.2 During tumorigenesis and progression, multiple genetic and epigenetic abnormalities synergistically disorder normal cell function, thus leading to NPC pathogenesis.3 However, the precise mechanism responsible for NPC progression remains not completely understood. Therefore, there is an urgent need to identify novel molecules involved in progression of NPC to provide biomarkers for prognosis and therapeutic targets for treatment.
Long non-coding RNAs (lncRNAs) are non-coding transcripts with over 200 nucleotides in length, most of which lack protein-coding capability. LncRNAs are actively implicated in regulation of gene expression, alternative splicing, histone modification and genes chromatin rearrangement.4 Importantly, accumulating evidence suggests that lncRNAs are involved in tumorigenesis and regulate various cancer-related events, such as proliferation, apoptosis, invasion and epithelial-mesenchymal transition (EMT).5,6 Metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) is a highly conserved lncRNA derived from chromosome 11q13 with more than 8000 nucleotides. MALAT1 has been demonstrated to be extremely abundant in many cancers, including bladder cancer.7, breast cancer8 and gastric cancer.9 Jin et al. 10 found that MALAT1 was significantly up-regulated in NPC tissues and cells. However, the underlying mechanism MALAT1 involved in NPC development is still unclear.
miR-124 generally acts as a tumor suppressor in several cancers, such as gastric cancer 11 and hepatocellular carcinoma.12 Moreover, miR-124 expression was down-regulated in NPC and overexpression of miR-124 inhibited proliferation, migration and invasion of NPC cells.13,14 However, how miR-124 expression was regulated in NPC should be further investigated.
In the present study, we attempted to explore the role of MALAT1 in NPC progression and its possible molecular basis.
Materials and methods
Cell culture
Human nasal epithelial cell line (HNEpC) and NPC cell lines (5-8F, CNE-2, C666-1 and HONE-1) were obtained from the American Type Culture Collection (ATCC) and cultured at 37°C in humidified 5% CO2. All cells were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from HNEpC or NPC cell lines using Trizol (Invitrogen). A high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) was used to generate the first-strand cDNA of MALAT1 and Capn4. cDNA of miR-124 was synthesized by using TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems). For MALAT1 and Capn4, qRT-PCR reaction was conducted using TaqMan gene expression assays (Applied Biosystems) with β-actin as an endogenous control. For miR-124, qRT-PCR procedure was performed by TaqMan microRNA assays (Applied Biosystems) with U6 snRNA as an internal reference. The relative level of RNAs was analyzed using the 2−ΔΔCt method.
Western blot analysis
Western blot analysis was performed as previously described.15 Antibody against Capn4 was obtained from LifeSpan Biosciences (Seattle, WA, USA). Antibodies including anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-Cyclin B1, anti-Cyclin D1, anti-Cyclin E, anti-Cyclin A, anti-p-CDC2 and anti-β-actin were purchased from Santa Cruz Biotechnology (SantaCruz, CA, USA). β-actin was used as an endogenous control. The protein bands were detected by an enhanced chemiluminescence substrate kit (Pierce Biotechnology, Rockford, IL, USA).
Cell transfection
MALAT1-overexpressing vector (pcDNA-MALAT1), Capn4-overexpressing vector (pcDNA-Capn4 vector), and their corresponding controls were obtained from Invitrogen. miR-124 mimics (miR-124) and scrambled mimic control (miR-NC) were synthesized by GenePharma (Shanghai, China). siRNAs specifically targeting MALAT1 (si-MALAT1) and control non-specific duplex siRNA (si-NC) were purchased from Dharmacon (Lafayette, CO, USA). Cell transfection was performed using Lipofectamine 2000 (Invitrogen).
Cell proliferation assay
5–8F and HONE-1 cells seeded into the 96-well plate were cultured for 24 h, and then different transfection treatments were carried out. Cell viability at indicated time points (24 h, 48 h and 72 h) was analyzed using MTT assay (Sigma, St. Louis, Missouri, USA). The absorbance at 450 nm was then determined using a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
Trypan blue cell viability assay
Transfected 5–8F and HONE-1 cells were inoculated into 24-well plates at a density of 5 × 105/well. At indicated time points (24 h, 48 h and 72 h), harvested cell suspension was mixed with the same volume of 0.4% trypan blue, followed by counting living cells and dead cells using a hemocytometer under a microscope. Cell viability was defined as the percentage of living cells to total number of cells.
Colony formation assay
Transfected 5–8F and HONE-1 cells were plated at a density of 500 cells/6 cm dish. After incubation for 14 days, cells were stained with 1% crystal violet. The number of colonies containing more than 50 cells was manually counted.
Cell invasion assay
Transwell chamber assay (Bection Dickinson, Bedford, MA, USA) was performed to determine the invasion ability of 5–8F and HONE-1 cells. Cells cultured in serum-free media was placed into the upper chamber with 10 μg/ml Matrigel and the cell culture media containing 10% FBS was added into the lower chamber. After 48 h, the invaded cells in the lower membrane were fixed in 100% methanol for 10 min, air-dried, stained in 0.1% crystal violet, and counted under a microscope (Olympus, Japan).
Luciferase reporter assays
To investigate the relationship between miR-124 and MALAT1, 5–8F and HONE-1 cells were co-transfected with miR-NC or miR-124 and luciferase reporter comprising the wild type or mutant MALAT1 fragment (MALAT1-WT or MALAT1-MUT). To explore the relationship among miR-124, MALAT1 and Capn4, 5–8F and HONE-1 cells were co-transfected with miR-NC, miR-124, miR-124 + pcDNA-control, or miR-124 + pcDNA-MALAT1 and wild type or mutant 3′ UTR (3′ UTR-WT or 3′ UTR-MUT) of Capn4. The luciferase activities were measured with the Dual-Luciferase Reporter Assay System (Promega, Madison WI, USA) 48 h post transfection.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA). All data were shown as means ± SD. The results were analyzed using Student's t-test or one-way ANOVA. Differences were considered to be significant when P < 0.05.
Results
MALAT1 and Capn4 expressions are upregulated, and miR-124 expression is downregulated in NPC cell lines
The expression of MALAT1, miR-124 and Capn4 mRNA was detected by qRT-PCR, and Capn4 protein level was measured using western blot in HNEpC or NPC cell lines (5-8F, CNE-2, C666-1 and HONE-1). MALAT1 expression (Fig. 1A), Capn4 expression at mRNA (Fig. 1C) and protein (Fig. 1D) was apparently increased in NPC cell lines compared with HNEpC. Conversely, miR-124 expression was extremely lowered in NPC cell lineswhen compared to HNEpC cells (Fig. 1B). These results suggested that aberrant expression of MALAT1, miR-124 and Capn4 may be involved in the pathogenesis of NPC.
Figure 1.
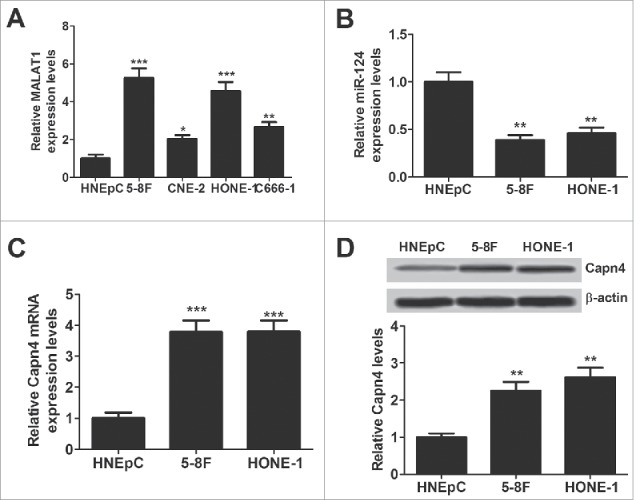
Expression of MALAT1, miR-124 and Capn4 in normal human nasal epithelial cell line (HNEpC) and NPC cell lines (5-8F, CNE-2, C666-1 and HONE-1). qRT-PCR analysis was performed to detect expression of MALAT1 (A), miR-124 (B) and CAPN4 mRNA (C) in HNEpC and NPC cells. (D) The protein level of Capn4 was detected in HNEpC, 5–8F and HONE-1 cells by western blot analysis. *P < 0.05, **P < 0.01, ***P < 0.001 vs. HNEpC.
MALAT1 knockdown inhibits proliferation, invasion and EMT of NPC cells
To explore the role of MALAT1 in NPC, 5–8F and HONE-1 cells were transfected with si-control or si-MALAT1. To explore the effect of MALAT1 on the proliferation of NPC cells, MTT assay, trypan blue exclusion method and colony formation analysis was performed. MTT results showed that knockdown of MALAT1 significantly suppressed cell growth of 5–8F (Fig. 2A) and HONE-1 cells (Fig. 2B) compared with the control groups. Trypan blue staining assay displayed that MALAT1 deficiency dramatically reduced cell viability in 5–8F (Fig. 2C) and HONE-1 cells (Fig. 2D). Moreover, the colony numbers of 5–8F (Fig. 2E) and HONE-1 cells (Fig. 2F) were significantly decreased following MALAT1 suppression. To examine the effect of MALAT1 on the invasion ability of NPC cells, transwell chamber assay was performed at 48 h after transfection. Compared with the control groups, transfection of si-MALAT1 significantly inhibited cell invasion in 5–8F (Fig. 2G) and HONE-1 cells (Fig. 2H).
Figure 2.
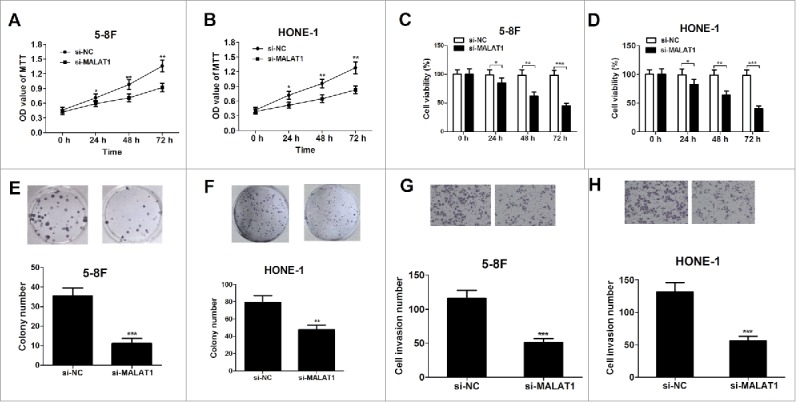
Knockdown of MALAT1 inhibits proliferation and invasion of NPC cell lines. 5–8F and HONE-1 cells were transfected with si-control or si-MALAT1. (A and B) MTT assay was performed to detect cell viability at 24, 48 and 72 h after transfection. (C and D) Trypan blue staining method was applied to determine cell viability at 24, 48 and 72 h after transfection. (E and F) The colony numbers of cell were determined by colony formation assay on day 14 after transfection. (G and H) Cell invasion capability was detected by transwell chamber assay at 48 h after transfection. *P < 0.05, **P < 0.01, ***P < 0.001 vs. si-NC.
To further investigate whether MALAT1 knockdown could influence the EMT process in NPC cells, western blot was conducted to examine expression of EMT-related proteins E-cadherin, N-cadherin and vimentin. The level of E-cadherin was increased and the expression of N-cadherin and vimentin was reduced in si-MALAT1 transfected 5–8F (Fig. 3A) and HONE-1 cells (Fig. 3B). The protein levels of cell cycle modulators (Cyclin A, Cyclin E, Cyclin B1, Cyclin D1, and p-CDC2) were further detected. The results of western blot analysis indicated that knockdown of MALAT1 remarkably decreased the expression of these proteins in 5–8F (Fig. 3C) and HONE-1 cells (Fig. 3D). These data suggested that MALAT1 knockdown suppressed proliferation, invasion and EMT of NPC cells.
Figure 3.
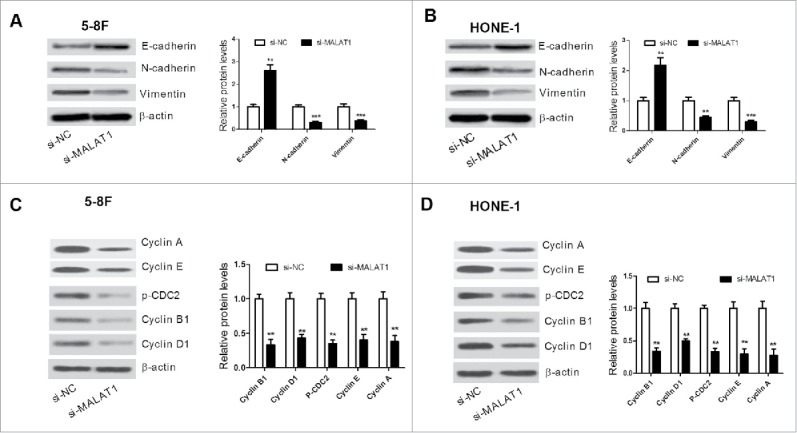
(A and B) The level of EMT-related proteins E-cadherin, N-cadherin and vimentin was detected by western blot in 5–8F and HONE-1 cells. (C and D) Western blot analysis was performed to measure the protein level of Cyclin A, Cyclin E, Cyclin B1, Cyclin D1, and p-CDC2 in 5–8F and HONE-1 cells. **P < 0.01, ***P < 0.001 vs. si-NC.
MALAT1 inhibits miR-124 expression in NPC cells
Recent studies have suggested that lncRNAs contain motifs with sequence complementary to miRNAs and weaken miRNAs expression and activity. Online bioinformatic tool starBase v2.0 was used to predict the potential miRNA for MALAT1. The results showed that MALAT1 contains two target binding site of miR-124 (Fig. 4A). Then, to confirm the interaction between miR-124 and MALAT1, dual luciferase reporter assay was performed. Results demonstrated that miR-124 overexpression significantly decreased the luciferase activity of the wild-type reporter (MALAT1-WT), but not the mutant reporter (MALAT1-MUT) compared with miR-control in 5–8F and HONE-1 cells (Fig. 4B). In order to further explore the actual influence of MALAT1 on miR-124 expression in NPC cells, qRT-PCR was performed to detect miR-124 expression in si-MALAT1- or pcDNA-MALAT1-transfected 5–8F and HONE-1 cells. As presented in Fig. 4C, miR-124 expression was obviously improved in NPC cells after introduction with si-MALAT1, while miR-124 expression was evidently diminished following pcDNA-MALAT1 transfection. All these data suggested that MALAT1 repressed miR-124 expression in NPC cells.
Figure 4.
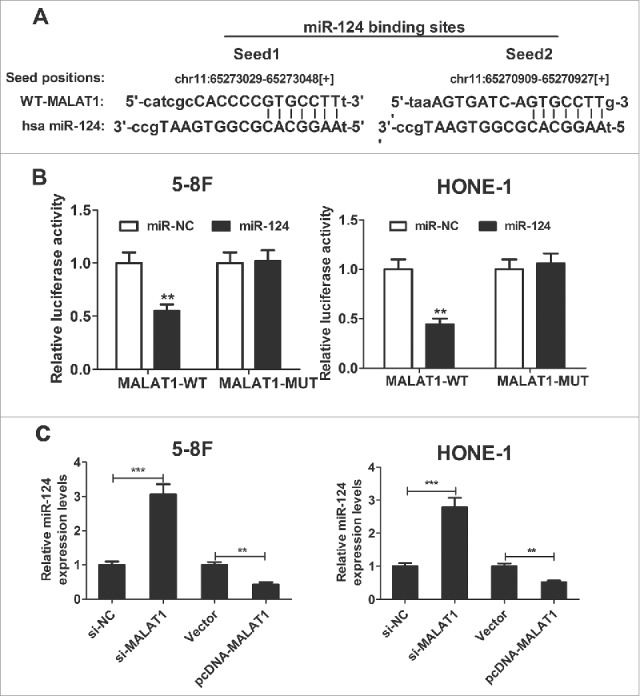
MALAT1 represses miR-124 expression in NPC cells. (A) Putative miR-124 binding sequence of MALAT1 is shown. (B) The relative luciferase activity was detected in 5–8F and HONE-1 cells co-transfected with MALAT1-WT or MALAT1-MUT reporter and miR-124 mimic or miR-NC. (C) qRT-PCR was performed to examine miR-124 expression in si-MALAT1- or pcDNA-MALAT1-transfected 5–8F and HONE-1 cells. **P < 0.01, ***P < 0.001 vs. controls.
MALAT1 overexpression reverses the inhibitory effect of miR-124 on proliferation, invasion and EMT of NPC cells
Considering the inhibitory effect of MALAT1 on miR-124, we tested whether MALAT1 exerted its function in NPC by regulating miR-124 expression. 5–8F and HONE-1 cells were transfected with miR-124, or co-transfected with miR-124 and pcDNA-MALAT1. MTT assay revealed that miR-124 evidently blocked 5–8F (Fig. 5A) and HONE-1 (Fig. 5B) cell proliferation, while restoring expression of MALAT1 attenuated this effect. Additionally, transwell chamber assay illuminated that miR-124 overexpression lead to an obvious decrease in invasive ability of 5–8F (Fig. 5C) and HONE-1 (Fig. 5D) cell, whereas, this effect was significantly reversed after co-transfection with pcDNA-MALAT1. Moreover, ectopic expression of miR-124 prominently promoted E-cadherin level and suppressed N-cadherin and vimentin expression in 5–8F (Fig. 5E) and HONE-1 (Fig. 5F) cells, which was substantially abated by MALAT1 overexpression. All these data suggested that MALAT1 promoted proliferation, invasion and EMT of NPC cells through inhibiting miR-124 expression.
Figure 5.
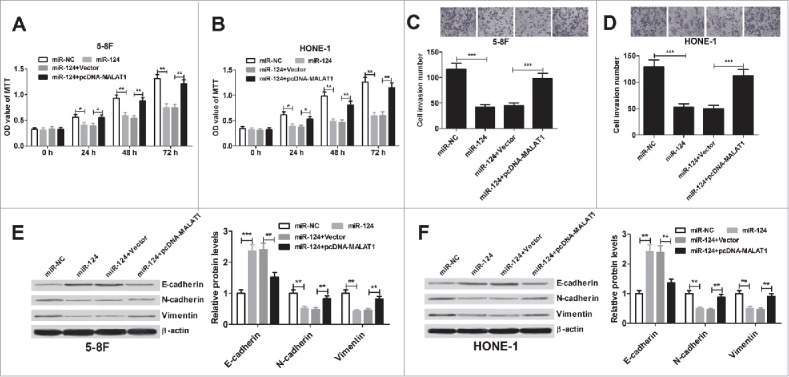
MALAT1 promotes proliferation, invasion and EMT of NPC cells by sponging miR-124. 5–8F and HONE-1 cells were introduced with miR-124 alone, or in combined with pcDNA-MALAT1. (A and B) MTT assay was performed to detect cell viability at 24, 48 and 72 h after transfection. (C and D) Transwell chamber assay was conducted to determine cell invasion at 48 h after transfection. (E and F) Expression of E-cadherin, N-cadherin and vimentin was measured by western blot analysis in 5–8F and HONE-1 cells 48 h post-transfection. **P < 0.01, ***P < 0.001 vs. controls.
MALAT1 upregulates Capn4 expression, a target of miR-124
It is well-known that lncRNAs could function as competing endogenous RNAs (ceRNAs) to sponge miRNAs, thus liberating the inhibited targets of these miRNAs. First, web-based software TargetScan was used to predict miR-124 target genes. The results revealed that 3′ UTR of Capn4 contained the binding sequence of miR-124 (Fig. 6A). Then dual luciferase reporter assay was performed to confirm whether Capn4 is the direct target of miR-124. Compared with miR-NC group, miR-124 significantly blocked the luciferase activity of Capn4-WT reporter in 5–8F (Fig. 6B) and HONE-1 (Fig. 6C) cells, however, MALAT1 overexpression led to a restoration of luciferase activity (Fig. 6B and C). Additionally, no significant changes were observed in luciferase activity of Capn4-MUT reporter in 5–8F and HONE-1 cells after any treatment. To further indentify the effect of miR-124 and MALAT1 on Capn4 expression, the protein level of Capn4 was detected by western blot in 5–8F and HONE-1 cells transfected with si-MALAT1, pcDNA-MALAT1, miR-124 or co-transfected with miR-124 and pcDNA-MALAT1. Compared with the controls, MALAT1 knockdown or miR-124 overexpression resulted in significant reduction of Capn4 expression, while MALAT1 overexpression markedly improved Capn4 level (Fig. 6D and E). Moreover, re-introduction of pcDNA-MALAT1 could partly restore the inhibited Capn4 expression caused by miR-124 (Fig. 6D and E). Overall, these data suggested that MALAT1 acted as an endogenous sponge by binding to miR-124, thus abolishing miR-124-induced suppression on Capn4.
Figure 6.
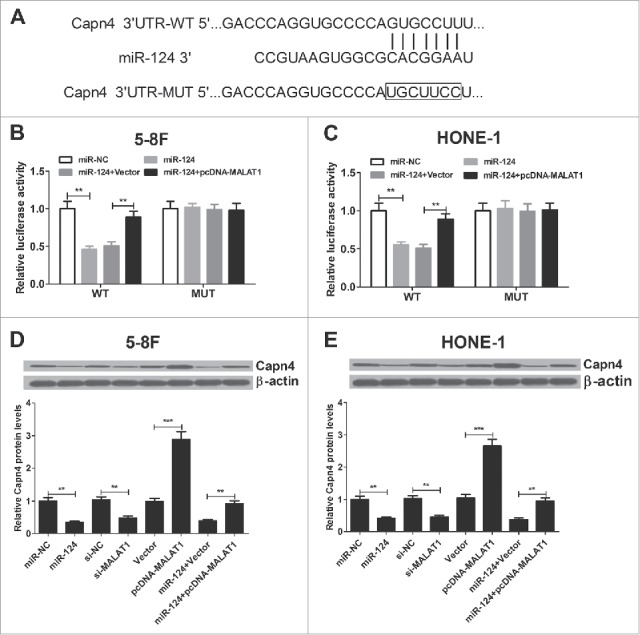
MALAT1 promotes Capn4 expression by sponging miR-124 in 5–8F and HONE-1 cells. (A) 3′ UTR binding sites of Capn4 within miR-124 are shown. (B and C) The relative luciferase activity was measured in 5–8F and HONE-1 cells co-transfected with Capn4-WT or Capn4-Mut reporter and miR-124 mimic or miR-124 mimic + pcDNA-MALAT1. (D and E) Western blot was performed to detect the Capn4 expression in 5–8F and HONE-1 cells transfected with si-MALAT1, pcDNA-MALAT1, miR-124 or co-transfected with miR-124 and pcDNA-MALAT1. **P < 0.01, ***P < 0.001 vs. controls.
Capn4 overexpression abrogates the effects of MALAT1 knockdown on proliferation, invasion and EMT of NPC cells
To further confirm the underlying mechanism of MALAT1 in NPC cell proliferation, invasion and EMT, 5–8F and HONE-1 cells were transfected with si-MALAT1 or in combined with pcDNA-Capn4. MTT assay revealed that Capn4 overexpression abated the inhibitory effect of MALAT1 knockdown on 5–8F (Fig. 7A) and HONE-1 (Fig. 7B) cell proliferation. Transwell chamber assay demonstrated that Capn4 upregulation abolished si-MALAT1-induced repression on invasion of 5–8F (Fig. 7C) and HONE-1 (Fig. 7D) cells. Moreover, regaining of Capn4 expression overturned the elevated E-cadherin level and declined N-cadherin and vimentin expression triggered by MALAT1 silencing in 5–8F (Fig. 7E) and HONE-1 (Fig. 7F) cells. All these data indicated that MALAT1 induced proliferation, invasion and EMT of NPC cells through de-repressing Capn4 via sponging miR-124.
Figure 7.
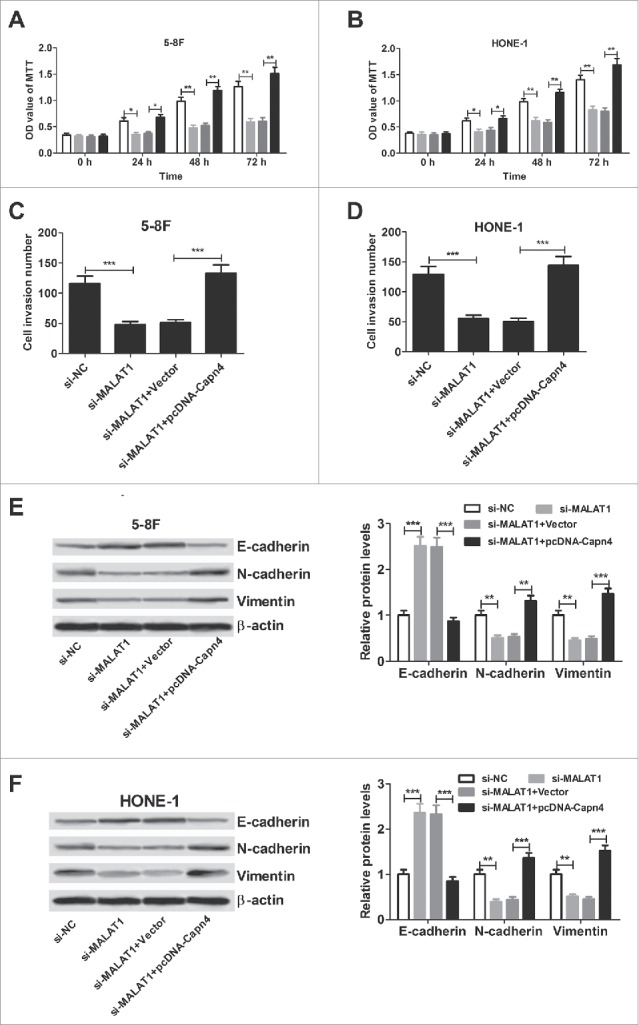
Capn4 overexpression abrogates the inhibitory effect of MALAT1 knockdown on proliferation, invasion and EMT of NPC cells. 5–8F and HONE-1 cells were introduced with si-MALAT1 or co-transfected with si-MALAT1 and pcDNA-Capn4. (A and B) MTT assay of viability in 5–8F and HONE-1 cells at 24, 48 and 72 h post-transfection. (C and D) Transwell chamber assay of invasive ability in 5–8F and HONE-1 cells 48 h after transfection. (E and F) Western blot analysis of E-cadherin, N-cadherin and vimentin in 5–8F and HONE-1 cells 48 h after transfection. *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls.
Discussion
Recently, increasing lncRNAs have been identified as tumor suppressor or oncogene involved in prevalent cancer types. Among these lncRNAs, MALAT1 is generally highly expressed in several cancers and functions as an oncogene.16 For instance, high MALAT1 expression resulted in increased growth and invasion of HR-HPV-positive cervical cancer cells.17 Upregulated MALAT1 promoted cell proliferation, migration, and invasion of colorectal cancer cells through PRKA kinase anchor protein 9.18 In non-small cell lung cancer, MALAT1 facilitated brain metastasis of cancer cells through inducing epithelial-mesenchymal transition.19 In the present study, we also confirmed that MALAT1 expression was upregulated in NPC cell lines. MALAT1 knockdown repressed cell proliferation, invasion and EMT in NPC. In consistence with our findings, Hua et al. 20 revealed that MALAT1 silencing suppressed NPC cellular proliferation, migration and invasion. However, the mechanism by which MALAT1 promoted NPC progression should be further studied.
In recent studies, lncRNAs are proposed to act as ceRNAs to insulate miRNAs from target mRNAs, or derepress target mRNAs expression through competitively binding to miRNAs.21 In this study, we provided further evidence of lncRNA as a ceRNA, involving mRNA post-transcriptional regulation in the pathogenesis of NPC. Based on the fact that MALAT1 RNA contains two target sites of miR-124 (Fig. 3A) and that the expression levels of MALAT1 and miR-124 were inverse in NPC cells (Fig. 1A and B), we suppose that MALAT1 might serve as a miRNA sponge to interact with miR-124. In support of this notion, the interaction of miR-124 and MALAT1 was validated by luciferase reporter assays. Moreover, MALAT1 knockdown increased miR-124 expression while MALAT1 upregulation inhibited miR-124 expression. Furthermore, MALAT1 overexpression reversed the inhibitory effect of miR-124 on NPC cell proliferation, invasion and EMT. Taken together, these data suggested that MALAT1 contributed to NPC progression by functioning as an endogenous sponge, suppressing miR-124 expression. Previous studies also indicated the carcinogenesis of MALAT1 as different miRNA sponges in diverse cancers, such as miR-200c in endometrioid endometrial carcinoma, miR-22 in melanoma and miR-206 in gallbladder cancer.22–24s
The calpains are a family of calcium-dependent neutral cysteine proteases.25 Calpain small subunit 1 (Capn4) is a small regulatory subunit of the family and plays an essential role in maintaining calpain stability and activity.26 Capn4 was documented to be as a cancer-causing gene in various tumors. For example, Capn4 downregulation mediated by miR-124 inhibited the invasion and migration of glioma cells.27 Capn4 enhanced the invasion ability of non-small cell lung cancer cells by upregulating the expression of matrix metalloproteinase 2.28 Moreover, Capn4 was highly expressed in NPC cell lines and Capn4 knockdown suppressed cell migration and invasion in vitro and in vivo.29 However, how Capn4 was regulated in NPC is unknown. In the present study, Canp4 was verified to be a target of miR-124 by dual luciferase reporter analysis and western blot analysis. MALAT1 could counteract the suppressive effect of miR-124 on Canp4, and MALAT1 knockdown reduced Canp4 expression in NPC cells. These data suggested that MALAT1 modulated the de-repression of Canp4 by sponging miR-124. Furthermore, the inhibitory effect of MALAT1 knockdown on NPC cell proliferation, invasion and EMT was abrogated by Canp4 overexpression. Taken together, MALAT1 could promote proliferation, invasion and EMT of NPC cells through de-repressing Canp4 by sponging miR-124. Therefore, the findings provide a new MALAT1/miR-124/Canp4 axis for understanding the pathogenesis of NPC. A previous report showed that MALAT1 regulated cancer stem cell activity and radioresistance by modulating miR-1/slug axis in NPC.10 In clear cell kidney carcinoma, MALAT1 promoted cancer cell proliferation and metastasis by sequestering miR-200s and releasing ZEB2.30 Moreover, MALAT1 promoted melanoma cell proliferation, invasion and migration through the de-repression of MMP14 and Snail by sponging miR-22.23 All these studies suggested that MALAT1 could function as a miRNA sponge to release the miRNA targets in cancers.
In summary, our study indicated that MALAT1 promoted proliferation, invasion and EMT of NPC cells through de-repressing Capn4 by sponging miR-124. The present study elucidated a novel MALAT1/miR-124/Capn4 regulatory axis in NPC, contributing to a better understanding of the pathogenesis of NPC and providing a promising therapeutic target for NPC patients. However, MALAT1 may modulate multiple miRNAs and one miRNA could control various target genes, thus we cannot preclude the possibility of functional contributions by other MALAT1 or miR-124 targets. Therefore, further efforts are needed to elucidate the function and mechanism of MALAT1 in the progression of NPC.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgments
Not applicable
Authors' contributions
Yandan Wang designed and performed the experiment. Fengfang Yin analyzed the data. Baoyuan Shi supervised the study and wrote the manuscript
References
- 1.Lee KT, Tan JK, Lam AK, Gan SY. MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: A critical review. Crit Rev Oncol Hematol. 2016;103:1-9. doi: 10.1016/j.critrevonc.2016.04.006. PMID:27179594 [DOI] [PubMed] [Google Scholar]
- 2.Chan KC, Chan LS, Ip JCY, Lo C, Yip TTC, Ngan RKC, Wong RNS, Lo KW, Ng WT, Lee AWM. Therapeutic targeting of CBP/[bgr]-catenin signaling reduces cancer stem-like population and synergistically suppresses growth of EBV-positive nasopharyngeal carcinoma cells with cisplatin. Sci Rep. 2015;5:9979. doi: 10.1038/srep09979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou J, Lin YC, Kim J, You L, Xu Z, He B, Jablons DM. Nasopharyngeal carcinoma-review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30:946-63. doi: 10.1002/hed.20833. PMID:18446839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vikram R, Ramachandran R, Abdul KSM. Functional significance of long non-coding RNAs in breast cancer. Breast Cancer. 2014;21:515-21. doi: 10.1007/s12282-014-0554-y. PMID:25038622 [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Zhang L, Zhang C. Long noncoding RNAs and tumorigenesis: genetic associations, molecular mechanisms, and therapeutic strategies. Tumor Biol. 2016;37:163-75. doi: 10.1007/s13277-015-4445-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097-109. doi: 10.1016/j.bbagrm.2014.08.012. PMID:25159663 [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531-41. doi: 10.1158/1078-0432.CCR-13-1455. PMID:24449823 [DOI] [PubMed] [Google Scholar]
- 8.Huang N, Chi Y, Xue J, Liu M, Huang S, Mo M, Zhou S, Wu J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) interacts with estrogen receptor and predicted poor survival in breast cancer. Oncotarget. 2016; 7:37957-65. doi: 10.18632/oncotarget.9364. PMID:27191888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014; 35:2731-9. doi: 10.1093/carcin/bgu200. PMID:25280565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin C, Yan B, Lu Q, Lin Y, Ma L. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumor Biol. 2016;37:4025-33. doi: 10.1007/s13277-015-4227-z. [DOI] [PubMed] [Google Scholar]
- 11.Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol. 2012;227:470-80. doi: 10.1002/path.4030. PMID:22450659 [DOI] [PubMed] [Google Scholar]
- 12.Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010; 31:766-76. doi: 10.1093/carcin/bgp250. PMID:19843643 [DOI] [PubMed] [Google Scholar]
- 13.Peng XH, Huang HR, Lu J, Liu X, Zhao FP, Zhang B, Lin SX, Wang L, Chen HH, Xu X. MiR-124 suppresses tumor growth and metastasis by targeting Foxq1 in nasopharyngeal carcinoma. Mol Cancer. 2014;13:1. doi: 10.1186/1476-4598-13-186. PMID:24387052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu S, Zhao N, Hui L, Song M, Miao ZW, Jiang XJ. MicroRNA-124-3p inhibits the growth and metastasis of nasopharyngeal carcinoma cells by targeting STAT3. Oncol Rep. 2016;35:1385-94. doi: 10.3892/or.2015.4524doi: 10.3892/or.2015.4524. PMID:26707908 [DOI] [PubMed] [Google Scholar]
- 15.Lluri G, Langlois GD, Soloway PD, Jaworski DM. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates myogenesis and β1 integrin expression in vitro. Exp Cell Res. 2008;314:11-24. doi: 10.1016/j.yexcr.2007.06.007. PMID:17678891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutschner T, Hämmerle M, Diederichs S. MALAT1-a paradigm for long noncoding RNA function in cancer. J Mol Med. 2013;91:791-801. doi: 10.1007/s00109-013-1028-y. PMID:23529762 [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Song L, Zeng S, Zhang L. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumor Biol. 2016;37:633-40. doi: 10.1007/s13277-015-3732-4. [DOI] [PubMed] [Google Scholar]
- 18.Yang MH, Hu ZY, Xu C, Xie LY, Wang XY, Chen SY, Li ZG. MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9. Biochim Biophys Acta. 2015;1852:166-74. doi: 10.1016/j.bbadis.2014.11.013. PMID:25446987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121:101-8. doi: 10.1007/s11060-014-1613-0. PMID:25217850 [DOI] [PubMed] [Google Scholar]
- 20.Hua WF, Zhong Q, Xia TL, Chen Q, Zhang MY, Zhou AJ, Tu ZW, Qu C, Li MZ, Xia YF. RBM24 suppresses cancer progression by upregulating miR-25 to target MALAT1 in nasopharyngeal carcinoma. Cell Death Dis. 2016;7:e2352. doi: 10.1038/cddis.2016.252. PMID:27584791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon JH, Abdelmohsen K, Gorospe M: Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9-14. doi: 10.1016/j.semcdb.2014.05.015. PMID:24965208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Zhang C, Chen R, Xiong H, Qiu F, Liu S, Zhang M, Wang F, Wang Y, Zhou X. Disrupting MALAT1/miR-200c sponge decreases invasion and migration in endometrioid endometrial carcinoma. Cancer Lett. 2016;383:28-40. doi: 10.1016/j.canlet.2016.09.019. PMID:27693631 [DOI] [PubMed] [Google Scholar]
- 23.Luan W, Li L, Shi Y, Bu X, Xia Y, Wang J, Djangmah HS, Liu X, You Y, Xu B. Long non-coding RNA MALAT1 acts as a competing endogenous RNA to promote malignant melanoma growth and metastasis by sponging miR-22. Oncotarget. 2016;7: 63901-12. doi: 10.18632/oncotarget.11564. PMID:27564100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Zhang W, Wu X, Zhang M, Weng M, Zhou D, Wang J, Quan Z. Long non-coding RNA Malat1 promotes gallbladder cancer development by acting as a molecular sponge to regulate miR-206. Oncotarget. 2016; ;7:37857-67. doi: 10.18632/oncotarget.9347. PMID:27191262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456:409-12. doi: 10.1038/nature07451. PMID:19020623 [DOI] [PubMed] [Google Scholar]
- 26.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer. 2011;11:364-74. doi: 10.1038/nrc3050. PMID:21508973 [DOI] [PubMed] [Google Scholar]
- 27.Cai JJ, Qi ZX, Chen LC, Yao Y, Gong Y, Mao Y. miR-124 suppresses the migration and invasion of glioma cells in vitro via Capn4. Oncol Rep. 2016; 35: 284-90. doi: 10.3892/or.2015.4355. PMID:26530859 [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Xu FK, Zhao GY, Lu CL, Lin ZW, Ding JY, Ge D. Capn4 promotes non-small cell lung cancer progression via upregulation of matrix metalloproteinase 2. Med oncol. 2015;32:1-8. doi: 10.1007/s12032-015-0500-7. [DOI] [PubMed] [Google Scholar]
- 29.Zheng PC, Chen X, Zhu HW, Zheng W, Mao LH, Lin C, Liu JN, Zheng M. Capn4 is a marker of poor clinical outcomes and promotes nasopharyngeal carcinoma metastasis via nuclear factor-κB-induced matrix metalloproteinase 2 expression. Cancer sci. 2014;105:630-8. doi: 10.1111/cas.12416. PMID:24703594 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng J, Xiao W, Yu G, Yao W, Zhou H. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget. 2015;6:38005. doi: 10.18632/oncotarget.5357. PMID:26461224 [DOI] [PMC free article] [PubMed] [Google Scholar]


