ABSTRACT
The present study aimed to investigate the significance of hedgehog signaling pathway in association with clinicopathology parameters and its effect on angiogenesis in oral squamous cell carcinoma (OSCC). The expression of Sonic Hh (Shh) and Gli1 were done on primary tumors and metastatic lymph nodes in OSCC samples from 80 patients by immunohistochemical analysis. The western blot was used to examine the expression of Shh in OSCC cell lines and OSCC-derived microvesicles (MVs). The role of Shh carried by MVs to induce endothelial cell angiogenesis was further investigated by matrigel assay. Our results indicated that the expression of Shh was positive associated with microvesseldentisty(MVD), TNM stage, tumor recurrence and lymph node metastasis. Moreover, Shh and Gli1 expression were higher in paired metastatic lymph nodes compared with expression of their primary tumors. The expression of Shh was abundant in Cal27, and present in SCC4, SCC9, and the amount of Shh protein in Cal27 targeting MVs was increased significantly than Cal27 cell group, up to ∼ fifth-fold. The Cal27 derived MVs increased significantly angiogenesis of HUVECs in vitro, and this effect was blocked with exoenzyme C3 transferase (C3) and shRNA targeting RhoA by suppressing RhoA expression and activation. The data suggested that OSCC derived Shh carried by MVs may facilitate the tumor growth and modulate the preparation of a vascular network in primary tumor and/or premetastatic niche.
KEYWORDS: angiogenesis, endothelial cells, Gli1, Oral cancer, microvesicles, Shh, RhoA
Introduction
The Sonic Hedgehog (Shh) pathway is vital for embryonic development, cellular differentiation, migration and angiogenesis, and its aberrant activation has been found in various cancer types. Constitutive expression of Shh on tumor cells can influence tumor microenvironment and favor tumor angiogenesis and metastasis.1-3 The aberrant activation of hedgehog pathway was observed in several cancers, and was positive associated with clinicopathological features, lymph metastasis, overall survival as well as tumor MVD.4-6 Considering that the overall survival of OSCC was not improved significantly in recent 10 years due to tumor relapse, metastasis and radioresistance, it is necessary to find out some effective biomarkers for evaluating the prognosis of patients with OSCC.
Recent studies showed that the clinical significance of hedgehog signaling pathway in tumors was closely related with angiogenesis. In gliomas, the aberrant expression of HH could regulate expressions of angiogenesis-related gene and was correlated positively with MVD in tumors.7 Although some investigations have showed the correlation between expression of hedgehog signaling molecules and clinicopathologic variables in oral squamous cell carcinoma(OSCC), the relationship between the Shh and tumor angiogenesis in OSCC was still uncertain. The mounting evidence showed that non-canonical hedgehog signaling pathway may be involved in Shh-mediated capillary formation of endothelial cells. Under oxygen-glucose (OGD) deprivation, Shh secreted by astrocytes was regulated significantly and the OGD-activated astrocyte-derived Shh directly induced angiogenesis in brain microvascular endothelial cells by RhoA/ROCK signaling pathway.8
It has been studied that microvesicles released by activated or apoptotic cells were considered as mediator of intercellular transfer of genetic information. Recent studies showed that tumor cells are able to generate large amount of MVs, which harbored genetic information, such as mRNAs and proteins9-11. Through transferring the proteins carried by MVs to microenvironment, MVs are able to modulate cancer progression, angiogenesis and formation of the premetastatic niche. In the animal model, CD105+ human renal cancer stem cells produced amount of MVs, and the tumor derived MVs further stimulated proliferation of endothelial cells and modulated formation of vascular network in lung premetastatic niche by activation of angiogenic phenotype of normal endothelial cell.12 Furthermore, increasing evidence showed that MVs were able to transport some of their contents to other cell types or into circulation. Skog et al. found that Glioblastoma-derived angiogenic proteins carried by MVs can be delivered to endothelial cells and promoted tubule formation in the tumor microenvironment. In addition, miRNA-21 was increased in serum MVs from the patients of Glioblastoma, and serum MVs containing miRNA-21 was considered as a valuable diagnostic biomarker.13
In this study, the correlations between expression of Shh, Gli1 and MVD in OSCC were studied. Our results showed that expression of Shh was positive associated with microvesseldentisty (MVD) and lymph node metastasis. Moreover, the expression of Shh and Gli-1 was higher in paired metastatic lymph nodes compared with expression of their primary tumors. The Cal27 derived MVs increased significantly angiogenesis of HUVECs in vitro, and this effect was blocked with exoenzyme C3 transferase (C3) and shRNA targeting RhoA by suppressing RhoA expression and activation. The data suggested that OSCC derived Shh carried by MVs may facilitate the tumor growth and modulate the preparation of a vascular network in primary tumor and/or premetastatic niche.
Materials and methods
Cell culture
Cal-27 and SCC-25 cell lines, which was purchased from American Type Culture Collection (ATCC, USA). The cells were cultured in DMEM and DMEM/F12 (Hyclone, USA) supplemented with 10% FBS (Gibco, USA), respectively. Primary HUVECs used in this study were kindly gifted by Professor Yi-Fang Zhao and cultured in EC basal Medium-2 (EBM-2, Lonza, MD) supplemented with 2% fetal bovine serum (FBS) and with EGM-2 growth factor mixture (Lonza). Passages 3 to passage 8 of HUVECs were used in this study. All cells were cultured at 37°C in an atmosphere containing 5% CO2.
Western blot analysis
Western blot was used to detect the protein expression of Shh, Gli1 and RhoA in the indicated groups. Proteins were harvested, loaded onto each well, and transferred onto nitrocellulose membranes for 2 h at 200 mA. Then, the membrane was blocked and incubated at 4°C overnight with indicated Shh antibody (1:1000, Abcam), Gli1 antibody (1:1500, Santa Cruz Biotechnology) and RhoA (1:1500, Abcam), and the bound antibody was detected with horseradish peroxide-conjugated secondary antibody at room temperature for 1 h.
RhoA activation assay
RhoA activation assay kit was used to selectively isolate and pull-down the active form of Rho from purified samples by Rhotekin RBD Agarose beads. Then, the precipitated GTP-Rho was evaluated by western blot analysis using an anti-RhoA specific primary antibody. Briefly, the cell lysates were collected after the indicated treatments, and Rho-GTP was precipitated with Rotekin RBD agarose beads at 4 °C for 1 h, then the amount of RhoA-GTP was detected by performing SDS PAGE with an anti-RhoA primary antibody (1:1500, Abcam). The levels of RhoA expression in each group were determined by normalizing the amount of RhoA-GTP to the total amount of RhoA.
Knockdown of RhoA by shRNA(h)-mediated strategy
A commercially available shRNA-mediated strategy (Santa Cruz, USA) was used to knockdown the RhoA expression in HUVEC cells. The shRNA(h) lentiviral particles reagents (sc-29471-V, Santa Cruz, USA) generally contained three to five expression constructs each encoding target-specific 19–25 nt (plus hairpin) shRNA designed to knockdown RhoA gene expression. The control shRNA lentiviral particles (sc-108080, Santa Cruz, USA) was used as the control knockdown in the transfection. For transduction, the lentiviral supernatants containing shRNA vector were added into the HUVECs. After 72h transduction, the transduced cells were collected to confirm the RhoA gene silencing efficency by using western blot.
Immunohistochemistry
Eighty cases of clinical specimens of OSCC were collected from surgeries performed between 2008 and 2014 at the affiliated hospital of Qingdao university. These specimens were fixed in 10% formalin and embedded in paraffin. The 4μm deparaffinized sections were retrieved by microwaving and blocked with 10% goat serum for 30 min, followed incubation with primary antibodies, Shh (1:100, Abcam), Gli1 (1:200, Santa Cruz Biotechnology) or CD34 (1:50, Maixin Biotech). After rinsing with PBS, the slides were detected with secondary antibody and avidin-peroxidase.
Immunohistochemical scoring and evaluation
The slides were randomly evaluated in 5 different regions under a low-power field. The total scores were calculated by combination of staining intensity with percentage of stained cells in our study. The staining intensity was scored according to the following criteria: 0 (no staining), 1 (mild), 2(moderate), 3 (strong). The percentage of positive cells was assessed as follows: 1 (positive cells <25%), 2 (positive cells = 25–50%); 3 (positive cells = 51–75%), and 4 (positive cells >75%), respectively. Scoring of ≤ 3 was considered as low staining, 4-5 was considered as moderate staining, 6–7 was taken as strong staining. MVD was estimated by depending on the method described by Weidner et al.14 The number of highest density of positive CD34 expressing cells was recorded as the value of MVD at low magnification. The median value of MVD in our study was used to distinguish between high and low.
MVs production
The production of MVs was performed according to the protocol in our previous study.15 Briefly, the sub-confluent Cal27 cells were cultured in DMEM/F12 containing with 1%FBS. After 24 h incubation, a supernatant was collected by centrifugation at 300 g for 5 min and then at 1500 g for 15 min. The supernatant was further filtered using a 0.1 μm pore-size filter (Millipore) before ultracentrifugation. MVs were produced by the following centrifugation step: centrifugation at 14,000 g for 2 h. After washing two times, the isolated MVs were suspended and stored in PBS.
In vitro tube formation assay
Re-suspended HUVECs were seeded on the matrigel (BD Bioscience) in 96-well plate, and then EBM-2 containing Cal27 derived MVs or Shh (5 ug/ml, Peprotech) alone or together with exoenzyme C3 transferase (100 ug/ml, Cytoskeleton) was added into the indicated wells. After 8 h incubation at 37°C, 5% CO2, the branch points of tube structures in each group were counted. The percentage of tube formation was used to quantify the ability of tube formation by taking the control group as 100% tube formation.
Statistical analysis
The results are expressed as the means ±SE from triplicate independent experiments and analyzed through Student's t test or ANOVA using the Statistical Product and Service Solutions (SPSS) 17 software. A P value of less than 0.05 was considered to be statistically significant.
Results
Immunochemical staining of Shh, Gli1, and CD34 in OSCC
The expression of Shh was mainly observed in cytoplasm of tumor cells in OSCC specimens (Fig. 1 A). Immunochemical staining of Shh was detected in 76 of 80 OSCC specimens. The expression of Gli1 was mainly detected in the nucleus, but the positive staining of Gli1 also can be observed in both nucleus and cytoplasm in some tumor cells (Fig. 1 B). We found that both Shh and Gli1 protein had higher immunoactivity in the paired metastatic lymph nodes than the immunostaining of their primary tumors (Fig. 2A,B), and score of Shh, Gli1 had a significant difference between in the primary tumor and in the paired metastatic lymph nodes (P < 0.01). We used the mean value of MVD to distinguish between high and low in this study. The MVD was detected by anti-CD34 antibody staining (Fig. 3A,B), and the mean value of MVD was 57 ± 18 in the OSCC specimens. The MVD was low in 43 of 80 OSCC specimens, and the MVD was high in 37 of 80 OSCC specimens.
Figure 1.
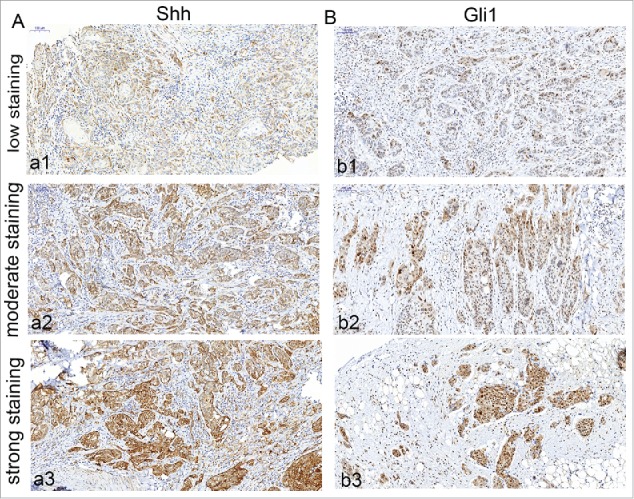
Immunohistochemical detection of Shh, Gli1 in OSCC specimens. A, cytoplasm expression of OSCC specimen for Shh (a1, low staining; a2, moderate staining; a3, strong staining); B, nuclear and cytoplasm expression of OSCC specimen for Gli1 (b1, low staining; b2, moderate staining; b3, strong staining). Bars indicate 100 um.
Figure 2.
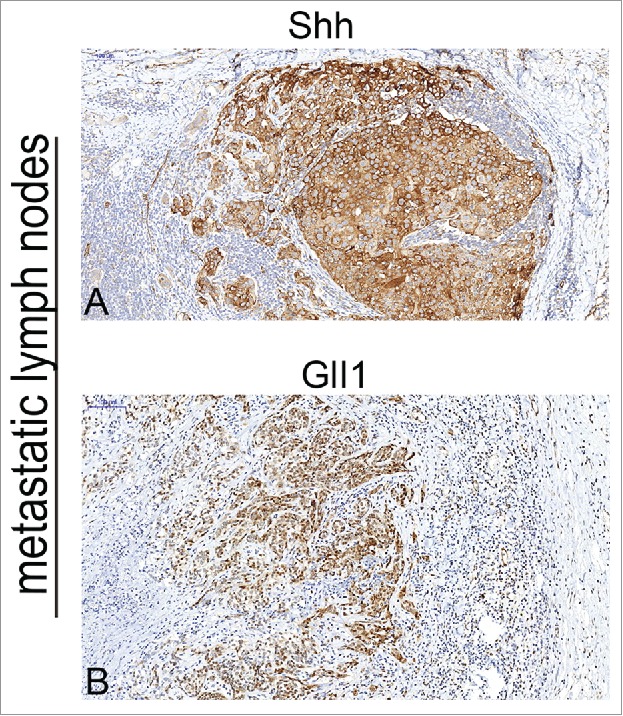
Immunohistochemical detection of Shh, Gli-1 in metastatic lymphnodes of OSCC specimens. A, cytoplasm of expression of metastatic lymphnodes for Shh (strong staining); B, nuclear and cytoplasm expression of metastatic lymphnodes for Gli1 (strong staining). Bars indicate 100 um.
Figure 3.
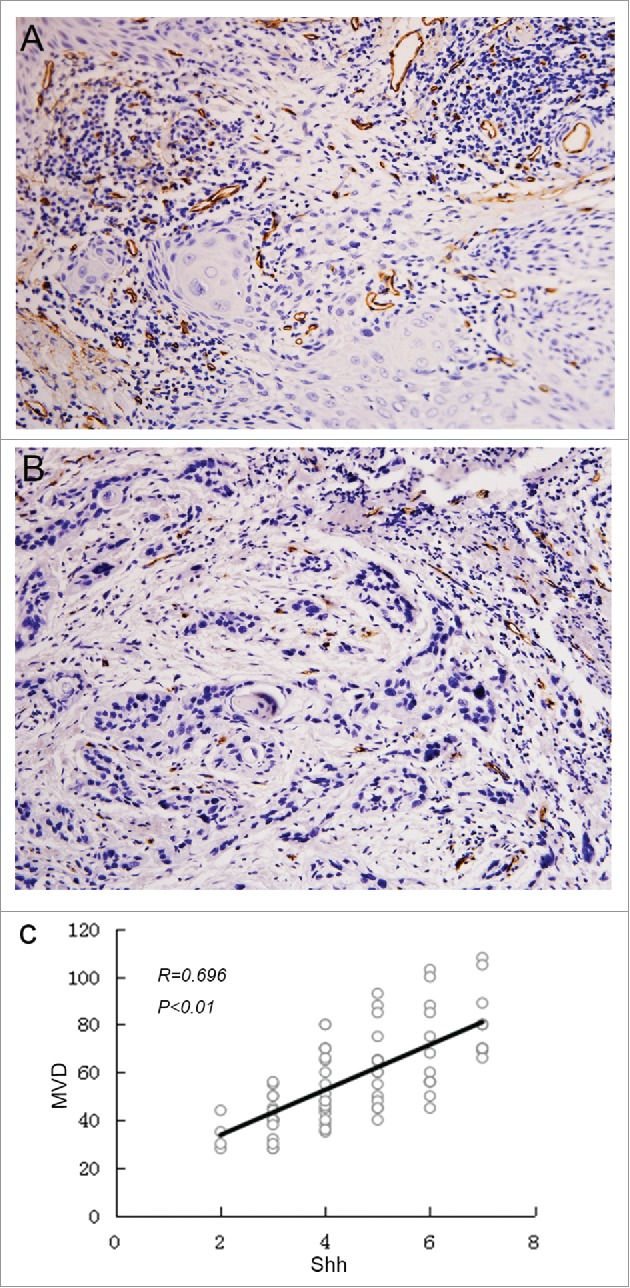
The correlation between MVD and Shh expression. Microvesseldentisty in OSCC specimens. A, the representative picture showed the value of MVD >57, and the MVD was high in 37 of 80 OSCC specimens; B, the representative picture showed the value of MVD <57, and the MVD was low in 43 of 80 OSCC specimens; C, Correlation between Shh and MVD expression. Spearman correlation and linear regression were used to determine the relationship between Shh and MVD. MVD correlated positively with Shh expression (P < 0.01).
Correlation among Shh, Gli1 and MVD expression
To investigate the relationship between Shh and MVD in OSCC specimen, the spearman correlation analysis and linear regression were done to detect the link between these two variables. Our data showed that there was significant linear correlation between Shh and MVD at P < 0.01 and R = 0.696(Fig. 3C). We also found that the expression of Shh was significantly correlated with expression of Gli1(P = 0.008)(Table 1).
Table 1.
The correlation between Shh and Gli1 expression.
| Gli1 expression |
||||
| |
total |
low(0–3) |
moderate and high(4–7) |
P |
| Shh(0–3) | 20 | 14 | 6 | 0.008 |
| Shh(4–7) | 60 | 20 | 40 | |
The expression of Shh was significantly correlated with expression of Gli1(P < 0.01).
Association among Shh, Gli1, MVD expression and clinicopathologic factors
Table 2 showed the correlation of several clinicopathologic factors with expression of Shh, Gli1 and MVD. The staining of Shh in primary tumor specimens was slight in 20 cases (25%), moderate and high in 60 cases (75%). The low Gli1 expression in primary tumor specimens was found in 34 cases (42.5%), moderate and high Gli1 expression in 46 cases (57.5%). The expression of Shh, Gli1 and MVD was significantly associated with TNM clinical stage, recurrence, lymph node metastasis (P< 0.05). The expression of Shh, Gli1 and MVD was not correlated with gender, age, and tumor size.
Table 2.
Correlation between Shh, Gli1, MVD expression and clinicopathologic factors.
| Shh |
Gli1 |
MVD |
||||||||
| |
n(%) |
0–3 |
4–7 |
P |
0–3 |
4–7 |
P |
≤57 |
>57 |
P |
| Age | ||||||||||
| ≤55 | 36(45%) | 12 | 24 | 0.123 | 15 | 21 | 0.893 | 23 | 13 | 0.102 |
| >55 | 44(55%) | 8 | 36 | 19 | 25 | 20 | 24 | |||
| Gender | ||||||||||
| Female | 47(59%) | 14 | 33 | 0.243 | 22 | 25 | 0.359 | 27 | 20 | 0.435 |
| Male | 33(41%) | 6 | 27 | 12 | 21 | 16 | 17 | |||
| Tumor size | ||||||||||
| ≤4cm | 50(63%) | 9 | 41 | 0.063 | 25 | 25 | 0.082 | 30 | 20 | 0.152 |
| >4cm | 30(37%) | 11 | 19 | 9 | 21 | 13 | 17 | |||
| TNM stage | ||||||||||
| I,II | 34(43%) | 14 | 20 | 0.004 | 20 | 14 | 0.011 | 24 | 10 | 0.009 |
| III, IV | 46(57%) | 6 | 40 | 14 | 32 | 19 | 27 | |||
| Lymphatic metastasis | ||||||||||
| Negtive | 30(37%) | 16 | 14 | 0.000 | 20 | 10 | 0.001 | 23 | 7 | 0.001 |
| Positive | 50(63%) | 4 | 46 | 14 | 36 | 20 | 30 | |||
| Tumor recurrence | ||||||||||
| Negative | 44(55%) | 16 | 28 | 0.009 | 24 | 20 | 0.016 | 30 | 14 | 0.004 |
| Positive | 36(45%) | 4 | 32 | 10 | 26 | 13 | 23 | |||
*P < 0.05 by X2 test.
Evaluation of Shh protein in OSCC and OSCC-derived MVs
Although the expression of Shh had been observed in OSCC specimens, whether the expression of Shh was present in some OSCC cell lines was unclear. In our study, we found that the expression of Shh was abundant in Cal27, and present in SCC4, SCC9 (Fig. 4A). Theoretically, MVs are able to harbor any genetic information contained in tumor cells. Thus, we further investigated the level of Shh protein in Cal27 derived MVs and Cal27 lysates, we found that the Shh protein was abundant in Cal27 derived MVs, and the amount of Shh protein in MVs was increased significantly than Cal27 cell group, up to ∼ fifth-fold(Fig. 4B).
Figure 4.
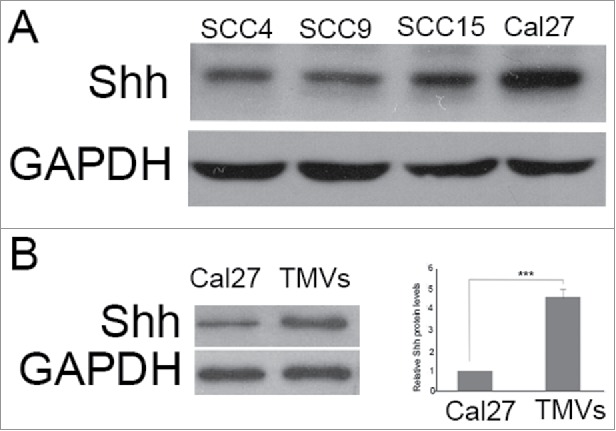
The expression of Shh in OSCC cell lines, Cal27 derived MVs A, the expression of Shh protein was detected in OSCC cell lines tested by western blot; B, western blot was used to detect the expression of Shh in Cal27 lysates and Cal27-derived MVs. A difference of P< 0.05 was considered significant.
OSCC-derived MVs induce tube formation through Shh/RhoA signaling pathway
To test the endothelial cell angiogenesis, the matrigel assay was used. Pretreatment with Cal27-derived MVs followed by culturing onto marigel, we found that the ability of tube formation of endothelial cell was significantly increased compared with control group after 8 h incubation (Fig. 5A). Our data showed that the MVs released by Cal27 cells carried mounts of Shh protein (Fig. 4B). To determine whether Shh/RhoA signaling pathway was involved in tube formation of endothelial cells induced by MVs, we examined the effect of RhoA inhibitor (exoenzyme C3 transferase,C3) on MVs-induced tube formation. We found that C3 reversed MVs-enhanced tube formation was related with GTP-RhoA expression (Fig. 5B). To confirm the role of RhoA in tube formation was correlated with Shh carried by MVs, we used the human recombinant Shh protein as the positive control. We found that the effect of tube formation evoked by Cal27 derived MVs was similar with human recombinant Shh protein, and both of these effect were inhibited by C3 treatment (Fig. 5A), suggesting that the presence of Shh/RhoA pathway were associated with endothelial function. In addition, our western blot data showed that expression of Gli1 was increased slightly in HUVECs after human recombinant Shh protein treatment compared with control group, and the effect of tube formation was reversed by RhoA inhibitor C3 without altering the expression of Gli1 significantly (Fig. 5C). We further used shRNA targeting RhoA to knockdown the RhoA expression and investigated the role of RhoA in angiogenesis after silencing the RhoA. We found that the effect of TMVs-enhanced angiogenesis was inhibited by silencing the RhoA expression and the role of knockdown of RhoA in TMVs-mediated angiogenesis was similar with the data using C3(Fig. 6).
Figure 5.
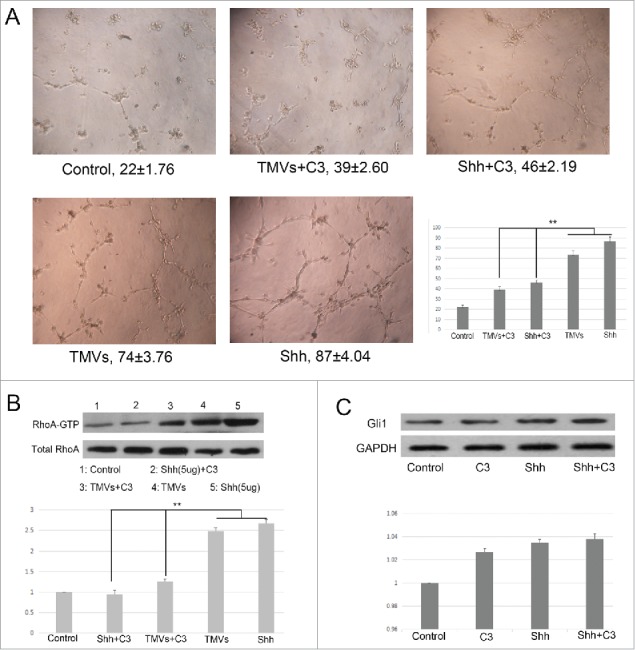
The efficacy of Shh/RhoA pathway on tube formation of HUVECs in vitro. A, Phase-contrast micrographs showed that MVs harboring Shh enhanced network formation of HUVECs on Matrigel. The efficacy of MVs-enhanced network formation was inhibited by C3 transferase (5 ug/ml). The Shh (5 ug/ml) was used as the positive control. The treatment condition and the actual number of branch points ± SEM were underneath the image. Branch points were used to quantify angiogenesis; B, Shh associated tube formation was related with RhoA activation; C, the expression of Gli1 in HUVECs after rh-shh (5 ug/ml) treatment. A difference of P < 0.05 was considered significant.
Figure 6.
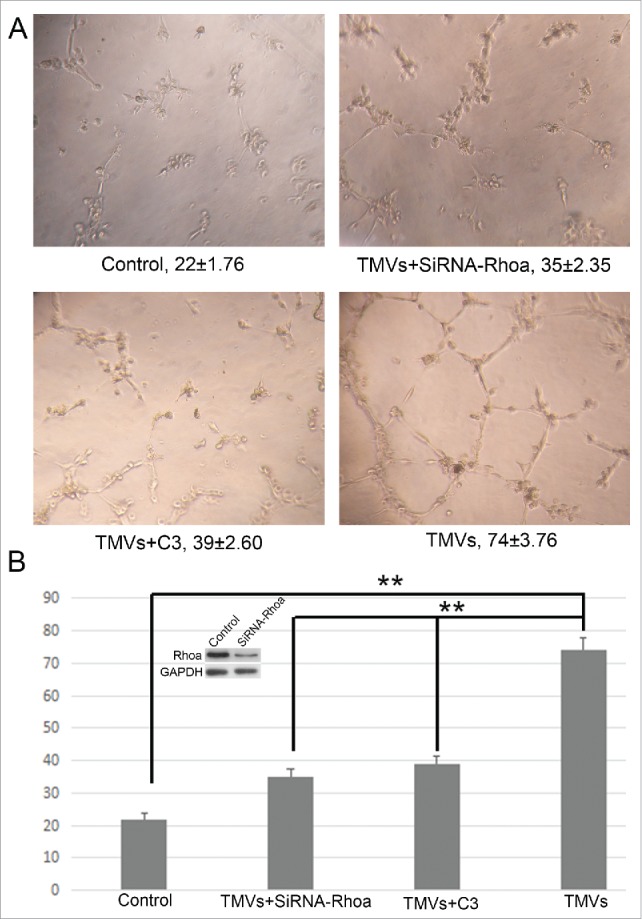
The effect of knockdown of RhoA on tube formation of HUVECs in vitro. A, The phase-contrast micrographs showed that TMVs enhanced network formation of HUVECs on Matrigel. The effect of TMVs-enhanced angiogenesis was inhibited by silencing the RhoA expression. The treatment condition and the actual number of branch points ± SEM were underneath the image. Branch points were used to quantify angiogenesis. B, As the histogram shown, the role of knockdown of RhoA in TMVs-mediated angiogenesis was similar with the data using C3. The western blot bands inserted in histogram showed the effect of silencing of RhoA. A difference of P < 0.05 was considered significant.
Discussion
Shh pathway has a critical role in tumor progression, metastasis and angiogenesis. Recent studies showed that the aberrant activation of hedgehog signaling pathway was positive associated with clinicopathological features, lymph metastasis, overall survival in several cancers, such as breast cancer, lung cancer.4-6 Because of low overall survival rate associated with Shh expression, many investigators aimed to evaluate the prognosis of patients with tumor by using Shh protein as the marker.16 In our study, the correlations among Shh, Gli1 and MVD expression in OSCC were studied. Our results agreed with previous studies,17,18 the upregulated expression of Shh and Gli1 in OSCC was associated significantly with lymph node metastasis. However, the Shh and Gli1 expression was not tested in metastatic lymph nodes in these studies. Our data showed that expression of Shh and Gli1 protein were higher in paired metastatic lymph nodes compared with expression of their primary tumor, indicating that the Shh or/and Gli1 high-expressing oral cancer cells was probably prone to metastasize to lymph nodes. In several tumors, the patients with Shh and Gli1 abundance were related significantly with the worst prognosis in the different expression status of Shh and Gli1. We found that both Shh and Gli1 expression were associated with lymph node metastasis, TNM stage and tumor recurrence, suggesting Shh and Gli1 protein could become the valuable biomarker in evaluating the lymph node metastasis in OSCC.
Although Shh derived from the tumor cells has been reported to modulate growth of the stroma, the mechanism of Shh protein promoted tumor angiogenesis in OSCC was unknown. Recent reports showed that Shh gene therapy favored neovascularization in mice ischemia models, and the role of Shh protein induced this effect can be inhibited partly by using cyclopamine, suggesting that hedgehog signaling pathway could regulate the angiogenesis in ischemia microenvionment.19 However, the role of Shh in pathologies associated with tumor progression in OSCC was not well studied, and what mechanism to mediate the crosstalk between tumor and endothelial cells was unclear. Our data showed that the Shh expression was significantly associated with MVD in OSCC, which provided a meaningful idea that whether hedgehog signaling pathway modulated the crosstalk between tumor and endothelial cells by regulating tumor angiogenesis or not. During the process of tumor progression, tumor cells generate a large amount of Shh, which was released into the tumor microenvironment to favor tumor growth. Recent studies showed that MVs, targeting genetic information, such as mRNAs and proteins, were considered as the mediator of intercellular transfer of genetic information.20 In our study, we found that Shh protein was expressed in both specimen of OSCC and OSCC cell lines such as SCC25 and Cal27. Theoretically, tumor derived MVs could carry any kind of mRNA or protein containing in the tumor cells. Thus, we hypothesized that the Shh protein derived from OSCC cells was transferred into the surrounding stroma or endothelial cells by MVs. In fact, we found that an abundance of Shh protein was detected in the Cal27 derived MVs. Tumor derived MVs has been shown to promote angiogenesis in mice ischemic models and regulate tumor progression, chemoresistance as well as angiogenesis in several tumors.12,21 A recent study suggested that the circulating MVs derived from serum of patients with OSCC were associated with tumor size and MVD, and these circulating MVs were able to increase the tube formation of endothelial cells in vitro.22 Additionally, it has been reported that recombinant Shh protein could induce tube formation of endothelial cells via activation of PI3k activity, this ability to induce tube formation could be markedly inhibited by both cyclopamine and PI3k inhibitor, LY294002.23 In our study, we demonstrated that the Cal27 derived MVs could enhance the formation of capillary-like structures on matrigel in vitro. Our observations are similar with the previous report,24 indicating that the role of shh carried by MVs was in correspondence with the effect of recombinant Shh protein in regulating angiogenesis of endothelial cells. Although a recent report had showed that Shh-expressing breast cancer cells activated the down-stream signaling component Gli1/CYR61 by autocrine and paracrine manner, leading to increased tumor vasculature as well as enhanced spontaneous metastases,25 the question raised is how Shh carried by MVs to modulate tube formation in our experimental system.
In recent years, the role of RhoA/Rock pathway in endothelial cell function has become a hotspot in life science and basic medical science.24,26,27 Renault et al. found that Shh promoted endothelial cell angiogenesis both in vitro and in vivo, and Shh activity in ECs is modulated directly by via RhoA/ROCK mediated OPN and MMP-9 expression.24 Our results demonstrated that MVs used in our study enhanced tube formation, and this effect could be inhibited by down-regulation of GTP-RhoA protein expression using C3 transferase. We further used shRNA targeting RhoA to knockdown the RhoA expression and investigated the role of RhoA in angiogenesis after silencing the total RhoA. We found that the effect of TMVs-enhanced angiogenesis was inhibited by silencing the RhoA expression and the role of knockdown of RhoA in TMVs-mediated angiogenesis was similar with the data using C3. Our western blot data showed the expression of Gli1 was increased slightly in HUVECs after recombinant Shh protein treatment compared with control group. In addition, the effect of rh-Shh induced tube formation was reversed by RhoA inhibitor C3 transferase without altering the expression of Gli1 significantly, suggesting that Shh related “non-classical” pathway modulated EC angiogenic activity. Furthermore, we found that the effect of rh-Shh and MVs induced tube formation was not completely inhibited by RhoA inhibitor C3, suggesting that shh also could mediate tube formation via other signaling pathway. Recent study demonstrated that Shh protein could induce the angiogenic activity by activating PI3k signaling pathway in vitro and tumor MVs-associated shh also could modulate angiogenesis via an indirect mechanism by inducing expression of adhesion molecules and production of proangiogenic factors.23,28
In summary, our results demonstrate that Shh and Gli1 protein were elevated at the primary tumor and metastatic lymph node in OSCC, which was related significantly with TNM stage, lymph node metastasis and recurrence. Moreover, Shh/RhoA signaling pathway may be a vital component to mediate tube formation of endothelial cells. Our data probably provided an effective biomarker to evaluate the lymph node metastasis and recurrence in OSCC. Inhibition of Shh/RhoA pathway could be a useful therapeutic strategy for treatment of patients with OSCC.
Conflict of interest statement
None declared.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 81502340] and Shandong Provincial Natural Science Foundation, China [grant number ZR2014HQ012].We are grateful to professor Yi-fang Zhao and Dr. Hai-xiao Zou for technical support (Department of Oral andMaxillofacial-Head and Neck oncology, School and Hospital of Stomatology, Wuhan University).
References
- 1.Delloye-Bourgeois C, Rama N, Brito J, Le Douarin N, Mehlen P. Sonic Hedgehog promotes the survival of neural crest cells by limiting apoptosis induced by the dependence receptor CDON during branchial arch development. Biochem Biophys Res Commun. 2014;452:655-60. doi: 10.1016/j.bbrc.2014.08.134. PMID:25193697 [DOI] [PubMed] [Google Scholar]
- 2.Li W, Miao S, Miao M, Li R, Cao X, Zhang K, Huang G, Fu B. Hedgehog Signaling Activation in Hepatic Stellate Cells Promotes Angiogenesis and Vascular Mimicry in Hepatocellular Carcinoma. Cancer Invest. 2016;34:424-30. doi: 10.1080/07357907.2016.1227442. PMID:27657189 [DOI] [PubMed] [Google Scholar]
- 3.Dai J, Ai K, Du Y, Chen G. Sonic hedgehog expression correlates with distant metastasis in pancreatic adenocarcinoma. Pancreas. 2011;40:233-6. doi: 10.1097/MPA.0b013e3181f7e09f. PMID:20938369 [DOI] [PubMed] [Google Scholar]
- 4.Yoshizaki A, Nakayama T, Naito S, Wen CY, Sekine I. Expressions of sonic hedgehog, patched, smoothened and Gli-1 in human intestinal stromal tumors and their correlation with prognosis. World J Gastroenterol. 2006;12:5687-91. doi: 10.3748/wjg.v12.i35.5687. PMID:17007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietanza MC, Litvak AM, Varghese AM, Krug LM, Fleisher M, Teitcher JB, Holodny AI, Sima CS, Woo KM, Ng KK, et al.. A phase I trial of the Hedgehog inhibitor, sonidegib (LDE225), in combination with etoposide and cisplatin for the initial treatment of extensive stage small cell lung cancer. Lung cancer. 2016;99:23-30. doi: 10.1016/j.lungcan.2016.04.014. PMID:27565909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takebe N, Hunsberger S, Yang SX. Expression of Gli1 in the hedgehog signaling pathway and breast cancer recurrence. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2012;24:257-8. doi: 10.1007/s11670-012-0260-2. PMID:23358885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui D, Chen X, Yin J, Wang W, Lou M, Gu S. Aberrant activation of Hedgehog/Gli1 pathway on angiogenesis in gliomas. Neurology India. 2012;60:589-96. doi: 10.4103/0028-3886.105192. PMID:23287320 [DOI] [PubMed] [Google Scholar]
- 8.He QW, Xia YP, Chen SC, Wang Y, Huang M, Huang Y, Li JY, Li YN, Gao Y, Mao L, et al.. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol. 2013;47:976-87. doi: 10.1007/s12035-013-8396-8. PMID:23325464 [DOI] [PubMed] [Google Scholar]
- 9.Hyun KA, Kwon K, Han H, Kim SI, Jung HI. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosensors & bioelectronics. 2013;40:206-12. doi: 10.1016/j.bios.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Menck K, Scharf C, Bleckmann A, Dyck L, Rost U, Wenzel D, Dhople VM, Siam L, Pukrop T, Binder C, et al.. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol. 2015;7:143-53. doi: 10.1093/jmcb/mju047. PMID:25503107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, Naoe T, Akao Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochimica et biophysica acta. 2014;1839:1256-72. doi: 10.1016/j.bbagrm.2014.09.002. PMID:25218966 [DOI] [PubMed] [Google Scholar]
- 12.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346-56. doi: 10.1158/0008-5472.CAN-11-0241. PMID:21670082 [DOI] [PubMed] [Google Scholar]
- 13.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-6. doi: 10.1038/ncb1800. PMID:19011622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. The New England journal of medicine. 1991;324:1-8. doi: 10.1056/NEJM199101033240101. PMID:1701519 [DOI] [PubMed] [Google Scholar]
- 15.Zhao XP, Wang M, Song Y, Song K, Yan TL, Wang L, Liu K, Shang ZJ. Membrane microvesicles as mediators for melanoma-fibroblasts communication: roles of the VCAM-1/VLA-4 axis and the ERK1/2 signal pathway. Cancer Lett. 2015;360:125-33. doi: 10.1016/j.canlet.2015.01.032. PMID:25661735 [DOI] [PubMed] [Google Scholar]
- 16.Honing J, Pavlov KV, Mul VE, Karrenbeld A, Meijer C, Faiz Z, Smit JK, Hospers GA, Burgerhof JG, Kruyt FA, et al.. CD44, SHH and SOX2 as novel biomarkers in esophageal cancer patients treated with neoadjuvant chemoradiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;117:152-8. doi: 10.1016/j.radonc.2015.08.031. PMID:26364884 [DOI] [PubMed] [Google Scholar]
- 17.Wang YF, Chang CJ, Lin CP, Chang SY, Chu PY, Tai SK, Li WY, Chao KS, Chen YJ. Expression of hedgehog signaling molecules as a prognostic indicator of oral squamous cell carcinoma. Head Neck. 2012;34:1556-61. doi: 10.1002/hed.21958. [DOI] [PubMed] [Google Scholar]
- 18.Fan HX, Wang S, Zhao H, Liu N, Chen D, Sun M, Zheng JH. Sonic hedgehog signaling may promote invasion and metastasis of oral squamous cell carcinoma by activating MMP-9 and E-cadherin expression. Med Oncol. 2014;31:41. doi: 10.1007/s12032-014-0041-5. PMID:24915900 [DOI] [PubMed] [Google Scholar]
- 19.Qin Y, He YH, Hou N, Zhang GS, Cai Y, Zhang GP, Xiao Q, He LS, Li SJ, Yi Q, et al.. Sonic hedgehog improves ischemia-induced neovascularization by enhancing endothelial progenitor cell function in type 1 diabetes. Mol Cell Endocrinol. 2016;423:30-9. doi: 10.1016/j.mce.2016.01.005. PMID:26773732 [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Zhang D, Zhu Z, Dela Cruz CS, Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:35250. doi: 10.1038/srep35250. PMID:27731391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang FF, Zhu YF, Zhao QN, Yang DT, Dong YP, Jiang L, Xing WX, Li XY, Xing H, Shi M, et al.. Microvesicles mediate transfer of P-glycoprotein to paclitaxel-sensitive A2780 human ovarian cancer cells, conferring paclitaxel-resistance. Eur J Pharmacol. 2014;738:83-90. doi: 10.1016/j.ejphar.2014.05.026. PMID:24877693 [DOI] [PubMed] [Google Scholar]
- 22.Ren JG, Zhang W, Liu B, Man QW, Xiong XP, Li C, Zhu JY, Wang WM, Jia J, Sun ZJ, et al.. Clinical Significance and Roles in Angiogenesis of Circulating Microparticles in Oral Cancer. J Dent Res. 2016;95:860-7. doi: 10.1177/0022034516641037. PMID:27013642 [DOI] [PubMed] [Google Scholar]
- 23.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244-9. doi: 10.1074/jbc.M210635200. PMID:12514186 [DOI] [PubMed] [Google Scholar]
- 24.Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, et al.. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49:490-8. doi: 10.1016/j.yjmcc.2010.05.003. PMID:20478312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris LG, Pannell LK, Singh S, Samant RS, Shevde LA. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene. 2012;31:3370-80. doi: 10.1038/onc.2011.496. PMID:22056874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao J, Ye Z, Tang H, Wang C, Peng H, Lai W, Li Y, Huang W, Lou T. The RhoA/ROCK Pathway Ameliorates Adhesion and Inflammatory Infiltration Induced by AGEs in Glomerular Endothelial Cells. Scientific reports. 2017;7:39727. doi: 10.1038/srep39727. PMID:28054559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbunova EE, Simons MJ, Gavrilovskaya IN, Mackow ER. The Andes Virus Nucleocapsid Protein Directs Basal Endothelial Cell Permeability by Activating RhoA. mBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, Martinez MC. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580-8. doi: 10.1093/carcin/bgp030. PMID:19168578 [DOI] [PubMed] [Google Scholar]


