Abstract
Introduction
Ig deposits identified on renal biopsy samples by paraffin immunofluorescence that show negative staining by routine immunofluorescence on frozen tissue have become known as “masked” deposits. Membranous-like glomerulopathy with masked IgG kappa (κ) deposits is a recently recognized pattern of immune complex deposition characterized by masked deposits that show IgG κ restriction and are subepithelial and mesangial by electron microscopy. Based on the frequent presence of C3-only staining by routine immunofluorescence microscopy (IF), these cases could be misdiagnosed as C3 glomerulonephritis in the absence of paraffin immunofluorescence evaluation.
Methods
The clinicopathologic details of all cases of membranous-like glomerulopathy with masked IgG κ deposits diagnosed in our laboratory were included, beginning with the initial recognition of this entity in 2011 through the end of 2015. Inclusion was based on renal biopsy sample morphologic features including glomerular deposits that stain for IgG κ and have a staining intensity that is significantly brighter by paraffin IF than by routine IF on frozen tissue.
Results
This pattern of immune complex deposition has been seen in 41 patients in our laboratory over a 5-year period. The patients with these biopsy findings are most commonly young female individuals with a mean age of 27.5 years, with 88% being less than 40 years. All patients had proteinuria with a mean 24-hour urine protein of 3.5 g (range 0.5−12.8 years) and 35% with nephrotic-range proteinuria. Hematuria was present in 88% of patients, and 29% had elevated serum creatinine at presentation. Autoimmune serologic tests were positive in 55% of patients, with a weakly positive antinuclear antibody being most common. Despite this, only 1 patient (2%) fulfilled the diagnostic criteria for systemic lupus erythematosus. The outcome data were mixed, as some patients showed spontaneous remission and mild disease whereas others progressed to end-stage renal disease. There was no apparent correlation between the treatment used and outcome in this retrospective analysis. One patient underwent transplantation and developed biopsy-proven recurrence of disease in the graft 42 months posttransplantation. The etiology of this entity remains unknown.
Discussion
We provide an expanded case series detailing the clinicopathologic findings of patients with membranous-like glomerulopathy with masked IgG κ deposits. Patients are most commonly young female individuals <40 years of age and commonly have positive autoimmune serologic studies such as antinuclear antibody, although few carry a diagnosis of any well-defined autoimmune disease such as lupus. The outcome data were mixed, as some patients showed spontaneous remission and mild disease whereas others progressed to ESRD. There was no apparent correlation between the treatment used and outcome in this retrospective analysis.
Keywords: C3 glomerulonephritis, masked deposits, membranous glomerulopathy, nephritis, proliferative glomerulonephritis with monoclonal IgG kappa deposits, renal biopsy
The classification for glomerulonephritis is based on findings identified by light, immunofluorescence, and electron microscopy in combination with clinical history. In most cases of glomerulonephritis, the renal biopsy diagnosis rendered is a morphologic pattern of injury rather than a specific disease. Careful clinicopathologic correlation and often extensive serologic testing are necessary to determine the specific underlying etiologic disease state. A good example is the renal biopsy diagnosis of membranous glomerulopathy, an immune complex−mediated glomerulopathy in which the immune deposits are predominantly subepithelial and IgG is the principal Ig. This disease may be primary or secondary. Secondary associations are a broad and diverse group that includes drugs, malignancy, infection, and autoimmune disease, among others.1, 2 The renal biopsy diagnosis of membranous glomerulopathy provides a framework for the workup that should be initiated and also has prognostic and treatment implications. In addition, it clusters the patients into meaningful groups that can be further studied, enabling breakthroughs in our understanding of the underlying disease such as the recent recognition of PLA2R and THSD7A autoantibodies as the etiology of most cases of primary membranous glomerulopathy.3, 4
In 2014, we reported 14 cases of an immune complex−mediated form of glomerulopathy with a unique pattern of immune deposition that was termed “membranous-like glomerulopathy with masked IgG kappa deposits” (MGMID).5 The renal biopsy findings were distinctive in that the deposits showed false-negative staining for Igs by routine immunofluorescence, with strong staining for IgG and kappa (κ) (but not lambda [λ]) by paraffin immunofluorescence. The deposits were subepithelial and/or mesangial by electron microscopy. The entity was also characterized by unique clinical findings compared to other glomerular diseases with monoclonal deposits, in that the patients affected were relatively young female individuals who often had some evidence of autoimmune disease.6, 7 Here, we expand this cohort to describe the clinicopathologic findings in 41 patients with MGMID, with follow-up data also presented.
Materials and Methods
All cases were processed by light, immunofluorescence, and electron microscopy using routine techniques.8 Briefly, kidney biopsy samples were fixed in buffered formalin, dehydrated in graded alcohols, and embedded in paraffin using standard techniques. Serial 3-μm-thick sections were cut and treated with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid−Schiff reagent. All data were collected according to protocols approved by Schulman Institutional Review Board.
Immunofluorescence
Samples were transported in Michel’s media, washed in buffer, and frozen in a cryostat. Sections, cut at 5 μm, were rinsed in buffer and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C4, C1q, fibrinogen, and κ-, and λ-light chains (all from Dako, Carpenteria, CA) for 1 hour, rinsed, and a coverslip applied using aqueous mounting media. IgA, IgG, IgM, κ, and λ were detected in paraffin-embedded sections using fluorescein-tagged polyclonal rabbit anti-human antibodies (all from Dako, Carpenteria, CA). The stains were evaluated by standard immunofluorescence microscopy using a Leica L5 filter cube and scored on a scale of 0 to 3. PLA2R was performed on paraffin-embedded sections following protease digestion using rabbit polyclonal anti-PLA2R antibodies (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:50, followed by highly cross-adsorbed Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, Carlsbad, CA) at a dilution of 1:100 as previously described.2 Immunoperoxidase staining for THSD7A was performed in paraffin-embedded sections using rabbit polyclonal anti-THSD7A antibodies (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:800, as previously described.9
Paraffin immunofluorescence was performed as described elsewhere.7, 10 Briefly, sections were cut at 3 μm and deparaffinized in xylene, followed by alcohol gradient, and proteinase K (Dako, Carpenteria, CA) was applied for 30 minutes at room temperature. The sections were then rinsed in buffer and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, κ-, and λ-light chains (all from Dako, Carpenteria, CA) at room temperature, rinsed, and a coverslip applied using aqueous mounting media.
Electron Microscopy
The ends of the renal biopsy specimen were removed as 1-mm cubes, dehydrated using graded alcohols, and embedded in epon/araldite resin. Sections 1-μm thick were cut using an ultramicrotome, stained with toluidine blue, and examined with a light microscope. Thin sections were examined in a Jeol JEM-1011 or a Jeol 1400 electron microscope (Jeol, Tokyo, Japan). “Hump-like” deposits were defined as large deposits with a rounded configuration present on the surface of the glomerular basement membrane, with minimal associated basement membrane response. In general, these deposits are as high above the glomerular basement membrane as they are wide along its surface. Hinge deposits were defined as hump-like deposits overlying the mesangium between folds of glomerular basement membrane.
Clinical Definitions
Hematuria was defined as >5 red blood cells per high-power field on microscopic examination of urine sediment. Nephrotic syndrome was defined as 24-hour urine protein > 3.5 g/d, hypoalbuminemia (<3.5 g/dl), and peripheral edema. Elevated serum creatinine was defined as a serum creatinine > 1.2 mg/dl. The following definitions were applied for the purposes of outcome analysis and are similar to those used by Nasr et al.6: complete remission, remission of proteinuria to <500 mg/d with normal renal function; partial remission, reduction in proteinuria by ≥50% and to <2 g/d with stable renal function (no more than a 20% increase in serum Cr); persistent kidney dysfunction, failure to meet criteria for complete or partial remission but not reaching end-stage kidney disease (incudes patients with unremitting proteinuria or progressive chronic kidney disease); and end-stage kidney disease, requiring renal replacement therapy.
Results
Clinical Features
All cases of MGMID diagnosed in our laboratory were included, beginning with the initial recognition of this entity in 2011 through the end of 2015. Inclusion was based on renal biopsy sample morphologic features, including glomerular deposits that stain for IgG κ and have a staining intensity that is significantly brighter by paraffin IF than by routine IF on frozen tissue. By electron microscopy, the deposits must be predominantly subepithelial and/or mesangial. Cases with a membranoproliferative pattern by light microscopy were excluded. There were 41 patients out of 42,711 total kidney biopsy samples (0.1%) processed during this 5-year study period (including 14 cases that were previously published5).
The patients were commonly young, with 88% being <40 years of age (Table 1), although there were 2 elderly patients (70 and 73 years). The youngest patient was 10 years of age. Females were affected more than males, with a ratio of 3.6 to 1. Cases were identified in multiple ethnicities, although individuals of white ethnicity were most common at 63%. Proteinuria was the indication for biopsy in 36 of 41 patients (88%), and the mean proteinuria was 3.5 g/d. In all, 14 of 40 patients (35%) with known quantitative proteinuria at the time of biopsy had proteinuria in the nephrotic range (>3.5 g/day). Of the 40 patients, 35 (85%) had hematuria.
Table 1.
Clinical characteristics of patients with membranous-like glomerulopathy with masked IgG κ deposits
| Characteristic | Value |
|---|---|
| Mean age, yr (range) | 27.5 (10−73) |
| ≤40, n (%) | 36 (88%) |
| 40−55, n (%) | 3 (7%) |
| >55, n (%) | 2 (5%) |
| Female/male, n (%) | 32 (78%)/9 (22%) |
| Race/ethnicity | |
| White, n (%) | 26 (63%) |
| Hispanic, n (%) | 9 (22%) |
| African American, n (%) | 3 (7%) |
| Other, n (%) | 3 (7%) |
| Mean serum Cr at biopsy, mg/dl (range) | 1.4 (0.5−6.4) |
| Elevated serum Cr at presentation, n (%) | 12 (29%) |
| Mean quantitative urine protein, g/24 h (range) | 3.5 (0.5−12.8) |
| Proteinuria < 1 g/24 h, n (%) | 4/40 (10%) |
| Proteinuria 1−3.5 g/24 h, n (%) | 22/40 (55%) |
| Proteinuria > 3.5 g/24 h, n (%) | 14/40 (35%) |
| Hematuria, n (%) | 35/40 (88%) |
| Positive autoimmune serologic test, n (%) | 22/40 (55%) |
| Positive ANA, n (%) | 20/38 (53%) |
| Positive dsDNA, n (%) | 9/39 (23%) |
| Positive ANA and dsDNA, n (%) | 8/40 (20%) |
| Normal C3 and C4, n (%) | 33/38 (87%) |
| Fulfills diagnostic criteria for SLE, n (%) | 1/41 (2%) |
ANA, antinuclear antibody; SLE, systemic lupus erythematosus.
Positive autoimmune serologic test results were common, with 55% of patients showing some positivity (Table 1). The most frequent positive serologic tests were antinuclear antibody (ANA) (53%) and dsDNA (23%). Two of 17 patients tested for anti-neutrophil cytoplasmic antibody (ANCA) were positive (12%), but all 7 cases with crescent formation were negative for ANCA. Two of 23 cases tested for rheumatoid factor were positive (9%). Thirty-seven cases were tested for hepatitis B and hepatitis C, and all were negative. Most patients showed normal C3 and C4 (87%), although there were 5 cases with hypocomplementemia including 1 case with low C3, 2 cases with low C4, and 2 cases with low C3 and C4. Serum protein electrophoresis was negative for paraproteins in 25 of 26 patients tested (96%). One patient, a 55-year-old man, had a small M-spike on serum protein electrophoresis but no evidence of a hematopoietic malignancy on further evaluation.
Pathologic Findings
Light Microscopy
Biopsy samples undergoing light microscopy had a mean of 18.8 glomeruli (range 8−47) with a mean of 4.3 (mean 23%) globally sclerotic. Approximately 50% of the cases had normocellular glomeruli by light microscopy, with the remaining 50% showing mesangial hypercellularity, focal segmental glomerulosclerosis, or focal/diffuse crescentic glomerulonephritis (Table 2, Figure 1). At least segmental glomerular basement membrane “spikes” and/or “holes” were present in most cases. There were no cases with endocapillary proliferation or a membranoproliferative pattern. No wire loops or hyaline thrombi were present in any case. Most of the cases (73%) showed no to mild interstitial fibrosis. None of the cases had morphologic evidence of a second underlying kidney disease. No cases showed evidence of acute or chronic thrombotic microangiopathy, and there was no evidence of arteritis.
Table 2.
Light microscopy results
| Finding | Value |
|---|---|
| Total number of glomeruli, mean n (range) | 18.8 (8−47) |
| Number of globally sclerotic glomeruli, mean n (%) | 4.3 (23%) |
| Predominant glomerular pattern | |
| Normal, n (%) | 20 (49%) |
| Mesangial hypercellularity, n (%) | 8 (20%) |
| FSGS, n (%) | 6 (15%) |
| Crescentic/focal crescentic, n (%) | 7 (17%) |
| Focal, n (%) | 4 (10%) |
| Diffuse, n (%) | 3 (7%) |
| Interstitial fibrosis | |
| None, n (%) | 18 (44%) |
| Mild, n (%) | 12 (29%) |
| Moderate, n (%) | 6 (15%) |
| Severe, n (%) | 5 (12%) |
FSGS, focal segmental glomerulosclerosis.
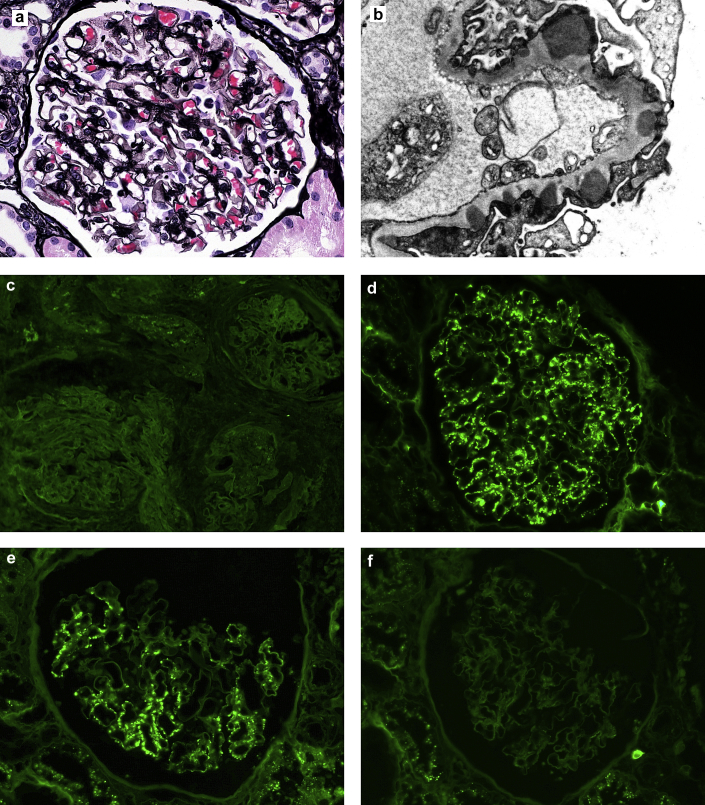
Figure 1.
Renal biopsy findings in membranous-like glomerulopathy with masked IgG κ deposits. (a) Segmental “spikes” and “holes” are present along the glomerular basement membranes by Jones methenamine silver stain (original magnification ×400). (b) Electron microscopy reveals numerous large subepithelial deposits present along the glomerular basement membrane (unstained, original magnification ×12,000). (c) Glomeruli stain negative for IgG by routine immunofluorescence on fresh tissue. (d) Glomeruli from the same case stain positive on paraffin-embedded tissue after protease digestion (direct immunofluorescence; original magnification ×400). (e) Glomeruli show staining for κ and (f) not λ on the protease-digested, paraffin-embedded tissue (direct immunofluorescence; original magnification ×400).
Immunofluorescence Microscopy
The most common antibody positive by routine, frozen immunofluorescence microscopy (IF) was C3, which was present in 32 cases (78%), with a mean intensity of 2+ (Table 3). A total of 28 cases (68%) had C3 intensity ≥2 orders of magnitude more than any other immune reactant or were C3 only (scale of 0−3) and therefore could have been misclassified as C3 glomerulonephritis. Twelve cases (29%) had staining for Ig by frozen IF that had a mean intensity of ≤1+. This included 6 cases (15%) with 1+ IgG staining by frozen IF. Five of these 6 with IgG staining had C3 predominance by frozen IF. IgG subclass staining was performed in each of these cases, and all showed exclusively IgG1-only staining (negative for IgG2, IgG3, and IgG4). The paraffin IF showed a similar staining pattern for all antibodies except IgG and κ. These were strongly positive in all cases (mean intensity 2.9+). The deposits were diffuse (present in more than half of glomeruli) in all cases. Mesangial staining was present in all cases. There was segmental (<50% loop involvement) staining of the capillary loops in 13 cases (32%) and global (>50% loop involvement) staining in 27 (68%). However, these cases with a global pattern had incomplete involvement of capillary loops in virtually all cases. PLA2R and THSD7A stains were performed and were negative in all cases tested (29 and 5 cases, respectively).
Table 3.
Immunofluorescence results
| Antibody | Immunofluorescence, frozena % positive (mean intensity among positive) |
Immunofluorescence, paraffina % positive (mean intensity among positive) |
|---|---|---|
| IgA | 5% (1+) | 5% (1+) |
| IgM | 17% (0.9+) | 22% (0.9+) |
| IgG | 15% (0.8+) | 100% (2.9+) |
| C3 | 78% (2+) | NA |
| Kappa (κ) | 17% (1+) | 100% (2.9+) |
| Lambda (λ) | 10% (1+) | 12% (1+) |
| PLA2R (n = 29) | NA | 0 |
| THSD7A (n = 5) | NA | 0 |
Scale 0, trace, 1+, 2+, 3+.
Electron Microscopy
Electron microscopy was available for all 41 cases. Almost all cases showed evidence of subepithelial and mesangial deposits (Table 4). There was 1 case of an 18-year-old girl with numerous mesangial-only deposits that showed 3+ C3 staining without Igs by frozen IF and, by paraffin IF, there was intense IgG and κ staining. Two cases had subepithelial deposits without mesangial deposition. Subendothelial deposits were identified in 3 cases but were rare, when present. There were 30 cases (73%) that had either hump-like and/or hinge region deposits, including 6 cases with only hump-like deposits, 6 cases with only hinge-region deposits, and 18 cases with both.
Table 4.
Electron microscopy results
| Electron microscopy finding | No. of cases (% of cases), n = 41 |
|---|---|
| Subepithelial deposits | 40 (98%) |
| Mesangial deposits | 39 (95%) |
| Subendothelial deposits | 3 (10%) |
| Subepithelial “humps” | 24 (59%) |
| Hinge-region deposits | 24 (59%) |
Treatment and Clinical Outcome
Follow-up was available in 27 patients for a mean of 22 months (range 6-51 months). These patients were treated with a variety of regimens (Table 5). The outcomes were mixed, with 15 patients undergoing complete or partial remission and 12 patients who had persistent disease on follow-up or progressed to ESRD (Table 6). There was no correlation between the treatment used and the clinical outcome. One of the patients who progressed to ESRD underwent renal transplantation and had biopsy-proven recurrence of disease in the graft 42 months after transplantation. The indication for the transplant biopsy was proteinuria. The biopsy from the transplant had a membranous pattern of immune complex deposition. The deposits were masked in both the native and allograft biopsy, showing negative staining for all immune reactants by routine immunofluorescence and strong staining for IgG and κ by paraffin IF.
Table 5.
Treatments used in patients with MGMID
| Treatment | No. of patients (% of patients), n = 27 |
|---|---|
| None | 6 (22%) |
| RAS blockade alone | 6 (22%) |
| Steroids and calcineurin inhibitor | 4 (15%) |
| Steroids and mycophenolate | 7 (26%) |
| Steroids and plaquenil | 1 (4%) |
| Steroids, calcineurin inhibitor, and rituximab | 1 (4%) |
| Steroids and azathioprine | 1 (4%) |
| Rituximab alone | 1 (4%) |
MGMID, membranous-like glomerulopathy with masked IgG κ deposits; RAS, renin−angiotensin system.
Table 6.
Clinical outcome by treatment
| Treatment | Complete Remission | Partial remission | Persistent disease | ESRD |
|---|---|---|---|---|
| None (n = 3) | 1 | 2 | 0 | 0 |
| RAS blockade alone (n = 6) | 3 | 0 | 3 | 0 |
| Immunosuppression (n = 15) | 7 | 2 | 6 | 0 |
| None; ESRD on presentation (n = 3) | 0 | 0 | 0 | 3 |
ESRD, end-stage renal disease; RAS, renin−angiotensin system.
Discussion
Most renal pathology laboratories in the United States perform evaluation for the presence of immune deposits using direct immunofluorescence on frozen unfixed tissue. “Masked deposits” refer to Igs that show false-negative staining by routine frozen immunofluorescence but that can be detected when immunofluorescence is repeated on formalin-fixed, paraffin-embedded tissue. Paraffin immunofluorescence is a valuable technique for the detection of Igs in renal biopsy samples, which has largely been used as a salvage technique when inadequate tissue was available to perform the testing on frozen unfixed tissue.11, 12, 13, 14 However, the study by Nasr et al.11 includes the first description of masked deposits revealed by this technique. Specifically, the authors found 10 cases of crystalline light chain proximal tubulopathy that all had staining for light chains by paraffin IF but only 4 of 10 with positive staining by routine IF. This phenomenon of masked light chain crystals in proximal tubulopathy has been confirmed in multiple subsequent papers and is now well established.15, 16 The initial description of MGMID5 was the first report of masked Igs in the glomeruli.5 Other glomerulonephritides subsequently described to show masked deposits include membranoproliferative glomerulonephritis with masked monotypic Ig deposits7 and cryoglobulinemic glomerulonephritis.10 The entities with masked deposits described thus far are heterogeneous both clinically and pathologically. However, the common feature in these disparate entities is the presence of a monotypic Ig molecule, raising the possibility that this plays an important role in the disease.
MGMID is unique in many ways but also has clinicopathologic features in common with other forms of glomerulonephritis. These similarities and differences with other diseases that have known underlying etiologies allow speculation as to the etiology of MGMID. The deposits are subepithelial and mesangial in almost all cases, and the granular staining pattern that is present by paraffin IF is diffuse; however, unlike PLA2R- or THSD7A-associated membranous glomerulopathy, it typically shows incomplete involvement of the glomerular capillary walls. This suggests that the Igs depositing in the glomeruli are more likely to be circulating immune complexes trapped in the subepithelial space than in situ immune complex formation directed against a podocyte antigen. The presence of mesangial deposits and the frequent finding of large hump-like deposits also support this hypothesis. The latter are most classically associated with trapping of circulating immune complexes in the setting of infection17 but have also been described in experimental models of serum sickness.18 The positive serologic studies such as ANA in many of the patients suggest the presence of some immune dysregulation such as an underlying autoimmune process. The light chain restriction in glomerular deposits is unusual, but is not necessarily indicative of an underlying hematologic aberrancy, as other forms of glomerulonephritis with this change commonly do not show associated disease.6, 19 It is also possible that the immune response underlying this disease is actually polyclonal but that only the IgG molecules with κ light chains have the physiochemical qualities that lead to trapping and deposition in the glomeruli. Therefore, based on what we know of MGMID and other diseases with more well-defined etiologies, we hypothesize that this disease is driven by glomerular deposition of circulating immune complexes in which the IgG κ molecules possess the appropriate properties to become trapped in the glomeruli. We are currently collecting the biospecimens necessary to further investigate this possibility.
The discovery of new diagnostic entities provides a subclassification of patients thought to have a common underlying pathogenesis. These attempts usually prove to be imperfect until the underlying etiology of disease is discovered. Prior to this, separating out disease categories based on shared clinical and morphologic parameters enables guidance for treatment, prognostication, and the ability to perform the studies necessary to identify the true pathogenesis. MGMID is at the early stage of disease classification, and, currently, the diagnostic features of this disease used as inclusion criteria are based solely on renal biopsy morphologic findings. Clearly, this is an imperfect system that brings up obvious questions. For example, should masking really be required for diagnosis? What about cases with masked λ restriction? Nevertheless, we have found that patients who have biopsy samples with the findings described as MGMID share common clinical features. They tend to be young female individuals, and frequently have nonspecific positive serologic test results indicating some role for autoimmune disease; and, perhaps the most curious aspect of this disease, the biopsy samples show monotypic Ig deposition despite the young age and absence of underlying hematologic neoplasia. We believe that the presence of these commonalities strengthens the possibility that patients with these biopsy findings share a common molecular pathogenic mechanism of their disease. We are at the advent of precision medicine with unprecedented tools available to study these pathogenic processes on a molecular level. When the underlying etiology of this disease is discovered, the diagnostic features and nomenclature that we have described will likely be replaced by terminology and diagnostic assays that more specifically define the disease. In this regard, we view the current classification criteria for this disease as a bridge to move cases that were formerly diagnosed as numerous disparate (and often incorrect) categories into a common pool that can be further studied in an effort to understand the pathogenic mechanisms of disease.
In summary, we provide an expanded case series detailing the clinicopathologic findings of patients with MGMID. This entity is newly recognized as a result of the more frequent use of paraffin IF in the renal pathology laboratory in recent years.10, 11 Based on the frequent presence of C3-only staining by routine IF, many cases would likely have been misplaced in the category of either C3 glomerulonephritis or infection-associated glomerulonephritis in the past. Diagnosis of MGMID is based on renal biopsy findings of subepithelial and/or mesangial deposits that stain for IgG κ and show significantly brighter staining by paraffin IF. We recommend performing paraffin IF to evaluate for the presence of this form of glomerulonephritis in cases that show mesangial and/or subepithelial deposits by electron microscopy with little to no Ig staining by routine IF and a lack of endocapillary proliferation by light microscopy. The patients are most commonly young female individuals <40 years of age with positive autoimmune serologic study results such as ANA, although a few carry a diagnosis of any well-defined autoimmune disease such as lupus. The outcome data were mixed, as some patients showed spontaneous remission and mild disease, whereas others progressed to ESRD. There was no apparent correlation between the treatment used and outcome in this retrospective analysis. Additional studies are needed to further define the etiology and ideal treatment regimen for this disease.
Disclosure
All the authors declared no competing interests.
References
- 1.Glassock R.J. The pathogenesis of membranous nephropathy: evolution and revolution. Curr Opin Nephrol Hypertens. 2012;21:235–242. doi: 10.1097/MNH.0b013e3283522ea8. [DOI] [PubMed] [Google Scholar]
- 2.Larsen C.P., Messias N.C., Silva F.G. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor (PLA2R1) staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 3.Beck L.H., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomas N.M., Beck L.H.J., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen C.P., Ambruzs J.M., Bonsib S.M. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86:154–161. doi: 10.1038/ki.2013.548. [DOI] [PubMed] [Google Scholar]
- 6.Nasr S.H., Satoskar A., Markowitz G. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen C.P., Messias N.C., Walker P.D. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015;88:867–873. doi: 10.1038/ki.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker P.D., Cavallo T., Bonsib S.M. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 9.Larsen C.P., Cossey L.N., Beck L.H. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol. 2016;29:421–426. doi: 10.1038/modpathol.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messias N.C., Walker P.D., Larsen C.P. Paraffin immunofluorescence in the renal pathology laboratory: more than a salvage technique. Mod Pathol. 2015;28:854–860. doi: 10.1038/modpathol.2015.1. [DOI] [PubMed] [Google Scholar]
- 11.Nasr S.H., Galgano S.J., Markowitz G.S. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70:2148–2151. doi: 10.1038/sj.ki.5001990. [DOI] [PubMed] [Google Scholar]
- 12.Qualman S.J., Keren D.F. Immunofluorescence of deparaffinized, trypsin-treated renal tissues. Preservation of antigens as an adjunct to diagnosis of disease. Lab Investig. 1979;41:483–489. [PubMed] [Google Scholar]
- 13.Fogazzi G.B., Bajetta M., Banfi G. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract. 1989;185:225–230. doi: 10.1016/S0344-0338(89)80256-0. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y.J., Reiner L. Immunofluorescence of renal lesions in paraffin-embedded and fresh-frozen sections. Am J Clin Pathol. 1980;73:116–119. doi: 10.1093/ajcp/73.1.116. [DOI] [PubMed] [Google Scholar]
- 15.Larsen C.P., Bell J.M., Harris A.A. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol. 2011;24:1462–1469. doi: 10.1038/modpathol.2011.104. [DOI] [PubMed] [Google Scholar]
- 16.Stokes M.B., Valeri A.M., Herlitz L. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorger K., Balun J., Hubner F.K. The garland type of acute postinfectious glomerulonephritis: morphological characteristics and follow-up studies. Clin Nephrol. 1983;20:17–26. [PubMed] [Google Scholar]
- 18.Fish A.J., Michael A.F., Vernier R.L. Acute serum sickness nephritis in the rabbit. An immune deposit disease. Am J Pathol. 1966;49:997–1022. [PMC free article] [PubMed] [Google Scholar]
- 19.Lai K.N., Lai F.M., Lo S.T. Light chain composition of IgA in IgA nephropathy. Am J Kidney Dis. 1988;11:425–429. doi: 10.1016/s0272-6386(88)80056-8. [DOI] [PubMed] [Google Scholar]