Abstract
Obstructive sleep apnea (OSA) is a highly prevalent chronic disease, especially in elderly and obese populations. Despite constituting a serious health, social and economic problem, most patients remain undiagnosed and untreated due to limitations in current equipment. In this work, we propose a novel method to diagnose OSA and monitor therapy adherence and effectiveness at home in a non-invasive and inexpensive way: combining acoustic analysis of breathing and snoring sounds with oral appliance therapy (OA). Audiodontics has introduced a new sensor, a tooth microphone coupled to an OA device, which is the main pillar of this system. The objective of this work is to characterize the response of this sensor, comparing it with a commercial tracheal microphone (Biopac transducer). Signals containing OSA-related sounds were acquired simultaneously with the two microphones for that purpose. They were processed and analyzed in time, frequency and time-frequency domains, in a custom MATLAB interface. We carried out a single-event approach focused on breaths, snores and apnea episodes. We found that the quality of the signals obtained by both microphones was quite similar, although the tooth microphone spectrum concentrated more energy at the high-frequency band. This opens a new field of study about high-frequency components of snores and breathing sounds. These characteristics, together with its intraoral position, wireless option and combination with customizable OAs, give the tooth microphone a great potential to reduce the impact of sleep disorders, by enabling prompt detection and continuous monitoring of patients at home.
I. Introduction
Obstructive sleep apnea (OSA) is one of the most common and serious sleep disorders, afflicting at least 25 million adults in the U.S [1], especially elderly and obese subjects. It is characterized by brief and repeated breathing pauses during sleep, which lead to hypoxia episodes and microarousals, thus disturbing the rest quality of the patient. This increases the cerebrovascular and cardiovascular mortality and morbidity, apart from the risk of traffic, domestic or work-related accidents due to daytime sleepiness. However, despite the severe implications of this disease, most patients remain undiagnosed and untreated. Reasons rely on multiple aspects, including limitations in diagnostic and therapeutic equipment.
Oral appliance devices (OA) are the main non-invasive alternative to Continuous Positive Airway Pressure (CPAP) ventilators as OSA treatment. The most common OA are mandibular-advancement-devices which produce a forward displacement of the lower jaw to increase the upper-airways (UA) space and hence avoid collapse [2,3]. Thus, this therapy recognizes the importance of the UA anatomy in the pathophysiology of OSA [4]. According to previous studies, OAs present lower efficacy but higher patients’ adherence, which can result in an equivalent therapeutic effect. Besides, their cost is usually lower than CPAP.
Regarding OSA diagnosis, the gold-standard technique is nocturnal polysomnography (PSG). It consists of recording a large amount of physiological signals from the patient during a night in the hospital, under the supervision of qualified professionals, to obtain a diagnostic index. However, PSG limitations, mainly its complexity and high cost, have led to a clear need of alternative diagnostic methods. In this context, the analysis of acoustic snoring signals has emerged as a promising approach. Snores are one of the most common and earliest symptoms of OSA and, as they are sounds produced by the vibration of anatomical structures in the UA, they contain valuable information about the degree of obstruction [5]. For this reason, several previous works have reported the use of contact or ambient microphones [5,6] to acquire full-night recordings and analyze, through different methods, the snores and apnea episodes and hence extract relevant information to detect and classify OSA patients.
In this paper we introduce a novel approach: the integration of tooth microphones into OA devices. The tooth microphone (Tooth Phone™ System for Sleep Apnea Monitoring, Audiodontics, LLC, Bethesda, MD, USA) is an intraoral audio sensor, attachable to almost any OA device. Previously, it was developed to record the vibration of teeth and dental implants when the skull is stimulated by a bone conduction hearing aid or by speech and respiratory sounds [7]. Through audio recording of nighttime respiratory sounds, this system could monitor daily patient usage and effectiveness of OA treatments [7]. At present, there is no suitable way to do that.
The objective of this work is to perform a preliminary characterization of a system which combines the tooth microphone hardware with sophisticated software that analyzes acoustic signals. We will compare these recordings with those obtained simultaneously with a commercial contact tracheal microphone. If the performance of the system is confirmed, this could lead to an enhanced simple, cheap, portable and automatic solution for accurate OSA screening and therapy monitoring.
II. Materials and Methods
A. Dataset and Acquisition Protocol
Short preliminary recordings (3–8 min) from a healthy subject, containing speech and simulated OSA-related sounds were taken. The dataset was completed with some short sleep tests (24–204 min) from the same healthy subject and one OSA patient being treated with an OA device. Breathing, snoring, and apnea events were extracted from these recordings and studied to characterize the microphones as acoustic sensors for sleep apnea detection.
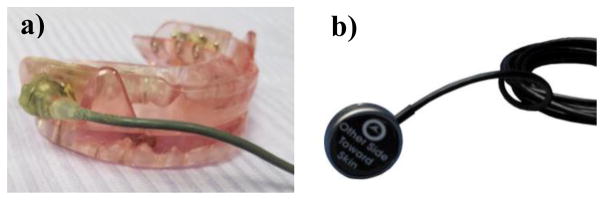
Signals were recorded in a certified sleep laboratory. The tooth microphone (also referred to as OA microphone) was integrated into the subject’s personal OA (Fig. 1a). The throat or tracheal microphone (Biopac contact sensor - TSD108, Fig. 1b) was attached to the neck of the subject 4mm off the mid-line at the crico-thyroid notch, with Littmann stethoscope double-stick disks (3M, St. Paul MN USA). Recordings were performed simultaneously with the two microphones, at a sampling frequency of 44.1 kHz and 16-bit resolution, and captured by Adobe Audition CC (release 2016). Gain to the signals (to both microphones) was independently provided by two custom Amalfi Acoustics preamplifiers prior to the capture by Audition. In order to perform the experiments with a repeatable and known gain, a previously-defined calibration procedure was carried out.
Figure 1.
Tooth microphone integrated into an OA (a). Biopac transducer (b)
B. Signals Processing and Conditioning
Signals were processed and analyzed offline, using a custom General User Interface (GUI) in MATLAB environment (Mathworks). They were downsampled to 5 kHz, applying an anti-aliasing Chebyshev low-pass filter with a cut-frequency of 2500 Hz. Power-line noise (60 Hz and harmonics) was removed using a Notch filter and an 8th order Butterworth band-pass filter between 70 Hz and 2 kHz was applied to remove cardiac and high-frequency noise and keep the band of interest for snores and breathing sounds.
C. Signals Analysis and Microphones Characterization
The quality of the signals was evaluated in time, frequency, and time-frequency domains. The signal-to-noise-ratio (SNR) was computed as the ratio of the root-mean-squared (RMS) value of the signal (in breathing and snore events) and apneas or flat regions where no sound was found.
The frequency response of each sensor was studied using the Fast Fourier Transform (FFT) calculated at 1024 points (NFFT=1024). Spectrograms were used as time-frequency (TF) representations (NFFT=1024, Hanning window of 500 points and overlap of 450) to further explore the signals.
Signals were also analyzed using an automatic snoring detector [8] to identify snores and apnea episodes. Apneas detected at each channel were compared with the manual labels assigned by a technician as a reference. A study of single snores was carried out by computing the Welch periodogram (Hanning window of 1000 points and 50% overlap) of each isolated episode as its power spectral density (PSD) representation and extracting a set of parameters from them to study the energy distribution (Table I).
TABLE I.
PARAMETERS EXTRACTED FROM SNORES PSD
| Parameter | Description |
|---|---|
| %PSD <500 Hz | Percentage of PSD energy below 500 Hz |
| %PSD 100–500 Hz | Percentage of PSD energy between 100 and 500 Hz |
| %PSD >800 Hz | Percentage of PSD energy over 800 Hz |
| ratiohl | High-low ratio (Energy at the band fs/4<f<fs/2 divided by the energy at the band 0<f<fs/4) |
| fmax | Maximum frequency: frequency accumulating 95% of the PSD energy |
D. Statistical Analysis
SNR is expressed as mean±standard deviation, while boxplots are presented for snores parameters. Mann-Whitney U-test was used to study whether there were significant differences in the SNR of each channel and in the extracted spectral parameters of snores.
III. Results
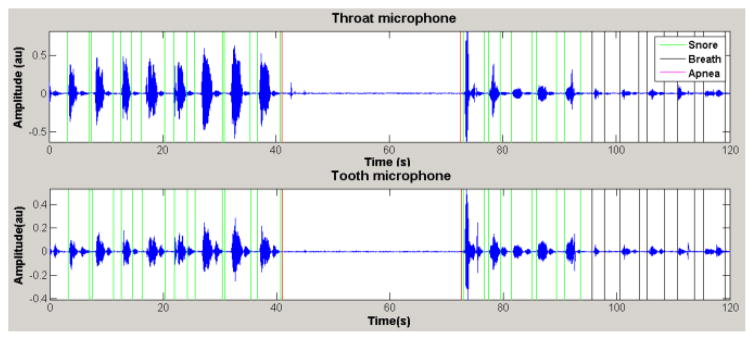
As expected, signals in time domain were quite similar in both channels, although certain differences were present in some segments due to the sensor responses. Amplitude differences were especially noticeable in regions corresponding to speech or swallow and not so great in breathing and snoring parts, which are the sounds of interest.
On the other hand, more high peaks corresponding to impulsive noise were found in the tooth channel. As it can be seen in the example of Fig. 2, in general terms, the quality of the two signals was almost equivalent. Raw signals from both channels presented remarkable electrical interference, but it was easily filtered. Once processed, their SNR clearly improved and was found to be of 14±5 dB in breathing and 28±6 dB in snoring for the tooth channel, compared with the 16±8 dB and 33±9 dB for the throat channel, respectively. No significant differences were found in the SNR between the microphones according to the statistical test.
Figure 2.
Segment of the full-night recording of the OSA patient, showing snores (green), an apnea event (red) and breathing episodes (black). Both channels have a similar SNR but some morphology differences.
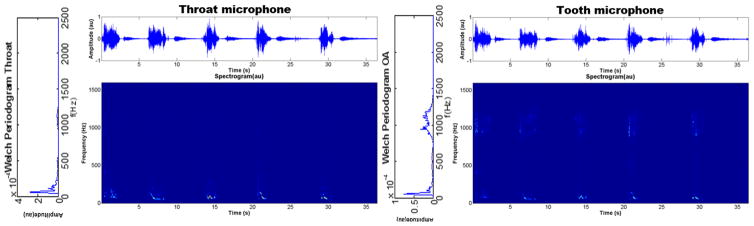
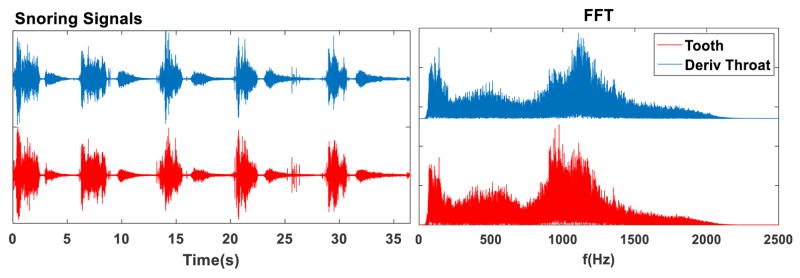
Regarding the frequency response of the sensors, while at low frequencies their patterns were similar, an interesting difference was found in the high-frequency band. PSD and TF representations of the signals from the OA channel showed a large amount of power in the band around 1–1.5 kHz, which was not present for the tracheal channel (Fig. 3). This suggests that the intraoral position of the tooth microphone induces the capture of higher frequencies which cannot be recorded with an external contact microphone. In fact, the temporal and spectral patterns of the signals from the OA microphones are like the ones of the tracheal microphone after applying a high-pass filter (Fig. 4). This will be further discussed below.
Figure 3.
Segment of simulated snores from a healthy subject, together with their time-frequency representation and the Welch periodogram of one snore (left). Higher frequency components (around 1 kHz) are captured by the tooth microphone.
Figure 4.
Result of applying a derivative filter (acting as a high-pass filter) to the tracheal channel in time (left) and frequency (right) domains. The two channels and their FFT become much more similar due to the high-pass effect.
On the other hand, the automatic detector [8] was used to identify snores and apneas (Fig. 2). Most apneic events were correctly identified in both channels, but the tooth channel was found to be much more sensitive (Table II). A reasonable snore detection was also obtained, although a few more episodes were missing in the tooth channel. It was expected because the detector had been designed for a tracheal microphone with a different frequency response.
TABLE II.
APNEA DETECTION IN BOTH CHANNELS*
| Channel | True Positives | False Positives | False Negatives |
|---|---|---|---|
| Throat | 43 | 1 | 16 |
| Tooth | 57 | 2 | 2 |
Technician manual inspection as a reference
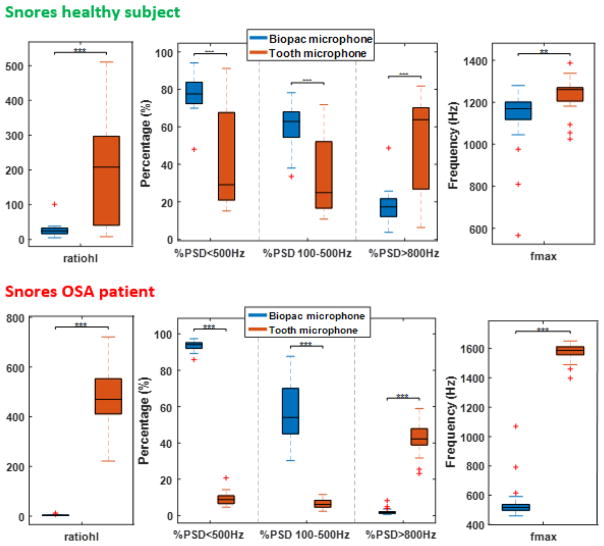
Once snores had been isolated, the PSD of each one was computed (i.e. Fig. 3) and a series of quantitative spectral parameters were extracted (Fig. 5). These values indicate the clear higher contribution of the tooth microphone at higher frequencies. Differences are even greater in the snores of the OSA subject. At the low-frequency band (<500–600 Hz), where snores components concentrate most of their power (at least, as it has been described historically by analysis of throat microphone signals), both sensors show a similar behavior. As our aim was to compare the microphones, the differences between the snores of the healthy and the OSA subjects are not listed in this work.
Figure 5.
Boxplots of the parameters extracted from the Welch periodograms of single snores from the healthy subject (top) and the OSA patient (bottom). These distributions indicate significant differences (**: p-value=0.01, ***: p-value=0.001) in the frequency response of the sensors. The tooth microphone captures a higher amount of high-frequency components.
IV. Discussion
Finding alternative systems for OSA diagnosis and treatment is essential to reduce the overall impact of this disease related to public health and economic costs. In this work, we are characterizing a system which combines novel screening and diagnosis strategies (acoustic analysis of snoring) with alternative non-invasive therapeutic options (OA treatment). The sensor which supports this approach is a tooth microphone.
We studied the response of this sensor in the time, frequency, and time-frequency domains, in comparison with a commercial tracheal microphone (Biopac transducer), using some preliminary test signals containing OSA-related sounds. As we are interested in the use of the tooth microphone for OSA monitoring, we carried out a study of single-episodes focused on snores and apnea events, which contain the most valuable diagnostic information.
We found that the temporal patterns of the signals provided by both microphones were quite similar, presenting comparable SNR. This indicates that the new sensor, apart from having a broad dynamic range, is able to provide quality acoustic signal as good as the ones of currently used contact microphones. The OA channel showed more peaks of impulsive and high-frequency noise, what could be expected since any noise inside the mouth (i.e. any “click” of the device or teeth grinding action) are closer to this microphone. However, this noise can be reduced by appropriate advanced filtering and the intraoral location may have other advantages such as a more accurate recording of snores and respiratory sounds. Its benefits also include a better and more repeatable human coupling, since being integrated into the OA it functions as if rigidly attached to the skull, does not move throughout the night and can be calibrated.
The SNR is lower in normal breathing than in snores, due to the obvious difference in the sound intensity. Having a sensor which is able to distinguish even low-amplitude breaths from background noise is critical for apnea detection in diagnostic applications. This has to do not only with the quality of the sensor but also with an accurate filtering stage. In our raw signals at 44.1 kHz (for both microphones), many breathing events were completely masked by noise. However, after resampling and filtering, keeping just this range between 70 and 2000 Hz as previous works [8–9], these respiratory episodes are no longer confused with apneas.
One of the most interesting points of this study relies on the frequency response of the tooth microphone. Probably because of its intraoral position, it captures high-frequency components, which could be either noise or intrinsic information from snores. Our hypothesis is that the internal vibration of snores could be rich in harmonics, but the impedance of the internal oropharyngeal tissues and external muscle and skin attenuates them, acting as a low-pass filter, so the vibrations do not reach the external microphone. This would explain why currently used tracheal sensors are not able to capture these components, and thus why they have not been reported before. We tried to check this hypothesis by filtering the throat signal using a first-order moving average derivative filter and normalizing the result. As shown in Fig. 4, the obtained signal is quite similar both in time and frequency domains to the one of the OA microphone. This finding could open up new opportunities to study the vibration patterns of breath and snores and how they are altered in case of pathological obstructions.
V. Conclusion and Future work
The proposed system, comprised of a tooth microphone integrated into an OA device, can enhance OSA patients monitoring by acoustic analysis of snoring and respiratory sounds. In this work, we have shown that it is a feasible solution to record OSA-related sounds, providing signals of a good SNR, comparable to that of commercial tracheal microphones. Moreover, it captures high-frequency components that are not found with external sensors and which could contain acoustic information relevant for the study of the disease. Whether this has a physiological origin or is simply amplified noise should be further explored.
The main limitation of this study rely on the number of available samples and patients. Future work will include testing this system with full-night signals, both from healthy and OSA subjects, and comparing it with PSG, the gold-standard technique. Efforts will also focus on exploring the high-frequency components of snores and checking if an advanced filtering or different detection algorithm are required to adapt to the new sensor. Even so, this preliminary characterization has shown that the tooth microphone is able to perform at least as well as other commercial models when recording speech, breathing and snoring sounds. Moreover, its intraoral position, wireless potential and coupling to a customizable OA give this novel sensor a valuable chance for personalized medicine. The resulting combination of acoustic analysis of snoring and OA therapy can constitute an improved and inexpensive method of monitoring OSA patients at home, thus reducing the serious health burdens of this disease.
Acknowledgments
This work was supported in part by Audiodontics LLC; the National Heart, Lung and Blood Institute (SBIR-NIH grant, ref.1R43HL123090-01A1); Secretariat of Universities and Research of the Department of Economy and Knowledge of the Government of Catalonia (Consolidated research group GRC 2014 GR 1569), the CERCA Programme/Generalitat de Catalunya and by the Spanish Ministry of Economy and Competitiveness through the project DPI2015-68820-R (MINECO/FEDER).
Footnotes
Financial Disclosure and Competing Interests
The funders had no role in study design, data collection and analysis or preparation of the manuscript. The co-authors from IBEC (YC, DB and RJ) led and develop this study as an independent research to compare different microphones for monitoring OSA patients. The co-authors from Audiodontics participated to acquire the signals, but not in the signal processing or data interpretation. Therefore, the authors have declared that no competing interests exist in this paper.
Contributor Information
Yolanda Castillo, Institute for Bioengineering of Catalonia (IBEC) and CIBER de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Barcelona, Spain.
Dolores Blanco-Almazán, IBEC, CIBER-BBN and ESAII Department, Universitat Politècnica-Barcelona Tech (UPC), Spain.
James Whitney, Amalfi Acoustics, LLC, Baltimore, MD and Audiodontics, Bethesda, MD, US.
Barry Mersky, Audiodontics, Bethesda, MD, US.
Raimon Jané, IBEC, CIBER-BBN and ESAII Department, Universitat Politècnica-Barcelona Tech (UPC), Spain.
References
- 1.Darien I. Rising prevalence of sleep apnea in U.S. threatens public health. National Healthy Sleep Awareness Project AASM News Archive. 2014 Sep; http://www.aasmnet.org/articles.aspx?id=5043.
- 2.Sutherland K, Vanderveken O, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014. 2015 Feb;10(2):215–227. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramar K, Dort L, Katz S, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 2015. 2015 Jul;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007 Aug;132(2):123–125. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 5.Pevernagie D, Aarts RM, De Meyer M. The acoustics of snoring. Sleep Med Rev. 2010 Apr;14(2):131–144. doi: 10.1016/j.smrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Jin H, Lee LA, Song L, et al. Acoustic analysis of snoring in the diagnosis of obstructive sleep apnea syndrome: a call for more rigorous studies. J Clin Sleep Med. 2015 Jul;11(7):765–771. doi: 10.5664/jcsm.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mersky BL, Masri R, Tan K, Kempler J, Hwang-Lee H, Whitney JE. Audiodontics, Bethesda MD, Dental School, University of MD-Balto, Morgan State Univ, Balto MD. “Abstract: Recording breath sounds with a novel tooth microphone”. 22nd Annual Meeting of the American Academy of Dental Sleep Medicine; 2013. [Google Scholar]
- 8.Jané R, Fiz JA, Solà-Soler J, et al. Automatic snoring signal analysis in sleep studies. Conf Proc IEEE Eng Med Biol Soc 2003. 1:366–369. [Google Scholar]
- 9.Fiz JA, Jané R, Solà-Soler J, et al. Continuous analysis and monitoring of snores and their relationship to the apnea-hypopnea index. Laryngoscope. 2010 Apr;120(4):854–862. doi: 10.1002/lary.20815. [DOI] [PubMed] [Google Scholar]