SUMMARY
Traumatic brain injury (TBI) is a leading cause of morbidity and disability, with a considerable socioeconomic burden. Heterogeneity of pathoanatomical subtypes and diversity in the pathogenesis and extent of injury contribute to differences in the course and outcome of TBI. Following the primary injury, extensive and lasting damage is sustained through a complex cascade of events referred to as “secondary injury”. Neuroinflammation is proposed as an important manipulable aspect of secondary injury in animal and human studies. Because neuroinflammation can be detrimental or beneficial, before developing immunomodulatory therapies, it is necessary to better understand the timing and complexity of the immune responses that follows TBI. With a rapidly increasing body of literature, there is a need for a clear summary of TBI neuroimmunology. This review presents our current understanding of the immune response to TBI in a chronological and compartment-based manner, highlighting early changes in gene expression and initial signaling pathways that lead to activation of innate and adaptive immunity. Based on recent advances in our understanding of innate immune cell activation, we propose a new paradigm to study innate immune cells following TBI that moves away from the existing M1/M2 classification of activation states towards a stimulus and disease-specific understanding of polarization state based on transcriptomic and proteomic profiling.
Keywords: TBI, Microglia, Astrocytes, Neutrophils, Models, Transcriptome, Neurodegeneration, M1, M2, Neuroimmunology, Neuroinflammation
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of death and disability worldwide, with a high incidence in both military and civilian populations (Maas et al., 2008). About 1.7 million people in the United States are afflicted by TBI annually, which contributes to 30% of all injury-related deaths and an annual cost of around $60 billion (Langlois et al., 2006; Nguyen et al., 2016; Roozenbeek et al., 2013). Traumatic brain injuries are especially challenging to treat because they are heterogeneous in nature and often induce complex pathogenesis pathways. The mechanical effects of trauma initiates injurious biochemical cascades collectively referred to as the “secondary injury” (Bramlett and Dietrich, 2015). However, because of the heterogeneity of the initital trauma, it is often difficult to reconstruct the precise events leading from primary to secondary injury (Blumbergs PC, 2008). Clinical outcome following TBI is determined by the nature and severity of the primary injury as well as additional insults such as hypoxemia, hypotension, and intracranial fluid dynamics, among others.
Because of all these contributing factors, no effective interventions were shown to improve functional outcome in survivors despite extensive effort to develop neuroprotective therapies for TBI patients (Gruenbaum et al., 2016; Janowitz and Menon, 2010; McConeghy et al., 2012). To better understand TBI pathogenesis and develop treatments, experimental animal models are routinely used (Marklund and Hillered, 2011; Xiong et al., 2013). The challenge with animal models is that each one reflects a specific pathoanatomic type of injury and is influenced by age, gender, and genetic background of the species under investigation, among other factors. No single model can fully recapitulate all types of primary and secondary damage observed following human TBI, as well as the complex diversity of injury mechanisms and other factors that contribute to outcome in a given individual (Saatman et al., 2008). Inadequate modeling of TBI may be one reason why therapies that showed promise in preclinical studies have failed in clinical trials (Chakraborty et al., 2016; Hawryluk and Bullock, 2016; Marklund et al., 2006; Simon et al., 2017), as diverse pathoanatomic injury subtypes make it challenging to stratify patients in clinical trials (Margulies et al., 2009) and devise effective therapies.
The key to developing effective therapies for TBI is to better understand and identify the precise mechanisms underlying TBI-related primary vs. secondary pathology. Primary injury occurs at the time of head impact and causes direct damage to neural tissue (Maas et al., 2008). Focal intracranial hemorrhage, epidural and subdural hematoma, brain contusion, and direct axonal damage are all examples of primary lesions (Maas et al., 2008). By contrast, secondary injury develops minutes to months following the mechanical insult, progressively contributing to neurological impairment. At the cellular level, secondary injury is mediated by several pathways including, but not limited to: (a) excitotoxicity caused by an excess of the neurotransmitter glutamate (Dorsett et al., 2017; Faden et al., 1989), (b) free radical generation causing damage to proteins and phospholipid membranes of neural cells (Anthonymuthu et al., 2016), and (c) the neuroinflammatory response comprised of local and systemic immune activation (Simon et al., 2017).
There is increasing interest in the role that the immune system plays in TBI pathogenesis. Some have proposed that immune modulation might significantly change the clinical outcome in TBI patients (Bergold, 2016). A sterile immune response develops within minutes of TBI and includes local signaling in neurons, glia, and recruited peripheral immune cells that induces an inflammatory cascade (Figure 1) (Corps et al., 2015). The activation of immune cells evolves over time, possessing both beneficial and pathogenic components, which might explain why attempts to broadly target immune activation have been unsuccessful in altering clinical outcome in TBI patients (Edwards et al., 2005; Roberts et al., 2004). It is therefore of utmost importance to understand the mechanisms underlying immune activation and function following TBI. A basic premise is that inflammation is associated with almost all brain injuries. In this review, we provide a panoramic view of the immunological events that follow TBI and highlight the beneficial vs. pathogenic aspects of the response. Based on recent advances in our understanding of innate immune cell activation and classification of macrophage / microglia polarization states, we propose a new paradigm to study innate immune cells in TBI that moves away from the simplistic M1/M2 classification scheme towards a stimulus and disease-specific delineation of innate immune functions based on transcriptomic and proteomic profiling. We also highlight several knowledge gaps in our understanding of how the immune response is temporally influenced by various types of brain injury and how filling such gaps can guide development of future TBI therapies.
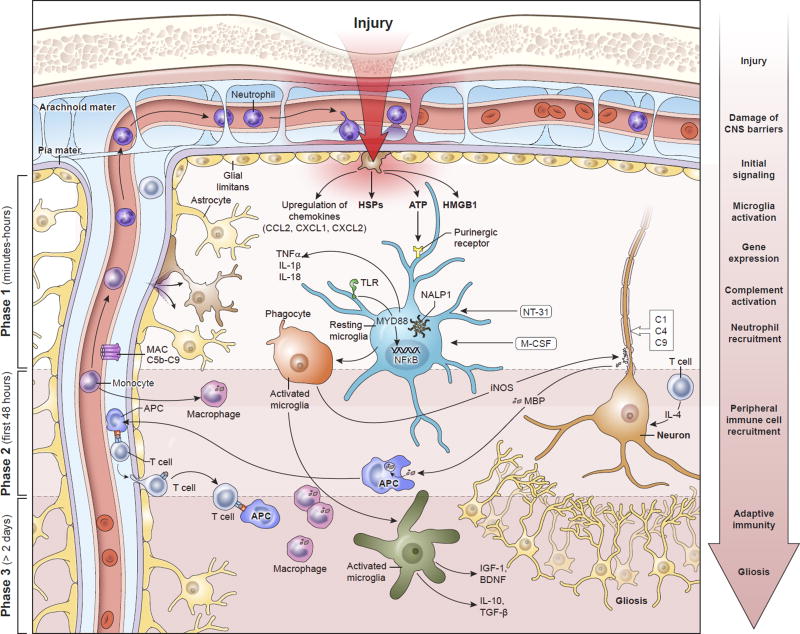
Figure 1. Temporal progression of the immune response to contusion TBI.
Phase I begins within minutes of brain injury due to the release of alarmins from the damaged meninges, glial limitans, and parenchyma, such as ATP, HSPs, HGMB1, etc. These signals bind to PAMP and DAMP sensors like TLRs and purinergic receptors that induce immediate activation of resident myeloid cells (e.g. microglia) and inflammasome assembly (NALP1) that promotes the generation of mature IL-1β and IL-18. In addition, NFκB translocates to the nuclei of these cells and induces an immunological program involving cellular proliferation and the release inflammatory amplifiers such as chemokines, cytokines, ROS, and NO, among others. Phase 1 also includes complement activation and the recruitment of neutrophils to the meninges and perivascular spaces. Neutrophil recruitment depends in part on purinergic receptor signaling. Secondary damage to CNS tissue occurs in Phase 1 and can continue into Phase 2. This can be mediated by inflammatory cytokines, complement, and ROS. T cells and monocytes are recruited to the damage site in Phase 2, where monocytes convert into macrophages and T cells have the ability to produce neuroprotective cytokines in response to alarmins. Macrophages participate in the cleanup of debris and damaged cells. Based on their state of functional activation, they can either promote further damage or initiate the process of inflammatory resolution and tissue repair. Inflammation can continue for an extended period of time into Phase 3. Self-antigens released from damaged neural cells can be presented by local APCs to T cells. The ideal outcome during Phase 3 is resolution of the inflammatory response, release of trophic factors, and isolation damaged areas via astrocytes. However, this is does not always occur following TBI and chronic inflammation can persist. Abbreviations: APC, antigen-presenting cell; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CCL2, chemokine (C-C motif) ligand 2; CXCL1, chemokine (C-X-C motif) ligand 1; CXCL2, chemokine (C-X-C motif) ligand 2; DAMP, damage associated molecular pattern molecules; HSPs, heat shock proteins; HMGB1, high mobility group box 1 protein; IGF-1, insulin-like growth factor-1; IL-1, interleukin-1; IL-4, interleukin-4; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; MyD88, myeloid differentiation primary response gene 88; NALP1, NAcht leucine-rich repeat protein 1; NT-3, neurotrophin-3; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; MBP, myelin basic protein; M-CSF, macrophage colony stimulating factor; NO, nitric oxide; PAMP, pathogen-associated molecular pattern; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; TLR, toll-like receptors; TNF-α, tumor necrosis factor-alpha
Innate and adaptive immunity
The immune system is used as a defense against many different pathogens and insults. It can also participate in the process of wound healing following injury. One arm of the immune system is classified as “innate” and defined by its ability to rapidly respond in a non-specific manner, with a limited capacity to remember the antigens it encounters. In addition to anatomical (e.g. skin) and physiological (e.g. temperature, pH) barriers, the innate immune system includes cellular effectors such as phagocytes (e.g. neutrophils, macrophages, microglia, dendritic cells), granulocytes (e.g. eosinophils, basophils, mast cells, neutrophils), innate lymphoid cells (e.g. natural killer cells), and unconventional T lymphocytes (e.g. γδ T cells) (Chaplin, 2010; Sonnenberg and Artis, 2015). These effector cells often use pattern recognition molecules such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), and scavenger receptors to sense pathogens and danger signals that inititate innate immune responses (Chaplin, 2010; PrabhuDas et al., 2017). Innate immune cells can then release chemokines and cytokines that amplify the immune response by recruiting other immune cells and promoting inter-cellular signaling at the site of injury or infection and distal signaling via the circulatory system. Another important component of innate immunity is complement, which is a family of proteins that have diverse roles in inflammatory processes, including pro-inflammatory signaling (anaphylatoxins), marking cells for uptake by other cells (opsonization), and formation of membrane attack complexes that cause direct cellular damage (Chaplin, 2010; Noris and Remuzzi, 2013). Collectively, the innate system possesses a diverse array of cellular and molecular defense strategies that slow the progress of invading pathogens. However, it is important to note that elements of this same system also participate in tissue remodeling and wound-healing following injury.
The innate immune response sets the stage and is followed by “adaptive” immunity, which is a more tailored response that typically involves the activation and expansion of T and / or B lymphocytes. CD8 and CD4 T lymphocytes recognize peptide antigens displayed in major histocompatibility complexes and can have cytotoxic, helper, or regulatory functions. B lymphocytes, on the other hand, produce immunoglobulins and can participate in the activation of T cells (Warrington et al., 2011). Two major characteristics of the adaptive immune system include antigen-specificity and the ability to “remember” previously encountered antigens. The latter permits rapid recall responses upon secondary pathogen challenge (Schenkel and Masopust, 2014; Weisel and Shlomchik, 2017). While the adaptive immune system plays a critical role in our defense against pathogens, in autoimmunity it can also be directed against self-antigens. Autoimmune reactions cause tissue pathology and disease, but some forms of transient autoimmunity that develop after tissue injury can be beneficial (Schwartz and Raposo, 2014).
Traumatic brain injury models
Human TBI is a heterogenous disease and includes cerebral contusion, concussion, and blast injury. Cerebral contusion is one of the major subtypes of human TBI modeled in animals. It is produced by focal brain injury and modeled in the laboratory by weight drop, lateral fluid percussion, or controlled cortical impact (Marklund et al., 2006; Xiong et al., 2013). The resulting focal contusions are characterized by parenchymal/extra-axial bleeding, blood brain barrier (BBB) damage, edema, progressive neuronal death, axon injury, and a robust parenchymal inflammatory response featuring leukocyte infiltration. On the other end of the pathological spectrum is concussion, defined as a functional alteration of the brain with less obvious structural damage on routine conventional imaging, often resulting from impact/acceleration forces (McCrory et al., 2017). A recent study, for example, demonstrated evidence of meningeal vascular leakage in ~50% of concussed patients that were determined to be otherwise normal based on computerized tomography (CT) scans (Roth et al., 2014). A source of confusion in the TBI literature is the use of the term “mild TBI”, which is often used interchangeably to describe contusion and concussion models. Because the inflammatory response to these two pathoanatomical subtypes might be quite different, meningeal and brain inflammation should be considered in the context of the specific TBI lesion as well as injury severity. Thus, mild and severe contusion models should be expected to produce a different inflammatory response than mild or severe concussion models. In general, concussion models feature gliosis, but a less robust inflammatory response than contusion. Inflammation is not as well characterized in concussion models. The bias towards using focal brain injury models to describe inflammation in TBI risks inappropriately generalizing these data to concussion, blast, and other pathoanatomic TBI models that may have their own unique inflammatory responses. This point is underscored by the finding that targeting the same inflammatory pathways in experimental contusion and concussion models can result in opposite effects on neurological outcomes (Bermpohl et al., 2007; Khuman et al., 2011; Park et al., 2012; Zhu et al., 2014). Another type of TBI is induced by blast, which results in axonal injury / neurodegeneration and has inflammatory features that differ from contusion and concussion (Xiong et al., 2013). Because of such differences, we will focus throughout this review only on the concussion and contusion forms of TBI and frame our discussion of inflammatory responses by including commentary about the specific TBI model under investigation.
Initial signaling
Expression of inflammatory factors occurs early after TBI, which helps orchestrate the activities of local and peripherally-derived immune cells. The following is a synopsis of some key mediators in this process.
Danger signals
Immune signaling in the damaged or infected central nervous system (CNS) is mediated in part by molecules referred to as damage and pathogen associated molecules patterns (DAMPs including alarmins and PAMPs, respectively) (Bianchi, 2007). These molecules interact with receptors such as Toll-like receptors (TLRs) nucleotide-binding oligomerization domain (NOD) like receptors (NLRs) and scavenger receptors that serve as “danger” sensors and help initiate the inflammatory cascade (Jounai et al., 2012; Matzinger, 1994; PrabhuDas et al., 2017). TLR4, which recognizes lipopolysaccharides and multiple endogenous proteins such as high mobility group box 1 protein (HMGB1), heat shock proteins (HSPs), low-density lipoprotein, etc., is upregulated in neural and immune cells following TBI (Lee et al., 2013). Brain injury in humans also induces expression of the TLR adaptor protein, myeloid differentiation primary response gene 88 (MyD88) (Li et al., 2013). After recognition of endogenous proteins released by damaged cells (such as alarmins (Bianchi, 2007) and other DAMPs), a cellular response is triggered that involves several kinases, including NFκB-inducing kinase (Lee et al., 2013). NFκB expression is elevated after TBI and associated with increased levels of inflammatory cytokines such as IL-6 and TNF-α (Hang et al., 2005). The functional role of TLR4 in this process was examined using controlled cortical impact (CCI), a model of cerebral contusion TBI. In this study, TLR4-deficient mice had smaller brain lesions and a reduced expression of inflammatory markers after CCI when compared to wild type controls (Ahmad et al., 2013). Similarly, a highly selective TLR4 blocker, VGX1027, lessened cerebral edema after TBI in mice, presumably by blocking HMGB1-induced IL-6 release from microglia and subsequent upregulation of the water channel, aquaporin 4 (AQP4), on astrocytes (Laird et al., 2014). HMGB1 is a dual function protein that acts as a cytokine and binds chromatin that serves as a danger signal following tissue injury (Goodwin et al., 1973; Klune et al., 2008; Scaffidi et al., 2002). Immune cells release this protein (acting as a cytokine), which can bind to TLR4 or the Receptor for Advanced Glycation End products (RAGE) and initiate an inflammatory response (Klune et al., 2008; Parker et al., 2017; Wang et al., 1999). HGMB1 and RAGE are expressed in animals and humans following brain injury (Gao et al., 2012), and inhibition of these pathways reduces BBB breakdown and inflammation (Okuma et al., 2012) as well as pulmonary dysfunction following cerebral contusion (Weber et al., 2014). Whether HMGB1 plays a role in concussion TBI remains to be explored.
Heat shock proteins (HSPs) represent another important protein family involved in the response to tissue damage. HSPs are protein chaperones induced upon cellular stress, especially during states of injury and wound healing (Binder, 2014; Zuo et al., 2016). HSPs are known to participate in early TLR / NLR signaling and can even serve as TLR ligands. Interestingly, HSPs can have both pro- and anti-inflammatory effects. For example, HSPs can stimulate immune responses by playing an active role in antigen-presentation (Binder, 2014). On the other hand, HSP70 can suppress the lethality associated with tumor necrosis factor-α (TNFα) by inhibiting IL-6 and nitric oxide (NO) production (Van Molle et al., 2002). Following CCI-induced TBI, overexpression of HSP70 reduced brain lesion size, hemorrhage, and expression of metalloproteinases, whereas the opposite was observed in HSP70-deficient mice (Kim et al., 2013). Deficiency in HSP110 similarly resulted in enhanced brain damage following TBI (Eroglu et al., 2014). Modulation of HSPs therefore has the potential to improve outcome in TBI patients. Along these lines, several studies in animals have shown that therapeutic induction of HSP70/110 is indeed neuroprotective (Eroglu et al., 2014; Kim et al., 2015). One recent study used 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), which is a potent HSP90 antagonist known to increase HSP70 levels (Kim et al., 2015). Administration of 17-AAG to mice after CCI enhanced expression of HSP70 in microglia and neurons, reduced brain lesion size, and improved neurological function. Collectively, these data indicate that HSPs may represent viable therapeutic targets following contusion TBI (Kim et al., 2012); however, additional studies are required to determine the temporal contributions of HSPs to brain injury responses in different TBI models as well as how specific HSPs guide sterile inflammation and wound healing.
Studies showed that TLRs require the presence of co-receptors such as the scavenger receptors SCARF1 and CD36, to promote DAMP signaling (Means et al., 2009; PrabhuDas et al., 2017; Stewart et al., 2010). The role of scavenger receptors in the setting of TBI is an area where much future work needs to be focused. This may have therapeutic implications as scavenger receptors have been proposed as targets for therapy in a variety of CNS conditions including Alzheimer’s Disease (Frenkel et al., 2013; Wilkinson et al., 2011). Of particular interest is the effect of targeting the scavenger receptors CD36, MEGF10, and SCARF on the clearance of debris and apototic cells in the CNS (Iram et al., 2016; Loov et al., 2012; Prabhudas et al., 2014; PrabhuDas et al., 2017; Ramirez-Ortiz et al., 2013). Although danger signals and their potential receptors are well studied in focal contusion models, their role, if any, in concussion models is less well investigated and represents a future direction in need of further investigation.
Purinergic receptors also play an important role in sterile injury responses following CNS injury (Corps et al., 2015; Davalos et al., 2005; Lou et al., 2016; Nimmerjahn et al., 2005; Roth et al., 2014; Wang et al., 2004). Tissue damage induces adenosine triphosphate (ATP) release into the extracellular milieu, which serves as an alarmin that activates the immune system (Eltzschig et al., 2012; Junger, 2011). The effects of ATP are regulated in part by glial cells that can promote a stepwise conversion of ATP to adenosine diphosphate (ADP) and ultimately adenosine. This is achieved by two different ectoenzymes referred to as ectonucleoside triphosphate diphosphohydrolase1 (CD39) and ecto-5′-nucleotidase (CD73). Adenosine binds to P1 purinergic receptors, whereas ATP and ADP are the ligands for P2 receptors. The responses elicited by purinergic receptor signaling are diverse and dictated by the receptor expression pattern, type of immune cells present, nature of the injury and time post injury. For example, purinergic receptor signaling plays a critical role in the early activation and morphological transformation of microglia following laser and mild TBI induced brain injury (Davalos et al., 2005; Nimmerjahn et al., 2005; Roth et al., 2014). Antagonism of early microglial responses through purinergic receptor blockade resulted in more damage in models of mild TBI (Lou et al., 2016; Roth et al., 2014). In this context, inhibiting P2RY12 function can have detrimental secondary effects on the BBB (Lou et al., 2016). Microglia also express P2RY6, a UDP receptor that regulates the phagocytic capacity of these cells (Koizumi et al., 2007), and a recent study demonstrated in a model of focal cortical contusion that inhibition of this receptor impeded the conversion of microglia into phagocytes and elevated the number of dead cells in the neocortex (Roth et al., 2014). Collectively, these data demonstrate purinergic receptor signaling helps to shape the function of microglia following TBI.
In addition to its effects on microglia, the purinergic system also promotes recruitment of neutrophils to sites of damage (McDonald et al., 2010; Roth et al., 2014). In a focal TBI model, P2X7 signaling was shown to promote neutrophil recruitment to the damaged meninges within 1-3 hours (Roth et al., 2014). These cells migrated exclusively to the meninges, and transcranial administration of a P2X7 antagonist resulted in enhanced meningeal damage. These data suggest that early purinergic receptor signaling can promote neuroprotective immune reactions following CNS injury. Thus, it might be beneficial to therapeutically agonize these responses or avoid interfering with them in the acute phase of injury. It remains to be determined how purinergic receptor signaling contributes to the later phases of the TBI immune response.
The role of the purinergic system is not restricted to microglia and neutrophils. Astrocytes also express purinergic receptors such as P2RY1. In fact, stimulation of P2RY1 in a closed head injury model significantly reduced edema, neuronal swelling, and reactive gliosis via a IP(3)-signaling pathway (Talley Watts et al., 2013). This was attributed to expression of P2RY1 on astrocytes. In addition to the above pathways, purinergic signaling can also contribute to activation of inflammasomes. Extracellular ATP released by dying or dead neurons activates P2X7 receptors on microglia, and possibly astrocytes, that in turn activate the NLRP3 inflammasome (Kimbler et al., 2012; Mariathasan et al., 2006; Roth et al., 2014).
Inflammasomes
NLRs are cytosolic receptors for PAMPs and DAMPs. Upon recognition of DAMPs, a subset of NLR (e.g. NLRP1, NLRC4, NLRC5, NLRP6, etc.) and non-NLR (e.g. absent in melanoma 2; AIM2) proteins can assemble into a macromolecular complex referred to as an inflammasome (Gold and El Khoury, 2015; Martinon et al., 2002). This structure then promotes cleavage of pro-capase 1 into its active form (capase 1) via interactions with caspase activation and recruitment domains (CARD) located within the inflammasome or in association with an adaptor protein referred to as apoptosis-associated speck-like protein containing a CARD (ASC) (Freeman and Ting, 2016; Martinon et al., 2002). Active caspase 1 then induces the generation of mature interleukin-1β (IL-1β) and IL-18, which play an important role in inducing sterile immune responses following brain injury (de Rivero Vaccari et al., 2009; Freeman and Ting, 2016; Walsh et al., 2014).
Inflammasomes can be assembled in multiple CNS cell populations, such as microglia, macrophages, astrocytes, and neurons, and upon activation can participate in the generation of pro-inflammatory cytokines (Liu et al., 2013; Walsh et al., 2014). Interestingly, inflammasome proteins such as NLRP1, ASC, and caspase-1 have been detected in the CSF of patients with moderate and severe TBI, and heightened levels of these proteins correlated with a more unfavorable neurological outcome (Adamczak et al., 2012). Thus, inflammasome proteins might serve as a useful biomarker for inflammation and injury severity in TBI patients. To date, few studies have focused on the role of inflammasome assembly and activation at different stages of TBI. Using the fluid percussion animal model of TBI, one study demonstrated that intracerebroventricular injection of anti-ASC antibodies immediately after injury reduced capase-1 activation as well as IL-1β production and significantly reduced the brain lesion volume at 3 days post-injury (de Rivero Vaccari et al., 2009). These data indicate that interference with the inflammasome pathway is neuroprotective in fluid percussion TBI. However, a recent study showed no benefit of NLRP1 and ASC knockout on histopathology and motor recovery in a mouse CCI model (Brickler et al., 2016). Thus, additional studies are required to determine if all inflammasome activation is uniformly pathogenic following TBI, or whether the effects of inflammasome activation may be cell type specific (e.g., neurons-NLRP1) (de Rivero Vaccari et al., 2009) vs. (astrocytes-NRLP3) (Hailer, 2008) and TBI model-dependent.
Inflammatory gene expression
Following detection of DAMPs and alarmins, a program of acute inflammatory gene expression is induced that guides the development and functionality of the ensuing immune response (Lagraoui et al., 2012). Using whole brain tissue, the pattern of inflammatory gene expression is surprisingly similar between mild brain injury such as craniotomy alone and craniotomy followed by cerebral contusion (i.e. CCI) (Lagraoui et al., 2012). Genes associated with chemotaxis (CCL2, CCL3, CCL4, CXCL1, CXCL4), cytokine signaling (IL-1β, IL-6, IL-12, IFNγ, IL-10, TGF-β), antigen presentation (MHC II, CD74, CD86), phagocytosis (C3, C4, FCGR1, FCGR2, FCGR4), and astrocyte activation (GFAP, AQP4), among others are all upregulated following craniotomy alone and craniotomy with CCI (Lagraoui et al., 2012). The primary difference between these two models of brain injury was the magnitude and duration of inflammatory gene expression. Expression levels were lower and returned to baseline more quickly (within 10 days) following craniotomy alone. Of note is that the differences observed were measured in whole brain tissues and not in individual cells. It is not clear if different cells have different gene expression profiles kinetics. In a separate study comparing fluid percussion injury (FPI) with CCI, 89% of the differentially regulated mRNA observed after mild FPI, including genes involved in inflammation, were also altered in mild CCI (Redell et al., 2013). These data suggest that the duration and magnitude of brain inflammation following TBI may be linked in part to the severity and extent of the initial injury.
Expression profiling of brain tissue in TBI has been mostly limited to a small number of genes selected for potential role in the inflammatory response (Morganti et al., 2016) or focused on whole brain tissue not on individual cells (Lagraoui et al., 2012; Meng et al., 2017). Global gene expression of individual cells such as microglia, macrophages, monocytes, or astrocytes has not been evaluated using new methodologies including single cell RNAseq and thus remains a knowledge gap in the field that needs to be addressed. It is also critical to understand the temporal patterns of inflammatory gene expression and immune cell infiltration in all major TBI models (contusion, concussion, repetitive concussion, blast injury, rotational acceleration, etc.), because it is possible that each model will reveal unique inflammatory features. Inflammatory profiles that are initially neuroprotective may change over time to become maladaptive (or, vice versa) in some models, but not others. Recent studies indicate that inflammatory gene expression does indeed change over time, and differences have been noted between the site of injury and distal brain regions (Almeida-Suhett et al., 2014; Graber et al., 2015; Lagraoui et al., 2012). There is evidence supporting that a unilateral brain injury resulting in deformation of the cortex can cause inflammation in ipsilateral and contralateral hippocampus (Almeida-Suhett et al., 2014). These changes in the hippocampus following focal trauma included upregulation of CC chemokine ligand 2 (CCL2) and 7 (CCL7), lipocalin-2 (LCN2) and tissue inhibitor of metalloproteinase 1 (TIMP1) within 24 hrs, followed by increased expression of low affinity immunoglobulin gamma Fc region receptor II (FCGR2), C3, MHC II, CD74, and Kruppel-like factor 4 (KLF4) at a later time points. Lasting alterations in CNS gene expression might explain some of the neurological sequelae observed in TBI patients.
Resident microglial activation
Microglia are sentinel CNS residents and are usually among the first responders to brain injury (Davalos et al., 2005; Fourgeaud et al., 2016; Kitamura et al., 1978; Nayak et al., 2014; Nayak et al., 2012; Nimmerjahn et al., 2005; Roth et al., 2014). They are ontologically distinct from bone-marrow-derived monocytes / macrophages and are derived instead from primitive macrophages coming from the embryonic yolk sac during development (Ginhoux et al., 2010). Under steady state conditions, microglia express a cluster of genes that allow them to sense and screen their surroundings for inflammatory cues, promote neuronal survival, contribute to activity-dependent synaptic remodeling, and phagocytose damaged cells (Hickman et al., 2013; Nayak et al., 2014; Tremblay et al., 2011). After focal brain injury, microglia usually respond within minutes by projecting processes toward the sites of damage (Davalos et al., 2005; Fourgeaud et al., 2016; Nimmerjahn et al., 2005; Roth et al., 2014), and noninvasive imaging studies in humans suggest that microglia activation can be sustained for many years after traumatic brain injury (Ramlackhansingh et al., 2011). Even in concussion models that lack acute cell death, BBB damage, brain edema, hemorrhage, parenchymal neutrophil infiltration, and significant axon injury, robust microgliosis and astrocytosis was observed in injured brain at early and at chronic time points after injury (Khuman et al., 2011; Kondo et al., 2015; Mannix et al., 2013). Blast injury models also feature microglial activation (Huber et al., 2016). The microglial response to TBI has multiple phases that include morphological transformation, electrophysiological changes, proliferation, migration, release of cytokines / chemokines, and phagocytosis (Chhor et al., 2016; Ramlackhansingh et al., 2011). The role of microglia following TBI is often debated, with some suggesting that they are primarily neurotoxic (Hailer, 2008). However, it is clear that microglia can be neuroprotective following brain injury. They participate in the cleanup of dead cells in the parenchyma and help maintain integrity of CNS barrier structures such as the glial limitans and vasculature (Lou et al., 2016; Roth et al., 2014). Microglia can also release neurotrophins that may play a role in rebuilding the nervous system following injury (Elkabes et al., 1996; Nagamoto-Combs et al., 2007). Thus, microglia are not inherently neurotoxic, and their contribution to TBI lesions should be considered temporally and contextually.
One of the earliest observable changes that occurs in microglia following mild focal cortical brain injury is a morphological transformation (Figure 2). Resting state microglia have small stationary cell bodies with highly dynamic arbors that continually survey the extracellular space. These arbors rapidly extend toward injury sites as well as the damaged glial limitans, and this is dependent on purinergic receptor (P2Y6, P2Y12, P2X4) and TAM receptor tyrosine kinase (Axl, Mer) mediated signaling (Davalos et al., 2005; Fourgeaud et al., 2016; Nimmerjahn et al., 2005; Roth et al., 2014). Interference with these signaling pathways impedes microglial process extension and their ability to converge onto the sites of damage. The early phase response to brain injury enables microglia to engage the lesion and they have been shown to help maintain the integrity of critical CNS barrier structures such as the glial limitans and vasculature (Figure 2) (Lou et al., 2016; Roth et al., 2014). Another crucial role played by microglia in the acute lesion is phagocytosis. This is mediated in part by release of uridine diphosphate (UDP) from dead cells, which is detected by P2Y6 on activated microglia (Koizumi et al., 2007; Roth et al., 2014). P2Y6 signaling in microglia facilitates phagocytosis and increases their motility. Recent imaging studies have shown large phagocytic networks of microglia forming along the damaged glial limitans after mild focal TBI (Figure 2) (Roth et al., 2014). These cells localized to damaged glial limitans astrocytes and participated in maintenance of the glial limitans barrier as well as debris clearance. In this mild focal brain injury model, antagonism of these early purinergic receptor-dependent microglial responses results in increased brain damage (Roth et al., 2014).
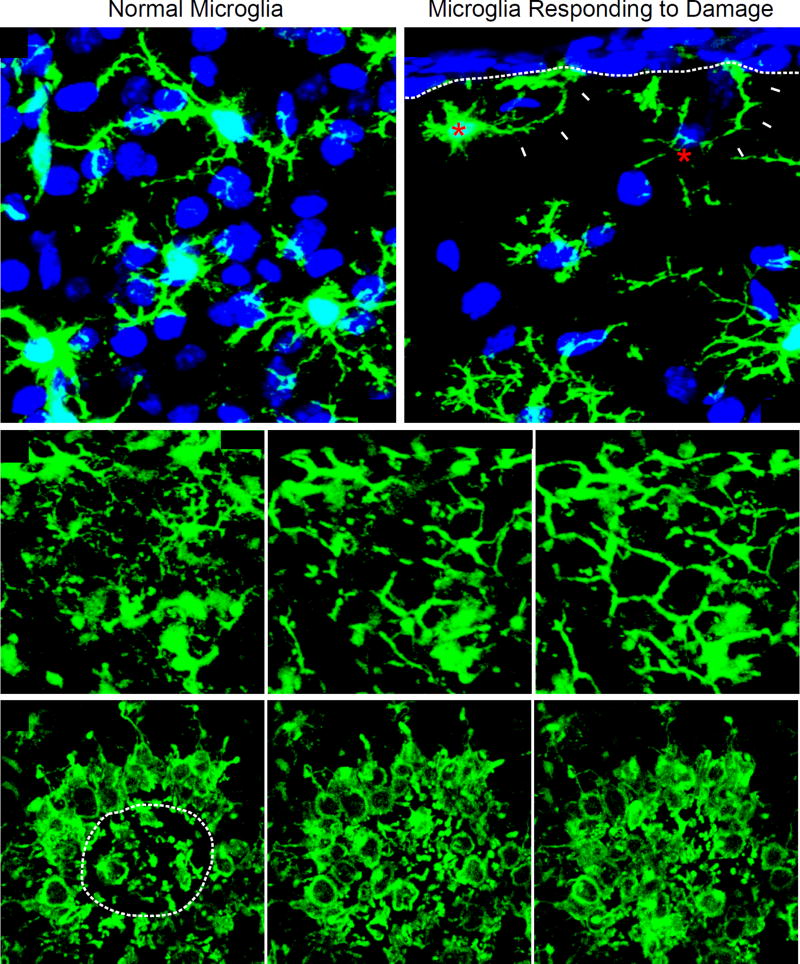
Figure 2. Acute microglial dynamics following mild focal cortical injury.
(A, B) Representative confocal images captured within the brain of a CX3CR1gfp/+ (green) mouse 3 hours following cortical injury show two microglia (red asterisks; panel B) extending processes (white arrowheads) toward the injured glial limitans (white dotted line) (Roth et al., 2014). Uninvolved, ramified microglia beneath the area of injury are shown in panel A for comparison. Cell nuclei are blue. (C, D) Representative time lapses captured by intravital two-photon microscopy through the thinned skull window of CX3CR1gfp/+ mice following mTBI. Panel C is a time lapse (beginning 5 min post-injury) depicting the morphological transformation of ramified microglia into “honeycomb” like structures that circumscribe individual astrocytes (white asterisks) within the injured glial limitans. These structures provide barrier support. Panel D shows the convergence of phagocytic “jellyfish” microglia into an area of heavy brain damage (white dotted line). These cells participate in the phagocytic clearance of debris.
Brain injury also induces microglial proliferation that starts within 24 hours and can continue for several weeks depending on the type of lesion (Giordana et al., 1994; Hailer et al., 1999; Kitamura et al., 1978; Susarla et al., 2014). This can occur following the release of many factors in the injured tissue such as macrophage colony-stimulating factor (M-CSF), brain derived neurotrophic factor (BDNF), neurotrophin (NT)-3, IL-1, and CX3CL1 (Ambrosini and Aloisi, 2004; Elkabes et al., 1996; Hicks et al., 1997; Mitrasinovic et al., 2001). Purinergic receptor signaling via P2X7 also has the potential to promote microglial proliferation (Bianco et al., 2006; Monif et al., 2009). One potent inhibitor of microglia proliferation is transforming growth factor (TGF)-β1. While this cytokine has been shown to be required for development of microglia in vitro (Butovsky et al., 2014), its expression increases following different types of CNS injury, and it may inhibit the proliferation of both astrocytes and microglia (Kiefer et al., 1995; Lindholm et al., 1992; Suzumura et al., 1993). Future studies are required to determine how microglial proliferation contributes to TBI lesion development and the precise role of TGF-β1 in this process before deciding whether to promote or block this response.
In addition to purinergic and TAM receptor signaling, microglia have the ability to sense DAMPs released from damaged cells via TLRs and NLRs (Hanisch and Kettenmann, 2007). They can also respond to a variety of other stimuli such as cytokines, chemokines, complement, neurotrophic factors, glutamate, and ions, among others (Hanisch and Kettenmann, 2007). Following activation, microglia produce a plethora of inflammatory mediators such as IL-1β, IL-6, IL-12, TNF-α, metalloproteinases, nitric oxide, and reactive oxygen species (Colton, 2009; Hernandez-Ontiveros et al., 2013). Production of these mediators can promote the inflammatory reaction by increasing BBB permeability and facilitating recruitment of peripheral immune cells (Shlosberg et al., 2010; Thal and Neuhaus, 2014). However, it should be noted that microglia are highly plastic cells, and how they contribute to a sterile immune reaction is dictated by their state of activation, lesion severity, interactions with neighboring cells, and the composition of the immune infiltrate (Saijo and Glass, 2011). In addition, all of these variables can change with time. Thus, no universal statements can be made about microglia following TBI. It is always important to focus on the context in which microglia reside, as they harbor the capacity to both repair and harm the injured CNS.
Several studies have attempted to therapeutically manipulate microglia in animal models of TBI; however, the interpretation of these studies is usually confounded by the failure to modulate specific microglia functions. For example, minocycline is a broad spectrum tetracycline antibiotic often used for its potent anti-inflammatory properties as well as its ability to suppress microglia / macrophage activation and function (Zemke and Majid, 2004). Minocycline can inhibit 5-lipoxygenase, NFκB nuclear translocation, and inflammatory cytokine production. Treatment of mice with minocycline following closed head injury revealed no detectable improvement in neurological outcome at day 4, although a modest improvement was noted at day 1 post-injury (Bye et al., 2007). By contrast, minocycline showed some benefit in a weight drop model of TBI (Homsi et al., 2010). Early treatment resulted in reduced microglial activation, brain lesion size, and locomotor hyperactivity, although the mechanism of neuroprotection in this study is unclear. Given the pleiotropic actions of minocycline, it will be important in future studies to interrogate the role of microglia in TBI using more specific genetic tools (Wieghofer et al., 2015). In this regard, the effect(s) of targeted deletion of inflammatory genes on the TBI pathogenesis needs to be conducted in a manner similar to that described in Alzheimer’s disease models (El Khoury et al., 2007; Frenkel et al., 2013). Alternatively, targeted deletion of specific genes in microglia using a Cre-lox approach and microglia specific promoters would also provide deeper insights into the role of microglial genes during TBI (Parkhurst et al., 2013). It will also be important to determine how anti-inflammatory cytokines, such as IL-10 and TGF-β1, specifically influence microglia functions during different stages of TBI (Guillot-Sestier et al., 2015; Kremlev and Palmer, 2005; Tyor et al., 2002). Microglia are major participants in brain injury and can certainly promote wound-healing through the release of many factors, including neurotrophins (Elkabes et al., 1996). Further insights into the contribution of microglia to TBI lesions will require more precise experimentation using conditional and inducible microglia promoter-specific gene manipulation.
Two important confounding factors that also need to be addressed when examining the role of microglia in TBI are the effects of gender and age on the development, progression and outcome of injury. The literature looking at the impact of sex disparities is limited (Caplan et al., 2017). In a recent study, mild-moderate CCI caused cortical microglia/macrophage activation in male mice peaking between 1 and 7 days. In contrast, CCI caused a less robust microglia/ macrophage response in females with a biphasic pro-inflammatory pattern that peaked at 4 h and 7 days, and a delayed anti-inflammatory peak at 30 days (Villapol et al., 2017). In a different study using moderate to severe CCI in female rats the inflammatory response peaked between 3–7 days post injury (Turtzo et al., 2014) In order to develop personalized therapies for TBI, more studies to understand the extent of sex differences, impact of sex on myeloid cells responses, and influence of circulatory hormonal changes, as well as other specific mechanisms like cerebral autoregulation, on the inflammatory response and functional outcomes after brain injury are warranted.
With regard to age, microglial ability to respond to new challenges is reduced with aging at the level of gene expression as well as functionally (Damani et al., 2011; Hickman et al., 2013). Young microglia are highly ramified and able to respond vigorously to purinergic stimuli released after injury. In contrast, aged microglia are less ramified and respond poorly to injury. It is not clear how these aging related changes would influence the response to brain injury in young versus aging adults andadditional studies are needed to clarify the role of age in TBI.
Microglia polarization: Time for reassessment
The existing classification of myeloid cell (e.g. monocytes, macrophages, microglia) polarization states is based on culturing these cells in vitro, stimulating them with individual cytokines such as IFNγ, IL-4, IL-10 or IL-13, and then measuring the expression of a limited number of genes (Martinez et al., 2006). However, myeloid cells do not encounter single cytokines in this way in vivo. During complex disease states, the polarization of myeloid cells is influenced by a mixture of up and/or downregulated cytokines, adhesion molecules, cell maturity, and the composition of the surrounding matrix and cellular partners. Thus, myeloid cell polarization in vivo depends on a variety of factors and interactions (Martinez and Gordon, 2014). For example, incubation of cultured human monocyte-derived macrophages with different cytokines / stimulants resulted in identification of at least nine different macrophage activation programs (Xue et al., 2014). It is therefore unlikely that the response to a single cytokine will reflect the actual polarization state of myeloid cells in vivo. While there are clear differences between M1 and M2 microglia in vitro (e.g. phagocytosis, microbial killing, secretory functions), there is also a considerable degree of functional overlap (Ransohoff, 2016). Oversimplifying the polarization state into the M1 / M2 paradigm does not reflect the true diversity of microglia / macrophage functions during complex diseases such as TBI. Microglia, for example, within the same injured brain tissue may exist in different functional states defined by expression of adhesion, maturation, effector, and chemoattractant molecules, among others. These states are linked in part to the anatomical position of the microglia in relation to injured neural structures such as vasculature, the glia limitans, neurons, white matter, etc. Expression analysis based on a small subset of genes (labeled M1 or M2) cannot reflect the different functional configurations of microglia. To accurately capture functional diversity, RNAseq at the single cell level can now be used to establish definitive profiles for microglia and other CNS macrophages during different stages of TBI. A clear illustration of this approach is the analysis of global gene expression of microglia in aging (Hickman et al., 2013). In this study, we observed that only 62% of M1 markers were significantly upregulated in microglia from aged mice, whereas 32% were not significantly changed, and two markers were downregulated. In contrast, aging was associated with downregulation or no change in 58% of M1 markers. These findings indicate that even in normal physiological aging, the polarization of microglia represents a mixed and complex state. We expect such complexity to appear in several disease states, including TBI.
The role of astrocytes in TBI
Astrocytes are involved in homeostatic functions of the CNS and blood flow control (Sofroniew and Vinters, 2010). Astrocytes form a functional barrier via the interaction of their foot processes with the parenchymal basement membrane, termed the glia limitans. This barrier separates the CNS from blood vessels, perivascular spaces, and the meninges. It also provides a checkpoint that can control influx of blood-borne immune cells (Sofroniew, 2015). Mechanical shear forces can elicit responses from astroctye mechanoreceptors that drive transmembrane ion flux, such as potassium efflux, calcium influx as well as secretion of ATP and glutamate. These ion and small molecule fluxes initiate cytotoxic pathways and signaling to recruit other immune cells (Burda et al., 2016). Reactive astrogliosis is also important in scar formation following injury that helps to contain the damage and inflammatory response to the injured area, thereby limiting spread of those changes to unaffected CNS areas. In addition, astrocytes also play a role in repairing the BBB and maintaining homeostasis by providing metabolic support for neurons and their synapses (Burda et al., 2016; Sofroniew, 2015). Following TBI, reactive astrocytes participate in the inflammatory response through the HMGB1-RAGE axis by activation of the NFkB pathway (Gao et al., 2012). This leads to secretion of different chemokines / cytokines and enhanced astrocyte phagocytic activity (Gao et al., 2012; Pan et al., 2012). Astrocytes also secrete MMP9 which has a role in BBB alterations after TBI (Pan et al., 2012). Astrocytes interact with other immune cells, such as microglia, through cytokine production. Both cells cooperate to release various growth factors like IGF1 and nerve growth factor that may promote healing after TBI (Burda and Sofroniew, 2014).
Peripheral innate immune activation
The inflammatory response following TBI is not confined to the CNS (Keel and Trentz, 2005; Lu et al., 2009; Weaver et al., 2015; Wilcockson et al., 2002). It is now known that an isolated brain injury can cause complex alterations in the systemic immune system. Disruption of CNS vasculature and critical barrier structures following TBI results in the leakage of debris and inflammatory mediators into the periphery, contributing to a complication referred to as the systemic inflammatory response syndrome (SIRS) (Lu et al., 2009; Plog et al., 2015; Wilcockson et al., 2002). This syndrome is defined by alterations in circulating leukocyte numbers, complement proteins, coagulants, inflammatory cytokines, etc. (Lu et al., 2009; Wilcockson et al., 2002). The body in turn attempts to compensate for SIRS through the release of anti-inflammatory cytokines that can promote immune dysfunction and / or enhance susceptibility to infection in peripheral tissues. Global systemic immune dysfunction further complicates the already difficult scenario of dealing with the primary brain injury and the local inflammatory response.
One family of key mediators of the systemic innate inflammatory response is the complement system. There are three basic pathways that can initiate the complement cascade (classical, alternative, and lectin), and these all give rise to a C3 convertase that promotes activation of C5 and ultimately assembly of a membrane attack complex consisting of C5b to C9 (MAC) (Holers, 2014). The complement system can profoundly amplify inflammatory reactions and participates in many functions, including opsonization, phagocytosis, immune cell chemotaxis, and cell lysis, among others. Various complement components such as C3, factor B, and C5b-9 have been found in the CSF of TBI patients (Kossmann et al., 1997; Stahel et al., 2001), and even correlate with the severity of BBB dysfunction (Stahel et al., 2001). Antagonism of the complement cascade in various animal models of TBI has been uniformly neuroprotective, including components such as C4 independent of terminal complement activation (Fluiter et al., 2014; Leinhase et al., 2006a; Leinhase et al., 2007; Leinhase et al., 2006b; Longhi et al., 2009; Rich et al., 2016; Stahel et al., 2009; You et al., 2007). Even though the complement system plays a positive role in the development and maintenance of the CNS under steady state conditions (Stephan et al., 2012; Stevens et al., 2007), it appears that complement activation and complement components such as C4 are primarily pathogenic following TBI.
Alerting the periphery is a standard CNS injury response. It is, however, the magnitude, localization, and quality of this response that influence outcome following TBI. After activation of CNS resident myeloid cells, neutrophils are usually among the first peripheral immune cells to arrive in contused brain and do so within just a few hours (Carlos et al., 1997; Clark et al., 1994; Roth et al., 2014; Szmydynger-Chodobska et al., 2009). They enter by extravasating across inflamed vessels, via leptomeninges, or through the choroid plexus located in the ventricular system (Carlos et al., 1997; Szmydynger-Chodobska et al., 2009).
After TBI, there is a noticeable increase in leukocytes in peripheral circulation, related to catecholamine and cortisol surges as well as an increase in their life span and numbers. A recent study has shown there is a significant surge in the number of peripheral neutrophils in the early hours after TBI that lasts until 48 hrs post-injury (Rhind et al., 2010). The infiltration of neutrophils into the CNS is directed by purines, cytokines, and chemokines. Elevated expression of cytokines, such as TNF-α and IL-1β, from damaged tissue after TBI induces the secretion of neutrophil chemoattractant molecules (e.g. CXCL1, 2, 3) by the choroid plexus epithelium (Szmydynger-Chodobska et al., 2009). This combined with expression of adhesion molecules (e.g. ICAM-1) facilitates migration of neutrophils across the blood-CSF-barrier and sometimes into the brain parenchyma. There is also more recent evidence that neutrophils and monocytes can enter the subarachnoid space via pial microvessels near the site of brain injury (Szmydynger-Chodobska et al., 2016).
The anatomical position of neutrophils within the lesion is likely determined by the time and the extent of damage. For example, a recent intravital imaging study demonstrated that following cortical injury neutrophils localized almost exclusively to the meninges and perivascular spaces (not the brain parenchyma) within the first 24 hrs (Roth et al., 2014). A similar perivascular neutrophil distribution was observed at 24 hrs in human TBI patients (Holmin et al., 1998). Parenchymal invasion was not seen until 3–5 days post-injury, concurrent with the arrival of a more diversified immune response, consisting of monocytes / macrophages and T cells. The contribution of neutrophils to TBI pathogenesis varies based on the experimental model. In one study of CCI, neutrophil depletion reduced edema, microglia activation, and activated caspase-3+ cells (a surrogate for cell death) in mice at 24 hrs (Kenne et al., 2012). Depletion did not, however, affect BBB leakage (Kenne et al., 2012; Whalen et al., 1999). Similar results were obtained in mice genetically deficient in CXCR2, a chemokine receptor important for neutrophil recruitment (Semple et al., 2010b). While these data suggest that neutrophils are inherently pathogenic, it is important to note that neutrophils can play a beneficial role in wound-healing responses (Lammermann et al., 2013) and even promote neurological recovery following injury (Stirling et al., 2009). Neutrophils can be recruited to sites of sterile injury via P2X7 and formyl-peptide receptor dependent signaling (McDonald et al., 2010), and antagonism of this response following cortical injury was shown to enhance meningeal cell death in a mild focal brain injury model (Roth et al., 2014). Future studies are required to determine how early neutrophil responses influence subsequent tissue repair after TBI and why neutrophils are injurious in some TBI models but not others. Blocking the influx of neutrophils after TBI can be neuroprotective. A recent study showed that blocking CD11d, a marker of both neutrophils and macrophages, after moderate and consecutive mild fluid percussion injuries in rats improved neurological outcomes (Weaver et al., 2015). Depleting neutrophils was found to be associated with decreased brain edema, tissue loss, and monocyte activation in a CCI model of TBI (Kenne et al., 2012). Functionally, the pathogenic capacity of neutrophils might be linked to changes in oxidative activity (i.e., ROS), which is evident early after human TBI with altered expression of the enzymes iNOS and NADPH oxidase (Liao et al., 2013).
In rat CCI models, modulation of systemic neutrophils did not affect brain edema and only modestly affected BBB damage (Whalen et al., 1999; Whalen et al., 2000), but antagonism of neutrophil elastase was beneficial in an immature rodent CCI model (Semple et al., 2015). Neutrophil recruitment to the injured brain is typically followed by the arrival of monocytes that convert into macrophages. Macrophages can contribute to both tissue injury and repair depending on their functional properties (Rua and McGavern, 2015; Wynn and Vannella, 2016). Following CNS damage, infiltrating monocyte-derived macrophages will often contribute to the injury response alongside yolk-sac derived tissue-resident myeloid cells like microglia. If successful these responses should help foster tissue remodeling and perhaps even regeneration; however, sustained pro-inflammatory macrophage activity is considered maladaptive and can actually promote further damage (Wynn and Vannella, 2016).
In TBI patients, monocytes enter the perivascular spaces and brain parenchyma within 1–2 days, differentiate into macrophages and can remain there for weeks after the injury (Beschorner et al., 2002). One mechanism of monocyte recruitment following TBI is reliant on local production of the chemokine CCL2, which can be produced by choroid plexus epithelium and is found in the CSF of TBI patients (Semple et al., 2010a; Szmydynger-Chodobska et al., 2012). This promotes the recruitment of CCR2+ monocytes, and interference with this pathway decreases lesion size and promotes neurological recovery in animal models of TBI (Gyoneva et al., 2015; Hsieh et al., 2014; Morganti et al., 2015; Semple et al., 2010a). These data suggest CCR2+ pro-inflammatory monocytes exacerbate TBI pathogenesis. Given that macrophages also have the capacity to promote repair following CNS injury in a TREM2-dependent manner (Saber et al., 2016; Shechter et al., 2009; Wattananit et al., 2016), it will be important in future studies to identify the specific monocyte subsets and the molecular mechanisms that give rise to pathogenic vs. non-pathogenic responses in TBI models that feature macrophages as a relevant pathogenic entity. Despite significant advances in understanding the role of monocyte subsets in experimental spinal cord injury (Blomster et al., 2013; Huang et al., 2014; Thawer et al., 2013), and more recently experimental stroke (Grosse et al., 2016; Kraft et al., 2015), similar data defining the functional roles of monocyte subsets in TBI models are currently lacking. Moreover, while it is clear that monocytes contribute robustly to cerebral contusion, it remains unknown whether or not monocytes infiltrate the brain and contribute to the pathogenesis of concussion TBI, which represents the vast majority of TBI cases. This important question remains to be addressed in animal models and humans with concussion.
The ability to distinguish microglia from invading peripheral monocytes has only recently become practical and easily done. Levels of CD45 expression have been used in the past, but it is not clear that one marker alone can distinguish between the two cell types. In this regard, recent transcriptomic data defining the molecular signature of microglia vs. monocytes and macrophages will help address this concern in future TBI studies (Gautier et al., 2012; Goldmann et al., 2016; Hickman et al., 2013). This has important clinical and therapeutic aplications since circulating monocytes are more amenable to pharmacologic manipulation than microglia, which reside behind the BBB.
Cytokines and other inflammatory proteins
Cytokines
Cytokines can be secreted by various cells in the CNS following TBI, and can have either pro- and/or anti-inflammatory properties (Ziebell and Morganti-Kossmann, 2010). As mentioned, IL-1β and IL-18 are both downstream of inflammasome activation and are often produced during sterile injury responses. IL-1β, for example, is elevated in the CSF of severe TBI patients for at least 24 hrs before declining, and is associated with increased intracranial pressure and a more unfavorable outcome (Hayakata et al., 2004; Shiozaki et al., 2005). A pathogenic role for IL-1β was suggested in animal studies, which revealed that administration of an IL-1 receptor antagonist reduced TBI lesion volumes and improved neurological function (Jones et al., 2005; Sanderson et al., 1999). Similar findings were uncovered with IL-18, which was detected early following TBI in humans and rodents (Yatsiv et al., 2002). Therapeutic administration of an IL-18 antagonist to mice one hour after brain injury enhanced neurological recovery (Yatsiv et al., 2002). These data suggest that IL-1β and IL-18 both exacerbate TBI pathogenesis in cerebral contusion models.
IL-6 and tumor necrosis factor alpha (TNF-α) are two additional pro-inflammatory cytokines up-regulated after TBI; however, these cytokines can foster both positive and negative outcomes. Interestingly, levels of IL-6 in the CSF were associated with a favorable outcome in children with severe TBI (Chiaretti et al., 2008). This finding is supported by murine studies showing that IL-6 deficiency slowed recovery from TBI, whereas transgenic overexpression of IL-6 in astrocytes hastened the healing process through a mechanism thought to depend on improved re-vascularization of the injury site (Swartz et al., 2001). TNF-α, on the other hand, is also elevated in TBI patients (Goodman et al., 1990), and several studies suggested that early blockade of this cytokine after injury was neuroprotective (Chio et al., 2010; Shohami et al., 1996). However, it is important to note that mice genetically deficient in TNF-α showed less severe neurological deficits 7 days after TBI, yet the opposite was observed at 2-4 weeks post-injury. At these later time points, TNF-α-deficient mice had larger lesions and more impaired motor functions relative to wild type control mice (Scherbel et al., 1999). These data suggest that TNF-α has a dual role following cerebral contusion, contributing to acute pathogenesis as well as tissue repair and recovery of neurological function. Interestingly, concomitant genetic inhibition of TNF-α and Fas receptor reduced tissue damage and improved functional outcome after CCI, but worsened functional outcome in a mouse concussion model characterized by impact and head acceleration without structural brain damage (Bermpohl et al., 2007; Yang et al., 2010) These findings underscore the important concept that specific molecular mechanisms can have divergent functions in different pathoanatomical models of TBI, and suggest that targeting TNF-α/Fas in patients with cerebral contusion may be beneficial but harmful in those with concussion.
Inflammatory responses are usually counterbalanced by anti-inflammatory cytokines such as IL-10 and TGF-β. IL-10 is a potent anti-inflammatory cytokine that signals through STAT3, is found in the CSF of TBI patients (Csuka et al., 1999; Shiozaki et al., 2005), and has been associated with circulating monocytes (Shimonkevitz et al., 1999). Intravenous (but not intrathecal) injection of IL-10 prior to injury was shown to reduce TNF-α expression and improve neurological recovery in rats following fluid percussion injury (Knoblach and Faden, 1998). These data indicate that IL-10 can be neuroprotective when administered early after injury and that it may need to act on the peripheral immune system. TGF-β is another pleiotropic cytokine with immunoregulatory properties that is found in CSF of TBI patients (Csuka et al., 1999; Morganti-Kossmann et al., 1999). Following weight drop TBI in rats, TGF-β1 was expressed in pericontusional neurons and astrocytes (Wang et al., 2015). Knockdown of TGF-β1 in the pericontusional area using a shRNA approach resulted in increased neuronal cell death, reduced astrogliosis, and enhanced neurological deficits. These data indicate that TGF-β1 is neuroprotective and promotes astrogliosis in this rat TBI model.
Matrix metalloproteinases (MMPs)
MMPs are a family of calcium-dependent zinc-containing endopeptidases that have diverse physiological and pathological functions, including degradation of extracellular matrix, regulation of cytokines/chemokines, cleavage of surface receptors, etc. (Verma and Hansch, 2007). These enzymes are made as zymogens that must be converted into an active form by removal of a pro-peptide domain. Once active, MMPs act on many different substrates to mediate their biological effects. The pattern of MMP expression may in some instances even serve as an inflammatory biomarker. For example, analysis of extracellular brain fluid from severe TBI patients revealed a temporal expression pattern of MMPs, with MMP-8,9 appearing first, followed by MMP-2,3, and finally MMP-7 (Roberts et al., 2013). Interestingly, expression of the neutrophil collagenase, MMP-8, was associated with increasing intracranial pressure and was higher in patients that died after TBI. MMP expression also appears to vary anatomically in TBI patients. Quantification of MMPs in TBI patients with contusions revealed heightened expression of MMP-9 (a gelatinase) in pericontusional brain within 72 hrs of injury, leading the investigators to suggest its participation in hemorrhagic progression and vasogenic edema (Guilfoyle et al., 2015). However, it is unclear based on these associative data whether MMP-9 is pathogenic or neuroprotective in this context.
To gain additional insights into the role of MMPs during TBI, several studies have focused on animal models. CCI in mice was shown to elevate MMP-9 expression in brain lesions for up to one week (Wang et al., 2000). Genetic deletion of MMP-9 in mice resulted in reduced brain lesions and motor deficits when compared to wild type controls (Wang et al., 2000). These genetic findings were confirmed using an MMP-9 inhibitor that reduced microglia activation, astrogliosis, brain lesion volumes, neuronal loss, and neurological dysfunction (Hadass et al., 2013). Mechanistically, it is thought that MMP-9 enhances TBI pathogenesis by fostering BBB breakdown and promoting vasogenic edema (Mori et al., 2002; Shigemori et al., 2006). Thus, MMP-9 antagonism might offer some therapeutic benefit in TBI subtypes with these histopathological features. How other MMPs influence TBI pathology and immune function remains to be determined.
In addition to the roles of MMP-2,9, tissue inhibitors of metalloproteinases (TIMPs) 1 and 9 were reported to be increased following CCI in rats (White et al., 2013). Interestingly, in humans TIMP-1 levels correlated with increased mortality and served as a possible biomarker for injury severity (Lorente et al., 2014). TIMP-3 is another member of this family that was found to be elevated in both injured and uninjured brain hemispheres after fluid percussion injury in rats - a response that was blocked by mild hypothermia (Jia et al., 2014).
Adaptive immunity
The role of adaptive immunity following TBI is not entirely clear. In general, tissue damage throughout the body results in activation of the immune system by endogenous DAMPs and presentation of self-antigens. This, however, does not necessarily result in pathogenic autoimmunity. Studies have shown that autoreactive T cells specific to CNS antigens (e.g. myelin basic protein) can be isolated from the blood of healthy individuals (Burns et al., 1983) as well as those with TBI (Cox et al., 2006). TBI can induce a cell-mediated immune response (Clausen et al., 2007; Czigner et al., 2007; Lenzlinger et al., 2001), with T cells being recruited to the CNS independent of their specificity (Hirschberg et al., 1998). Paradoxically, autoreactive CD4+ T cells specific to CNS antigens were shown to protect injured axons following damage (Moalem et al., 1999; Schwartz, 2000). This might be due in part to the ability of CD4+ T cells to release IL-4 in areas of CNS injury (Gadani et al., 2012). In fact, a recent study demonstrated in a model of spinal cord injury that infiltrating CD4+ T cells can sense DAMPS and produce IL-4 in an MHC II-independent manner, which then acts via neuronal IL-4 receptors to potentiate neurotrophin signaling and promote recovery of injured neurons (Walsh et al., 2015). These data demonstrate that T cells can be neuroprotective even in the absence of T cell receptor engagement; however, it remains to be determined whether a similar type of neuroprotective response can be elicited following TBI. Even humoral immune responses have been observed following head injury (Rudehill et al., 2006), although a study focused on a closed skull model of head injury revealed no difference in injury severity or neurological impairment between wild type and recombination-activating gene 1 (RAG-1) deficient mice (Weckbach et al., 2012). Because RAG-1−/− mice have no T or B cells, these data indicate that adaptive immunity plays little role in this model of head injury (at least within the first week of injury). Additional studies are required to evaluate the role of adaptive immune cells in other models of TBI and at later time points post-injury.
TBI-induced chronic neuroinflammation
There is growing evidence to support that TBI is a major risk factor for developing many neurological disorders, including Alzheimer’s disease (AD), chronic traumatic encephalopathy (CTE), and other neurodegenerative diseases (Bloom, 2014; Chen et al., 2007; Giunta et al., 2012; McKee et al., 2009). Repeated mild traumatic brain injury (mTBI) can cause sustained cognitive and psychiatric changes as well as neurodegeneration, but the underlying mechanisms remain unclear. Chronic inflammation induced by brain injury is proposed to be a major player in the pathophysiology of neurodegenerative disorders (Aungst et al., 2014; Johnson et al., 2013).
Many studies have linked TBI to AD and suggest a dose-dependent relationship between head injury and the predisposition to dementia. The pathological mechanisms underlying this predisposition include disruption of white matter tracts and neural networks, reduced cognitive reserves, and release / deposition of amyloid beta (Aβ) and tau (Bloom, 2014; Mendez, 2017). The accumulation of neurotoxic forms of Aβ and tau are believed to cause neuroinflammatory responses that drive AD pathogenesis after TBI (Heneka et al., 2015). Future studies to target chronic inflammatory responses after brain trauma might offer a therapeutic intervention to limit the risk of AD development in TBI patients (Loane et al., 2009).
TBI is also linked to a delayed onset progressive neurodegenerative disease, referred to as chronic traumatic encephalopathy (CTE), which is associated with a prolonged history of repetitive head injuries (both concussive and subconcussive) and has been reported in sports athletes as well as soldiers exposed to blasts (Reams et al., 2016). Clinically, CTE is post-mortem diagnosis that associates with behavioral changes, including executive and cognitive impairments (McKee et al., 2013; Reams et al., 2016). Pathologically, CTE is characterized by frontal and temporal lobe atrophy, neuronal and axonal loss, and abnormal deposits of proteins, including phosphorylated tau (pTau) and 43 kDa TAR deoxyribonucleic acid (DNA)-binding protein (TDP-43) (McKee et al., 2014; McKee et al., 2013).
Studies have demonstrated increased densities of activated microglial and tau pathology after repetitive head injuries, suggesting a role for a persistent neuroinflammatory response in the development of TBI-induced CTE (Loane et al., 2014; McKee et al., 2014). This is further supported by recent in vivo brain imaging studies of football athletes, which revealed evidence of chronic microglial activation (Cherry et al., 2016; Coughlin et al., 2017). Interestingly, research in murine AD models has shown that neuroinflammatory cytokines and reactive microglia can promote tau pathology and contribute to the spread of pTau, which might explain the link between TBI-induced inflammation and CTE (Ghosh et al., 2013; Maphis et al., 2015). Additional studies are required to investigate the mechanism(s) underlying tau accumulation and the role of microglia / astrocytes in the neurodegenerative diseases that develop following TBI. A comprehensive understanding of the relationship between TBI and neurodegenerative diseases will require development of novel animal models that enable us to study the factors that initiate Aβ accumulation as well as tau phosphorylation and aggregation. In addition, more advanced clinical neuroimaging (e.g. PET imaging to detect pathological tau and amyloid deposition) is needed to explore the mechanisms driving chronic neurodegeneration after TBI in humans and selecting patients for potential future therapies.
FUTURE DIRECTIONS
In future studies, it will be exciting to use recent advances in our understanding of microglial biology during development, aging, neurodegeneration, and CNS repair to better dissect the role and function of microglia following TBI. A baseline transcriptome has been established for microglia in the healthy and aged brain using RNA-Seq technology (Hickman et al., 2013), and a similar approach was used to define the transcriptome for other brain resident myeloid cell populations such as meningeal, perivascular, and choroid plexus macrophages (Goldmann et al., 2016). It will be important to use this information to define the unique transcriptional changes that occur in CNS resident myeloid cells at different stages of TBI and in different TBI models. It will also be important to consider the anatomical position of the responding myeloid cells and the location(s) of the brain injury in order to refine our mechanistic understanding of the factors that give rise to TBI pathogenesis and recovery. In this regard, we propose to steer away from the M1 vs. M2 classification for microglia and other myeloid cells during TBI (Ransohoff, 2016) and define a TBI subset-specific profile similar to those established for microglia in amyotrophic lateral sclerosis (Chiu et al., 2013) and aging (Hickman et al., 2013). In fact, the majority of published papers that classify microglia as M1 vs M2 rely on a handful of transcripts that are up or down (Kumar et al., 2016; Loane and Kumar, 2016). We believe that a more global view of the microglia is necessary, as M1 vs M2 does not necessarily reflect all aspects of microglial function and should not be used to guide the development of potential therapies. Instead, we propose that specific transcriptional networks for microglia in TBI should be defined and immunomodulatory therapies developed against pathways that dampen neurogenerative programs while promoting tissue remodeling and homeostasis. Identification of TBI-specific myeloid cell signatures based on anatomical location and the stage / type of injury will significantly advance the TBI field and aid in the development of therapies to improve clinical outcomes by modulating myeloid cell target genes.
Traditionally, immune cell subsets are divided into brain resident and peripherally-derived. For example, one common division in the TBI community is to consider microglia separately from monocytes. This is indeed an important distinction, but it is important to consider that there are several different subsets of monocytes (e.g. classical, non-classical) as well as brain resident myeloid cells (e.g. microglia, meningeal macrophages, perivascular macrophages, choroid plexus macrophages). Thus, it is essential to develop and utilize new genetic tools to study these distinct populations in the context of TBI. Progress has already been made in this area and new tools are always under development. Inducible CX3CR1-GFP-Cre mice have been used to delete specific genes from microglial and other myeloid cell populations (Parkhurst et al., 2013). Studies have shown, for example, that microglia participate in synaptic pruning during learning (Parkhurst et al., 2013) as well as in pathological conditions such Alzheimer’s disease (Vasek et al., 2016). While these mice have proven useful, CX3CR1 is expressed in most myeloid cell populations throughout the body, making it challenging to delete genes from individual populations. More recently, Sall1 was identified as a gene expressed specifically in microglia, and an inducible Sall1-cre mouse was generated to remove genes from this cell population alone (Buttgereit et al., 2016). This type of approach should be used to interrogate all of the different myeloid cell subsets as well as other innate immune populations in different models of TBI, as there is still much to learn about the role of innate immunity during brain damage and repair.
Another critical area of future study is the delineation of inflammatory processes that mobilize in response to pathoanatomically distinct types of TBI (e.g. focal contusion vs. diffuse concussion vs. meningeal damage) (Xiong et al., 2013). This is an important area of investigation for several reasons. First, knowing the aspects of a region-specific inflammatory response that mediate damage vs. repair in different types of TBI will aid in the design of tailored clinical trials for TBI patients with the appropriate pathoanatomic lesions and at the appropriate stage of injury. Second, inflammation-targeted therapies may have differential effects based on the type (e.g. focal vs. diffuse) and stage (acute vs. chronic) of injury (Bermpohl et al., 2007; Khuman et al., 2011; Park et al., 2012; Zhu et al., 2014); thus, stratifying TBI data based on anatomical features, time, and severity may help to better predict the efficacy of inflammation-based therapies that enter clinical trials.
CONCLUDING REMARKS
Experimental and human TBI induces an immune response comprised of local and peripherally-derived participants. These responses are initiated within minutes and can continue for decades if the injury is not resolved or gives rise to a chronic disorder such as chronic traumatic encephalopathy (CTE) (McKee et al., 2015). Sterile immune responses are evolutionarily conserved reactions designed to ward off pathogens and promote wound-healing. Thus, the immunological response to TBI should not be considered inherently pathogenic. Studies have clearly shown beneficial aspects of CNS immunity following brain injury such as preservation of barrier function, clearance of debris, resolution of inflammation, and the release of trophic factors. Innate and adaptive immune cells can play an essential role in successful wound-healing responses and the restoration of tissue homeostasis. On the other hand, there are maladaptive aspects to CNS injury responses that can exacerbate TBI pathology and promote a chronic inflammatory state. The failure to resolve a robust pro-inflammatory reaction can negate the beneficial aspects of an immune-mediated wound-healing response. Identifying the factors that sustain pathogenic pro-inflammatory reactions is critical to the development of efficacious TBI therapies in humans. Rather than completely ablate the immune response to TBI, future studies should focus on therapeutically guiding TBI immunity toward a favorable wound-healing response that rapidly stabilizes or even restores CNS function.
Highlights.
Traumatic brain injury (TBI) is a leading cause of morbidity and disability.
Neuroinflammation is an important manipulable aspect of secondary injury following TBI.
A sterile immune response develops within minutes of TBI and have lasting effects.
A new paradigm that moves away from the M1/M2 classification is needed.
Acknowledgments
This work was supported by the NIH intramural program (DBM and YJ), NIA grant RF1AG051506 (JEK), and NINDS 1R21HD086385 (MW). We thank Alan Hoofring in the NIH Medical Arts Design Section for his help with the illustration shown in Figure 1, Matthew Russo (NINDS) for providing the images shown in Figure 2.
Funding sources
Yasir N Jassam MD: Reports no disclosures.
Saef Izzy MD: Reports no disclosures.
Michael Whalen, MD.: NINDS 1R21HD086385
Dorian McGavern, Ph.D.: Supported by the NIH intramural program.
Joseph El Khoury MD: NIA Grant 1RF1AG051506.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
Yasir N Jassam MD and Saef Izzy MD equally contributed to manuscript concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Michael Whalen, MD.: Acquisition of data, analysis and interprestation, critical revision of the manuscript for important intellectual content, study supervision.
Dorian McGavern, Ph.D.: manuscript concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Joseph El Khoury MD: manuscript concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
References
- Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Crupi R, Campolo M, Genovese T, Esposito E, Cuzzocrea S. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 2013;8:e57208. doi: 10.1371/journal.pone.0057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Suhett CP, Li Z, Marini AM, Braga MF, Eiden LE. Temporal course of changes in gene expression suggests a cytokine-related mechanism for long-term hippocampal alteration after controlled cortical impact. J Neurotrauma. 2014;31:683–690. doi: 10.1089/neu.2013.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Bayir H. Therapies targeting lipid peroxidation in traumatic brain injury. Brain Res. 2016;1640:57–76. doi: 10.1016/j.brainres.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergold PJ. Treatment of traumatic brain injury with anti-inflammatory drugs. Experimental neurology. 2016;275(Pt 3):367–380. doi: 10.1016/j.expneurol.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99:745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193:5765–5771. doi: 10.4049/jimmunol.1401417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomster LV, Brennan FH, Lao HW, Harle DW, Harvey AR, Ruitenberg MJ. Mobilisation of the splenic monocyte reservoir and peripheral CX(3)CR1 deficiency adversely affects recovery from spinal cord injury. Experimental neurology. 2013;247:226–240. doi: 10.1016/j.expneurol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Blumbergs PCRP, Vink R. Trauma. 2008;1 [Google Scholar]
- Bramlett HM, Dietrich WD. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J Neurotrauma. 2015;32:1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickler T, Gresham K, Meza A, Coutermarsh-Ott S, Williams TM, Rothschild DE, Allen IC, Theus MH. Nonessential Role for the NLRP1 Inflammasome Complex in a Murine Model of Traumatic Brain Injury. Mediators Inflamm. 2016;2016:6373506. doi: 10.1155/2016/6373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Experimental neurology. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Rosenzweig A, Zweiman B, Lisak RP. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell Immunol. 1983;81:435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit A, Lelios I, Yu X, Vrohlings M, Krakoski NR, Gautier EL, Nishinakamura R, Becher B, Greter M. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17:1397–1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Experimental neurology. 2007;204:220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Caplan HW, Cox CS, Bedi SS. Do microglia play a role in sex differences in TBI? J Neurosci Res. 2017;95:509–517. doi: 10.1002/jnr.23854. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Skolnick B, Narayan RK. Neuroprotection Trials in Traumatic Brain Injury. Curr Neurol Neurosci Rep. 2016;16:29. doi: 10.1007/s11910-016-0625-x. [DOI] [PubMed] [Google Scholar]
- Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3–23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016;4:112. doi: 10.1186/s40478-016-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor V, Moretti R, Le Charpentier T, Sigaut S, Lebon S, Schwendimann L, Ore MV, Zuiani C, Milan V, Josserand J, et al. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR, Yang CZ, Chang CP. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010;115:921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- Clausen F, Lorant T, Lewen A, Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Minn I, Bienko N, Ambinder EB, Xu X, Peters ME, Dougherty JW, Vranesic M, Koo SM, et al. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017;74:67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AL, Coles AJ, Nortje J, Bradley PG, Chatfield DA, Thompson SJ, Menon DK. An investigation of auto-reactivity after head injury. J Neuroimmunol. 2006;174:180–186. doi: 10.1016/j.jneuroim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- Czigner A, Mihaly A, Farkas O, Buki A, Krisztin-Peva B, Dobo E, Barzo P. Kinetics of the cellular immune response following closed head injury. Acta Neurochir (Wien) 2007;149:281–289. doi: 10.1007/s00701-006-1095-8. [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett CR, McGuire JL, DePasquale EA, Gardner AE, Floyd CL, McCullumsmith RE. Glutamate Neurotransmission in Rodent Models of Traumatic Brain Injury. J Neurotrauma. 2017;34:263–272. doi: 10.1089/neu.2015.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, Fernandes J, Gogichaisvili T, Golden N, Hartzenberg B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu B, Kimbler DE, Pang J, Choi J, Moskophidis D, Yanasak N, Dhandapani KM, Mivechi NF. Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J Neurochem. 2014;130:626–641. doi: 10.1111/jnc.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Fluiter K, Opperhuizen AL, Morgan BP, Baas F, Ramaglia V. Inhibition of the membrane attack complex of the complement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J Immunol. 2014;192:2339–2348. doi: 10.4049/jimmunol.1302793. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136(Suppl 1):29–38. doi: 10.1111/jnc.13217. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, El Khoury J. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer's-like disease progression. Nat Commun. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, Shao HJ, Jin ZF, Fu ZJ. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J Trauma Acute Care Surg. 2012;72:643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, O'Banion MK. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordana MT, Attanasio A, Cavalla P, Migheli A, Vigliani MC, Schiffer D. Reactive cell proliferation and microglia following injury to the rat brain. Neuropathol Appl Neurobiol. 1994;20:163–174. doi: 10.1111/j.1365-2990.1994.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Giunta B, Obregon D, Velisetty R, Sanberg PR, Borlongan CV, Tan J. The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. J Neuroinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, El Khoury J. beta-amyloid, microglia, and the inflammasome in Alzheimer's disease. Semin Immunopathol. 2015;37:607–611. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Graber DJ, Costine BA, Hickey WF. Early inflammatory mediator gene expression in two models of traumatic brain injury: ex vivo cortical slice in mice and in vivo cortical impact in piglets. J Neuroinflammation. 2015;12:76. doi: 10.1186/s12974-015-0298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse GM, Schulz-Schaeffer WJ, Teebken OE, Schuppner R, Dirks M, Worthmann H, Lichtinghagen R, Maye G, Limbourg FP, Weissenborn K. Monocyte Subsets and Related Chemokines in Carotid Artery Stenosis and Ischemic Stroke. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum SE, Zlotnik A, Gruenbaum BF, Hersey D, Bilotta F. Pharmacologic Neuroprotection for Functional Outcomes After Traumatic Brain Injury: A Systematic Review of the Clinical Literature. CNS Drugs. 2016;30:791–806. doi: 10.1007/s40263-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle MR, Carpenter KL, Helmy A, Pickard JD, Menon DK, Hutchinson PJ. Matrix Metalloproteinase Expression in Contusional Traumatic Brain Injury: A Paired Microdialysis Study. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J, Jr, Leung BP, Rezai-Zadeh K, Town T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Kim D, Katsumoto A, Kokiko-Cochran ON, Lamb BT, Ransohoff RM. Ccr2 deletion dissociates cavity size and tau pathology after mild traumatic brain injury. J Neuroinflammation. 2015;12:228. doi: 10.1186/s12974-015-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB, Purnell W, Robinson CR, 2nd, et al. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One. 2013;8:e76904. doi: 10.1371/journal.pone.0076904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Grampp A, Nitsch R. Proliferation of microglia and astrocytes in the dentate gyrus following entorhinal cortex lesion: a quantitative bromodeoxyuridine-labelling study. Eur J Neurosci. 1999;11:3359–3364. doi: 10.1046/j.1460-9568.1999.00808.x. [DOI] [PubMed] [Google Scholar]
- Hang CH, Shi JX, Li JS, Wu W, Yin HX. Concomitant upregulation of nuclear factor-kB activity, proinflammatory cytokines and ICAM-1 in the injured brain after cortical contusion trauma in a rat model. Neurol India. 2005;53:312–317. doi: 10.4103/0028-3886.16930. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Bullock MR. Past, Present, and Future of Traumatic Brain Injury Research. Neurosurg Clin N Am. 2016;27:375–396. doi: 10.1016/j.nec.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, et al. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's disease. Nat Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Hirschberg DL, Moalem G, He J, Mor F, Cohen IR, Schwartz M. Accumulation of passively transferred primed T cells independently of their antigen specificity following central nervous system trauma. J Neuroimmunol. 1998;89:88–96. doi: 10.1016/s0165-5728(98)00118-0. [DOI] [PubMed] [Google Scholar]
- Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- Holmin S, Soderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. discussion 298–299. [DOI] [PubMed] [Google Scholar]
- Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma. 2010;27:911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Niemi EC, Wang SH, Lee CC, Bingham D, Zhang J, Cozen ML, Charo I, Huang EJ, Liu J, Nakamura MC. CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J Neurotrauma. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Vodovotz Y, Kusturiss MB, Barclay D, Greenwald K, Boninger ML, Coen PM, Brienza D, Sowa G. Identification of distinct monocyte phenotypes and correlation with circulating cytokine profiles in acute response to spinal cord injury: a pilot study. PM R. 2014;6:332–341. doi: 10.1016/j.pmrj.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber BR, Meabon JS, Hoffer ZS, Zhang J, Hoekstra JG, Pagulayan KF, McMillan PJ, Mayer CL, Banks WA, Kraemer BC, et al. Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience. 2016;319:206–220. doi: 10.1016/j.neuroscience.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, Frenkel D, El Khoury J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016;36:5185–5192. doi: 10.1523/JNEUROSCI.3850-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med. 2010;2:27rv21. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- Jia F, Mao Q, Liang YM, Jiang JY. The effect of hypothermia on the expression of TIMP-3 after traumatic brain injury in rats. J Neurotrauma. 2014;31:387–394. doi: 10.1089/neu.2008.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Prior MJ, Burden-Teh E, Marsden CA, Morris PG, Murphy S. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci. 2005;22:72–78. doi: 10.1111/j.1460-9568.2005.04221.x. [DOI] [PubMed] [Google Scholar]
- Jounai N, Kobiyama K, Takeshita F, Ishii KJ. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol. 2012;2:168. doi: 10.3389/fcimb.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J Neuroinflammation. 2012;9:17. doi: 10.1186/1742-2094-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuman J, Meehan WP, 3rd, Zhu X, Qiu J, Hoffmann U, Zhang J, Giovannone E, Lo EH, Whalen MJ. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab. 2011;31:778–789. doi: 10.1038/jcbfm.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Streit WJ, Toyka KV, Kreutzberg GW, Hartung HP. Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. Int J Dev Neurosci. 1995;13:331–339. doi: 10.1016/0736-5748(94)00074-d. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol Dis. 2013;58:289–295. doi: 10.1016/j.nbd.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kim JY, Yenari MA. Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012;20:177–185. doi: 10.1007/s10787-011-0115-3. [DOI] [PubMed] [Google Scholar]
- Kim N, Kim JY, Yenari MA. Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience. 2015;284:912–919. doi: 10.1016/j.neuroscience.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Tsuchihashi Y, Fujita S. Initial response of silver-impregnated "resting microglia" to stab wounding in rabbit hippocampus. Acta Neuropathol. 1978;44:31–39. doi: 10.1007/BF00691636. [DOI] [PubMed] [Google Scholar]
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Experimental neurology. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523:431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Morganti-Kossmann MC, Jones JL, Barnum SR. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol. 1997;73:63–69. doi: 10.1016/s0165-5728(96)00164-6. [DOI] [PubMed] [Google Scholar]
- Kraft P, Drechsler C, Schuhmann MK, Gunreben I, Kleinschnitz C. Characterization of Peripheral Immune Cell Subsets in Patients with Acute and Chronic Cerebrovascular Disease: A Case-Control Study. Int J Mol Sci. 2015;16:25433–25449. doi: 10.3390/ijms161025433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremlev SG, Palmer C. Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol. 2005;162:71–80. doi: 10.1016/j.jneuroim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagraoui M, Latoche JR, Cartwright NG, Sukumar G, Dalgard CL, Schaefer BC. Controlled cortical impact and craniotomy induce strikingly similar profiles of inflammatory gene expression, but with distinct kinetics. Front Neurol. 2012;3:155. doi: 10.3389/fneur.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee S, Cho IH, Lee SJ. Toll-like receptors: sensor molecules for detecting damage to the nervous system. Curr Protein Pept Sci. 2013;14:33–42. doi: 10.2174/1389203711314010006. [DOI] [PubMed] [Google Scholar]
- Leinhase I, Holers VM, Thurman JM, Harhausen D, Schmidt OI, Pietzcker M, Taha ME, Rittirsch D, Huber-Lang M, Smith WR, et al. Reduced neuronal cell death after experimental brain injury in mice lacking a functional alternative pathway of complement activation. BMC Neurosci. 2006a;7:55. doi: 10.1186/1471-2202-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I, Rozanski M, Harhausen D, Thurman JM, Schmidt OI, Hossini AM, Taha ME, Rittirsch D, Ward PA, Holers VM, et al. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I, Schmidt OI, Thurman JM, Hossini AM, Rozanski M, Taha ME, Scheffler A, John T, Smith WR, Holers VM, Stahel PF. Pharmacological complement inhibition at the C3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Experimental neurology. 2006b;199:454–464. doi: 10.1016/j.expneurol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Lenzlinger PM, Hans VH, Joller-Jemelka HI, Trentz O, Morganti-Kossmann MC, Kossmann T. Markers for cell-mediated immune response are elevated in cerebrospinal fluid and serum after severe traumatic brain injury in humans. J Neurotrauma. 2001;18:479–489. doi: 10.1089/089771501300227288. [DOI] [PubMed] [Google Scholar]
- Li W, Liu HD, You WC, Zhou ML, Ling HP, Shen W, Zhu L, Hang CH. Enhanced cortical expression of myeloid differentiation primary response protein 88 (Myd88) in patients with traumatic brain injury. J Surg Res. 2013;180:133–139. doi: 10.1016/j.jss.2012.10.928. [DOI] [PubMed] [Google Scholar]
- Liao Y, Liu P, Guo F, Zhang ZY, Zhang Z. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013;8:e68963. doi: 10.1371/journal.pone.0068963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Castren E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, Zhou ML, Zhu L, Hang CH. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38:2072–2083. doi: 10.1007/s11064-013-1115-z. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Experimental neurology. 2016;275(Pt 3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi L, Perego C, Ortolano F, Zanier ER, Bianchi P, Stocchetti N, McIntosh TK, De Simoni MG. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit Care Med. 2009;37:659–665. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- Loov C, Hillered L, Ebendal T, Erlandsson A. Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS One. 2012;7:e33090. doi: 10.1371/journal.pone.0033090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, Martin MM, Lopez P, Ramos L, Blanquer J, Caceres JJ, Sole-Violan J, Solera J, Cabrera J, Argueso M, et al. Association between serum tissue inhibitor of matrix metalloproteinase-1 levels and mortality in patients with severe brain trauma injury. PLoS One. 2014;9:e94370. doi: 10.1371/journal.pone.0094370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113:1074–1079. doi: 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Frontiers in bioscience. 2009;14:3795–3813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J, Zhang J, Bryant J, Rezaie S, Chung JY, et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol. 2013;74:65–75. doi: 10.1002/ana.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, Lamb BT, Bhaskar K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S, Hicks R Combination Therapies for Traumatic Brain Injury Workshop, L. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des. 2006;12:1645–1680. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- McConeghy KW, Hatton J, Hughes L, Cook AM. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26:613–636. doi: 10.2165/11634020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October, 2016. Br J Sports Med. 2017 doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF. What is the Relationship of Traumatic Brain Injury to Dementia? J Alzheimers Dis. 2017;57:667–681. doi: 10.3233/JAD-161002. [DOI] [PubMed] [Google Scholar]
- Meng Q, Zhuang Y, Ying Z, Agrawal R, Yang X, Gomez-Pinilla F. Traumatic Brain Injury Induces Genome-Wide Transcriptomic, Methylomic, and Network Perturbations in Brain and Blood Predicting Neurological Disorders. EBioMedicine. 2017;16:184–194. doi: 10.1016/j.ebiom.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Perez GV, Zhao F, Lee YL, Poon C, Murphy GM., Jr Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. J Biol Chem. 2001;276:30142–30149. doi: 10.1074/jbc.M104265200. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Jopson TD, Liu S, Riparip LK, Guandique CK, Gupta N, Ferguson AR, Rosi S. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci. 2015;35:748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Riparip LK, Rosi S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PLoS One. 2016;11:e0148001. doi: 10.1371/journal.pone.0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma. 1999;16:617–628. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Aoki T, Lo EH. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma. 2002;19:1411–1419. doi: 10.1089/089771502320914642. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, et al. The International Incidence of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Can J Neurol Sci. 2016;43:774–785. doi: 10.1017/cjn.2016.290. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–384. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012;2012:217580. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, Whalen MJ. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2012;32:330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TM, Nguyen AH, Rabang JR, Patil AA, Agrawal DK. The danger zone: Systematic review of the role of HMGB1 danger signalling in traumatic brain injury. Brain Inj. 2017;31:2–8. doi: 10.1080/02699052.2016.1217045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudas M, Bowdish D, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, Means TK, Moestrup SK, et al. Standardizing scavenger receptor nomenclature. J Immunol. 2014;192:1997–2006. doi: 10.4049/jimmunol.1490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas MR, Baldwin CL, Bollyky PL, Bowdish DME, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J Immunol. 2017;198:3775–3789. doi: 10.4049/jimmunol.1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Pendergraft WF, 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, Luster AD, Hacohen N, El Khoury J, Means TK. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14:917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Reams N, Eckner JT, Almeida AA, Aagesen AL, Giordani B, Paulson H, Lorincz MT, Kutcher JS. A Clinical Approach to the Diagnosis of Traumatic Encephalopathy Syndrome: A Review. JAMA Neurol. 2016;73:743–749. doi: 10.1001/jamaneurol.2015.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Moore AN, Grill RJ, Johnson D, Zhao J, Liu Y, Dash PK. Analysis of functional pathways altered after mild traumatic brain injury. J Neurotrauma. 2013;30:752–764. doi: 10.1089/neu.2012.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind SG, Crnko NT, Baker AJ, Morrison LJ, Shek PN, Scarpelini S, Rizoli SB. Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J Neuroinflammation. 2010;7:5. doi: 10.1186/1742-2094-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MC, Keene CN, Neher MD, Johnson K, Yu ZX, Ganivet A, Holers VM, Stahel PF. Site-targeted complement inhibition by a complement receptor 2-conjugated inhibitor (mTT30) ameliorates post-injury neuropathology in mouse brains. Neurosci Lett. 2016;617:188–194. doi: 10.1016/j.neulet.2016.02.025. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Jenne CN, Leger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ, Yong VW, Kubes P, Zygun DA. A prospective evaluation of the temporal matrix metalloproteinase response after severe traumatic brain injury in humans. J Neurotrauma. 2013;30:1717–1726. doi: 10.1089/neu.2012.2841. [DOI] [PubMed] [Google Scholar]
- Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R, McGavern DB. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J Leukoc Biol. 2015;98:319–332. doi: 10.1189/jlb.4RI0115-006RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudehill S, Muhallab S, Wennersten A, von Gertten C, Al Nimer F, Sandberg-Nordqvist AC, Holmin S, Mathiesen T. Autoreactive antibodies against neurons and basal lamina found in serum following experimental brain contusion in rats. Acta Neurochir (Wien) 2006;148:199–205. doi: 10.1007/s00701-005-0673-5. discussion 205. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, Workshop Scientific T, Advisory Panel M. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber M, Kokiko-Cochran ON, Puntambekar S, Lathia J, Lamb BT. TREM2 deficiency alters acute macrophage distribution and improves recovery after TBI. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4401. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Beneficial autoimmune T cells and posttraumatic neuroprotection. Ann N Y Acad Sci. 2000;917:341–347. doi: 10.1111/j.1749-6632.2000.tb05400.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Raposo C. Protective Autoimmunity: A Unifying Model for the Immune Network Involved in CNS Repair. Neuroscientist. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010a;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Dis. 2010b;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Semple BD, Trivedi A, Gimlin K, Noble-Haeusslein LJ. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol Dis. 2015;74:263–280. doi: 10.1016/j.nbd.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–133. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R, Bar-Or D, Harris L, Dole K, McLaughlin L, Yukl R. Transient monocyte release of interleukin-10 in response to traumatic brain injury. Shock. 1999;12:10–16. doi: 10.1097/00024382-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Tasaki O, Hosotubo H, Fuijita K, Mouri T, Tajima G, Kajino K, Nakae H, Tanaka H, et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel PF, Flierl MA, Morgan BP, Persigehl I, Stoll C, Conrad C, Touban BM, Smith WR, Beauchamp K, Schmidt OI, et al. Absence of the complement regulatory molecule CD59a leads to exacerbated neuropathology after traumatic brain injury in mice. J Neuroinflammation. 2009;6:2. doi: 10.1186/1742-2094-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel PF, Morganti-Kossmann MC, Perez D, Redaelli C, Gloor B, Trentz O, Kossmann T. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29:753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susarla BT, Villapol S, Yi JH, Geller HM, Symes AJ. Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro. 2014;6:159–170. doi: 10.1042/AN20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–2158. [PubMed] [Google Scholar]
- Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86–95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Shan R, Thomasian N, Chodobski A. The Involvement of Pial Microvessels in Leukocyte Invasion after Mild Traumatic Brain Injury. PLoS One. 2016;11:e0167677. doi: 10.1371/journal.pone.0167677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Strazielle N, Gandy JR, Keefe TH, Zink BJ, Ghersi-Egea JF, Chodobski A. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab. 2012;32:93–104. doi: 10.1038/jcbfm.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1503–1516. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley Watts L, Sprague S, Zheng W, Garling RJ, Jimenez D, Digicaylioglu M, Lechleiter J. Purinergic 2Y1 receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J Neurotrauma. 2013;30:55–66. doi: 10.1089/neu.2012.2488. [DOI] [PubMed] [Google Scholar]
- Thal SC, Neuhaus W. The blood-brain barrier as a target in traumatic brain injury treatment. Archives of medical research. 2014;45:698–710. doi: 10.1016/j.arcmed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Thawer SG, Mawhinney L, Chadwick K, de Chickera SN, Weaver LC, Brown A, Dekaban GA. Temporal changes in monocyte and macrophage subsets and microglial macrophages following spinal cord injury in the Lys-Egfp-ki mouse model. J Neuroimmunol. 2013;261:7–20. doi: 10.1016/j.jneuroim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL. Treatment of spinal cord impact injury in the rat with transforming growth factor-beta. J Neurol Sci. 2002;200:33–41. doi: 10.1016/s0022-510x(02)00113-2. [DOI] [PubMed] [Google Scholar]
- Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–695. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, Burns MP. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 2017 doi: 10.1002/glia.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Golz G, Rosenberger K, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699–714. doi: 10.1172/JCI76210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Ba YC, Xiong LL, Li XL, Zou Y, Zhu YC, Zhou XF, Wang TH, Wang F, Tian HL, Li JT. Endogenous TGFbeta1 Plays a Crucial Role in Functional Recovery After Traumatic Brain Injury Associated with Smad3 Signal in Rats. Neurochem Res. 2015;40:1671–1680. doi: 10.1007/s11064-015-1634-x. [DOI] [PubMed] [Google Scholar]
- Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S1. doi: 10.1186/1710-1492-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Miskinyte G, Ge R, Ahlenius H, Lindvall O, et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J Neurosci. 2016;36:4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LC, Bao F, Dekaban GA, Hryciw T, Shultz SR, Cain DP, Brown A. CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats. Experimental neurology. 2015;271:409–422. doi: 10.1016/j.expneurol.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, Vittal R, Capitano ML, Kim Y, Allette YM, et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 2014;6:252ra124. doi: 10.1126/scitranslmed.3009443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Weckbach S, Neher M, Losacco JT, Bolden AL, Kulik L, Flierl MA, Bell SE, Holers VM, Stahel PF. Challenging the role of adaptive immunity in neurotrauma: Rag1(−/−) mice lacking mature B and T cells do not show neuroprotection after closed head injury. J Neurotrauma. 2012;29:1233–1242. doi: 10.1089/neu.2011.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel F, Shlomchik M. Memory B Cells of Mice and Humans. Annu Rev Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Kochanek PM, Clark RS, Heineman S, Schiding JK, Franicola D, Memarzadeh F, Lo W, Marion DW, Dekosky ST. Neutrophils do not mediate blood-brain barrier permeability early after controlled cortical impact in rats. J Neurotrauma. 1999;16:583–594. doi: 10.1089/neu.1999.16.583. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, Kochanek PM. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28:3710–3717. doi: 10.1097/00003246-200011000-00029. [DOI] [PubMed] [Google Scholar]
- White TE, Ford GD, Surles-Zeigler MC, Gates AS, Laplaca MC, Ford BD. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genomics. 2013;14:282. doi: 10.1186/1471-2164-14-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieghofer P, Knobeloch KP, Prinz M. Genetic targeting of microglia. Glia. 2015;63:1–22. doi: 10.1002/glia.22727. [DOI] [PubMed] [Google Scholar]
- Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab. 2002;22:318–326. doi: 10.1097/00004647-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Wilkinson K, Boyd JD, Glicksman M, Moore KJ, El Khoury J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. J Biol Chem. 2011;286:34914–34922. doi: 10.1074/jbc.M111.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, You Z, Kim HH, Hwang SK, Khuman J, Guo S, Lo EH, Whalen MJ. Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27:1037–1046. doi: 10.1089/neu.2009.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsiv I, Morganti-Kossmann MC, Perez D, Dinarello CA, Novick D, Rubinstein M, Otto VI, Rancan M, Kossmann T, Redaelli CA, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J Cereb Blood Flow Metab. 2002;22:971–978. doi: 10.1097/00004647-200208000-00008. [DOI] [PubMed] [Google Scholar]
- You Z, Yang J, Takahashi K, Yager PH, Kim HH, Qin T, Stahl GL, Ezekowitz RA, Carroll MC, Whalen MJ. Reduced tissue damage and improved recovery of motor function after traumatic brain injury in mice deficient in complement component C4. J Cereb Blood Flow Metab. 2007;27:1954–1964. doi: 10.1038/sj.jcbfm.9600497. [DOI] [PubMed] [Google Scholar]
- Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol. 2004;27:293–298. doi: 10.1097/01.wnf.0000150867.98887.3e. [DOI] [PubMed] [Google Scholar]
- Zhu X, Park J, Golinski J, Qiu J, Khuman J, Lee CC, Lo EH, Degterev A, Whalen MJ. Role of Akt and mammalian target of rapamycin in functional outcome after concussive brain injury in mice. J Cereb Blood Flow Metab. 2014;34:1531–1539. doi: 10.1038/jcbfm.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo D, Subjeck J, Wang XY. Unfolding the Role of Large Heat Shock Proteins: New Insights and Therapeutic Implications. Front Immunol. 2016;7:75. doi: 10.3389/fimmu.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]