Abstract
A renewed interest in the role of complement in the pathogenesis of glomerular diseases has improved our understanding of their basic, underlying physiology. All 3 complement pathways—classical, lectin, and alternative—have been implicated in glomerular lesions both rare (e.g., dense deposit disease) and common (e.g., IgA nephropathy). Here we review the basic function of these pathways and highlight, with a disease-specific focus, how activation can lead to glomerular injury. We end by exploring the promise of complement-targeted therapies as disease-specific interventions for glomerular diseases.
Keywords: complement, glomerulonephritis, nephrotic syndrome, membranous nephropathy, lupus, IgA nephropathy, C3 glomerulopathy
In clinical practice, a nephrologist evaluating a patient with a glomerular disease always begins with a simple, dichotomous classification scheme. The glomerular disease is considered either “primary” (intrinsic to the kidney) or “secondary” (a renal manifestation of a systemic disease that can also affect other organ systems). For example, in approximately 25% of patients whose kidney biopsies show the lesion of membranous nephropathy, a systemic disease process such as lupus, hepatitis B, or cancer will be identified as the underlying etiology of the lesion. Traditionally, the primary glomerular lesions have also been called idiopathic, often with the suggestion of an underlying, ill-defined autoimmune process. In recent years, advances in our understanding of the pathophysiology behind many of the glomerular diseases have gradually chipped away at this “idiopathic” nomenclature. One area of major advances has been through a heightened appreciation of the role of complement in glomerular injury. This complement-oriented approach has allowed nephrologists to come increasingly close to answering the question most commonly posed by patients—Why did this happen?—and has provided a guide to prognosis and treatment of a number of glomerular diseases.
Overview of Complement Pathways
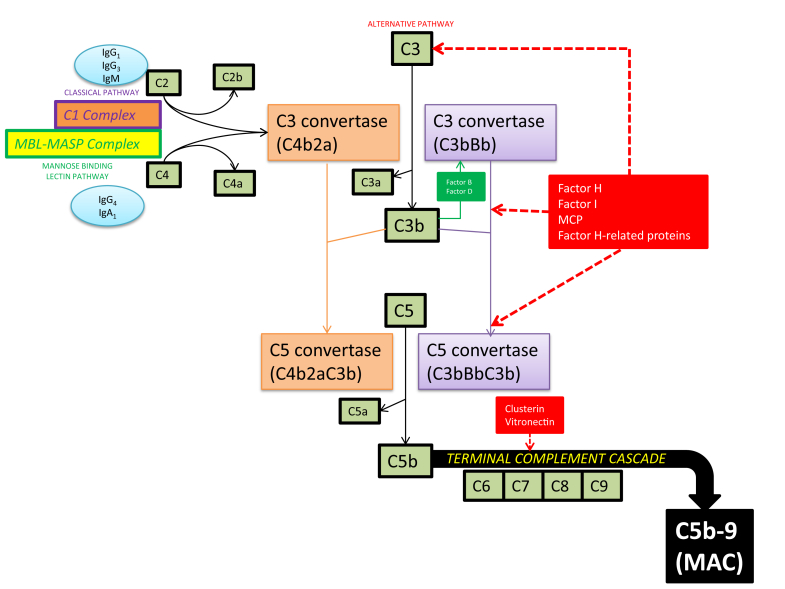
The complement system is divided into 3 initiating pathways—the classical, lectin, and alternative pathways (Figure 1). Proper functioning of each pathway is required for coordinated activity of innate and acquired immunity.1 The 3 initiating pathways all converge at C3 to generate an enzyme complex known as C3 convertase that cleaves C3 into C3a and C3b. The association of C3b with C3 convertase results in generation of C5 convertase, which cleaves C5 into C5a and C5b. This cleavage triggers the terminal complement cascade, the assembly of the membrane attack complex (MAC), which is also known as C5b-9, and subsequent cell lysis or sublytic cellular injury.
Figure 1.
An overview of the complement cascade. The classical, mannose-binding lectin and alternative complement pathways converge at C3 to generate an enzyme complex known as C3 convertase that cleaves C3 into C3a and C3b. However, the pathways are distinct in their points of origin. The classical complement pathway is activated by either IgG (predominantly IgG1 and IgG3) or IgM antibodies bound to antigen; this immune complex exposes a binding site on the Ig for the first component of the classical pathway, C1, to form a C1 complex that cleaves C2 and C4. The lectin pathway is initiated by the binding of mannose-binding lectin (MBL) to the polysaccharide surface of pathogenic bacteria. This binding results in the formation of a trimolecular complex with mannose-binding lectin-associated serine proteases (MASPs) and subsequent cleavage of C2 and C4. IgG4 and IgA1 autoantibodies can also bind MBL and activate the lectin complement pathway. The alternative pathway begins at the level of C3 and is constitutively active via spontaneous hydrolysis of C3 to C3b, which binds factor B to yield the C3 convertase (C3bBb) of this pathway. Hence, activation of this complement pathway is generally considered antibody independent. MAC, membrane attack complex; MCP, membrane cofactor protein.
Although all 3 pathways converge at a similar level and therefore have similar downstream targets, the pathways are distinct in their points of origin. The classical complement pathway, which plays a major role in humoral immunity, is “triggered” into action by either IgG or IgM antibodies bound to antigen. This immune complex formation of antigen and antibody exposes a binding site on the Ig for the first component of the classical pathway, C1. The lectin pathway is initiated by the binding of mannose-binding lectin to the polysaccharide surface of pathogenic bacteria. This binding results in the formation of a trimolecular complex with 2 serine proteases and subsequent cleavage of C4 and C2, the next complement proteins in the cascade. In this pathway, C4b and C3b can bind to antigen-associated Igs as well as to microbial surfaces. The alternative pathway begins at the level of C3. Although microbial antigens can activate this pathway, the alternative pathway is also constitutively active via spontaneous hydrolysis of C3 to C3b, which binds factor B to yield the C3 convertase (C3bBb) of this pathway.
This distinction, between the constitutively active alternative pathway versus the triggered classical and lectin pathways, is manifest on immunofluorescence (IF) studies, which are performed routinely on all medical kidney biopsy specimens (Table 1). Specifically, the presence of Ig staining (IgG, IgM, and/or IgA) alongside complement on IF microscopy implies that immune complexes of antigen-antibody have triggered consumption of the classical and/or lectin pathway proteins (Figure 1), whereas the presence of C3 staining alone without Ig suggests that the glomerular lesion is mediated by complement alone in an antibody-independent fashion, implicating the alternative complement pathway.2 For the treating nephrologist, these IF patterns in turn focus the work-up and treatment of the glomerular disease on (i) the trigger in classical or lectin pathway–mediated injuries, with attention toward infectious, autoimmune, malignant, or drug-induced etiologies, versus (ii) the “dysregulation” of the constitutively active alternative pathway in C3-mediated lesions, with attention toward genetic mutations or autoantibodies targeted at components of the alternative pathway.3
Table 1.
Typical IF staining patterns for IgG, C3, and C1q in different glomerular diseases
| Disease | IF staining |
||
|---|---|---|---|
| IgG | C3 | C1q | |
| Lupus nephritis | + | + | + |
| C3 glomerulopathy | – | + | – |
| Membranous nephropathy | + | + | – |
IF, immunofluorescence.
IF staining can highlight which complement pathway is involved in disease pathogenesis. In lupus nephritis, IF staining should be positive for IgG, C3, and C1q, indicating a role for IgG activation of the classical complement pathway. The classic IF pattern of C3 glomerulopathies is isolated staining of C3 with negative Ig and C1q staining, implicating alternative pathway activation. In primary membranous nephropathy, the presence of IgG and C3 staining on IF with absent C1q staining suggests a pathogenic role of the lectin pathway.
LN: A Classical Complement Pathway–Mediated Glomerulonephritis
Systemic lupus erythematosus is a chronic autoimmune disease that can affect multiple organs, including the skin, joints, brain, peripheral nervous system, heart, gastrointestinal tract, and kidneys. Renal involvement in systemic lupus erythematosus, generally termed lupus nephritis (LN), is a major contributor to disease morbidity and mortality. Up to 50% of patients with systemic lupus erythematosus will have clinically evident kidney disease at presentation; during follow-up, renal involvement occurs in up to 75% of patients, with an even greater representation among children and young adults. In addition to nonspecific laboratory findings of changes in serum creatinine level and presence of proteinuria and/or hematuria, measurement of serum complement components (C3, C4) are an important screening test for LN if kidney involvement is suspected. Both C3 and C4 are often low when disease is systemically active, as is usually the case with any severe, proliferative form of LN.4
The classic pattern of LN is an immune complex–mediated glomerulonephritis (GN) with a varied pathology that includes 6 distinct classes of disease.5 The glomerular deposits in LN stain dominantly on IF microscopy for IgG with codeposits of IgA, IgM, C3, and C1q (Table 1) in a so-called full house pattern. The role of the classical complement pathway in mediating glomerular injury in LN is critical in understanding these and other representative biopsy findings in LN.
The immune deposits in LN are primarily immune complexes of anti–double-stranded DNA antibodies directed against nucleosomal antigens. A smaller fraction of autoantibodies can also bind directly to chromatin in the glomerular basement membrane (GBM) and mesangium.6, 7 These immune complexes, when deposited in the mesangium and subendothelial space, are proximal to the GBM and in communication with the systemic circulation. Subsequent activation of the classical complement pathway, triggered by the DNA–anti-DNA antibody complex formation, generates the potent chemoattractants C3a and C5a, which elicit an influx of neutrophils and mononuclear cells. The pattern on light microscopy (LM) is a proliferative GN that can be mesangial (class II), focal endocapillary (class III), or diffuse endocapillary (class IV). Immune complex deposits in the subepithelial space can also activate complement but only locally; the chemoattractants (C3a and C5a) are separated from the circulation by the GBM, and hence no influx of inflammatory cells occurs into this space. The injury in this class V LN (so-called membranous LN) is limited to the glomerular epithelial cells, the primary clinical manifestation is proteinuria, and the histologic pattern on LM is similar to that of primary membranous nephropathy.
Primary Membranous Nephropathy and the Lectin Complement Pathway
Our understanding of the pathophysiology behind the membranous nephropathy (MN) lesion has made a seismic leap in the last decade with the identification of target antigens and their associated autoantibodies, which are responsible for the majority of primary MN cases. In 2009, Beck et al.8 reported that the M-type phospholipase A2 receptor (PLA2R) was the specific podocyte antigen responsible for eliciting immune complex formation with circulating autoantibodies. Anti-PLA2R antibodies were detected in approximately 75% of idiopathic MN cases and rarely found in secondary forms of MN (e.g., lupus-, hepatitis-, and tumor-associated MN).8 Additional alternative podocyte autoantigens—–mitochondrial superoxide dismutase 2, aldose reductase, alpha-enolase, neutral endopeptidase,9 and, most recently, thrombospondin type-1 domain-containing 7A10—have been reported in patients with primary MN, potentially filling in the missing gaps in PLA2R antibody–negative disease. These breakthroughs have established MN as a disease of autoantibodies and potentially made the term idiopathic defunct.
Membranous nephropathy occurs when circulating antibodies permeate the GBM and, in the subepithelial space, form immune complexes with epitopes on podocyte membranes. This in situ antigen-antibody interaction leads to activation of complement; the ensuing formation of the MAC inflicts sublytic damage on the podocyte (leading to foot process effacement) and induces secretion, from the damaged podocyte, of additional extracellular material that further expands the GBM. The important role of complement in glomerular injury in MN is supported by the consistent finding of C3 and C5b-9 in the subepithelial deposits that, along with dominant granular staining for IgG, define the disease. The dominance of IgG4 in primary MN mediated by anti-PLA2R, as compared with the general deficiency of IgG4 in cases of secondary MN (e.g., in the setting of malignancy, in which IgG1 is more commonly found), raises questions about which complement pathway is most prominently involved in the pathogenesis of the MN lesion.
IgG4 is incapable of binding C1q and therefore is unable to activate complement via the classical pathway. Indeed, positive C1q staining in membranous nephropathy, a signal of classical complement pathway activation, often suggests a secondary form of the lesion, such as lupus MN. In contrast, IF staining for C3 and C4d, a breakdown product of C4b, in the absence of C1q is characteristic of primary MN (see Table 1), and formation of the MAC at the site of glomerular injury is required for the development of proteinuria.11 The most reasonable explanation for these findings is that the C3, C4d, and the MAC detected in these biopsy specimens result largely from activation of the lectin complement pathway.12 Anti-PLA2R IgG4 autoantibodies can bind mannose-binding lectin and activate the lectin complement pathway. Presumably, the IgG4 autoantibodies directed at other, less common autoantigens associated with primary MN (e.g., anti–thrombospondin type-1 domain-containing 7A antibodies) can also trigger activation of the lectin pathway. Evidence of the lectin pathway’s role in MN has surfaced in biopsy specimens from affected children that show mannose-binding lectin codepositing with IgG4.13
Alternative Complement Pathway–Mediated GN: The C3 Glomerulopathies
The alternative complement pathway is constitutively active at a low level. The term tickover has been used to describe this basal physiologic activation of the alternative pathway by spontaneous hydrolysis of C3 and the production of C3b, which binds factor B to yield a fluid phase C3 convertase (C3bBb).14 This alternative pathway C3 convertase, though, is under tight control by soluble or membrane-bound regulating proteins, including complement factor H, complement factor I, and membrane cofactor protein (see Figure 1). Thus, a genetic or acquired (i.e., via autoantibodies or monoclonal gammopathies that interfere with complement regulatory proteins) defect in either the activation or regulation of the C3 convertase could lead to a transformation from low-grade physiologic activity (tickover) to unrestrained, hyperactivity (diseases of complement dysregulation). This loss of alternative pathway control can result in GN that on IF stains only (or dominantly) for C3, with complement proteins (and not immune complexes) mediating the glomerular injury.
The reclassification of idiopathic membranoproliferative GN (MPGN) highlights how nephrologists and pathologists have incorporated the role of complement into their approach to disease.15, 16 Traditionally, MPGN was categorized according to ultrastructural findings on electron microscopy. Subendothelial and mesangial deposits predominated in MPGN type I, highly electron dense intramembranous and mesangial deposits were the hallmark of type II, and in type III MPGN, deposits could be subendothelial and subepithelial (Burkholder subtype) or produce complex intramembranous, subendothelial, and subepithelial formations with fraying of the lamina densa (Strife and Anders subtype). A major drawback of the membranoproliferative GN–based classification scheme was that it was based on histopathologic patterns and not on pathophysiology of disease.17 For example, many patients with intramembranous dense deposits, characteristic of MPGN type II, lacked an MPGN pattern altogether on LM, which led to use of the more accurate and inclusive term dense deposit disease (DDD). In addition, some cases of MPGN type I and type III had only C3 immune deposits distinct from the more common variants of type I and type III MPGN that contain Ig. These cases resembled DDD and were rebranded as C3 GN. Currently, the term C3 glomerulopathy has been proposed as an umbrella classification for any GN (including non-MPGN LM patterns) with isolated or dominant C3 staining. The term encompasses both DDD and C3 GN and signals an etiology rooted in dysregulation of the alternative complement pathway.18
The C3 glomerulopathies overlap with atypical hemolytic uremic syndrome (aHUS [also termed complement-mediated thrombotic microangiopathy19]) in genetic abnormalities (e.g., mutations in factor H, factor I, membrane cofactor protein, C3, and factor B) and autoantibodies (e.g., anti–factor H autoantibodies) reported in these diseases.20, 21, 22, 23 Yet, aHUS differs from the C3 glomerulopathies where the alternative pathway dysregulation occurs. The alternative pathway consists of a network of complement proteins in either the fluid phase as soluble plasma proteins or in the solid phase as cell membrane proteins. The underlying defect in most instances of the C3 glomerulopathies is felt to be excessive activation of the alternative complement pathway in the fluid phase.3, 24, 25 In contrast, the endothelial damage that is the hallmark of aHUS is due to dysregulation at the level of the cell membrane or in the solid phase.14
Overlaps: Glomerular Lesions Mediated by More Than 1 Complement Pathway
It may turn out to be overly simplistic to think of only 1 complement pathway for a specific glomerular disease. For example, a French cohort study comparing patients with immune complex-mediated MPGN type I lesions (i.e., C3 and Ig staining on IF) and patients with C3 GN (i.e., C3 alone on IF) found virtually equal rates of genetic mutations and autoantibodies targeted at alternative complement pathway regulatory proteins. This suggests that a subgroup of patients with immune complex–mediated lesions, and subsequent classical pathway–mediated injury, also have an underlying defect in alternative pathway control, a defect that in turn may have made these patients more susceptible to formation of the antigen-antibody interactions that triggered their classical pathways and initiated subsequent disease.26 With regard to MN, a recent report from Bally et al. describes 5 patients with primary, PLA2R-associated MN and genotypes associated with mannose-binding lectin deficiency, suggesting that, in these patients, complement activity was more likely in the alternative pathway rather than in the lectin pathway.27
IgA nephropathy, the most common form of primary GN worldwide, appears to be a disease mediated by both the mannose-binding lectin and alternative complement pathways. A multihit pathogenesis model of IgA nephropathy has emerged: abnormal polymeric IgA1 with deficient O-linked glycosylation at the hinge region (galactose-deficient IgA1) forms immune complexes with IgG antibodies directed at the abnormal hinge region (antiglycan antibodies), and these immune complexes are then deposited in the mesangium.28 On LM, mesangial proliferation and matrix expansion are the typical findings of IgA nephropathy, and the diagnosis is established by dominant IgA staining on IF microscopy. The IF microscopy can also show codominant or subdominant staining of IgG, C3, C4d, and C5b-9 that colocalize with IgA. C1q staining, however, is generally absent, suggesting no role of the classical complement pathway in the pathogenesis of disease. Instead, these IF findings suggest a potentially important contribution from both the alternative and lectin complement pathways.29 IgA1 can activate both pathways in vitro, and pathway components are present in the mesangial deposits, including properdin and factor H of the alternative pathway and mannose-binding lectin, mannose-binding lectin–associated serine proteases 1 and 2, and C4d of the lectin pathway. Indeed, intensity of C3 staining and deposition of mannose-binding lectin, respectively, have both been shown in small studies to correlate with severity of IgA nephropathy.30, 31, 32
Postinfectious GN—a diffuse, exudative, and proliferative lesion that is mainly seen in response to bacterial infections (most commonly streptococcal or staphylococcal species)—classically follows an acute self-limited infection. The latency period from infection to GN, presumably the time required for antibodies directed at the offending bacteria to form immune complexes with intrinsic tissue that is in turn deposited in the glomerulus, is approximately 2 weeks. This rationale for the development of postinfectious GN implicates activation of the classical complement pathway by circulating immune complexes, which is supported by staining on IF for both C3 and IgG. However, staining of C3 is usually of equal or greater intensity than IgG, and low-intensity staining for C1q is seen in only a minority of cases, suggesting that some C3 deposition is independent of the classical pathway. The more complex explanation for the postinfectious GN lesion includes bacterial antigens—circulating outside the glomerulus as well as planted at the subepithelial and subendothelial spaces of the GBM—directly activating both the alternative and mannose-binding lectin complement pathways.33 Thus, the classical, alternative, and mannose-binding lectin complement pathways may all be operant to varying extents in individual patients with postinfectious GN.
The degree to which a complement-focused approach can transform our thinking about pathogenesis of disease is perhaps most evident in antineutrophil cytoplasmic autoantibody (ANCA)-associated GN. Although the hallmark finding on IF staining in ANCA-associated GN is a paucity of Ig and complement deposition, most cases have some focal complement deposition at sites of glomerular injury.34 In ANCA-mediated disease, ANCA IgG activates cytokine-primed neutrophils that, in turn, release factors that stimulate activity of the alternative complement pathway. In mice, administration of antimyeloperoxidase IgG leads to a necrotizing, crescentic GN with neutrophil and macrophage infiltration alongside low-level glomerular IgG and C3 deposition. In contrast, coadministration of antimyeloperoxidase IgG with cobra venom factor, which depletes C3, protects against the development of such a lesion.35 A similar “resistance” against anti-myeloperoxidase–mediated GN was shown in knockout mice deficient in C5 and factor B (both proteins of the alternative pathway) but not in mice deficient in C4 (an important protein of both the classical and lectin pathways).35 Therefore, complement targeting therapies (discussed later in this review) are now being tested as adjunct treatment in ANCA-associated disease.
Implications for Therapy
A better understanding of the role of complement in glomerular diseases in turn yields questions about targeting therapies at the complement pathways.36 The most logical target of therapy for diseases mediated by classical complement pathway activity is the trigger(s) or inciting event(s) that led to complement consumption—a documented infection, for example, or systemic lupus erythematosus. In cases in which a trigger is not apparent, or in glomerular lesions in which the lectin or alternative pathways appear to be playing the dominant role, complement-directed therapies may offer a more precise route of treatment than traditional use of nonspecific immunosuppression.
The first U.S. Food and Drug Administration–approved anticomplement therapy is eculizumab, a fully humanized monoclonal antibody that binds with high affinity to C5 and prevents the generation of MAC. Eculizumab was first approved for the treatment of paroxysmal nocturnal hemoglobinuria37 and, more recently, for treatment of aHUS.38 Both conditions are thought to be caused by abnormalities in alternative pathway regulation, usually via a mutation in a complement regulatory protein. A small but growing body of literature has emerged on using eculizumab for C3 glomerulopathies.39, 40, 41, 42, 43, 44 Only 1 of these reports was from a prospective trial, the open label study of eculizumab therapy in 6 subjects with C3 glomerulopathies that was published in 2 parts, 1 focusing on clinical response to therapy45 and 1 focusing on the results of biopsies done before and after treatment.46 All subjects were treated with eculizumab for 1 year with the same dosing schedule used for aHUS. After 1 year of therapy, 2 subjects demonstrated significantly reduced serum creatinine with decreased mesangial and/or endocapillary proliferation on LM, 1 subject attained remission of nephrotic syndrome with reduced mesangial proliferation on LM and partial resorption of deposits on electron microscopy, and 1 subject had stable laboratory parameters but significantly reduced mesangial and endocapillary proliferation on LM. The 2 remaining patients, however, demonstrated steeply declining renal function during treatment. The remainder of the literature consists of single case reports that are almost entirely positive, which may introduce publication bias.39, 40, 41 Overall, eculizumab’s efficacy in C3 glomerulopathies is still unclear and likely will remain unclear until an adequately powered, appropriately designed trial is performed.
By blocking at C5, eculizumab prevents formation of C5b, which initiates the formation of the MAC, and C5a, a potent anaphylatoxin. In theory, the benefits of eculizumab may result as much from its anti-C5a effects, which are anti-inflammatory, as from its prevention of MAC formation. A C5aR blocker, CCX168, is currently being studied as an adjunctive therapy for ANCA-associated GN on the basis of animal models clearly implicating a role of alternative pathway activity in vasculitis pathogenesis. Two randomized placebo-controlled phase II studies of CCX168 in ANCA-associated disease, 1 in Europe (NCT01363388) and 1 in the United States (NCT02222155), have recently concluded, and blocking specifically at the level of C5a may have relevance in other glomerular diseases.
Blockade at the level of C3 may, in theory, be more effective than anti-C5 therapy given that many abnormalities emerge at the level of abnormal C3 convertase activity of either the alternative (C3bBb) or classic/lectin pathways (C4b2a). Recently, Zhang et al. reported results using soluble complement receptor 1 (CR1) in mice deficient in factor H (a model for C3 GN) and transgenic for human CR1.47 CR1 is a cell surface protein expressed on a number of cells, including B cells, some T cells, dendritic cells, and podocytes, with regulatory activity at both the level of C3 convertase and C5 convertase. Additionally, CR1 is the only cofactor of factor I (other than factor H) that can cleave inactive C3b into smaller fragments, potentially clearing this breakdown product of the C3 convertase away from the GBM. Daily injections of soluble CR1 in factor H– and CR1-deficient mice led to increases in serum C3 levels and, histopathologically, to decreases in C3 deposition, with clearance of old C3 deposits demonstrated by reduced C3c staining. In this same report, Zhang et al. detailed the short-term use (7 doses) of soluble CR1 in an 8-year-old girl with end-stage renal disease due to DDD. Serum C3 levels rose and soluble MAC levels normalized transiently, although no differences were seen in specimens from biopsies performed before and after treatment. A small phase 1 trial (n = 5) of soluble CR1 (also called CDX-1135) in pediatric and adult patients with DDD (NCT01791686), however, was terminated early after enrolling only a single subject. Other C3-targeting agents have been evaluated in preclinical settings—either in animal models of complement-mediated GN or in vitro—including compstatin analog Cp40,48 recombinant forms of complement factor H (CR2-FH and FH1-5ˆ18-20),49, 50 and anti-C3 monoclonal antibodies.51
Conclusion
A heightened appreciation of the role of each of the complement pathways (classical, lectin, and alternative) in the pathogenesis of glomerular diseases has helped nephrologists and renal pathologists come closer to removing the term idiopathic from the lexicon of glomerular lesions. Moreover, this approach to pathogenesis inevitably spurs discussions of treatment directed at the complement system. The advent of therapies aimed at the complement cascade, now in its earliest phases, may promise breakthroughs in disease-specific treatments that change the natural history of disease.
Disclosure
ASB has received or expects to receive consulting honoraria from Omeros, Chemocentryx, and Achillion. GBA has received or expects to receive consulting honoraria from Omeros, Alexion, and Achillion. The other author declared no competing interests.
Acknowledgments
ASB is supported by National Institutes of Health-National Institute on Minority Health and Health Disparities grant R01-MD009223.
References
- 1.Chen M., Daha M.R., Kallenberg C.G. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–J286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S. Etiology-based diagnostic approach to proliferative glomerulonephritis. Am J Kidney Dis. 2014;63:561–566. doi: 10.1053/j.ajkd.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 4.Contreras G., Roth D., Pardo V., Striker L.G., Schultz D.R. Lupus nephritis: a clinical review for practicing nephrologists. Clin Nephrol. 2002;57:95–107. doi: 10.5414/cnp57095. [DOI] [PubMed] [Google Scholar]
- 5.Weening J.J., D'Agati V.D., Schwartz M.M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 6.Kalaaji M., Mortensen E., Jorgensen L., Olsen R., Rekvig O.P. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168:1779–1792. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan M.R., Wang C., Marion T.N. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 2012;82:184–192. doi: 10.1038/ki.2011.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck L.H., Jr., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murtas C., Bruschi M., Candiano G. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:1394–1400. doi: 10.2215/CJN.02170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S., Nasr S.H., De Vriese A.S., Fervenza F.C. C4d as a diagnostic tool in proliferative GN. J Am Soc Nephrol. 2015;26:2852–2859. doi: 10.1681/ASN.2014040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma H., Sandor D.G., Beck L.H., Jr. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segawa Y., Hisano S., Matsushita M. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010;25:1091–1099. doi: 10.1007/s00467-009-1439-8. [DOI] [PubMed] [Google Scholar]
- 14.Roumenina L.T., Loirat C., Dragon-Durey M.A., Halbwachs-Mecarelli L., Sautes-Fridman C., Fremeaux-Bacchi V. Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods. 2011;365:8–26. doi: 10.1016/j.jim.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31:341–348. doi: 10.1016/j.semnephrol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Bomback A.S., Appel G.B. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8:634–642. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]
- 17.D'Agati V.D., Bomback A.S. C3 glomerulopathy: what's in a name? Kidney Int. 2012;82:379–381. doi: 10.1038/ki.2012.80. [DOI] [PubMed] [Google Scholar]
- 18.Fakhouri F., Fremeaux-Bacchi V., Noel L.H., Cook H.T., Pickering M.C. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- 19.Cataland S.R., Wu H.M. Diagnosis and management of complement mediated thrombotic microangiopathies. Blood Rev. 2014;28:67–74. doi: 10.1016/j.blre.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Skerka C., Licht C., Mengel M. Autoimmune forms of thrombotic microangiopathy and membranoproliferative glomerulonephritis: indications for a disease spectrum and common pathogenic principles. Mol Immunol. 2009;46:2801–2807. doi: 10.1016/j.molimm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Noris M., Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 22.Maga T.K., Nishimura C.J., Weaver A.E., Frees K.L., Smith R.J. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31:E1445–E1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- 23.Lorcy N., Rioux-Leclercq N., Lombard M.L., Le Pogamp P., Vigneau C. Three kidneys, two diseases, one antibody? Nephrol Dial Transplant. 2011;26:3811–3813. doi: 10.1093/ndt/gfr436. [DOI] [PubMed] [Google Scholar]
- 24.Pickering M., Cook H.T. Complement and glomerular disease: new insights. Curr Opin Nephrol Hypertens. 2011;20:271–277. doi: 10.1097/MNH.0b013e328345848b. [DOI] [PubMed] [Google Scholar]
- 25.Smith R.J., Harris C.L., Pickering M.C. Dense deposit disease. Mol Immunol. 2011;48:1604–1610. doi: 10.1016/j.molimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Servais A., Noel L.H., Roumenina L.T. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 27.Bally S, Debiec H, Ponard D, et al. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency [e-pub ahead of print]. J Am Soc Nephrol. ASN.2015101155, accessed July 22, 2016. [DOI] [PMC free article] [PubMed]
- 28.Suzuki H., Kiryluk K., Novak J. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillard N., Wyatt R.J., Julian B.A. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa M., Ortega R., Sanchez M. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9:897–904. doi: 10.2215/CJN.09710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L.L., Liu N., Chen Y. Glomerular mannose-binding lectin deposition is a useful prognostic predictor in immunoglobulin A nephropathy. Clin Exp Immunol. 2013;174:152–160. doi: 10.1111/cei.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., Zhang Y., Duan X. C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy. J Clin Immunol. 2014;34:224–232. doi: 10.1007/s10875-013-9970-6. [DOI] [PubMed] [Google Scholar]
- 33.Hisano S., Matsushita M., Fujita T., Takeshita M., Iwasaki H. Activation of the lectin complement pathway in post-streptococcal acute glomerulonephritis. Pathol Int. 2007;57:351–357. doi: 10.1111/j.1440-1827.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 34.Jennette J.C., Wilkman A.S., Falk R.J. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–930. [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao H., Schreiber A., Heeringa P., Falk R.J., Jennette J.C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bomback A.S. Anti-complement therapy for glomerular diseases. Adv Chronic Kidney Dis. 2014;21:152–158. doi: 10.1053/j.ackd.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Hillmen P., Young N.S., Schubert J. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 38.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 39.Daina E., Noris M., Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161–1163. doi: 10.1056/NEJMc1112273. [DOI] [PubMed] [Google Scholar]
- 40.Vivarelli M., Pasini A., Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163–1165. doi: 10.1056/NEJMc1111953. [DOI] [PubMed] [Google Scholar]
- 41.Payette A., Patey N., Dragon-Durey M.A., Fremeaux-Bacchi V., Le Deist F., Lapeyraque A.L. A case of C3 glomerulonephritis successfully treated with eculizumab. Pediatr Nephrol. 2015;30:1033–1037. doi: 10.1007/s00467-015-3061-2. [DOI] [PubMed] [Google Scholar]
- 42.Oosterveld M.J., Garrelfs M.R., Hoppe B. Eculizumab in Pediatric Dense Deposit Disease. Clin J Am Soc Nephrol. 2015;10:1773–1782. doi: 10.2215/CJN.01360215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Quintrec M., Lionet A., Kandel C. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484–489. doi: 10.1053/j.ajkd.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Inman M., Prater G., Fatima H., Wallace E. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J. 2015;8:445–448. doi: 10.1093/ckj/sfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomback A.S., Smith R.J., Barile G.R. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herlitz L.C., Bomback A.S., Markowitz G.S. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229–1237. doi: 10.1681/ASN.2011121186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Nester C.M., Holanda D.G. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24:1820–1829. doi: 10.1681/ASN.2013010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Shao D., Ricklin D. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993–998. doi: 10.1016/j.imbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruseva M.M., Peng T., Lasaro M.A. Efficacy of targeted complement inhibition in experimental C3 glomerulopathy. J Am Soc Nephrol. 2016;27:405–416. doi: 10.1681/ASN.2014121195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols E.M., Barbour T.D., Pappworth I.Y. An extended mini-complement factor H molecule ameliorates experimental C3 glomerulopathy. Kidney Int. 2015;88:1314–1322. doi: 10.1038/ki.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paixao-Cavalcante D., Torreira E., Lindorfer M.A. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J Immunol. 2014;192:4844–4851. doi: 10.4049/jimmunol.1303131. [DOI] [PMC free article] [PubMed] [Google Scholar]