Abstract
Background
A dry powder inhaled formulation is used for the anti-influenza drug laninamivir octanoate hydrate (laninamivir). Although two successive inhalations (puffs) are recommended to minimize residual amounts of active ingredients, previous reports suggest that pediatric patients with low peak inspiratory flow are unable to inhale the active ingredient adequately. In the present study, we prospectively investigated the appropriate number of repeated inhalations of laninamivir dry powder and factors influencing the residual amount of ingredients in pediatric patients with influenza.
Methods
The study enrolled 64 patients receiving laninamivir dry powder inhaler (Inavir®) between January and March 2016 at Tsu emergency medical center/pediatric clinic and dental clinic. All patients enrolled used a laninamivir dry powder inhaler in four repeated inhalations, as instructed by a pharmacist. The residual amount of laninamivir dry powder was calculated by measuring the device weight before and after each inhalation and a residual amount of >20% was defined as an unsuccessful inhalation.
Results
The inadequate inhalation rate after two successive inhalations was 45%, and it decreased as number of inhalation repeats increased, reaching 23% after four successive inhalations. Peak inspiratory flow in patients with inadequate inhalation was significantly lower than that in patients with adequate inhalation, for all numbers of inhalation repeats analyzed. Receiver operating characteristic analyses indicated peak inspiratory flow cut-off values of 140, 120, 100, and 100 L/min at 1-4 successive inhalations, respectively.
Conclusions
The present findings suggest that a proportion of patients with low peak inspiratory flow were unable to inhale the active ingredient adequately when laninamivir dry powder inhaler was administered as two successive inhalations, as recommended in the instruction manual. Three or four repeated inhalations of laninamivir dry powder inhaler should be administered to pediatric patients with low peak inspiratory flow.
Electronic supplementary material
The online version of this article (10.1186/s40780-017-0094-7) contains supplementary material, which is available to authorized users.
Keywords: Inhalation, Influenza, Laninamivir, Peak inspiratory flow, Pediatric patients
Background
Inavir® (Daiichi Sankyo Co., Ltd., Tokyo, Japan) is a disposable dry powder inhaler (DPI) formulation of laninamivir octanoate hydrate (laninamivir), a long-acting neuraminidase inhibitor, featuring two plastic medicine compartments (TwinCaps®, Hovione, Loures, Portugal) [1]. Laninamivir DPI is widely used for treating influenza virus A and B infections in Japan because of the convenient single-use treatment course [2]. However, if laninamivir DPIs are not operated correctly, a sufficient therapeutic effect cannot be obtained [3]. Therefore, pharmacists are required to instruct patients about the proper inhalation technique and provide information on the appropriate use of the inhalers [4].
The pharmaceutical company manufacturing laninamivir DPI suggests that pharmacists confirm patient inhalation ability and proper inhalation technique using a special whistle provided by the company when laninamivir DPI is prescribed to pediatric patients. Nevertheless, pediatric patients with low peak inspiratory flow (PIF) and those aged 4-6 years have been reported to be unable to adequately inhale laninamivir [3, 5]. In addition, decreased effectiveness of laninamivir and prolonged disease duration have been observed in patients with >20% of residual drug after inhalation [3]. Furthermore, for zanamivir DPI (Relenza®, GlaxoSmithKline), another inhaled formulation of an anti-influenza drug, it was shown that pediatric patients (≥ 5 years old) found inhaling the entire amount in a single inhalation difficult, indicating that repeated inhalations are needed to achieve sufficient therapeutic effect [6]. Therefore, we hypothesized that a single inhalation of laninamivir DPI may not achieve sufficient inhalation of the active ingredient in pediatric patients with a predictably low PIF [7, 8]. In support of this hypothesis, two successive inhalations (puffs) of laninamivir DPI have been recommended in the instruction manual by the manufacturer to reduce residual amounts of the drug. However, the appropriate number of inhalation repeats and factors influencing the residual amount of laninamivir DPI ingredients in pediatric patients have not been reported. In addition, clinical trials of Inavir® performed by the pharmaceutical company were not conducted using the TwinCaps® device [9] and it is unclear whether pediatric patients can properly use the inhaler. In the present study, we prospectively investigated the appropriate number of inhalation repeats and factors influencing the residual amount of laninamivir DPI ingredients in pediatric patients with influenza.
Methods
Study design
This prospective study enrolled 65 patients who received laninamivir DPI (Inavir®) between January and March 2016 at Tsu emergency medical center/pediatric clinic and dental clinic (Mie, Japan). Written informed consent was obtained from all study participants or guardians. One patient was unable to receive treatment via inhalation and was excluded. A pharmacist (with 7 years of work experience) instructed patients and/or their guardians in proper inhalation technique using leaflets describing the structure and inhalation procedure of laninamivir DPI (INAIP00104_0DH, Daiichi Sankyo Co., Ltd., Tokyo). Following confirmation of patient inhalation ability by the pharmacist (using a whistle provided by Daiichi Sankyo Co., Ltd., Tokyo, Japan), patients received laninamivir DPI treatment.
Determination of the residual amounts of laninamivir dry powder
All enrolled patients performed four successive inhalations of laninamivir DPI following pharmacist instructions. One inhalation was defined as a single inhalation from two plastic dose compartments of the DPI. The residual amount of laninamivir dry powder was determined by measuring the device weight before and after each inhalation repeat using an electronic balance (LB-300 T, Takazono Co., Ltd., Tokyo, Japan). The residual amount of laninamivir dry powder was calculated by subtracting the device weight after inhalation from that before inhalation, as previously described [3]. If the residual amount was >20% compared to laninamivir dry powder content before use (0.1 g), inhalation was defined as inadequate.
Data collection on eligible patients
Patient characteristics (age, sex, Rohrer index, and body temperature) were extracted from clinical records. Respiratory rate was estimated by observing the respiratory movements of the thorax in a sitting position. Percutaneous arterial oxygen saturation (SpO2) and pulse rate were determined using a portable pulse oximeter (BO-600, Japan Precision Instruments Inc., Shibukawa, Japan). History of asthma was obtained from patient interviews. PIF in a state of maximal inspiration before inhalation of laninamivir DPI was measured using an In-Check® oral inspiratory flow meter (accuracy: ± 10%, Clement Clarke International Ltd., Essex, UK) without any adapters. A paper disposable mouthpiece was attached to the In-Check® device for each patient.
Statistical analyses
Statistical comparisons were performed using the Wilcoxon test with Bonferroni correction, Mann-Whitney U-test, and Fisher’s exact test for continuous and categorical variables, respectively. Cut-off values for continuous variables were determined by receiver operating characteristic (ROC) curve method. Statistical analyses were performed with JMP® version 12.0.1 (SAS Institute Inc., Cary, NC, USA). Significance was established at P < 0.05.
Results
Patient characteristics
Characteristics of the 64 enrolled participants are summarized in Table 1. The median age was 9 years [range: 5–15 years]. Thirty-seven patients (58%) were male. The median PIF value was 110 [range: 40–240 L/min].
Table 1.
Patient characteristics
| Characteristics | |
|---|---|
| Number of patients | 64 |
| Age (years) | 9 [5 – 15] |
| Male | 37 (58) |
| Rohrer index (kg/cm3) | 126 [83 – 246] |
| Body temperature (°C) | 38.4 [35.8 – 40.4] |
| History of asthma | 11 (17) |
| Pulse rate (beats/min) | 115 [73 – 150] |
| Respiratory rate (breaths/min) | 25 [14 – 50] |
| SpO2 (%) | 96 [93 – 99] |
| PIF (L/min) | 110 [40 – 240] |
Values are presented as median [range] or number (%)
SpO2: peripheral arterial oxygen saturation, PIF peak inspiratory flow
Residual amounts of laninamivir dry powder after repeated inhalations
Figure 1 shows the residual amounts of laninamivir dry powder after each inhalation repeat. The median residual amounts [range] corresponding to one to four inhalation repeats were 0.029 [0.003 – 0.078 g], 0.019 [0.002 – 0.058 g], 0.014 [0.002 – 0.041 g], and 0.013 [0.002–0.033 g], respectively. Significant differences in residual drug amount were observed between inhalation repeats, except when three and four successive inhalations were compared.
Fig. 1.
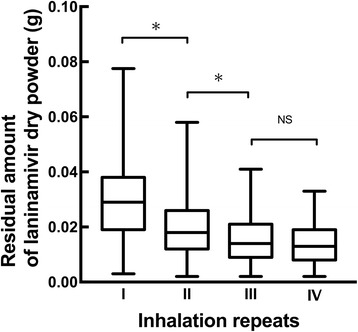
Residual amount of laninamivir dry powder after repeated inhalation of laninamivir DPI. Box plots represent the median with range. Statistical analyses were performed using the Wilcoxon test with Bonferroni correction. *: P < 0.05, NS: not significant
Inadequate inhalation rate after repeated inhalations
Figure 2 shows the rate of inadequate inhalation after each inhalation repeat. The inadequate inhalation rate was 70% after a single inhalation, and decreased with increased number of inhalation repeats (45, 28, and 23%, after two, three, and four inhalations, respectively).
Fig. 2.
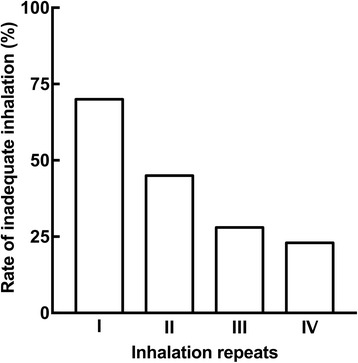
Rate of inadequate inhalation after repeated inhalations of laninamivir DPI
Comparisons of patient characteristics between adequate and inadequate inhalation groups after each inhalation repeat
Comparisons of patient characteristics between the adequate and inadequate inhalation groups after repeated inhalations are summarized in Table 2. PIF values in patients with inadequate inhalation were significantly lower than those in patients with adequate inhalation for each analyzed number of inhalation repeats. Moreover, the age of patients with inadequate inhalation was significantly lower than that of patients with adequate inhalation after two, three, and four repeated inhalations. Significantly fewer male patients displayed inadequate inhalation after one, two, or three successive inhalations. Furthermore, respiratory rates in patients who were unable to inhale adequately after a single inhalation were significantly higher than those in patients with adequate inhalation.
Table 2.
Comparisons of patient characteristics between adequate and inadequate inhalation groups after repeated inhalations
| Inhalation repeats | I | II | III | IV | ||||
|---|---|---|---|---|---|---|---|---|
| Groups by inhalation | Adequate | Inadequate | Adequate | Inadequate | Adequate | Inadequate | Adequate | Inadequate |
| (n = 19) | (n = 45) | (n = 35) | (n = 29) | (n = 46) | (n = 18) | (n = 49) | (n = 15) | |
| Age (years) | 10 [5-14] | 9 [5-15] | 10 [5-15] | 9 [5-14]* | 10 [5-15] | 7 [5-13]* | 10 [5-15] | 7 [5-13]* |
| Male | 15 (79) | 22 (49)* | 26 (74) | 11 (38)* | 31 (67) | 6 (33)* | 31 (63) | 6 (40) |
| Rohrer index (kg/cm3) | 134 [83-188] | 125 [98-246] | 128 [83-246] | 126 [98-190] | 126 [83-246] | 127 [100-190] | 126 [83-246] | 127 [100-190] |
| Body temperature (°C) | 38.4 [35.9-40.4] | 38.4 [35.8-40.3] | 38.4 [35.9-40.4] | 38.5 [35.8-40.1] | 38.4 [35.9-40.4] | 38.5 [35.8-40.0] | 38.4 [35.9-40.4] | 38.5 [35.8-40.0] |
| History of asthma | 2 (11) | 9 (20) | 7 (20) | 4 (14) | 10 (22) | 1 (6) | 10 (20) | 10 (67) |
| Pulse rate (beats/min) | 116 [88-143] | 115 [73-150] | 114 [73-143] | 117 [77-150] | 114 [73-143] | 119 [77-150] | 114 [73-143] | 119 [77-150] |
| Respiratory rate (breaths/min) | 19 [14-35] | 25 [14-50]* | 22 [14-50] | 25 [14-37] | 23 [14-50] | 26 [18-37] | 23 [14-50] | 26 [18-37] |
| SpO2 (%) | 96 [94-98] | 97 [93-99] | 96 [94-99] | 97 [93-99] | 96 [93-99] | 97 [94-99] | 96 [93-99] | 97 [94-99] |
| PIF (L/min) | 150 [80-240] | 110 [40-230]* | 140 [80-240] | 100 [40-200]* | 130 [80-240] | 85 [40-150]* | 130 [80-240] | 85 [40-150]* |
Values are presented as median [range] or number (%). Statistical analyses were performed using Fisher’s exact test or Mann-Whitney U-test. *: P < 0.05. SpO2: peripheral arterial oxygen saturation, PIF peak inspiratory flow
ROC analyses for PIF cut-off values after repeated inhalations
As significant differences in PIF values were observed for all numbers of inhalations analyzed, we calculated PIF cut-off values using ROC analyses. PIF cut-off values, area under the ROC curve (AUC), sensitivity, and specificity estimated by ROC analyses are summarized in Table 3. ROC analyses indicated PIF cut-off values of 140, 120, 100, and 100 L/min for one, two, three, and four inhalation repeats, respectively.
Table 3.
ROC analyses for PIF cut-off values after repeated inhalations
| Inhalation repeats | PIF cut-off value (L/min) | Sensitivity (%) | Specificity (%) | AUC | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| I | 140 | 84 | 53 | 0.69 | 81 | 59 |
| II | 120 | 86 | 69 | 0.83 | 69 | 86 |
| III | 100 | 89 | 76 | 0.89 | 59 | 95 |
| IV | 100 | 87 | 71 | 0.86 | 48 | 95 |
AUC area under the ROC curve
Discussion
The adequate number of inhalation repeats and factors influencing the residual amount of laninamivir dry powder when using laninamivir DPI have not been previously reported. Our results show that pediatric patients were often unable to adequately inhale the active ingredient with two successive inhalations of laninamivir DPI. In addition, this is the first study to demonstrate that repeated inhalation of laninamivir DPI (more than three times) could improve the rate of inhalation in pediatric patients with PIF > 100 L/min.
As shown in Fig. 2, the inadequate inhalation rate after two successive inhalations suggested by the manufacturer was 45%, revealing that approximately half of pediatric patients were unable to attain adequate inhalation. Furthermore, the inhalation rate improved with increasing inhalation repeats. Tabata et al. [6] reported that patients 5-16 years old could not inhale the entire dose amount of zanamivir (Relenza®), another inhaled anti-influenza agent. Moreover, Katsumi et al. [5] showed that some patients aged 4-6 years were not able to inhale the full amount of laninamivir with a single inhalation. These reports indicate that it may be difficult to achieve adequate inhalation of laninamivir in pediatric patients with a single inhalation.
To explore factors influencing the residual amount of laninamivir dry powder, patient characteristics were compared between adequate and inadequate inhalation groups for all numbers of inhalation repeats (Table 2). Patients with inadequate inhalation were significantly younger and significantly fewer were male, compared to patients with adequate inhalation, for almost all inhalation repeats analyzed (Table 2). Furthermore, the respiratory rate in patients with inadequate inhalation was higher than that in patients with adequate inhalation (Table 2). PIF was previously reported to be positively correlated with age in pediatric patients [8]. Moreover, it is considered that respiratory rate affects PIF value. Taylor et al. [10] reported sex as an independent factor contributing to PIF. In the present study, we confirmed a positive correlation between PIF and age (r = 0.514, P < 0.001, Additional file 1: Figure S1) as well as a negative correlation between PIF and respiratory rate (r = −0.403, P = 0.001, Additional file 2: Figure S2). Moreover, PIF values in female patients were significantly lower than those in male patients (P = 0.007, Additional file 3: Figure S3). Most importantly, PIF values in patients with inadequate inhalation were significantly lower than those in patients with adequate inhalation, regardless of the number of inhalation repeats. These observations suggest that PIF is the most suitable indicator for evaluating residual drug amounts. Additionally, as female or younger patients, and/or patients with a high respiratory rate, are unlikely to adequately inhale laninamivir DPI, inhalation should be monitored in these patients when PIF is not determined.
To determine the appropriate number of inhalation repeats and select values of PIF useful as a clinical indicator, PIF cut-off values were calculated using ROC analyses (Table 3). These results suggest that two successive inhalations are sufficient for patients with PIF > 120 L/min, whereas at least three inhalation repeats are required for patients with lower PIF (100-120 L/min).
This study indicated that inhalation of laninamivir dry powder appears insufficient in patients with PIF < 100 L/min (Table 3), in agreement with a previous study, which demonstrated that patients with PIF < 90 L/min could not adequately inhale laninamivir dry powder [3]. Considering the accuracy (± 10%) of the In-Check® inspiratory flow meter used, the differences in these PIF values are within expectations. As judging whether administration of treatment by inhalation is suitable using only the whistle designed for confirmation of inhalation ability is considered difficult, additional assessments based on PIF may allow more reliable evaluation of inhalation applicability.
The limitations of our present study include the small number of cases, not examining repeated inhalations according to patient’s individual understanding of the inhalation technique, and not evaluating the therapeutic efficacy of laninamivir.
Conclusions
Our present findings suggest that some pediatric patients are unable to adequately inhale the active ingredient after two successive inhalations of laninamivir DPI. Pediatric patients with low PIF may require more than three repeated inhalations of laninamivir DPI. These results provide useful information for the successful use of laninamivir DPI in pediatric patients with influenza.
Additional files
Correlation between PIF and age in pediatric patients receiving laninamivir dry powder inhaler (n = 64). Statistical analysis was performed using Spearman correlation coefficient. Each point represents the patients. PIF, peak inspiratory flow. (PDF 716 kb)
Correlation between PIF and respiratory rate in pediatric patients receiving laninamivir dry powder inhaler (n = 64). Statistical analysis was performed using Spearman correlation coefficient. Each point represents the patients. (PDF 562 kb)
Comparisons of PIF between male (n = 37) and female (n = 27) pediatric patients receiving laninamivir dry powder inhaler. Statistical analysis was performed using Mann-Whitney U test. Each point represents the patients and median are indicated by horizontal lines. PIF, peak inspiratory flow. (PDF 738 kb)
Acknowledgements
We are grateful to the staff of the Tsu City Office and Tsu medical center/pediatric clinic and dental clinic.
Funding
This study was supported by a new research project grant of the Mie University Graduate School of Medicine and a contribution by Tomopha Co., Ltd.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AUC
Area under the ROC curve
- DPI
Dry powder inhaler
- PIF
Peak inspiratory flow
- ROC
Receiver operating characteristic
- SpO2
Percutaneous arterial oxygen saturation
Authors’ contributions
TM, KI, TE, YM, and MO contributed to the study conception and design. TM was involved in data collection. TM, KI, TE, YM, MI, KT, TI, and MO were involved in analysis and interpretation of data and drafting the manuscript. KI, TI, and MO critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Mie University Graduate School of Medicine and Faculty of Medicine (No. 2854). Written informed consent was obtained from all study participants or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s40780-017-0094-7) contains supplementary material, which is available to authorized users.
Contributor Information
Toshiki Murasaka, Email: to.murasaka@gmail.com.
Kenji Ikemura, Email: ikemurak@clin.medic.mie-u.ac.jp.
Tomoyuki Enokiya, Email: tenokiya@clin.medic.mie-u.ac.jp.
Yuichi Muraki, Email: y-muraki@clin.medic.mie-u.ac.jp.
Mayumi Ikemura, Email: mayuco@doc.medic.mie-u.ac.jp.
Koji Terada, Email: kts_33_tmm@yahoo.co.jp.
Takuya Iwamoto, Email: taku-iwa@clin.medic.mie-u.ac.jp.
Masahiro Okuda, Phone: 059-231-5081, Email: okudam@clin.medic.mie-u.ac.jp.
References
- 1.Ikematsu H, Kawai N. Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev Anti Infect Ther. 2011;9:851–857. doi: 10.1586/eri.11.112. [DOI] [PubMed] [Google Scholar]
- 2.Ikematsu H, Kawai N, Iwaki N, Kashiwagi S. Duration of fever and other symptoms after the inhalation of laminamivir octanoate hydrate for influenza treatment; comparison among the four Japanese influenza seasons from 2011 - 2012 to 2014 - 2015. J Infect Chemother. 2016;22:605–610. doi: 10.1016/j.jiac.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida Y, Kawakubo T, Kaneyoshi Y, Sugihara Y. Investigation of inspiratory flow rate and post-administration residual volume of laninamivir. Gairai Syounika. 2013;16:148–153. [Google Scholar]
- 4.Okada M, Hara M, Hashida T, Okayama K, Morikawa K, Morikawa K, Shinada A, Matsushita R. Survey of the proper use of instructions and compliance for laminamivir octanoate dry powder inhalation in community pharmacies for treatment of influenza. J Jpn Primary Care Assoc. 2013;36:106–109. [Google Scholar]
- 5.Katsumi Y, Otabe O, Sakaue S, Tsuma Y, Yamaguchi M, Matsui F, Suematsu M, Miyagaki S, Ito H. Incomplete inhalation of laninamivir octanoate in children with influenza. Pediatr Ther. 2015;5:225. doi: 10.1542/peds.2011-2054. [DOI] [PubMed] [Google Scholar]
- 6.Tabata Y, Arashiki Y. Examination of the number of inhalations required for children to inhale one blister of anti-influenza agent zanamivir. Jpn J Pediatr. 2010;63:463–465. [Google Scholar]
- 7.Kamps AW, Brand PL, Roorda RJ. Variation of peak inspiratory flow through dry powder inhalers in children with stable and unstable asthma. Pediatr Pulmonol. 2004;37:65–70. doi: 10.1002/ppul.10410. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen KG, Auk IL, Bojsen K, Ifversen M, Klug B, Bisgaard H. Clinical effect of Diskus dry-powder inhaler at low and high inspiratory flowrates in asthmatic children. Eur Respir J. 1998;11:350–354. doi: 10.1183/09031936.98.11020350. [DOI] [PubMed] [Google Scholar]
- 9.Daiichi Sankyo Co., Ltd., Tokyo, Japan. General clinical evaluation: Dry powder inhaler of laninamivir octanoate. http://www.pmda.go.jp/drugs/2010/P201000050/430574000_22200AMX00925_G100_1.pdf. Accessed 26 May 2017.
- 10.Taylor TE, Holmes MS, Sulaiman I, Costello RW, Reilly RB. Influences of gender and anthropometric features on inspiratory inhaler acoustics and peak inspiratory flow rate. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2227–2230. doi: 10.1109/EMBC.2015.7318834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between PIF and age in pediatric patients receiving laninamivir dry powder inhaler (n = 64). Statistical analysis was performed using Spearman correlation coefficient. Each point represents the patients. PIF, peak inspiratory flow. (PDF 716 kb)
Correlation between PIF and respiratory rate in pediatric patients receiving laninamivir dry powder inhaler (n = 64). Statistical analysis was performed using Spearman correlation coefficient. Each point represents the patients. (PDF 562 kb)
Comparisons of PIF between male (n = 37) and female (n = 27) pediatric patients receiving laninamivir dry powder inhaler. Statistical analysis was performed using Mann-Whitney U test. Each point represents the patients and median are indicated by horizontal lines. PIF, peak inspiratory flow. (PDF 738 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


