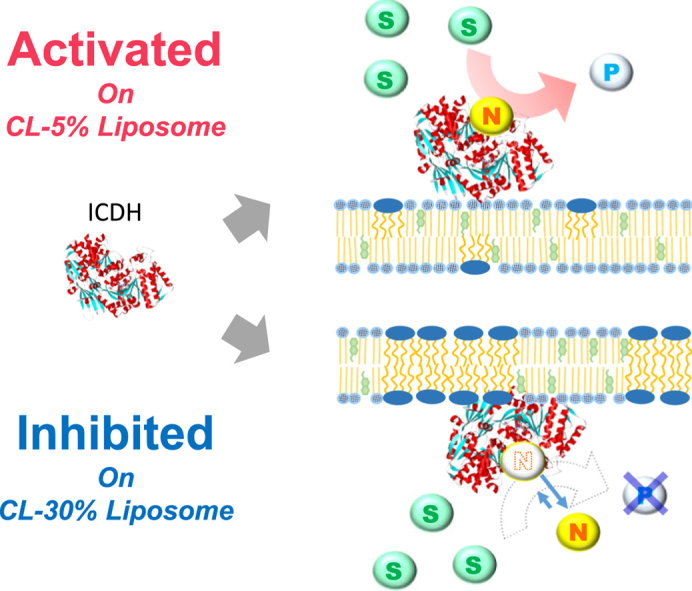
Abstract
Cardiolipin (CL) is a phospholipid found in the outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) in animal cells. Isocitrate dehydrogenase (ICDH) is an important catalytic enzyme that is localized at the cytosol and mitochondria; the metabolic pathway catalyzed by ICDH differs between the OMM and IMM. To estimate the possible role of lipid membrane in the enzymatic activity of NADP+-dependent ICDH, CL-modified liposomes were prepared using CL/1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)/cholesterol (Ch), and their characteristics were analyzed based on the fluorescent probe method. The relative enzymatic activity of ICDH decreased in the presence of CL/DPPC/Ch=(30/50/20) liposome, whereas activity increased in the presence of CL/DPPC/Ch=(5/75/20) liposome. NADP+ had the greatest substrate affinity and was dominant in the regulation of ICDH activity. Analysis of membrane properties indicated that membranes in CL-modified liposomes were dehydrated by ICDH binding. Using circular dichroism analysis, CL/DPPC/Ch=(30/50/20) liposome induced a conformational change in ICDH, indicating that CL-rich membrane domains could inhibit ICDH activity. These results suggest that lipid membranes, including CL molecules, could act as a platform to regulate ICDH-related metabolic pathways such as the tricarboxylic acid cycle and lipid synthesis.
Abbreviations: CL, cardiolipin; ICDH, isocitrate dehydrogenase; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; Ch, cholesterol; TCA, tricarboxylic acid; NADP+, β-nicotinamide-adenine dinucleotide phosphate oxidized form; NADPH, β-nicotinamide-adenine dinucleotide phosphate reduced form; MLV, multilamellar vesicles; LUV, large unilamellar vesicles; ld, liquid-disordered; lo, liquid-ordered; so, solid-ordered; PDB, protein data bank
Keywords: Cardiolipin, Liposome, Isocitrate dehydrogenase, Membranome, System biology
Graphical abstract
Highlights
-
•
Phosphatidylcholine liposomes were modified with cardiolipin and characterized.
-
•
DPPC liposomes did not affect the activity of ICDH.
-
•
ICDH activity was enhanced with liposomes at 5 mol% cardiolipin.
-
•
ICDH activity was lowered with liposomes at 30 mol% cardiolipin.
-
•
Liposomes with high content of cardiolipin led to conformational changes of ICDH.
1. Introduction
Interactions of proteins with biomembranes play important roles in many biological processes, which have been extensively studied, whereby membrane components and properties are assumed to be optimized to functionalize biomolecules (Wenk, 2005). Mitochondria are unique organelles with two membranes, an outer mitochondrial membrane (OMM) and an inner mitochondrial membrane (IMM), which differ in the lipid composition of cardiolipin (CL) (Horvath and Daum, 2013). CL is a specific phospholipid found in mitochondrial and bacterial membranes, which has four acyl chains and two negatively-charged phosphate groups. CL plays important roles, not only in the structural organization of the membrane, but also in the function of mitochondrial membrane-related processes (Ren et al., 2014, Li et al., 2007). Recently, interactions between CL and proteins have been reported (Nury et al., 2005, Zhang et al., 2005, Basova et al., 2007): for instance, cytochrome c binding to CL can induce conformational changes in the tertiary structure that alters the environment of the heme catalytic center (Kagan et al., 2005). The binding mechanism of cytochrome c to CL is mainly driven by electrostatic interaction, as well as by hydrophobic interaction (Belikova et al., 2006). In addition, lipid transfer (Schlattner et al., 2013), maintenance of mtDNA (Zhong et al., 2004), and apoptosis (Garcia Fernandez et al., 2000, Kirkland et al., 2002, Matsko et al., 2001; Ott et al., 2007), have been induced by the presence of CL in the membrane. From another point of view, the interaction of bio-macromolecules with the CL on the phospholipid membranes has been reported to induce the phase segregation of the CL-modified membranes (Luévano-Martínez et al., 2015, Sennato et al., 2005, Maniti et al., 2009). It is clearly suggested that lipid membranes, including CL, must affect the catalytic activity of enzymes located around mitochondria, where metabolic pathways such as the tricarboxylic acid (TCA) cycle and lipid synthesis can be controlled. Therefore, the physicochemical properties of CL-modified membranes are considered as a potential indication of the control of the metabolic flux generated by each biomolecule.
Isocitrate dehydrogenase (ICDH) is an enzyme catalyzing the oxidative decarboxylation of isocitrate to α-ketoglutarate and carbon dioxide. Several isoforms of ICDH are present in mammals that vary in function: NAD+-dependent ICDH has a catabolic role in the TCA cycle at the mitochondria in the energy production pathway. On the other hand, NADP+-dependent ICDH, which is not usually considered a member of the TCA cycle, has the anabolic function of providing biosynthetic intermediates, generating NADPH for the reductive biosynthesis of fatty acids and the regulation of oxidative damage, in cytosol and in mitochondria (Ceccarelli et al., 2002). NADP+-dependent ICDH isomers, localized at mitochondria, cytosol, and peroxisomes (Contreras-Shannon et al., 2005), are assumed to be key enzymes in several metabolic pathways. In regards to the microscopic environment around organelle membranes, the composition of membrane lipids differs from organelle to organelle (Jain and Wagner, 1980), and the role of the membrane in ICDH isomers must be dependent on the characteristics of the membrane. An essential role of biomembranes is to localize the function of biomolecules (Walde et al., 2014).
In our series of previous works, the activities of biomolecules have been regulated by their binding to membranes: fragmented superoxide dismutase (Tuan et al., 2008), in vitro transcription and translation (Bui et al., 2008, Suga et al., 2011), fibrillation of amyloid β (Shimanouchi et al., 2012a, Shimanouchi et al., 2012b), and hexokinase (Umakoshi and Nishida, 2012). It has also been reported that activities of metabolic enzymes could be regulated on outer or inner mitochondrial membranes (Denton, 2009, Laterveer et al., 1994). Owing to the diversity in phospholipid composition in organelle membranes (Jain and Wagner, 1980, Böttinger et al., 2012), it is assumed that membrane-localized ICDH must be regulated depending on the characteristics of membranes; CL molecules could play an important role in controlling the binding and activity of dehydrogenases by altering the membrane properties. Although NADP+-dependent ICDH has crucial roles in metabolic pathways, its interaction with membranes, especially with CL, is still unclear. It is therefore important to evaluate potential roles of lipid membranes in the metabolic flux.
In this study, the interaction between NADP+-dependent ICDH and CL-modified lipid membranes was examined to understand the possible role of the membrane in controlling the function of the enzyme. Based on previous reports (Ellis and Goldberg, 1971, Kvamme et al., 1991), the relative enzymatic activity, kcat/Km, and the substrate affinity, 1/Km, were calculated for ICDH in the presence and absence of liposomes. Liposomes, containing 0–30 mol% of CL, 20 mol% of cholesterol (Ch), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), were used as model-biomembranes and were characterized by previously reported methods using fluorescent probes (Hayashi et al., 2011, Suga and Umakoshi, 2013). The effect of physicochemical properties of CL-modified liposomes on the activity of ICDH and their interaction were investigated.
2. Materials and methods
2.1. Materials
1,1,2,2-Tetraoleoylcardiolipin (sodium salt) (CL) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL, USA). Cholesterol (Ch), isocitrate dehydrogenase (NADP+-dependent) from porcine heart, and DL-isocitrate (trisodium salt) were purchased from Sigma Aldrich (St. Louis, MO, USA). β-Nicotinamide-adenine dinucleotide phosphate oxidized form (NADP+) and other chemicals were purchased from Wako Pure Chemicals (Osaka, Japan). These chemicals were used without further purification.
2.2. Liposome preparation
Liposome suspensions were prepared on the basis of literatures (Suga and Umakoshi, 2013). Briefly, a chloroform solution containing 0–30 mol% of CL, 0–20 mol% of Ch, and DPPC was dried in a round-bottom flask by rotary evaporation under vacuum. The obtained lipid films were dissolved in chloroform, and the solvent was evaporated. The drying and dissolving were repeated 3 times. The lipid thin film obtained was kept under a high vacuum for at least 3 h, and then hydrated with PBS buffer at room temperature. The vesicle suspension was frozen at −80 °C and thawed at 50 °C to enhance the transformation of small vesicles into larger multilamellar vesicles (MLVs). This freeze–thaw cycle was performed 5 times. MLVs were used to prepare large unilamellar vesicles (LUVs) by extruding the MLV suspension 11 times through 2 layers of polycarbonate membranes with mean pore diameters of 100 nm using an extruding device (Liposofast; Avestin Inc., Ottawa, Canada).
2.3. Evaluation of membrane properties of liposomes
The fluorescent probe N,N-dimethyl-6-dodecanoyl-2-naphthylamine (Laurdan) is sensitive to the polarity around itself, which allows the surface polarity of lipid membrane to be determined (Parasassi et al., 1991). The solution containing Laurdan and liposome was incubated for 1 h at 37 °C. After that, ICDH was added to the solution, and the sample solution was incubated for 1 h at 37 °C. The sample solution was excited at 340 nm, and the fluorescent spectra were recorded by the fluorescence spectrophotometer FP-6500 (JASCO Co., Tokyo, Japan). The membrane polarity was evaluated by calculating the emission intensities (I440, I490) as follows: GP340=(I440−I490)/(I440+I490), where the GP340 represents the general polarization. The total concentrations of lipid and Laurdan were 100 µM and 1 µM, respectively. The fluidity in the interior of liposome membrane was evaluated by measuring the fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) incorporated in the liposome membrane, using the fluorescence spectrophotometer FP-6500 (JASCO, Tokyo, Japan). The solution containing DPH and liposome was incubated for 1 h at 37 °;C. After that, ICDH was added to the solution, and the sample solution was incubated for 1 h at 37 °;C. The sample solution was excited with vertically polarized light (360 nm), and the emission intensities both perpendicular (I⊥) (0°, 0°) and parallel (I∥) (0°, 90°) to the excited light were recorded at 430 nm. The polarization (P) of DPH was then calculated by using the following equations:
where i⊥ and i∥ are emission intensities perpendicular to the horizontally polarized light (90°, 0°) and parallel to the horizontally polarized light (90°, 90°), respectively, and G is the correction factor. The membrane fluidity was evaluated based on the reciprocal of polarization, 1/P. All the measurement was achieved at 37 °C. The total concentrations of lipid and DPH were 250 µM and 1 µM, respectively.
2.4. Measurement of enzymatic activity
A PBS buffer solution (pH 7.3) containing ICDH, MgCl2, NADP+ and liposome was incubated for 1 h at 37 °C. Then, the reaction was started by mixing the above solution (150 μL) and the substrate solution containing isocitrate (50 µL) at 37 °C, and the absorbance at 340 nm was recorded by xMark Microplate Spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). The activity of ICDH was monitored continuously by the reduction of NADP+ to NADPH (ε340=6.22×103 M−1 cm−1) at 37 °C (Bartholomae et al., 2014). The enzymatic parameters were calculated by Hanes–Woolf plot (Kvamme et al., 1991, Umakoshi and Nishida, 2012). Liposomes did not affect the NADP+ stability. The total concentrations of the materials were as follows; ICDH, 0.1 μM; MgCl2, 1 mM; lipid, 0.5 mM; and isocitrate, 6.7 mM. The initial rate of NADPH production (rP0), obtained in the presence of 0.3 mM of NADP+, was used as standard, and the relative rP0 values were measured for calculation of the enzymatic activity (kcat/Km) and substrate affinity for NADP+ (1/Km).
2.5. Conformation analysis of ICDH
The secondary structure of ICDH was determined by circular dichroism (CD) analysis using J-820W spectrometer (JASCO Co., Tokyo, Japan). A quartz cell with 0.1 cm-path length was used for the measurement, and the CD spectra were recorded from 200 to 250 nm. The samples containing 3.0 μM of ICDH were diluted in PBS buffer with or without liposomes and were, then, incubated for 1 h at 37 °C. Because the peaks at 222 and 209 nm were derived from α-helix structure, and the peak at 218 nm was from β-sheet structure (Chen et al., 1972), the conformation of ICDH was determined by the comparison of these peak intensities. CD measurements were carried out with the following parameters: 2 nm bandwidth; 50 nm/min run speeds; 0.1 nm step size; 2 s response times; and average of 4 runs. The total concentrations of ICDH and lipid were 3.0 μM and 0.5 mM, respectively.The intrinsic fluorescence of tryptophan (Trp) was measured to analyze the tertiary structural change of the enzymes (Umakoshi and Nishida, 2012), by measuring the fluorescent emission of Trp, which can be shifted by the surrounding dielectric environment (Zahid et al., 2013). The measurement was performed by using the fluorescence spectrophotometer FP-6500 (JASCO Co., Tokyo, Japan) at an excitation wavelength of 295 nm.
3. Results and discussion
In general, biological membranes are composed of saturated and unsaturated phospholipids. CL molecules are enriched in the IMM, whereas enrichment is poor in the OMM. Based on our series of works, liposomes composed of the ternary lipid mixture of unsaturated lipid (e.g., 1,2-dioleoyl-sn-glycero-3-phosphocholine), saturated lipid (e.g., DPPC), and Ch showed lower membrane fluidities than those composed of purely unsaturated lipids (Suga et al., 2013). This implies that biological membranes are in liquid phases, but the membranes would be kept in lower fluidities. Considering these membrane properties, CL-modified liposomes with different CL ratios were prepared using the ternary lipid mixture of CL/DPPC/Ch. Here, CL/DPPC/Ch=(30/50/20), CL/DPPC/Ch=(20/60/20), CL/DPPC/Ch=(5/75/20), and CL/DPPC/Ch=(0/100/0) are referred to as CL 30, CL 20, CL 5, and DPPC, respectively. Based on the analysis of physicochemical membrane properties, the effect of liposomes on the interaction and activity of ICDH was investigated.
3.1. Characterization of CL-modified liposomes
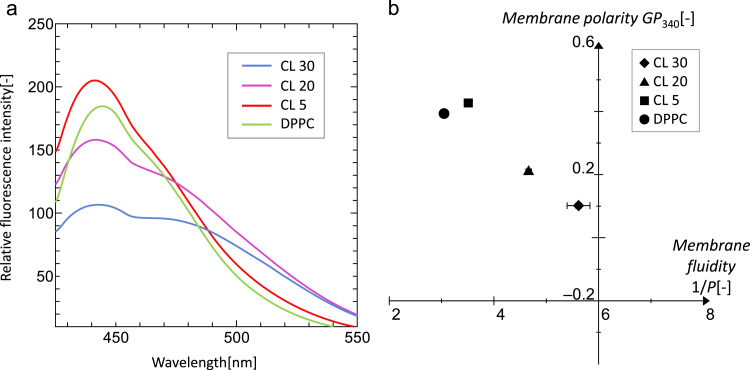
Laurdan is a microenvironment-sensitive fluorescent probe that is used to characterize the liposome membrane surface (Hayashi et al., 2011, Suga and Umakoshi, 2013). Fig. 1(a) shows the emission spectra of Laurdan for CL-modified liposomes. The spectra of CL 30 and CL 20 liposomes showed two emission peaks at 440 and 490 nm, derived from the ordered and disordered phases, respectively. In our previous work (Suga and Umakoshi, 2013), the LUVs that showed two emission peaks of Laurdan fluorescence could be in heterogeneous phases (liquid-disordered (ld)+liquid-ordered (lo), or (ld)+solid-ordered (so)). As CL and DPPC possess the unsaturated aliphatic chains and saturated chains, respectively, the disordered phases (Laurdan peak at 490 nm) that appeared in CL 30 and CL 20 liposomes were estimated to be CL-rich domains, whereas the ordered phases were DPPC and Ch-rich.By combining Laurdan and DPH analyses, the phase state of liposomes could be estimated. Fig. 1(b) shows a Cartesian diagram of CL-modified liposomes at 37 °C, where the x-axis and y-axis are membrane fluidity (DPH) and membrane polarity (Laurdan), respectively. CL 5 liposome showed properties similar to those of the DPPC liposome, indicating that CL 5 liposome could be in the homogeneously ordered phase. In proportion to the amount of CL, the 1/P values increased and the GP340 values decreased. It is suggested that CL 30 and CL 20 liposomes could be segregated in two phases, where CL molecules could form nano-domains in the disordered phases. It has been reported that the conical shape of CL molecules induce a negative curvature strain, so that bilayer polar region becomes more accessible to water (Ioffe et al., 2006). By using these liposomes, the enzymatic activity of ICDH and the possible interaction mechanism were determined in the following sessions.
Fig. 1.
(a) Fluorescence spectra of Laurdan. Emission peak at 440 and 490 nm are derived from the ordered and disordered phases, respectively. Experiments were performed with 100 µM lipid and 1 µM Laurdan at 37 °C. Lines indicate CL 30 (blue), CL 20 (purple), CL 5 (red), and DPPC (green). (b) Cartesian diagram (Suga and Umakoshi, 2013) of liposomes at 37 °C. 1/P and GP340 values indicate the membrane fluidity and polarity, respectively. When the liposome membrane becomes polar (GP340 decrease), its fluidity becomes higher (1/P increase). Symbols indicate CL 30 (closed diamond), CL 20 (closed triangle), CL 5 (closed square), and DPPC (closed circle). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Enzymatic activity of ICDH
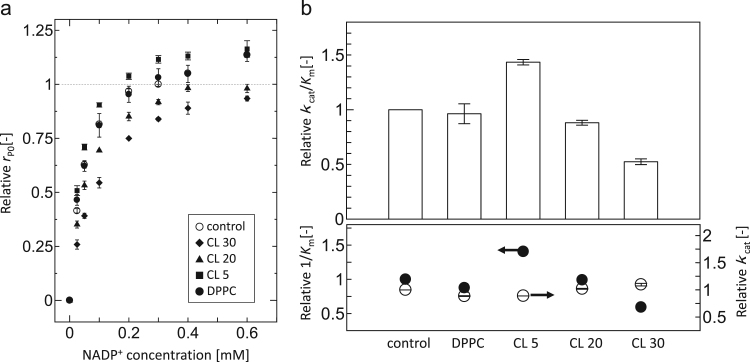
The initial rates of NADPH production (rP0) were measured at various concentrations of NADP+, while maintaining isocitrate at saturating concentrations, in the presence or absence of liposomes (Fig. 2(a)). The rP0 values decreased in the presence of CL 30 and CL 20 liposomes, compared to the control (in the absence of liposomes), whereas rP0 values increased in the presence of CL 5 liposome. DPPC liposome, which did not include CL molecules, did not significantly affect rP0 values. The kinetic parameters for NADP+ as a substrate were determined based on a Hanes-Woolf plot (Kvamme et al., 1991, Umakoshi and Nishida, 2012) to consider the kinetic constants for enzymatic activity with small measurement errors. The rP0 values were plotted against NADP+ concentration, and a linear relationship was obtained by plotting the data according to Hanes–Woolf plot, which allowed determining kcat and kcat/Km values from the slope and intercept (Supplementary Materials, Fig. S1). The relative enzymatic activity (kcat/Km) in the presence of CL 30 or CL 20 liposomes was lower than that of the control, in which the substrate affinity for NADP+ (1/Km) decreased (Fig. 2(b)). In contrast, the kcat/Km value in the presence of CL 5 liposome was higher than that of the control, whereas the 1/Km value increased. It has been reported that phospholipid membranes can act as regulators of localized activity (Walde, 2010, Walde et al., 2014), and a possible role of the membrane is to localize enzymes and substrates to their surface. In the case of CL 5 liposome, substrates and ICDH could accumulate on the membrane surface, without the denaturation of ICDH conformation. In the presence of DPPC liposomes, both kcat/Km and 1/Km values were not significantly affected. The kcat values were maintained in the presence of liposomes. These changes of relative activity were considered to be due to the changes in substrate affinity for NADP+, indicating that CL-modified liposome membrane surfaces could control substrate affinity. Using protein data bank (PDB) data analysis (PDB ID: 1LWD (Ceccarelli et al., 2002)), ICDH possesses a positive net charge in our experimental conditions (pH 7.3). It has been reported that CL clusters and membrane domain formation could be induced by mitochondrial proteins (Epand et al., 2007), such as cytochrome c (Mirkin et al., 2008) and creatine kinase (Fritz-Wolf et al., 1996). Such mitochondrial membrane binding proteins are usually positively-charged under physiological conditions, indicating that ICDH can also bind to negatively-charged membranes, such as CL-modified liposomes. However, the strong electrostatic interaction between protein (enzyme) and liposome may induce denaturation (Umakoshi and Nishida, 2012). In this work, total lipid concentration did not affect the enzymatic activities of ICDH, possibly because the concentration of ICDH (0.1 μM) was much lower than that of lipid (0.5 mM) or NADP+ (0.025–0.6 mM). In regards to the biological membrane composition of mitochondria, the OMM and IMM include <1 wt% and 18 wt% of CL of total phospholipids, respectively (Daum and Vance, 1997). It is speculated that membranes containing a significant amount of CL act to decrease ICDH activity, whereas membranes containing less CL do not show these inhibitory effects. These results suggest that the CL-modified liposomes may act as regulators of the flux of ICDH activity.
Fig. 2.
(a) Initial rate of NADPH production (rP0) in the presence of liposomes at 37 °C. The kinetic parameters were determined by varying the concentration of NADP+. Symbols indicate control (without liposomes) (open circle), with CL 30 (closed diamond), with CL 20 (closed triangle), with CL 5 (closed square), and with DPPC (closed circle). (b) Relative enzymatic activity of ICDH (top column) and relative 1/Km (y axis) or relative kcat (r axis) (bottom column). The data were calculated by using Hanes–Woolf plots, whereby the control measurements (“control”) is ICDH only (without liposomes). The total concentrations of ICDH, isocitrate, and lipid were 0.1 μM, 6.7 mM, and 0.5 mM, respectively, in all experiments.
3.3. Effect of ICDH on characteristics of liposome membranes
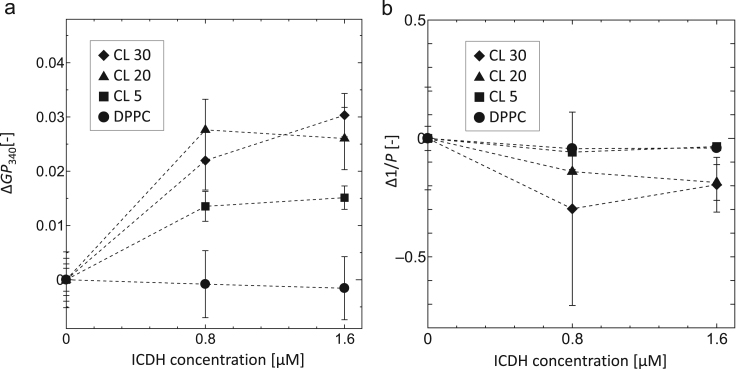
To evaluate the effect of ICDH on the characteristics of liposome membranes, variations in membrane polarity (ΔGP340) and membrane fluidity (Δ1/P) were investigated by using Laurdan and DPH, respectively. As Laurdan and DPH can monitor membrane properties at surface regions and membrane fluidities at hydrophobic interior regions, respectively, it is possible to estimate the ICDH binding region of the liposome membrane using these fluorescent probes. Laurdan, which is sensitive to the dielectric surroundings, can indicate a polar environment of liposome membranes, whereas the ΔGP340 value provides the degree of hydration at the membrane surface in the presence of a guest molecule (Parasassi et al., 1991, Hirsch-Lerner and Barenholz, 1999). The GP340 was calculated in the presence of ICDH and values are summarized in Fig. 3(a). The ΔGP340 values of CL 30 and CL 20 liposomes increased in the presence of ICDH, indicating that CL-modified liposome membrane surfaces were dehydrated. The ΔGP340 values of CL 5 also increased slightly, whereas those of DPPC did not vary with the coexistence of ICDH. In contrast, 1/P values were not affected by the coexistence of ICDH for these liposomes (Fig. 3(b)). The CL-rich domain was estimated to be in disordered phases (Fig. 1), as these membranes were more hydrophilic than those in the ordered phases. It has been reported that increases in ΔGP340 values indicate dehydration of the membrane surface, due to interactions with protein (Melo et al., 2014) and nucleic acid (Suga et al., 2013). Our results suggest that ICDH could located at the membrane surface, not but at the hydrophobic interior region. The degree of change in this membrane polarity would reflect the strength of the interaction between liposomes and ICDH. It was therefore considered that the CL-rich domain in CL 30 and CL 20 liposomes strongly interacted with ICDH. The interaction between the CL-rich domain and ICDH inhibit the substrate affinity for NADP+, resulting in a decrease in activity of ICDH.
Fig. 3.
(a) Variations of membrane polarity (ΔGP340) in the presence of ICDH at 37 °C. The total concentrations of lipid and Laurdan were 100 µM and 1 µM, respectively. ΔGP340 values were calculated as follows: ΔGP340=GP340, (+)ICDH−GP340, (−)ICDH. (b) Variations of membrane fluidity (Δ1/P) in the presence of ICDH at 37 °C. The total concentrations of lipid and DPH were 250 µM and 1 µM, respectively. Δ1/P values were calculated as follows: Δ1/P=1/P, (+)ICDH−1/P, (−)ICDH. Symbols indicate CL 30 (closed diamond), CL 20 (closed triangle), CL 5 (closed square), and DPPC (closed circle).
3.4. Effect of CL-modified liposomes on ICDH conformation
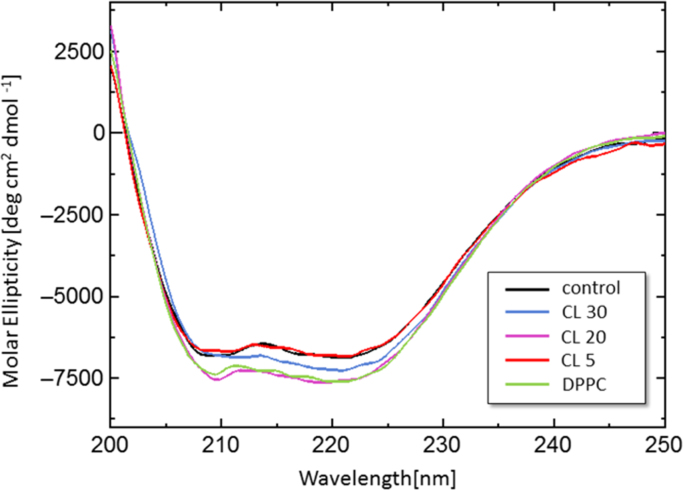
In general, the function of a protein or enzyme is strongly related to its conformation (Ghosh and Ray, 2013). It is expected that the regulation of ICDH activity could be induced by its conformational change at the liposome membrane surface. To investigate the effect of CL-modified liposomes on the ICDH conformation, the secondary structure of ICDH in the presence of liposomes was analyzed by circular dichroism (CD) spectroscopy (Fig. 4). Variation in the CD spectrum was observed in the presence of CL 30 liposome, indicating a conformational change in ICDH. In contrast, the CD spectrum of ICDH was not changed significantly in the presence of CL 5 liposome. Based on the fitting analysis (Greenfield and Fasman, 1969), the coexistence of CL 30 liposome and ICDH reduced the α-helix content of ICDH (Table 1), whereas the presence of CL 5 liposome did not. Therefore, the CL 30 liposome was likely to affect the conformation of ICDH, which was one of possible reasons for the decrease in 1/Km and inhibition of ICDH activity. As CL 30 liposomes could form the CL-rich domain and interact with ICDH strongly, compared to the CL 5 liposome (Fig. 3), it is suggested that the binding site of NADP+ in ICDH can interact with the CL-rich domain in disordered phases. It has been reported that ICDH loses its enzymatic activity in lower pH conditions (pH<5) (Huang et al., 2004). The surface of CL-rich membranes could be acidic, and the localization of ICDH could result in the inhibition of ICDH activity. In regards to the structure of ICDH, some parts of the amino acid sequence in ICDH are α-helix and positively charged (including arginine) (Supplementary Materials, Fig. S2), which were exposed to the bulk water. It was therefore assumed that CL-modified liposomes could make contact with such sequences, to induce conformational changes in ICDH. Considering the small conformational change of ICDH induced by DPPC liposome, it could be suggested that the DPPC liposome had no apparent impact on the ICDH activity, although further studies are required to understand the role of the liposome membrane.To get an insight into the inner conformational change of ICDH, the intrinsic fluorescence of Trp in ICDH was also evaluated in the presence of liposomes. It has been reported that the emission peak of Trp can be shifted by the surrounding dielectric environment: in a non-polar environment (e.g., organic solvent, interior of protein), the fluorescent peak of Trp appears at almost 330 nm, whereas this peak shifts to a higher frequency in a polar environment (e.g., water, surface of protein) (Konev, 1967, Umakoshi and Nishida, 2012). In this study, no peak shift of Trp emission was observed in the presence of liposomes (Table 1), which indicated that the intrinsic Trp surroundings were not affected by CL-modified liposome interactions, therefore the inner conformational change of ICDH did not occur. It is therefore investigated that these CL-modified liposomes did not induce the denaturation of the intrinsic conformation of ICDH.
Fig. 4.
CD spectra of ICDH in the presence or absence of liposomes. The total concentrations of ICDH and lipid were 3.0 μM and 0.5 mM, respectively. Lines indicate control (without liposome) (black), with CL 30 (blue), with CL 20 (purple), with CL 5 (red), and with DPPC (green). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Conformation analysis of ICDH in the presence of liposomes.
| Helix [%]a | Sheet [%]a | Trp fluorescent peak [nm]b | |
|---|---|---|---|
| Control | 82.9±0.3 | 17.1±0.3 | 343.6±0.4 |
| CL 30c | 77.3±0.6 | 22.7±0.6 | 342.6±0.4 |
| CL 20c | 82.2±0.5 | 17.7±0.5 | 344.0±1.2 |
| CL 5c | 82.5±0.8 | 17.5±0.8 | 343.6±0.8 |
| DPPCc | 80.8±0.4 | 19.2±0.4 | 343.6±0.4 |
Evaluated by CD spectrum fitting. All experiments were conducted at least 6 times. Average and error values were shown.
Evaluated by the fluorescence emission of intrinsic Trp of ICDH (excitation wavelength was 340 nm). All experiments were conducted at least 3 times. Average and error values were shown.
Experiments were performed with 0.1 µM ICDH and 0.5 mM liposomes at 37 °C.
4. Conclusions
The enzymatic activity of ICDH was regulated at the surface of CL-modified liposome membranes. Based on fluorescent probe analysis (Laurdan and DPH), the physicochemical membrane properties of CL 30, CL 20, and CL 5 liposomes were investigated: CL 30 and CL 20 liposomes could form a CL-rich domain in liquid-disordered phases, whereas the CL 5 liposome was in a homogeneously liquid-ordered phase. The CL 5 liposome enhanced the ICDH activity, whereas CL 30 and CL 20 liposomes inhibited this activity. The dominant factor was the substrate affinity for NADP+, 1/Km in our experimental conditions. By investigating liposome membrane polarities in the absence and presence of ICDH, membrane surfaces of liposomes forming CL-rich domains were found to be more dehydrated by ICDH binding than CL 5 liposome. Therefore, ICDH can interact with CL-rich domains in disordered phases. CD spectroscopic analysis showed the conformational change of ICDH in the presence of CL 30 liposome, but not in the presence of CL 5 liposome. These results suggest that the CL-rich domain in liposomes can interact with ICDH, inducing a decrease in substrate affinity for NADP+ via a conformational change in ICDH. It is assumed that the diversity of membrane lipids and related membrane property variations must be implied in regulating this metabolic flux. Therefore, designed liposome membranes can be applied to understand the possible roles of CL molecules in organelle membranes. Based on our findings, concerted, consecutive, and competitive enzymatic reactions, involving multi-enzymes, are expected to be controlled at the membrane surface, depending on the characteristics of the membranes.
Acknowledgments
We thank Dr. Y. Okamoto (Graduate School of Engineering Science, Osaka University) for his constructive comments and technical support. This work was supported by the Funding Program for Next Generation World-Leading Researchers of the Council for Science and Technology Policy (CSTP) (GR066), JSPS Grant-in-Aid for Scientific Research A (26249116), JSPS Grant-in-Aid for Research Activity Start-up (25889039), and JSPS Grant-in-Aid for Challenging Exploratory Research (T15K142040).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.meteno.2015.11.002.
Appendix A. Supplementary material
Supplementary material
References
- Bartholomae M., Meyer F.M., Commichau F.M., Burkovski A., Hillen W., Seidel G. Complex formation between malate dehydrogenase and isocitrate dehydrogenase from Bacillus subtilis is regulated by tricarboxylic acid cycle metabolites. FEBS J. 2014;281:1132–1143. doi: 10.1111/febs.12679. [DOI] [PubMed] [Google Scholar]
- Basova L.V., Kurnikov I.V., Wang L., Ritov V.B., Belikova N.A., Vlasova I.I., Pacheco A.A., Winnica D.E., Peterson J., Bayir H., Waldeck D.H., Kagan V.E. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikova N.A., Vladimirov Y.A., Osipov A.N., Kapralov A.A., Tyurin V.A., Potapovich M.V., Basova L.V., Peterson J., Kurnikov I.V., Kagan V.E. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttinger L., Horvath S.E., Kleinschroth T., Hunte C., Daum G., Pfanner N., Becker T. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J. Mol. Biol. 2012;423:677–686. doi: 10.1016/j.jmb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui H.T., Umakoshi H., Nishida M., Shimanouchi T., Kuboi R. Liposome membrane itself can regulate gene expression in cell free translation system. Langmuir. 2008;24:10537–10542. doi: 10.1021/la801962j. [DOI] [PubMed] [Google Scholar]
- Ceccarelli C., Grodsky N.B., Ariyaratne N., Colman R.F., Bahnson B.J. Crystal structure of porcine mitochondrial NADP+-dependent isocitrate dehydrogenase complexed with Mn 2+ and isocitrate: insights into the enzyme mechanism. J. Biol. Chem. 2002;277:43454–43462. doi: 10.1074/jbc.M207306200. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H., Yang J.T., Martinez H.M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972;11:4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Contreras-Shannon V., Lin A.-P., McCammon M.T., McAlister-Henn L. Kinetic properties and metabolic contributions of yeast mitochondrial and cytosolic NADP+-specific isocitrate dehydrogenases. J. Biol. Chem. 2005;280:4469–4475. doi: 10.1074/jbc.M410140200. [DOI] [PubMed] [Google Scholar]
- Daum G., Vance J.E. Import of lipids into mitochondria. Prog. Lipid. Res. 1997;36:103–130. doi: 10.1016/s0163-7827(97)00006-4. [DOI] [PubMed] [Google Scholar]
- Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta – Bioenerg. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Ellis G., Goldberg D.M. An improved manual and semi-automatic assay for NADP-dependent isocitrate dehydrogenase activity, with a description of some kinetic properties of human liver and serum enzyme. Clin. Biochem. 1971;4:175–185. doi: 10.1016/s0009-9120(71)91363-4. [DOI] [PubMed] [Google Scholar]
- Epand R.F., Tokarska-Schlattner M., Schlattner U., Wallimann T., Epand R.M. Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J. Mol. Biol. 2007;365:968–980. doi: 10.1016/j.jmb.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Fritz-Wolf K., Schnyder T., Wallimann T., Kabsch W. Structure of mitochondrial creatine kinase. Nature. 1996;381:341–345. doi: 10.1038/381341a0. [DOI] [PubMed] [Google Scholar]
- Garcia Fernandez M., Troiano L., Moretti L., Pedrazzi J., Salvioli S., Castilla-Cortazar I., Cossarizza A. Changes in intramitochondrial cardiolipin distribution in apoptosis-resistant HCW-2 cells, derived from the human promyelocytic leukemia HL-60. FEBS Lett. 2000;478:290–294. doi: 10.1016/s0014-5793(00)01861-5. [DOI] [PubMed] [Google Scholar]
- Ghosh M.C., Ray A.K. Membrane phospholipid augments cytochrome P4501a enzymatic activity by modulating structural conformation during detoxification of xenobiotics. PLoS One. 2013;8:e57919. doi: 10.1371/journal.pone.0057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8:4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Shimanouchi T., Kato K., Miyazaki T., Nakamura A., Umaksoshi H. Span 80 vesicles have a more fluid, flexible and “wet” surface than phospholipid liposomes. Colloids Surf. B: Biointerfaces. 2011;87:28–35. doi: 10.1016/j.colsurfb.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Hirsch-Lerner D., Barenholz Y. Hydration of lipoplexes commonly used in gene delivery: Follow-up by laurdan fluorescence changes and quantification by differential scanning calorimetry. Biochim. Biophys. Acta – Biomembr. 1999;1461:47–57. doi: 10.1016/s0005-2736(99)00145-5. [DOI] [PubMed] [Google Scholar]
- Horvath S.E., Daum G. Lipids of mitochondria. Prog. Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Huang Y.C., Grodsky N.B., Kim T.-K., Colman R.F. Ligands of the Mn2+ bound to porcine mitochondrial NADP-dependent isocitrate dehydrogenase, as assessed by mutagenesis. Biochemistry. 2004;43:2821–2828. doi: 10.1021/bi030253f. [DOI] [PubMed] [Google Scholar]
- Ioffe V.M., Gorbenko G.P., Domanov Y.A., Tatarets A.L., Patsenker L.D., Terpetsching E.A., Dyubko T.S. A new fluorescent squaraine probe for the measurement of membrane polarity. J. Fluoresc. 2006;16:47–52. doi: 10.1007/s10895-005-0018-2. [DOI] [PubMed] [Google Scholar]
- Jain M.K., Wagner R.C. John Wiley; New York: 1980. Introduction to Biological Membranes. [Google Scholar]
- Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Kini V., Vlasova I.I., Zhao Q., Zou M., Di P., Svistunenko D.A., Kurnikov I.V., Borisenko G.G. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kirkland R.A., Adibhatla R.M., Hatcher J.F., Franklin J.L. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115:587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- Konev S.V. Plenum Press; New York: 1967. Fluorescence and Phosphorescence of Proteins and Nucleic Acids; p. 21. [Google Scholar]
- Kvamme E., Torgner I.A., Roberg B. Evidence indicating that pig renal phosphate-activated glutaminase has a functionally predominant external localization in the inner mitochondrial membrane. J. Biol. Chem. 1991;266:13185–13192. [PubMed] [Google Scholar]
- Laterveer F.D., Van der Heijden R., Toonen M., Nicolay K. The kinetic consequences of binding of hexokinase-I to the mitochondrial outer membrane. Biochim. Biophys. Acta – Bioenerg. 1994;1188:251–259. [Google Scholar]
- Li G., Chen S., Thompson M.N., Greenberg M.L. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim. Biophys. Acta. 2007;1771:432–441. doi: 10.1016/j.bbalip.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Luévano-Martínez L.A., Forni M.F., Dos Santos V.T., Souza-Pinto N.C., Kowaltowski A.J. Cardiolipin is a key determinant for mtDNA stability and segregation during mitochondrial stress. Biochim. Biophys. Acta – Bioenerg. 2015;1847:587–598. doi: 10.1016/j.bbabio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Maniti O., Lecompte M.-F., Marcillat O., Desbat B., Buchet R., Vial C., Granjon T. Mitochondrial creatine kinase binding to phospholipid monolayers induces cardiolipin segregation. Biophys. J. 2009;96:2428–2438. doi: 10.1016/j.bpj.2008.12.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsko C.M., Hunter O.C., Rabinowich H., Lotze M.T., Amoscato A.A. Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem. Biophys. Res. Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- Melo A.M., Loura L.M.S., Fernandes F., Villalaín J., Prieto M., Coutinho A. Electrostatically driven lipid–lysozyme mixed fibers display a multilamellar structure without amyloid features. Soft Matter. 2014;10:840–850. doi: 10.1039/c3sm52586d. [DOI] [PubMed] [Google Scholar]
- Mirkin N., Jaconcic J., Stojanoff V., Moreno A. High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins: Struct. Funct. Genet. 2008;70:83–92. doi: 10.1002/prot.21452. [DOI] [PubMed] [Google Scholar]
- Nury H., Dahout-Gonzalez C., Trézéguet V., Lauquin G., Brandolin G., Pebay-Peyroula E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 2005;579:6031–6036. doi: 10.1016/j.febslet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Ott M., Zhivotovsky B., Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R.M., Gratton. E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Phoon C.K.L., Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014;55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Schlattner U., Tokarska-Schlattner M., Ramirez S., Tyurina Y.Y., Amoscato A.A., Mohammadyani D., Huang Z., Jiang J., Yanamala N., Seffouh A., Boissan M., Epand R.F., Epand R.M., Klein-Seetharaman J., Lacombe M.-L., Kagan V.E. Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. J. Biol. Chem. 2013;288:111–121. doi: 10.1074/jbc.M112.408633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennato S., Bordi F., Cametti C., Coluzza C., Desideri A., Rufini S. Evidence of domain formation in cardiolipin–glycerophospholipid mixed monolayers. A thermodynamic and AFM study. J. Phys. Chem. 2005;109:15950–15957. doi: 10.1021/jp051893q. [DOI] [PubMed] [Google Scholar]
- Shimanouchi T., Kitaura N., Onishi R., Umakoshi H., Kuboi R. Secondary nucleation of Aβ Fibrils on liposome membrane. AIChE J. 2012;58:3625–3632. [Google Scholar]
- Shimanouchi T., Shimauchi N., Ohnishi R., Kitaura N., Yagi H., Goto Y., Umakoshi H., Kuboi R. Formation of spherulitic amyloid β aggregate by anionic liposomes. Biochem. Biophys. Res. Commun. 2012;426:165–171. doi: 10.1016/j.bbrc.2012.07.107. [DOI] [PubMed] [Google Scholar]
- Suga K., Tanabe T., Tomita H., Shimanouchi T., Umakoshi H. Conformational change of single-stranded RNAs induced by liposome binding. Nucleic Acid Res. 2011;39:8891–8900. doi: 10.1093/nar/gkr568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga K., Tanabe T., Umakoshi H. Heterogeneous cationic liposomes modified with 3β-{N-[(N′,N′-Dimethylamino)ethyl]carbamoyl}cholesterol can induce partial conformational changes in messenger RNA and regulate translation in an Escherichia coli cell-free translation system. Langmuir. 2013;29:1899–1907. doi: 10.1021/la3050576. [DOI] [PubMed] [Google Scholar]
- Suga K., Umakoshi H. Detection of nanosized ordered domains in DOPC/DPPC and DOPC/Ch binary lipid mixture systems of large unilamellar vesicles using a TEMPO quenching method. Langmuir. 2013;29:4830–4837. doi: 10.1021/la304768f. [DOI] [PubMed] [Google Scholar]
- Tuan L.Q., Umakoshi H., Shimanouchi T., Kuboi R. Liposome-recruited activity of oxidized and fragmented superoxide dismutase. Langmuir. 2008;24:350–354. doi: 10.1021/la702690a. [DOI] [PubMed] [Google Scholar]
- Umakoshi H., Nishida A. Modulation of yeast hexokinase on bio-inspired membranes. Biochem. Eng. J. 2012;69:138–143. [Google Scholar]
- Walde P. Phospholipid membranes as regulators of localized activity. Chem. Biol. 2010;17:922–923. doi: 10.1016/j.chembiol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Walde P., Umakoshi H., Stano P., Mavelli F. Emergent properties arising from the assembly of amphiphiles. Artificial vesicle membranes as reaction promoters and regulators. Chem. Commun. 2014;50:10177–10197. doi: 10.1039/c4cc02812k. [DOI] [PubMed] [Google Scholar]
- Wenk M.R. The emerging field of lipidomics. Nat. Rev. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- Zahid N.I., Abou-Zied O.K., Hashim R. Evidence of basic medium in the polar nanochannels of the inverse bicontinuous cubic phase of a guerbet glycolipid: a steady-state and time-resolved fluorescence study. J. Phys. Chem. C. 2013;117:26636–26643. [Google Scholar]
- Zhang M., Mileykovskaya E., Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q., Gohil V.M., Ma L., Greenberg M.L. Absence of cardiolipin results in temperature sensitivity, respiratory defects, and mitochondrial DNA instability independent of pet56. J. Biol. Chem. 2004;279:32294–32300. doi: 10.1074/jbc.M403275200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material