Abstract
Silencing of virally transduced genes by promoter methylation and histone deacetylation has been a chronic problem both experimentally and therapeutically. We observed frequent silencing of the tetracycline-inducible Tet-On promoter borne by the Tripz lentivirus in mammary epithelial cell lines. We found that silencing could be prevented by continuous induction, but uninduced Tet-On gradually became uninducible, suggesting promoter modification. Accordingly, silencing was reversible by a common inhibitor of histone deacetylases, sodium butyrate. The effect was cell-line dependent, as HEK293 cells exhibited only moderate silencing that could be partly reversed by extended induction. These results indicate the need to test individual cell lines prior to using this system for studies that require induction after long periods of repression such as in animal models or RNA interference screens.
Keywords: pTripz, Tet-On, Inducibility, Promoter silencing, Sodium butyrate, Histone deacetylase, Breast cancer
Highlights
-
•
Loss of inducibility of Tet-On transgenes in cancer cell lines is reported.
-
•
Expression can be restored by an HDAC inhibitor.
-
•
Expression can be maintained by continuous induction.
-
•
Loss of expression is cell-line dependent.
1. Introduction
Downregulation of transgenes following viral transduction continues to slow application of transgenic technologies (Papadakis et al., 2004; Johansen et al., 2002). Strong viral promoters in particular are susceptible to silencing by cellular antiviral responses, leading to methylation, deacetylation, and chromatin condensation (Papadakis et al., 2004). One motivation for the development of tetracycline-regulated promoters was the hope that they might escape this mechanism while repressed, thus allowing robust expression upon induction (Johansen et al., 2002).
Since their invention by Bujard and Gossen over 20 years ago, many variations of tetracycline-regulated gene expression systems have been developed and employed widely in genetic engineering of living systems (Gossen and Bujard, 1992; Bockamp et al., 2002). The classic Tet-Off system relies on the bacterial tet repressor fused to the VP16 transactivator domain, known as tTA. Tet operator segments are embedded in a CMV core promoter, and expression is constitutive in the absence of tetracycline or its analog doxycycline. Tetracycline binding blocks this activity. In the Tet-On version, reverse tTA (rtTA), the tet repressor has been modified so that tetracycline induces rtTA binding to the operator and subsequent transcriptional activation. Additional variants have been developed that greatly enhance induction levels, such as rtTA3 (Zhou et al., 2006). While rtTA was originally encoded on a separate vector from its target, necessitating two rounds of antibiotic selection and screening of clones for well regulated expression, more recently both elements have been combined into a single vector in a lentiviral backbone allowing selection of a population of such cells and obviating cloning (Szulc et al., 2006). Since lentiviruses tend to integrate into active chromatin, this design would theoretically be expected to minimize silencing by adjacent heterochromatin, a problem that limited utility of the original system (Ciuffi, 2008).
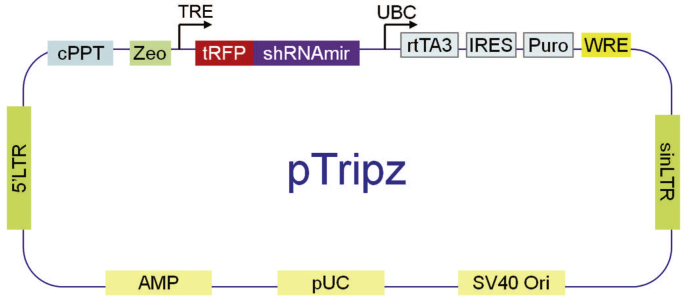
The single-vector, doxycycline-inducible shRNAmir lentiviral system Tripz (Fig. 1) (http://dharmacon.gelifesciences.com/uploadedfiles/resources/ptripz-inducible-lentiviral-manual.pdf) developed by OpenBiosystems has many desirable features and is widely used in biomedical research, with 565 citations listed by Google Scholar. The synthetic transcription factor rtTA3 is expressed constitutively from the UBC promoter to drive universal expression. Puromycin-resistance is encoded by the same transcript, ensuring that any resistant cell also encodes rtTA3. The target of rtTA3, the TRE promoter, drives expression of red fluorescent protein (RFP) with a gene-specific small hairpin RNA (shRNA) encoded in its tail. Thus red cells must express the shRNA and downregulate the target gene. Conversely, removal of the inducer should result in rapid loss of RFP and reversal of knockdown; and re-addition of the inducer should restore RFP expression and knockdown. Theoretically, this strategy allows the expansion of cells in the absence of toxic effects of knockdown and allows comparison of populations that differ only in attenuation of a single gene. In our experience reported here, the system lost inducibility in cells of mammary epithelial lineage.
Fig. 1.
Structure of pTripz Plasmid. The synthetic transcription factor rtTA3 is activated by tetracycline or doxycycline to bind the tetracycline response element (TRE). TRE drives expression of RFP and shRNA in the same transcript. Puromycin resistance is constitutively driven by the UBC promoter independently of doxycycline.
2. Methods
MCF7, MDA-MB-231, HEK293, and HEK293T cells were purchased from ATCC. The immortalized human mammary epithelial cell line HMLE was a kind gift from Robert Weinberg. The lentiviral vector Tripz containing shRNA inserts was purchased from OpenBiosystems and packaged by co-transfection into HEK293T with p8.74 and pMD2.G (kind gifts from Didier Trono). Lentiviral supernatants were collected 48 h later, filtered, and applied to cells in the presence of 8 µg/ml polybrene. Target cells were infected at a multiplicity of infection of about 0.5 infectious units per cell. After 48 h, 0.5 µg/ml doxycycline was added to determine the infection efficiency and maintained unless otherwise noted. After 72 h, puromycin was added (1 µg/ml) and maintained throughout the experiments unless otherwise noted. Uninfected cells were killed by 4 days of selection, and all surviving cells were RFP-positive. Puromycin-resistant cells were pooled and analyzed as populations rather than clones. Sodium butyrate (Sigma) was resuspended in water, filtered, and applied at a final concentration of 1 mM. Transcriptional induction was measured by RT-qPCR as described (Walia et al., 2011). P values were obtained by Student's two-tailed t test.
3. Results
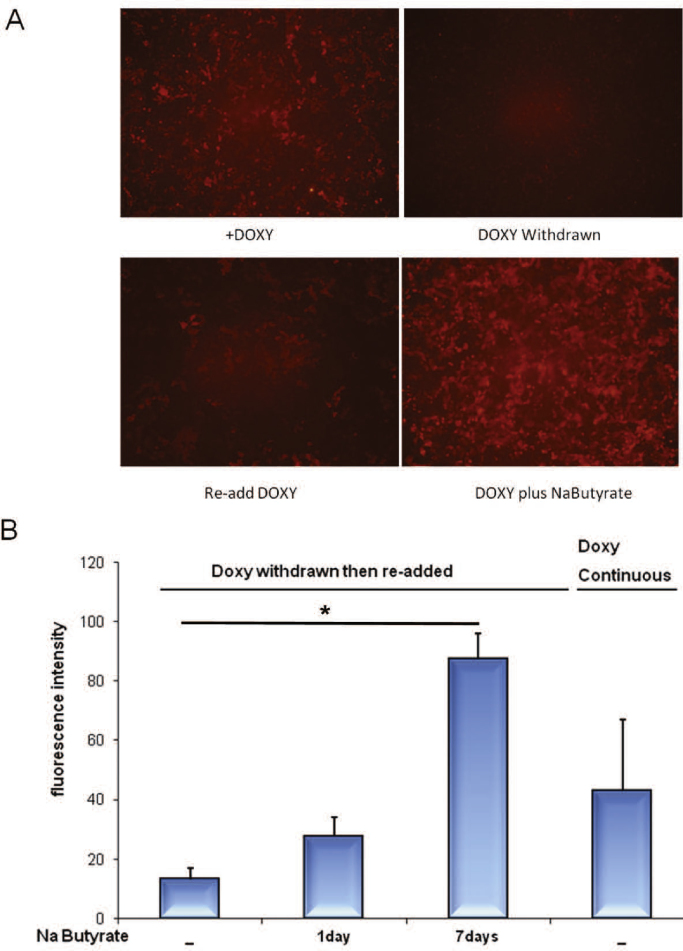
We found that when Tripz was transduced into breast cancer cell line MCF7, the system initially produced high fluorescence upon induction with doxycycline (Fig. 2A). As expected, after one week in the absence of inducer, red fluorescence was nearly undetectable. However, upon reintroduction of doxycycline, fluorescence was much fainter than originally observed (Fig. 2A, lower). We found that this silencing effect could be prevented by maintaining the cells in doxycycline inducer continuously (Fig. 2B), suggesting that silencing was due to chromatin modification of the inactive promoter.
Fig. 2.
Rescue of doxycycline-inducibility by sodium butyrate (Na Butyrate). A) Microimages of MCF7 cells transduced with pTripz. RFP expression was induced with 0.5 µg/ml doxycycline (+Doxy), then withdrawn for one week. Cells were then seeded into a 96 well plate, 14,000 per well. Re-addition of doxycycline did not result in full expression unless Na Butyrate was included. All images were collected at the same exposure time and magnification, 200×. B) Fluorimetric quantification of RFP. After doxycycline induction for 24 h, doxycycline was withdrawn for one week then restored in the absence or presence of Na Butyrate for one or seven days. Control had continuous exposure to doxycycline. To measure RFP, 14,000 cells were transferred to a 96 well plate the day before reading it. Puromycin selection (1 µg/ml) was maintained throughout the experiment. *p=0.04.
To test this, we treated cells with sodium butyrate (Na Butyrate), a well known inhibitor of histone deacetylases (HDAC), that causes histone hyperacetylation, chromatin decondensation, and activation of silenced promoters (Monneret, 2005). We found that 24 h of exposure to Na Butyrate increased RFP expression by twofold, and 7 days exposure increased it by sixfold, completely reversing the silencing effect (Fig. 2B).
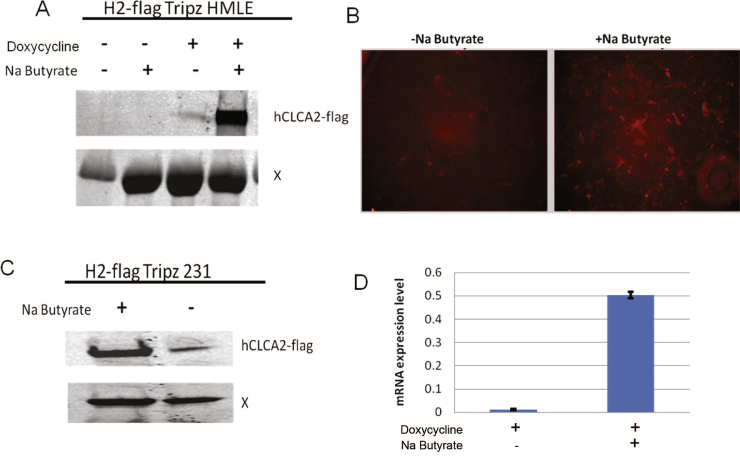
A similar loss of inducibility was observed when the Tripz vector was used to express a toxic protein. We replaced the RFP-shRNA cassette with a cDNA encoding hCLCA2 bearing a Flag tag. The resulting lentivirus pTripz-h2-flag was packaged, and HMLE cells were infected and selected with puromycin for approximately one week. Immunoblotting revealed low expression of the transgene after induction (Fig. 3A, lane 3), and less than 1% of the cells were positive by immunofluorescence (Fig. 3B). Addition of 1 mM Na Butyrate for 3 days dramatically increased expression. Similar results were obtained in breast cancer cell line MDA-MB-231 (Fig. 3C). Quantification by RT-qPCR confirmed that induction was at the transcriptional level (Fig. 3D).
Fig. 3.
Improved induction of hCLCA2 after Na Butyrate treatment. A) Immunoblot of flag-tagged hCLCA2 (H2-flag) Tripz-transduced HMLE. X, nonspecific band serves as loading control. B) Immunofluorescence of doxy-induced cells treated with 1 mM Na Butyrate and probed with anti-Flag and Alexa568-labeled secondary antibody. C) Immunoblot of hCLCA2-flag-Tripz transduced MDA-MB-231 cells. D) Enhancement of transcriptional induction in cells from C. Cells were treated with 1 µg/ml doxycycline. Induced mRNA was measured by RT-qPCR and normalized to beta actin mRNA. In A and C, protein was immunoprecipitated with anti-Flag antibody M2 before electrophoresis.
These results demonstrate that promoter silencing remains a major problem with transgenes driven by tetracycline-regulated promoters. The cells remained puromycin-resistant so they must still have expressed the rtTA3 transactivator. However, we noticed that if selection was omitted for a few passages then restored, most of the cells were killed, suggesting that the UBC promoter driving puromycin resistance was also subject to silencing. Silencing was time-dependent: the longer the inducer was removed, the lower the RFP expression upon re-induction. This was so in two breast cancer and one immortalized breast epithelial cell lines. In contrast, we have never observed loss of expression in cells transduced with pGIPZ lentivirus, which drives expression of GFP and shRNA from a constitutive promoter (OpenBiosystems).
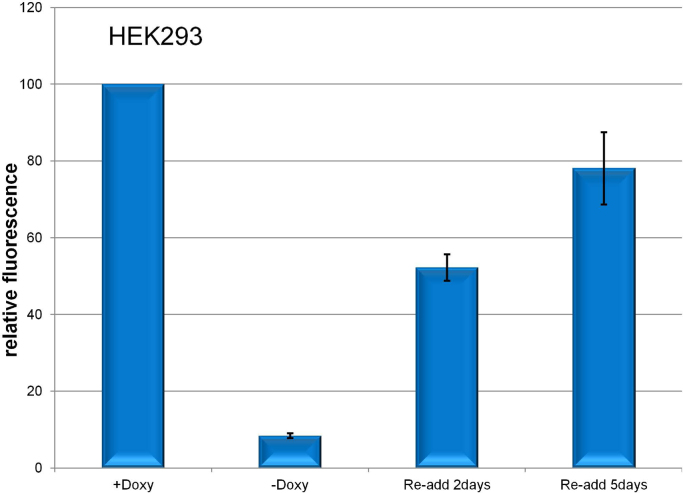
Importantly, the extent of silencing was cell-line dependent. While all breast and breast cancer cell lines tested showed dramatic silencing, human embryonic kidney 293 (HEK) cells repressed for one week regained 52% of their original expression level by 48 h and 78% by 5 days after doxycycline addition (Fig. 4).
Fig. 4.
Silencing is cell-line dependent. Levels of RFP were measured in HEK293-Tripz cells exposed to doxycycline continuously (100%) or repressed then re-exposed for 2 days or 5 days. P value for Doxy plus vs. 2 days=0.002; for Doxy plus vs. 5 days=0.06.
4. Discussion
Problems with silencing of lentivirally transduced genes, including Tet-On constructs, have been reported previously (Papadakis et al., 2004; Johansen et al., 2002). Strategies to increase the efficiency of repression may unintentionally lead to an uninducible transgene. For example, fusing the Tet repressor to the KRAB repressor domain resulted in better repression in the “off” state, but the construct became uninducible when it was repressed through early embryogenesis in mouse (Wiznerowicz et al. 2007). Silencing was found to result from heterochromatinization not only of the Tet-regulated promoter but of several kilobases flanking it as well. This could explain the co-silencing of the Ubc promoter observed here. Similar problems were observed with use of the Tet-Off system, both in vivo and in cell culture. Silencing was linked to histone deacetylation and was proportional to the time span of repression (Oyer et al., 2009). In animals, this produced mosaic effects (Kues et al. 2006).
Many strategies have been devised to avert promoter silencing. Since viral promoters are targets for downregulation, host promoters for structural genes such as spectrin and EF1alpha have been substituted with some success (Papadakis et al., 2004; Vilaboa and Voellmy, 2006). Very recently, promoter elements termed ubiquitously acting chromatin opening elements (UCOE) were discovered that are resistant to silencing. They have been used successfully to drive transgene expression in hematopoetic cells but have not been applied to inducible systems thus far (Zhang et al., 2007). In some cases, downregulation may be due to position effects, and it may be possible to isolate individual clones that maintain expression (Papadakis et al., 2004). Alternatively, following viral infection, cells can be split into two populations for puromycin selection. One is treated with doxycycline continuously, the other not. We have found that RFP expression and knockdown remain robust indefinitely in the presence of the inducer (Walia et al., 2011). We have also found periodic sorting of the cells for RFP by flow cytometry helpful in maintaining a uniformly inducible population.
We were unable to find previous reports of cancer cell-line dependent effects on tet-regulated expression, but it is not surprising. During cancer progression, promoters are frequently inactivated by interdependent CpG methylation and histone deacetylation (Momparler, 2003). The methyl transferases and histone deacetylases responsible are upregulated or dysregulated with tumor progression, resulting in downregulation of multiple growth-limiting genes (Momparler, 2003; Montezuma, 2015). Thus, normal tissue and benign tumors show low or infrequent promoter methylation, while it may be rampant in adjacent carcinomas (Nuovo et al., 1999). HEK293 is an immortalized kidney neuroendocrine cell line that was generated by transduction of adenoviral E1A into primary cells, while MCF7 and MDA-MB-231 are autochthonous breast cancer cell lines (Shaw et al., 2002). Thus, MCF7 and MDA-MB-231 have undergone in vivo selection for activation of promoter silencing machinery, while HEK293 has not. Indeed, HDAC6 was found to be upregulated in MCF7 and contribute to its malignant phenotype (Aldana et al., 2011). Accordingly, it is expected that heterochromatinization of inducible promoters should be more severe in tumor cells that are more advanced on the spectrum of malignancy. Investigators designing screens based on regulated knockdown by a transduced shRNA library in a cancer cell line should first determine whether irreversible silencing of the Tet-On promoter occurs in the target cell line. This is an even more crucial issue for the success of animal and gene therapy studies that rely on this system (Stieger et al., 2009).
Acknowledgments
This study was supported in part by National Institutes of Health Grant R15CA151094 to RCE.
References
- Aldana-Masangkay G.I., Sakamoto K.M. The role of HDAC6 in cancer. J. Biomed. Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. PubMed PMID: 21076528; PubMed Central PMCID: PMC2975074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockamp E., Maringer M., Spangenberg C., Fees S., Fraser S., Eshkind L., Oesch F., Zabel B. Of mice and models: improved animal models for biomedical research. Physiol. Genom. 2002;11(3):115–132. doi: 10.1152/physiolgenomics.00067.2002. Epub 2002 Dec 3. Review. PubMed PMID: 12464688. [DOI] [PubMed] [Google Scholar]
- Ciuffi A. Mechanisms governing lentivirus integration site selection. Curr. Gene. Ther. 2008;8(6):419–429. doi: 10.2174/156652308786848021. Review. PubMed PMID: 19075625. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA. 1992;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. PMC 49329. PMID 1319065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K., Rosenblad C., Andsberg K., Møller A., Lundberg C., Björlund A. Evaluation of Tet-on system to avoid transgene down-regulation in ex vivo gene transfer to the CNS. Gene Ther. 2002;9:1291–1301. doi: 10.1038/sj.gt.3301778. [DOI] [PubMed] [Google Scholar]
- Kues W.A., Schwinzer R., Wirth D., Verhoeyen E., Lemme E., Herrmann D., Barg-Kues B., Hauser H., Wonigeit K., Niemann H. Epigenetic silencing and tissue independent expression of a novel tetracycline inducible system in double-transgenic pigs. FASEB J. 2006;20(8):1200–1202. doi: 10.1096/fj.05-5415fje. Epub 2006 May 9. PubMed PMID: 16684801. [DOI] [PubMed] [Google Scholar]
- Momparler R.L. Cancer epigenetics. Oncogene. 2003;22(42):6479–6483. doi: 10.1038/sj.onc.1206774. Review. PubMed PMID: 14528271. [DOI] [PubMed] [Google Scholar]
- Monneret C. Histone deacetylase inhibitors. Eur. J. Med. Chem. 2005;40:1–13. doi: 10.1016/j.ejmech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Montezuma D., Henrique R.M., Jerónimo C. Altered expression of histone deacetylases in cancer. Crit. Rev. Oncog. 2015;20(1–2):19–34. doi: 10.1615/critrevoncog.2014012554. Review. PubMed PMID: 25746102. [DOI] [PubMed] [Google Scholar]
- Nuovo G.J., Plaia T.W., Belinsky S.A., Baylin S.B., Herman J.G. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Poc. Natl. Acad. Sci. USA. 1999;96(22):12754–12759. doi: 10.1073/pnas.96.22.12754. PubMed PMID: 10535995; PubMed Central PMCID: PMC23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyer J.A., Chu A., Brar S., Turker M.S. Aberrant epigenetic silencing is triggered by a transient reduction in gene expression. PLoS One. 2009;4(3):e4832. doi: 10.1371/journal.pone.0004832. Epub 2009 Mar 12. PubMed PMID: 19279688; PubMed Central PMCID: PMC2654015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis E.D., Nicklin S.A., Baker A.H., White S.J. Promoters and control elements: designing expression cassettes for gene therapy. Curr. Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- Shaw G., Morse S., Ararat M., Graham F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16(8):869–871. doi: 10.1096/fj.01-0995fje. Epub 2002 Apr 10. PubMed PMID: 11967234. [DOI] [PubMed] [Google Scholar]
- Stieger K., Belbellaa B., Le Guiner C., Moullier P., Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv. Drug. Deliv. Rev. 2009;2:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc J., Wiznerowicz M., Sauvain M.O., Trono D., Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat. Methods. 2006;3(2):109–116. doi: 10.1038/nmeth846. PubMed PMID: 16432520. [DOI] [PubMed] [Google Scholar]
- Vilaboa N., Voellmy R. Regulatable gene expression systems for gene therapy. Curr. Gene Ther. 2006;6:421–438. doi: 10.2174/156652306777934829. [DOI] [PubMed] [Google Scholar]
- Walia V., Yu Y., Cao D., Sun M., McLean J.R., Hollier B.G., Cheng J., Mani S.A., Rao K., Premkumar L., Elble R.C. Loss of breast epithelial marker hCLCA2 promotes epithelial to mesenchymal transition and indicates higher risk of metastasis. Oncogene. 2011;31:2237–2246. doi: 10.1038/onc.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznerowicz M., Jakobsson J., Szulc J., Liao S., Quazzola A., Beermann F., Aebischer P., Trono D. The Kruppel-associated box repressor domain can trigger de novo promoter methylation during mouse early embryogenesis. J. Biol. Chem. 2007;282:34535–34541. doi: 10.1074/jbc.M705898200. [DOI] [PubMed] [Google Scholar]
- Zhang F., Thornhill S.I., Howe S.J., Ulaganathan M., Schambach A., Sinclair J., Kinnon C., Gaspar H.B., Antoniou M., Thrasher A.J. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Vink M., Klave B., Berkhout B., Das A.T. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 2006;13(19):1382–1390. doi: 10.1038/sj.gt.3302780. PMID 16724096. [DOI] [PubMed] [Google Scholar]