Abstract
It is theoretically possible to engineer Saccharomyces cerevisiae strains in which isobutanol is the predominant catabolic product and high-yielding isobutanol-producing strains are already reported by industry. Conversely, isobutanol yields of engineered S. cerevisiae strains reported in the scientific literature typically remain far below 10% of the theoretical maximum. This study explores possible reasons for these suboptimal yields by a mass-balancing approach. A cytosolically located, cofactor-balanced isobutanol pathway, consisting of a mosaic of bacterial enzymes whose in vivo functionality was confirmed by complementation of null mutations in branched-chain amino acid metabolism, was expressed in S. cerevisiae. Product formation by the engineered strain was analysed in shake flasks and bioreactors. In aerobic cultures, the pathway intermediate isobutyraldehyde was oxidized to isobutyrate rather than reduced to isobutanol. Moreover, significant concentrations of the pathway intermediates 2,3-dihydroxyisovalerate and α-ketoisovalerate, as well as diacetyl and acetoin, accumulated extracellularly. While the engineered strain could not grow anaerobically, micro-aerobic cultivation resulted in isobutanol formation at a yield of 0.018±0.003 mol/mol glucose. Simultaneously, 2,3-butanediol was produced at a yield of 0.649±0.067 mol/mol glucose. These results identify massive accumulation of pathway intermediates, as well as overflow metabolites derived from acetolactate, as an important, previously underestimated contributor to the suboptimal yields of ‘academic’ isobutanol strains. The observed patterns of by-product formation is consistent with the notion that in vivo activity of the iron–sulphur-cluster-requiring enzyme dihydroxyacid dehydratase is a key bottleneck in the present and previously described ‘academic’ isobutanol-producing yeast strains.
Keywords: Saccharomyces cerevisiae; Isobutanol; Catabolic pathway; By-product formation; 2,3-butanediol; Diacetyl
Highlights
-
•
Catabolic isobutanol production in S. cerevisiae is limited by by-product formation.
-
•
Main by-products are pathway intermediates and acetatolactate degradation products.
-
•
Saccharomyces cerevisiae harbours efficient BCAA intermediates export systems.
-
•
Iron–sulphur cluster containing dihydroxyacid dehydratase may be the main bottleneck.
1. Introduction
Biofuels produced from renewable feedstocks offer a promising alternative for current fossil-oil based transport fuels. In comparison with bioethanol, currently the single largest product of microbial fermentation (Weber et al., 2010), isobutanol offers several advantages: i) a higher energy content, similar to that of conventional gasoline (Kolodziej and Scheib, 2012), ii) a lower volatility, resulting in lower greenhouse gas emission and iii) a lower water miscibility, which facilitates storage and distribution in existing petrochemical infrastructure and use as a pure or blended fuel in existing combustion engines (Kolodziej and Scheib, 2012). Furthermore, isobutanol can be enzymatically or chemically converted to a wide range of economically relevant compounds, including isobutyl acetate (Altiokka and Citak, 2003), p-xylene (Peters et al., 2010), polyisobutylene (Wettling et al., 2013), kerosene (Ilika, 2010), and polyethylene terephthalate (PET) (Kolodziej and Scheib, 2012). When produced from cellulosic biomass, isobutanol can meet the specifications required to qualify as an advanced biofuel, with an over 50% lower greenhouse gas emission than conventional gasoline (Brat and Boles, 2013, Generoso et al., 2015, Kolodziej and Scheib, 2012).
Saccharomyces cerevisiae naturally produces isobutanol as an end product of valine catabolism via the Ehrlich pathway (Ehrlich, 1907, Dickinson et al., 1998, Hazelwood et al., 2008). As this yeast can, moreover, convert pyruvate, the product of glycolysis, into valine via its mitochondrial valine biosynthesis pathway (Ryan and Kohlhaw, 1974), it contains all genetic information required for de novo isobutanol production from glucose (Fig. 1). However, when grown on ammonium sulphate as sole nitrogen source, tight regulation of the valine biosynthetic pathway prevents isobutanol formation (Jones and Fink, 1982, Vuralhan et al., 2005).
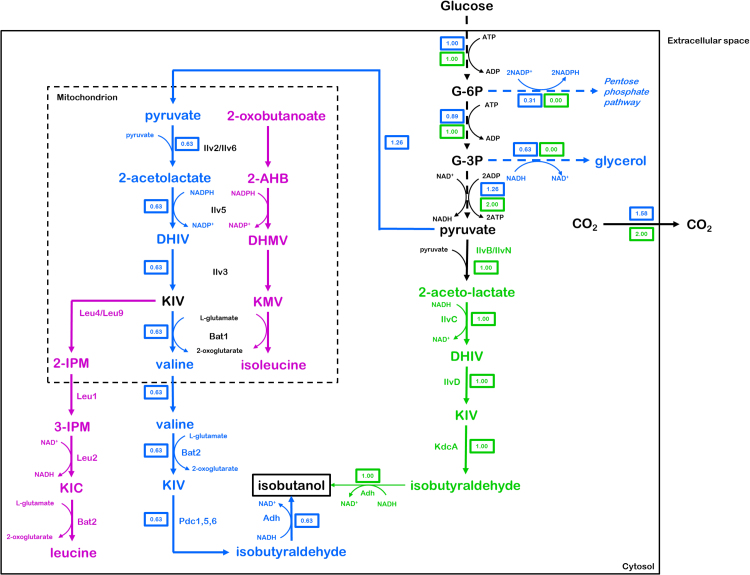
Fig. 1.
Schematic representation of branched-chain amino acid biosynthesis and isobutanol production in S. cerevisiae. Blue: Theoretical isobutanol production pathway using native S. cerevisiae reactions, with concomitant ribulose-5-phosphate production (via the oxidative pentose phosphate pathway) to regenerate NADPH consumed by Ilv5 and glycerol production to regenerate NAD+ consumed in lower glycolysis. Green: Redox-cofactor-balanced catabolic isobutanol production pathway with regeneration of NAD+ consumed in lower glycolysis by IlvC and Adh. Purple: Native pathway for the biosynthesis of leucine and isoleucine. Black: reactions common to all pathways. Dashed arrows represent multiple enzyme-catalysed reactions. Numbered boxes represent distribution of glucose flux in case of theoretically maximum product yields for the native and redox-balanced catabolic pathways (expressed in mol) as determined by stoichiometric modelling. G-6P: glucose-6-phosphate, G-3P: glyceraldehyde-3-phosphate, DHIV: 2,3-dihydroxyisovalerate, KIV: α-ketoisovalerate, 2-AHB: 2-aceto-2-hydroxybutyrate, DHMV: 2,3-dihydroxymethylvalerate, KMV: α-ketomethylvalerate, 2-IPM: 2-isopropylmalate, 3-IPM: 3-isopropylmalate, KIC: α-ketoisocaproate. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
After many years of research, academic studies on isobutanol production by S. cerevisiae have generated yields that remain far below the theoretical maximum yield of 1 mol isobutanol/mol glucose (reviewed by (Generoso et al., 2015). For example, overexpression of the native S. cerevisiae valine biosynthesis and degradation pathways led to isobutanol yields of only 0.0059 mol/mol glucose (Chen et al., 2011), while additional elimination of competing enzymes such as Bat1, Leu2, Ald6, Ecm31, Pdc1 and Lpd1 resulted in significant but moderate increases of isobutanol yields (Ida et al., 2015, Kondo et al., 2012, Matsuda et al., 2013, Park et al., 2014). Another challenge in engineering the native yeast valine pathway is its distribution over the cytosol and mitochondria. To circumvent problems related to intracellular metabolite transport and redox co-factor balancing, two studies explored expression of complete isobutanol pathway localization into either the mitochondria (Avalos et al., 2013) or cytosol (Brat et al., 2012). The relatively small improvements in isobutanol production resulting from these strategies indicate the existence of other, significant constraints. However, a lack of mass balances and quantitative data on concentrations of pathway intermediates made it difficult to identify potential rate-controlling reactions in previously described engineered strains. While academic literature has consistently reported isobutanol yields far below the maximum theoretical yield, industrial research has already resulted in S. cerevisiae strains that produce isobutanol at 85% of the maximum theoretical yield (Ryan, 2015). While the cryptic nature of patent literature makes it difficult to define the exact engineering strategies, the near-theoretical yields indicate that isobutanol is produced as the main catabolic product in these strains. Akin ethanol biosynthesis under anaerobic conditions, a catabolic pathway requires a net generation of ATP, sufficient pathway flux to support cellular maintenance and growth, and efficient redox cofactor balancing without the need for external electron acceptors. With respect to the latter, the set of native S. cerevisiae reactions that forms the basis for previous academic studies is not in itself redox balanced due to the use of an NADPH-dependent acetohydroxyacid reductoisomerase (AHAR, encoded by ILV5) to catalyse the conversion of acetolactate to 2,3-dihydroxyisovalerate. Using a heterologous NADH-dependent AHAR as well as an NADH-dependent alcohol dehydrogenase offers the possibility to regenerate the NADH cofactors produced during the conversion of glucose to pyruvate (glycolysis) (Fig. 1).
This study aims to investigate the reason for the low product yields in previous academic reports on engineered, isobutanol-producing S. cerevisiae strains. To this end, S. cerevisiae was engineered to cytosolically express a redox-cofactor balanced, ATP-yielding isobutanol pathway. Subsequently, a complete analysis of the production of pathway intermediates and derived metabolites was performed in aerobic and micro-aerobic cultures. The results of this analysis were used to quantify fluxes towards isobutanol and by-products.
2. Materials and methods
2.1. Media, strains and maintenance
All S. cerevisiae strains used in this study (Table 1) share the CEN.PK genetic background (Entian and Kötter, 2007, Nijkamp et al., 2012). Frozen stocks of Escherichia coli and S. cerevisiae strains were prepared by addition of glycerol (30% (v/v)) to exponentially growing cells and aseptically storing 1 mL aliquots at −80 °C. Cultures were grown in synthetic medium (SM) [3 g/L KH2PO4, 0.5 g/L MgSO47H2O and 5 g/L (NH4)2SO4] (Verduyn et al., 1992) with appropriate growth factors added (Pronk, 2002) and the pH adjusted to 6.0. Cultures were also grown in complex YP medium [10 g/L yeast extract, 20 g/L peptone]. Synthetic medium and complex medium with glucose as sole carbon source (SMG/YPD) contained 20 g/L glucose. Tween-80 (420 mg/L) and ergosterol (10 mg/L) were added were added to media for anaerobic cultures. Synthetic medium agar plates were prepared as described above but with the addition of 20 g/L agar (Becton Dickinson B.V. Breda, The Netherlands).
Table 1.
S. cerevisiae strains used in this study.
| Name | Relevant genotype | Origin |
|---|---|---|
| CEN.PK113-3B | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 | (Entian and Kötter, 2007) |
| IME169 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 pUDE189 | This study |
| IMK463 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv2::loxP-natNT2-loxP | This study |
| IMK464 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv3::loxP-natNT2-loxP | This study |
| IMK465 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv5::loxP-natNT2-loxP | This study |
| IMK466 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv6::loxP-natNT2-loxP | This study |
| IMZ346 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv2::loxP-natNT2-loxP pUDE189 | This study |
| IMZ347 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv3::loxP-natNT2-loxP pUDE189 | This study |
| IMZ348 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv5::loxP-natNT2-loxP pUDE189 | This study |
| IMZ349 | MATa ura3-52 his3-Δ1 MAL2-8c SUC2 ilv6::loxP-natNT2-loxP pUDE189 | This study |
| IMZ500 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::loxP MTH1∆T p426GPD | This study |
| IMI302 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ade2::PDC1-amdS | (Milne et al., 2015) |
| IMX708 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ilv2Δ::coilvB hphNT1coilvC6E6 coilvDcoilvNM13 | This study |
| IME305 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ilv2Δ::coilvB hphNT1 coilvC6E6 coilvDcoilvNM13 p426-GPD | This study |
| IME306 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ilv2Δ::coilvB hphNT1 coilvC6E6 coilvDcoilvNM13 pUDE001 | This study |
| IME307 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ilv2Δ::coilvB hphNT1 coilvC6E6 coilvDcoilvNM13 pUDE321 | This study |
| IME308 | MATa ura3-52 HIS3 MAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::cas9-tagA-loxP-natNT2-loxP MTH1∆T ilv2Δ::coilvB hphNT1 coilvC6E6 coilvDcoilvNM13 pUDE336 | This study |
2.2. Strain and plasmid construction
2.2.1. Expression cassettes for isobutanol biosynthetic genes
DNA coding sequences of Corynebacterium glutamicum ilvNM13 and C. glutamicum ilvB (Elisakova et al., 2005), E. coli ilvC6E6 (Bastian et al., 2011) and Lactococcus lactis ilvD (Urano et al., 2012) were codon optimised for S. cerevisiae using the JCat algorithm (Grote et al., 2005) (Supplementary materials). Custom synthesized cassettes cloned into pUC57 (Y14837.1) were provided by BaseClear (Leiden, The Netherlands). In these vectors, the codon optimized genes (co) were flanked by strong constitutive promoters and terminators from S. cerevisiae glycolytic genes. Each cassette was further flanked with 60 bp tags (labelled A through I) with homology to an adjacent cassette. These tags have no significant homology to the S. cerevisiae genome, ensuring that each cassette can only recombine with an adjacent cassette using homologous recombination (Kuijpers et al., 2013). Custom synthesis resulted in plasmids pUD220 (D-TEF1P-coilvNM13-CYC1t-C), pUD221 (B-TPI1P-coilvB-ADH1t-C), pUD222 (D-ADH1P-coilvC6E6-PYK1t-F) and pUD223 (G-PGK1P-coilvD-TEF1t-I). Each plasmid was transformed into chemically competent E. coli (T3001, Zymo Research, Irvine, CA) according to the manufacturer’s instructions, and the gene sequences confirmed by Sanger sequencing (BaseClear). The gene cassettes from each plasmid were used to assemble the plasmid pUDE189, in association with cassettes encoding a URA3 yeast selection marker (pUD192: A-URA3-B), a CEN6-ARS4 yeast replicon (pUD193: F-CEN6-ARS4-G), and a fragment containing the bla (AmpR) ampicillin resistance marker and E. coli origin of replication (pUD195: I-AmpR-A) to allow selection and propagation in both S. cerevisiae and E. coli (Kozak et al., 2014b) (Table 2). Plasmids propagated in E. coli were isolated with Sigma GenElute Plasmid Kit (Sigma Aldrich, Zwijndrecht, The Netherlands). Each cassette was flanked by unique restriction sites allowing them to be excised from the plasmid backbone to generate fragments to use in the assembly of pUDE189 by vector assembly via homologous recombination. For digestion of each plasmid, high fidelity restriction endonucleases (Thermo Scientific, Waltham, MA) were used according to the manufacturer’s instructions. pUD220, pUD222 and pUD223 were digested with ApaI and BamHI, pUDE221 was digested with XmnI and BamHI, pUD192 was digested with XhoI, pUD194 was digested with SacII and pUD195 was digested with NotI. After digestion, each fragment was purified by gel electrophoresis using 1% (w/v) agarose (Sigma Aldrich) in TAE buffer (40 mM Tris-acetate pH 8.0 and 1 mM EDTA). Isolation of agarose trapped DNA fragments was performed using Zymoclean Gel DNA Recovery Kit (Zymo Research). Equimolar amounts of each fragment were transformed into CEN.PK113-3B (ura3-52, his3-Δ1) allowing for in vivo vector assembly of the fragments by homologous recombination. Correctly assembled transformants were selected on SMG agar supplemented with histidine (0.125 g/L). A single colony isolate was stocked as IME166 (Table 1). Correct plasmid assembly was verified using primer pairs which bound in each of the gene cassettes and amplified the 60 bp homologous tags (Table 3). The plasmid was extracted from IME166, named as pUDE189 and transformed into E. coli DH5α by electroporation in 2 mM cuvettes (BioRad, Hercules, CA) using a Gene PulserXcell electroporation system (BioRad) following the manufacturer’s protocol and stocked in the E. coli host.
Table 2.
Plasmids used in this study
| Name | Characteristics | Origin |
|---|---|---|
| pUC57 | bla (ApR), rep (pMB1 E. coli replicon) (NCBI accession number: Y14837.1) | BaseClear |
| pUD192 | pUC57+URA3 | (Kozak et al., 2014b) |
| pUD194 | pUC57+2 µm replicon | (Kozak et al., 2014b) |
| pUD195 | bla (ApR), rep (pMB1) | (Kozak et al., 2014a) |
| pUD220 | pUC57+TEF1P-coilvNM13-CYC1t | This study |
| pUD221 | pUC57+TPI1P-coilvB-ADH1t | This study |
| pUD222 | pUC57+ADH1P-coilvC6E6-PYK1t | This study |
| pUD223 | pUC57+PGK1P-coilvD-TEF1t | This study |
| pUDE189 | 2µm ori, bla (ApR), URA3 TEF1P-coilvNM13-CYC1tTPI1P-coilvB-ADH1t, ADH1P-coilvC6E6-PYK1t PGK1P-coilvD-TEF1t | This study |
| p426GPD | 2µm ori, URA3 TDH3P-CYC1t | (Mumberg et al., 1995) |
| pUDE001 | 2µm ori, URA3 TDH3P-ARO10-CYC1t | (Vuralhan et al., 2005) |
| pUDE321 | 2µm ori, URA3 TDH3P-cokdcA-CYC1t | (Milne et al., 2015) |
| pUDE336 | 2µm ori, URA3 TDH3P-cokivD-CYC1t | (Milne et al., 2015) |
| pUGnatNT2 | 2µm ori, URA3 TEF2P-natNT1-TEF2t | (de Kok et al., 2012) |
co: Codon optimised.
Table 3.
Oligonucleotide primers used in this study
| Name | Sequence (5′→3′) |
|---|---|
| Plasmid/integration conformation | |
| A-tag amp fwd | AAATAAACAAATAGGGGTTCCGC |
| A-tag amp rev | GAAATGCTGGATGGGAAGCG |
| B-tag amp fwd | TCCCATATGATTGTCTCCGTAAGCTCG |
| B-tag amp rev | ACTCTGTCATATACATCTGCCGCAC |
| C-tag amp fwd | GCAAATGCCTGCAAATCG |
| C-tag amp rev | CGCGTGTACGCATGTAAC |
| D-tag amp fwd | GCTAAATGTACGGGCGACAG |
| D-tag amp rev | GCCTTCATGCTCCTTGATTTCC |
| F-tag amp fwd | GTCGTCATAACGATGAGGTGTTGC |
| F-tag amp rev | ATGAAGCACAGATTCTTCGTTG |
| G-tag amp fwd | GAGAAGAACGGCATAGTGCGTG |
| G-tag amp rev | GTAAGTTTCACGAGGTTCTAC |
| I-tag amp fwd | GCGTCAATCGTATGTGAATGC |
| I-tag amp rev | GCCTTTGAGTGAGCTGATACC |
| ILV2 upstream fwd | TCCTTTCTCCACCATCCCTA |
| ILV2 downstream rev | CGTGTCCGACGAGTTAAAAC |
| Knockout cassette amplification | |
| ILV2 KO fwd | TTTACAAAATCTAAACCCTTTGAGCTAAGAGGAGATAAATACAACAGAATCAATTTTCAACAGCTGAAGCTTCGTACGC |
| ILV2 KO rev | AATAATAATAAAGTCTGCATTTTTTACTGAAAATGCTTTTGAAATAAATGTTTTTGAAATGCATAGGCCACTAGTGGATCTG |
| ILV3 KO fwd | CTGTAATCTTTAGTAACGGATTCTTGTATTTTTTTGTAAACAGCCAAGAAAAAAGTAGAGCAGCTGAAGCTTCGTACGC |
| ILV3 KO rev | AAAGATGATGGAAAAGGAGAATCTCTATATATATATTCATCGATTGGGGCCTATAATGCAGCATAGGCCACTAGTGGATCTG |
| ILV5 KO fwd | AACCTATTCCTAGGAGTTATATTTTTTTACCCTACCAGCAATATAAGTAAAAAATAAAACCAGCTGAAGCTTCGTACGC |
| ILV5 KO rev | ACTTGATGTTGCAAAAATTCCAAGAGAAAAAGTTTCCAGCACTTGATATTATTTTCCTCTGCATAGGCCACTAGTGGATCTG |
| ILV6 KO fwd | TACATAGTTCGTATATACAGAATCTTTAGAACATCTGAGCTCACTAACCCAGTCTTTCTACAGCTGAAGCTTCGTACGC |
| ILV6 KO rev | TACGTTATATAGATGTATAGAGGAGAGTCCCGAGGGCGATCGCAAGGCCGAGAGACTAACGCATAGGCCACTAGTGGATCTG |
| Knockout conformation | |
| ILV2 upstream fwd | TCCTTTCTCCACCATCCCTA |
| ILV2 downstream rev | CGTGTCCGACGAGTTAAAAC |
| ILV3 upstream fwd | CCCTCTTGTATCCATTCC |
| ILV3 downstream rev | CTTTAGTGGCAGCAAAGC |
| ILV5 upstream fwd | GTTGTGCGCGTGCACATTTC |
| ILV5 downstream rev | AATCGTAGCTGTCCCGATGAGG |
| ILV6 upstream fwd | GCACATCCAACGAATCACCTCACCGTTATC |
| ILV6 downstream rev | CGCGTCACCTCGTACAAACGTACAATC |
| Verification of plasmid transformation | |
| GPD1 promoter Fwd | GGGATGTGCTGCAAGGCGATTAAGTTGG |
| CYC1 terminator Rev | GGCAGTGAGCGCAACGCAATTAATGTGAG |
| Cassette integration | |
| ilvD amp with ILV2 hom fwd | TTTACAAAATCTAAACCCTTTGAGCTAAGAGGAGATAAATACAACAGAATCAATTTTCAAGCCAGAGGTATAGACATAGCCAGAC |
| ilvD amp (I-tag rev) | AGACGTCGCGGTGAGTTCAG |
| hphNT1 amp with I-tag hom fwd | TATTCACGTAGACGGATAGGTATAGCCAGACATCAGCAGCATACTTCGGGAACCGTAGGCCCAGCTGAAGCTTCGTACGC |
| hphNT1 amp with B-tag hom rev | GTTGAACATTCTTAGGCTGGTCGAATCATTTAGACACGGGCATCGTCCTCTCGAAAGGTGGCATAGGCCACTAGTGGATCTG |
| ilvB amp (B-tag fwd) | TACTCGCCGATAGTGGAAAC |
| ilvB amp (C-tag rev) | CGCGTGTACGCATGTAAC |
| ilvN amp (C-tag fwd) | GCAAATGCCTGCAAATCG |
| ilvN amp (D-tag rev) | GCCTTCATGCTCCTTGATTTCC |
| ilvC amp (D-tag fwd) | GCTAAATGTACGGGCGACAG |
| ilvC amp with ILV2 hom rev | AATAATAATAAAGTCTGCATTTTTTACTGAAAATGCTTTTGAAATAAATGTTTTTGAAATTGCCGAACTTTCCCTGTATGAAGC |
2.2.2. Branched-chain amino acid pathway gene deletions
ILV2, ILV3, ILV5 and ILV6 deletion cassettes were constructed by amplifying the natNT2 cassette from pUGnatNT2 (de Kok et al., 2012) using primers with added homology to the upstream and downstream region of each respective gene (Table 3). Each individual natNT2 deletion cassette was transformed into CEN.PK113-3B (ura3-52, his3-Δ1) yielding strains IMK463 (ilv2Δ), IMK464 (ilv3Δ), IMK465 (ilv5Δ) and IMK466 (ilv6Δ). Transformants were selected on complex medium agar (YPD) supplemented with 100 mg/L nourseothricin (Jena Bioscience, Jena, Germany). Each strain was then transformed with pUDE189 yielding strains IMZ346 (ilv2Δ, pUDE189), IMZ347 (ilv3Δ, pUDE189), IMZ348 (ilv5Δ, pUDE189) and IMZ349 (ilv6Δ, pUDE189) (Table 1). Transformants were selected on SMG agar supplemented with histidine (0.125 g/L) and nourseothricin (100 mg/L).
2.2.3. Construction of heterologous pathway strains
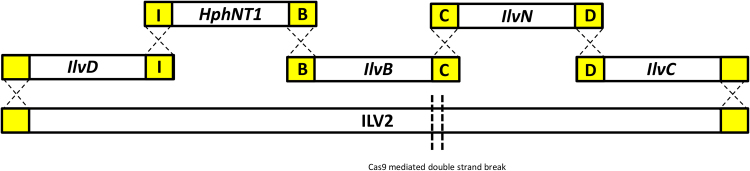
S. cerevisiae IMX708 was constructed by integrating the coilvB, coilvC6E6, coilvD and coilvNM13 overexpression cassettes along with a hphNT1 dominant selection marker conferring resistance to hygromycin (Goldstein and McCusker, 1999) at the ILV2 locus of IMI302 (Milne et al., 2015) using the CRISPR-Cas system (Mans et al., 2015). Cassettes were amplified by PCR using primers which either bound in the already introduced 60bp tags of each cassette, or primers with added homology to an adjacent 60 bp cassette or to the flanking regions of the ILV2 locus in order to allow in vivo assembly of adjacent cassettes and subsequent integration. The coilvD cassette was amplified from pUD223 with a primer that introduced homology to the upstream ILV2 region and a primer which bound in the 60 bp I-tag already flanking the cassette (ilvD amp with ILV2 hom fwd/ ilvD amp (I-tag rev)). The coilvC6E6 cassette was amplified from pUD222 with a primer which bound in the 60 bp D-tag already flanking the cassette and a primer that introduced homology to the downstream ILV2 region (ilvC amp (D-tag fwd)/ ilvC amp with ILV2 hom rev). The coilvB cassette was amplified from pUD221 with primers that bound in the B and C tags already flanking the cassette (ilvB amp (B-tag fwd)/ ilvB amp (C-tag rev)). The coilvNM13 cassette was amplified from pUD220 using primers which annealed in the C and D tags already flanking the cassette (ilvN amp (C-tag fwd)/ ilvN amp (D-tag rev)). Finally the hphNT1 cassette was amplified from pUGhphNT1 with primers that introduced homology to the I and B tags (hphNT1 amp with I-tag hom fwd/ hphNT1 amp with B-tag hom rev). Targeted integration of these cassettes at the ILV2 locus was facilitated by the CRISPR-Cas system according to the in vivo plasmid assembly protocol described by (Mans et al., 2015). Assembly of the required plasmid containing the ILV2 specific guide RNA and subsequent Cas9 mediated removal of the ILV2 gene was achieved in a single in vivo homologous recombination reaction step. Transformation of the CRISPR plasmid backbone, the ILV2 specific guide RNA fragment and the homologously linked expression cassettes resulted in the in vivo assembly of the plasmid, a Cas9 mediated double strand break in the ILV2 gene, and repair of that break using the homologously assembled expression cassettes with homology to the upstream and downstream regions of ILV2 (Fig. 3). Correctly assembled transformants were first selected on SMG agar plates supplemented 0.5 g/L valine, leucine and isoleucine as well as 200 mg/L hygromycin and in the absence of adenine supplementation to induce the loss of the transient PDC1 cassette (Milne et al., 2015). Single colonies were then streaked 3 times onto SMG agar plates containing 200 mg/L hygromycin, 1 g/L 5-fluoorotic acid (5’FOA) and 0.150 g/L uracil to induce the loss of the ILV2 targeting in vivo assembled CRISPR plasmid, without valine, leucine and isoleucine supplementation. A single colony isolate with restored branched chain amino acid biosynthesis was stocked and labelled as IMX708. The uracil auxotrophy of this strain was then complemented by transformation with p426GPD and pUDE321 resulting in strains IME305 (URA3), and IME307 (cokdcA URA3) respectively. The Pdc- control strain IMZ500 was constructed by transforming IMI244 with the p426GPD (URA3) plasmid.
Fig. 3.
Construction and assembly of a heterologous KIV biosynthesis pathway in S. cerevisiae using CRISPR-Cas guided ILV2 gene disruption and integration of the heterologous gene cassettes via homologous combination with 60 bp overlapping tags. A specific guide RNA was used to target cas9 to ILV2. The resulting double-strand break at the ILV2 locus was then repaired by the assembly and integration, by in vivo homologous recombination, of the expression cassettes for the codon-optimized heterologous genes that together formed the new KIV biosynthesis pathway.
In all cases PCR amplification of the gene cassettes was performed using Phusion® Hot Start II High Fidelity Polymerase (Thermo scientific) according to the manufactures instructions using HPLC or PAGE purified, custom synthesized oligonucleotide primers (Sigma Aldrich) in a Biometra TGradient Thermocycler (Biometra, Gottingen, Germany). Conformation of plasmid assembly/transformation, gene knockout and genome integration was achieved using the diagnostic primers listed in Table using DreamTaq (Thermo scientific) and desalted primers (Sigma Aldrich) in a Biometra TGradient Thermocycler (Biometra).
2.3. Shake flask cultivation, bioreactor-batch fermentation and micro-aerobic high cell density cultivation
All S. cerevisiae strains were grown in complex medium (YPD) or synthetic medium (SMG) (Verduyn et al., 1990) containing 20 g/L glucose. If required, 125 mg/L histidine and/or 150mg/L uracil was added to the synthetic media in order to complement a histidine and/or uracil auxotrophy. Cultures were grown in either 250 mL or 500 mL shake flasks containing 50 mL or 100 mL medium with incubation at 30 °C in an Innova incubator shaker (New Brunswick Scientific, Edison, NJ) at 200 rpm. Optical density at 660 nm was measured using a Libra S11 spectrophotometer (Biochrom, Cambridge, United Kingdom).
Controlled aerobic batch cultivation was carried out at 30 °C in 2 L bioreactors (Applikon, Schiedam, The Netherlands) with a working volume of 1 L. Synthetic medium was supplemented with 20 g/L glucose and 0.2 g/L of Pluronic antifoam (BASF, Ludwigshaven, Germany). The pH was kept constant at pH 5.0 by automatic addition of 2 M KOH. The stirrer speed was constant at 800 rpm and the aeration rate kept at 500 mL/min.
Micro-aerobic high-cell-density cultures were studied in SMG medium supplemented with Tween-80 (420 mg/L) and ergosterol (10 mg/L) in a total volume of 25 mL in 30 mL rubber stopper serum bottles. In contrast to pH control batch fermentation and to prevent a too fast acidification of the culture medium the initial pH was set to 6.0.with 2 M KOH. High-cell-density cultures were prepared by growing each strain in a 1 L aerobic batch fermentation setup. Cell cultures were harvested then centrifuged at 4700 g for 5 min then resuspended to a final OD660 of ~50. After inoculation into 30 mL serum stopper bottles the cap was tightly sealed to create micro-aerobic conditions. Rubber stoppers were pierced with a 0.6 mM Microlance needle (Becton Dickinson) to prevent pressure build-up. Each needle head also contained a cotton plug to prevent contamination. Cultures were incubated at 30 °C. Samples were taken to determine extracellular metabolite concentrations, OD660 and pH over the linear phase of glucose consumption. To limit the introduction of oxygen into the cultures during sampling, liquid samples were taken by attaching a sterile syringe to the pierced needle, inverting the serum bottle and withdrawing ~200 µL. The biomass concentration of each culture was estimated by taking the average OD 660 value and assuming 1 g/L of cell biomass equates to an OD660 value of 4.02.
2.4. Analytical methods
Biomass dry weight from bioreactors was determined by filtration of 10 mL broth over pre-dried and weighed 0.45 µm nitrocellulose filters (Gelman Laboratory, Ann Arbor, MI). After filtration the filters were dried for 20 min in a microwave at 350 W. To determine general extracellular metabolite concentrations, culture samples were spun down at 3500 g and the supernatant was collected. Metabolites were analysed using an Agilent 1260 Affinity HPLC machine (Agilent Technologies, Amstelveen, The Netherlands) with an Aminex HPX-87H ion exchange column (BioRad) operated at 60 °C with a mobile phase of 5 mM H2SO4 and a flow rate of 0.6 mL/min. Extracellular diacetyl was determined using static headspace gas chromatography. 5 mL of supernatant sample with 20 mg/L 2,3-hexandione as internal standard was heated to 65 °C for 30 min prior to injection using a CTC Combi Pal headspace autoinjector (CTC Analytics AG, Zwingen, Switzerland). Samples were analysed using a 7890A Agilent GC (Agilent Technologies) with an electron capture detector on a CP-Sil 8 CB (50 m×530 µm×1 µm) capillary column (Agilent Technologies). The split ratio was 1:1 with a split flow of 8 mL nitrogen per minute. The injector was set at 120 °C and an oven temperature profile of 35 °C for 3 min followed by an increase of 10 °C/min to 95 °C was used. The ECD detector was set at 150 °C was a make-up flow of 10 mL/min of nitrogen gas.
Samples for intracellular metabolite measurements were collected in pre-weighed tubes containing 30 mL 100% methanol kept at −40 °C. Approximately 6 mL of broth (~2 mg biomass) were quenched in methanol and the tubes weighed again to determine the exact volume added and vortexed. The samples were then centrifuged for 5 min at 10,000 g at −19 °C. The supernatant was discarded and the cell pellet was resuspended in 6 mL 100% methanol, and centrifuged again for 5 min at 10,000 g at −19 °C. The supernatant was discarded and 120 µL of 13C cell extract (as internal standard) was added to the cell pellet and the mix was resuspended in 2.5 mL pre-cooled 50% (v/v) aqueous methanol and 2.5 mL pre-cooled 100% chloroform. Samples were vigorously shaken for 45 min in an orbital shaker using a custom-made tube adaptor at −40 °C. Samples were then centrifuged for 5 min at 5000 g at −19 °C. The resulting upper layer (water/methanol) containing the metabolites of interest was transferred. To transfer putative remainders in the chloroform phase, the extraction was repeated by adding 2.5 mL water/methanol to the remaining chloroform layer. Excess liquid was removed using the Rapidvap system (Labconco, Kansas city, MO) and the dried samples were resuspended in 600 µL MilliQ water and stored until analysis at −80 °C. Samples for extracellular amino acid determination were prepared by passing broth through a filter and collecting the filtrate. The amino acid concentrations were determined using the N-Methyl-N-tert-butyldimethylsilytrifluoroacetamide (MTBSTFA) derivatization method according to (Dauner and Sauer, 2000) using 100 µL of intracellular sample or 10 µL of extracellular sample.
2.5. Stoichiometric modelling and metabolic flux analysis
The metabolic model was set up based on the pathway stoichiometries from MetaCyc (Caspi et al., 2008). To obtain a compact model, linear reactions were lumped. The lumped reactions included were glycolysis (simplified), the pentose-phosphate pathway and the TCA cycle (included as a single mitochondrial localized reaction). Furthermore, the electron transport chain and oxidative phosphorylation were included to represent and estimate a putative oxygen consumption rate. With different compartments, transporters and carriers have a major influence on the network functionality. To account for this, a lumped exchange reaction for NADPH/NADP, derived from the assumption of an active citrate/α-ketoglutarate shuttle together with NADP-dependent isocitrate-dehydrogenase and transport of the two acids was included. Also included was a lumped reaction for the exchange of NAD/NADH based on a malate/aspartate shuttle working together with aspartate transaminase and malate dehydrogenase (Palmieri et al., 2006). Additionally, a glutamate/α-ketoglutarate shuttle and valine transporter were included, as well as pyruvate transport via mitochondrial pyruvate carriers (MPC) (Herzig et al., 2012). A complete list of the metabolic network reactions can be found in Supplementary material. With this reaction network, “wild-type” and catabolic variants of the isobutanol pathway were included and the resultant metabolic flux and maximum yield determined using the software CellNetAnalyzer 2015.1 (Klamt et al., 2007). The flux map of the networks was created using Omix (Droste et al., 2013) (Supplementary material).
For the theoretical yield, the isobutanol production flux was set as only target and the glucose uptake rate was set to 100. For the estimation of intracellular fluxes based on experimental data the respective genotype was taken into account (i.e. knock out of ILV2 encoding the native mitochondrial Ilv2). The experimental standard deviation was used to weight the single measurements and the resulting flux map created using Omix (Supplementary material).
3. Results
3.1. Design of a catabolic route to isobutanol
Due to non-matching redox-cofactor specificities, a pathway that solely consists of native S. cerevisiae enzymes cannot support anaerobic isobutanol formation without the need for concomitant glycerol production. This redox issue limits the theoretical maximum yield of such a pathway to 0.63 mol/mol glucose and, moreover, imposes a requirement for aerobic respiration to supply ATP for cellular maintenance and growth. Production of isobutanol as sole catabolic product, with a maximum theoretical yield of 1 mol/mol glucose, requires several genetic modifications (Fig. 1). In this study, design of a catabolic isobutanol pathway was based on the following genetic interventions: 1) inactivation of the native alcoholic fermentation pathway by deletion of the pyruvate-decarboxylase genes PDC1, PDC5 and PDC6 and introduction of an internal deletion in MTH1 to restore growth on glucose (Oud et al., 2012, van Maris et al., 2004a); 2) introduction of a cytosolic isobutanol pathway comprising (i) a feedback-insensitive regulatory subunit (IlvNM13) (Elisakova et al., 2005) and catalytic subunit (IlvB) (Cordes et al., 1992) of Corynebacterium glutamicum acetolactate synthase; (ii) an E. coli acetohydroxyacid reductoisomerase (EC 1.1.1.86) engineered for use of NADH as redox cofactor (IlvC6E6) (Bastian et al., 2011, Holmberg and Petersen, 1988); (iii) a dihydroxyacid dehydratase (EC 4.2.1.9) from L. lactis (IlvD), previously shown to be active in the S. cerevisiae cytosol (Urano et al., 2012); (iv) a 2-oxo acid decarboxylase from L. lactis (KdcA) with a high specificity and activity towards α-ketoisovalerate upon expression in S. cerevisiae (Milne et al., 2015); and (v) endogenous S. cerevisiae NADH-dependent alcohol dehydrogenase(s) Adh2 with affinity towards isobutyraldehyde (Brat et al., 2012). Provided that a sufficiently high flux through this cytosolic, redox-cofactor-balanced and ATP-yielding pathway (Fig. 1) can be achieved in vivo, it should allow for formation of isobutanol as sole catabolic product in anaerobic cultures.
3.2. In vivo activity of a heterologous branched-chain amino-acid pathway in S. cerevisiae
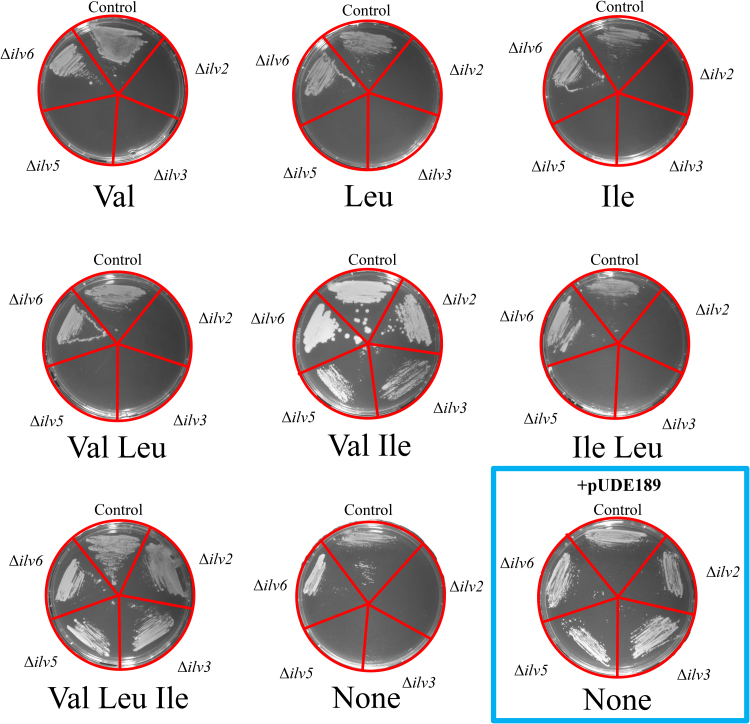
In vivo activity of the heterologous enzymes involved in the conversion of pyruvate to α-ketoisovalerate (KIV) via the pathway design described above was tested by complementation of S. cerevisiae mutants lacking key enzymes in the native branched-chain amino-acid biosynthesis pathway (Fig. 1). Consistent with earlier studies (Kingsbury and McCusker, 2010, Velasco et al., 1993, Zelenayatroitskaya et al., 1995) strains containing deletions of individual ‘catalytic’ genes IMK463 (ilv2Δ), IMK464 (ilv3Δ), IMK465 (ilv5Δ) did not grow on media lacking both valine and isoleucine (Fig. 2). In these strains, the presence of valine is sufficient to restore leucine synthesis since KIV formed by transamination of valine can feed leucine biosynthesis (Fig. 1). Deletion of ILV6 (strain IMK466) did not lead to auxotrophy (Fig. 2) due to its non-essential role as regulatory subunit of acetolactate synthase (Cullin et al., 1996, Pang and Duggleby, 1999). These single deletion mutants were transformed with plasmid pUDE189, carrying the heterologous coilvB, coilvNM13 (C. glutamicum), coilvC6E6 (E. coli), and coilvD (L. lactis) genes under the control of strong constitutive promoters. The resulting strains IMZ346 (ilv2Δ pUDE189), IMZ347 (ilv3Δ pUDE189), IMZ348 (ilv5Δ pUDE189), and IMZ349 (ilv6Δ pUDE189) readily grew on synthetic medium without branched-chain amino acid supplementation (Fig. 2), thereby demonstrating functional replacement of the native, mitochondrial yeast enzymes by their cytosolically expressed heterologous orthologs.
Fig. 2.
Complementation of S. cerevisiae deletion mutants affected in branched chain-amino acid biosynthesis with a heterologous pathway. Strains CEN.PK113-3B (control), IMK463 (Δilv2), IMK464 (Δilv3), IMK465 (Δilv5), IMK466 (Δilv6) were grown in SMG medium supplemented with histidine (0.150 g/L) and uracil (0.125 g/L). The corresponding strains complemented with the heterologous branched chain amino acid biosynthesis pathway IME169 (control+pUDE189) IMZ346 (Δilv2+pUDE189), IMZ347 (Δilv3+pUDE189), IMZ348 (Δilv5+pUDE189) and IMZ349 (Δilv6) were grown in SMG medium supplemented with histidine. Cells were then washed with water and streaked onto SMG agar plates supplemented with histidine and uracil (if required) and 5 g/L of valine (Val), leucine (Leu) and/or isoleucine (Ile) as indicated. Plates were incubated at 30 °C for 3 days.
To further investigate in vivo activity of the engineered pathway, coilvB, coilvNM13, coilvC6E6 and coilvD gene cassettes were integrated at the ILV2 locus of strain IMI302 (Fig. 3), which carries a triple PDC deletion, combined with an MTH1 internal deletion, to eliminate unwanted ethanol formation and allow growth on glucose (Oud et al., 2012). The resulting strain IMX708 (Δpdc1,5,6 Δilv2 MTH1ΔT coilvNM13, coilvB, coilvC6E6, coilvD, ura3-52) was subsequently transformed with the p426GPD plasmid to obtain the uracil prototrophic strain IME305 (Δpdc156 Δilv2 MTH1ΔT coilvNM13coilvB coilvC6E6 coilvD p426GPD).
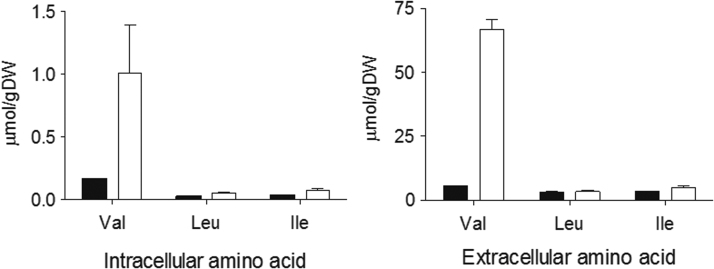
To investigate whether expression of the cytosolic pathway led to branched-chain amino acid accumulation, strain IME305 and the Pdc− reference strain IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD) were grown in shake flask cultures on SMG medium, followed by analysis of intracellular and extracellular amino acid concentrations. In the reference strain IMZ500, concentrations of valine, leucine and isoleucine for IMZ500 were lower than intracellular branched chain amino acid concentrations measured precedently in a Pdc+ S. cerevisiae strain (Luttik et al. 2008) (Fig. 4). Significantly higher intra- and extracellular concentrations of valine, leucine and isoleucine were observed in cultures of strain IME305. In particular, intra- and extracellular valine concentrations were 6-fold and 12-fold higher, respectively, than in cultures of the reference strain IMZ500. These observations further confirmed the functionality of the engineered cytosolic pathway and, in particular, the successful bypassing of regulatory mechanisms that prevent valine accumulation in wild-type S. cerevisiae (Elisakova et al., 2005, Ljungdahl and Daignan-Fornier, 2012).
Fig. 4.
Intracellular and extracellular branched-chain amino acid pools in S. cerevisiae IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD) (black bars) and IME305 (Δpdc1,5,6 Δilv2 MTH1ΔTcoilvBCDN p426GPD) (white bars) expressed in µmol/gDW. Both strains were grown in SMG medium and samples taken over the course of exponential phase for analysis. Data are presented as averages and standard deviations of duplicate experiments.
3.3. Physiological characterization of an engineered isobutanol pathway in S. cerevisiae
To complete the catabolic isobutanol pathway, a codon-optimized version of the L. lactis 2-oxo acid decarboxylase gene kdcA (Smit et al., 2005), which yields an active KIV decarboxylase upon expression in S. cerevisiae (Milne et al., 2015), was expressed from the episomal plasmid pUDE321 in IMX708. The resulting strain IME307 (Δpdc1,5,6 Δilv2 MTH1ΔT coilvNM13 coilvB coilvC6E6 coilvD cokdcA) was then compared with strain IME305 and the Pdc- reference strain IMZ500 in aerobic shake flask cultures on SMG medium.
Introduction of the heterologous pathway, either with or without kdcA, resulted in a 2.7 fold decrease of the specific growth rate relative to that of strain IMZ500 (Table 4). Presence of the heterologous pathway resulted in the formation of low quantities of isobutyrate as pictured by the calculated yield (Table 4). Although this result was far from the theoretical yield, it was however in full agreement with yields obtained in previous isobutanol engineering attempts in S. cerevisiae (Chen et al., 2011, Ida et al., 2015, Kondo et al., 2012, Matsuda et al., 2013, Park et al., 2014). Under aerobic conditions, S. cerevisiae preferably oxidizes isobutyraldehyde to isobutyrate (Hazelwood et al., 2008), which therefore can be taken as a proxy for isobutanol in these experiments. Consistent with an earlier report (van Maris et al., 2004a) the Pdc− strain IMZ500 converted a large fraction of the consumed glucose to pyruvate (0.289±0.071 mol/mol glucose). Conversely, only trace amounts of pyruvate were detected extracellularly in cultures of strains IME305 and IME307 (<0.02 mol/mol glucose). Instead, these strains, which express the heterologous valine pathway, produced substantial concentrations of metabolites derived from the branched-chain amino acid pathway. In particular, they produced high extracellular concentrations of the pathway intermediate dihydroxyisovalerate (DHIV) and, in strain IME305, of KIV (Table 4). Additionally, diacetyl (derived from spontaneous oxidative decarboxylation of acetolactate (Suomalainen and Ronkainen, 1968)) and acetoin (produced from diacetyl by a diacetyl reductase (Ehsani et al., 2009)) were detected extracellularly (Table 4).
Table 4.
Maximum specific growth rates, final optical density at 660 nm (OD660) and metabolite yields of aerobic shake flask cultures of S. cerevisiae strains IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD), IME305 (Δpdc1,5,6 Δilv2 MTH1ΔTcoilvBCDN p426GPD) and IME307 (Δpdc1,5,6 Δilv2MTH1ΔT coilvBCDNcokdcA). Cells were grown in SMG medium and samples taken for analysis over the course of the exponential phase. Data are presented as averages and mean deviation of duplicate experiments. *Sum total of extracellular diacetyl and acetolactate, BD: Below detection limit of analytical methods, DHIV: 2,3-dihydroxy-isovalerate, KIV: α-keto-isovalerate.
| IMZ500 | IME305 | IME307 | |
|---|---|---|---|
| (pdc minus control) | (p426GPD) | (kdcA) | |
| µmax (h−1) | 0.094±0.015 | 0.035±0.001 | 0.034±0.00 |
| Final OD 660 | 4.07±0.31 | 4.84±0.27 | 2.78±0.11 |
| Pyruvate (mol/mol glucose) | 0.29±0.071 | 0.017±0.000 | 0.005±0.002 |
| Diacetyl* (mol/mol glucose) | 7.1.10−5±1.5.10−5 | 0.055±0.007 | 0.031±0.012 |
| Acetoin (mol/mol glucose) | BD | 0.18±0,00 | 0.09±0.02 |
| DHIV (mol/mol glucose) | BD | 0.31±0.00 | 0.19±0.07 |
| KIV (mol/mol glucose) | BD | 0.15±0.01 | BD |
| Isobutyrate (mol/mol glucose) | BD | 0.02±0.00 | 0.05±0.00 |
| Isobutanol (mol/mol glucose) | BD | BD | BD |
Quantitative comparison of the strains in shake flasks was complicated by accumulation of organic acids (e.g. pyruvate, DHIV, KIV and isobutyrate), which led to acidification and cessation of growth before glucose was fully consumed. Therefore, aerobic, pH-controlled bioreactor cultures were performed with strains IME307 (Δpdc156 Δilv2 MTH1ΔT coilvNM13 coilvB coilvC6E6 coilvD cokdcA) and IMZ500 (Δpdc156 MTH1ΔT p426GPD) to quantify metabolic fluxes through the cytosolic isobutanol pathway and towards the observed by-products. In the bioreactor cultures, glucose was completely consumed by both strains. As well as a decreased growth rate, IME307 displayed a substantially lower biomass yield (0.136±0.021 g/g glucose) than IMZ500 (0.422±0.012 g/g glucose), and a concomitant decrease in qCO2 (0.089±0.019 g/g biomass/h for IME307 versus 0.165±0.012 g/g biomass/h for IMZ500) (Table 5). In general, metabolite profiles of the two strains in bioreactors strongly resembled those observed in shake flasks, with the exception of the production of some α-ketoisovalerate and a higher acetoin yield in strain IME307.
Table 5.
Physiology and metabolite production of S. cerevisiae strains IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD) and IME307 (Δpdc1,5,6 Δilv2 MTH1ΔTcoilvBCDNcokdcA) in aerobic batch cultures on SMG medium maintained at pH 5.0. Data are presented as average and mean deviation of duplicate experiments. *Sum total of extracellular diacetyl and acetolactate, BD: Below detection limit of analytical methods, DHIV: 2,3-dihydroxy-isovalerate, KIV: α-keto-isovalerate.
| IMZ500 | IME307 | |
|---|---|---|
| Growth rate (h−1) | 0.115±0.010 | 0.020±0.001 |
| YX/S (g/g glucose) | 0.422±0.012 | 0.136±0.021 |
| qGlucose (g/g biomass/h) | −0.273±0.030 | −0.152±0.031 |
| qCO2 (g/g biomass/h) | 0.165±0.012 | 0.089±0.019 |
| Pyruvate yield (mol/mol glucose) | 0.330±0.001 | 0.001±0.000 |
| Ethanol yield (mol/mol glucose) | BD | BD |
| Diacetyl yield* (mol/mol glucose) | 0.001±0.000 | 0.040±0.002 |
| Acetoin yield (mol/mol glucose) | BD | 0.053±0.002 |
| DHIV yield (mol/mol glucose) | BD | 0.201±0.010 |
| KIV yield (mol/mol glucose) | BD | 0.033±0.001 |
| Isobutyrate yield (mol/mol glucose) | BD | 0.021±0.004 |
| Isobutanol yield (mol/mol glucose) | BD | BD |
| Carbon recovery (%) | 103.4±4.7 | 103.2±2.8 |
3.4. Distribution of carbon flux in micro-aerobic cultures
Oxygen availability not only affects the conversion of isobutyraldehyde to either isobutanol or isobutyrate (Hazelwood et al., 2008), but also influences ATP generation and NADH oxidation via respiration. Although the isobutanol pathway used in this study was designed to function as a catabolic pathway, strain IME307, which expresses the complete pathway, did not show growth on glucose in anaerobic cultures. Growth remained absent when cultures were incubated for several weeks in an attempt to select for spontaneous mutants in which the capacity and/or other characteristics of the engineered pathway had improved sufficiently to sustain anaerobic growth. Therefore, further analysis of the pathway was performed in micro-aerobic high-cell-density cultures, using biomass from aerobic, pH-controlled bioreactor cultures. The absence of growth in these micro-aerobic cultures facilitated a stoichiometric analysis of flux distribution. Micro-aerobically, isobutanol production was observed in IME307, but at a very low yield (0.018±0.003 mol/mol glucose) (Table 6). However, isobutyrate was still produced at higher yields (0.065±0.005 mol/mol glucose). Acetoin was not detected in the micro-aerobic cultures. Instead, 2,3-butanediol, the product of acetoin reduction, was produced at very high yields (0.65±0.07 mol/mol glucose), indicating that the micro-aerobic conditions favoured reduction of acetoin to 2,3-butanediol. Glycerol production was observed in both strains. In strain IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD), glycerol production can be attributed to the need to re-oxidize the NADH formed as a result of pyruvate accumulation. In strain IME307 (Δpdc1,5,6 Δilv2 MTH1ΔT coilvNM13 coilvB coilvC6E6 coilvD cokdcA), which produced much lower concentrations of pyruvate, the NADH required for glycerol production was likely derived from the formation of oxidised products DHIV, isobutyrate and CO2. In strain IME307, a low but significant production of ethanol was observed, consistent with the low affinity of KdcA towards pyruvate (Milne et al., 2015).
Table 6.
Metabolite production from glucose bio-conversion in micro-aerobic cultures of IMZ500 (Δpdc1,5,6 MTH1ΔT p426GPD) and IME307 (Δpdc1,5,6 Δilv2 MTH1ΔTcoilvBCDNcokdcA). Cells were first grown in SMG medium in aerobic, pH-controlled bioreactors, then washed with water and resuspended to a final cell density of ~12 g/L in SMG medium supplemented with Tween-80 (420 mg/L) and ergosterol (10 mg/L) with the initial pH set to 6.0. Cells were then incubated micro-aerobically at 30 °C and metabolite concentrations were measured during linear glucose consumption. Data are presented as average and mean deviation of duplicate experiments. *Sum total of extracellular diacetyl and acetolactate, BD: Below detection limit of analytical methods, NA: Not applicable, ND: Not determined, DHIV: 2,3-dihydroxy-isovalerate, KIV: α-keto-isovalerate.
| IMZ500 |
IME307 |
|||
|---|---|---|---|---|
| Rate | Yield | Rate | Yield | |
| (µmol/g biomass/h) | (mol/mol glucose) | (µmol/g biomass/h) | (mol/mol glucose) | |
| Glucose | −155.74±11.00 | NA | −25.95±4.57 | NA |
| Pyruvate | 116.66±0.94 | 0.770±0.101 | 1.08±0.28 | 0.041±0.013 |
| Ethanol | BD | BD | 3.37±0.75 | 0.131±0.039 |
| Glycerol | 123.42±25.36 | 0.76±0.00 | 11.73±3.41 | 0.51±0.00 |
| Acetoin | BD | BD | BD | BD |
| 2,3-butanediol | BD | BD | 13.66±1.70 | 0.649±0.067 |
| DHIV | BD | BD | 1.71±0.30 | 0.070±0.000 |
| KIV | BD | BD | BD | ND |
| Isobutyrate | BD | BD | 1.40±0.12 | 0.065±0.005 |
| Isobutanol | BD | BD | 0.41±0.09 | 0.018±0.003 |
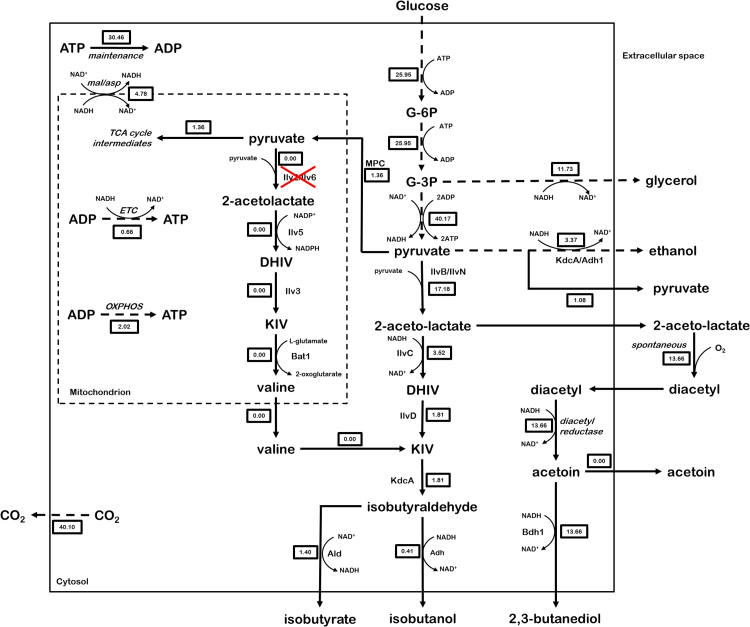
A stoichiometric metabolic model was set up to visualize the distribution of glucose carbon over the intracellular pathways leading to the surprisingly large set of (by-)products observed in these experiments. Using the biomass-specific rates of extracellular product formation as input (Table 6), the model allowed for the construction of detailed intracellular flux maps (Fig. 5). These flux maps indicated a low activity of the TCA cycle, respiration and oxidative phosphorylation in the micro-aerobic cultures. Approximately 45% of the total carbon flux was diverted to glycerol in order to maintain a redox-cofactor balance. At the acetolactate branch point, a significant fraction (80%) of the remaining carbon flux was diverted away from the isobutanol pathway and into the 2,3-butanediol pathway. The model enabled an estimation of the specific rate of ATP synthesis from glycolysis and oxidative phosphorylation of 0.03 mMol/g biomass/h. With an estimated ATP requirement for cellular maintenance of anaerobic S. cerevisiae cultures of ca. 1 mMol/g biomass/h (Boender et al., 2009), the in vivo rate of ATP production from the engineered isobutanol pathway was clearly too low to sustain anaerobic growth on glucose.
Fig. 5.
Flux distribution maps for S. cerevisiae IME307 (Δpdc1,5,6 Δilv2 MTH1ΔTcoilvBCDNcokdcA) grown in micro-aerobic cultures (see Table 6), calculated using CellNetAnalyzer. Dashed arrows represent multiple enzyme-catalysed reactions. Numbered boxes represent the modelled metabolic flux through each reaction (expressed in µmol/g biomass/h). Mal/asp: malate/aspartate shuttle, ETC: Electron transport chain, OXPHOS: Oxidative phosphorylation, G-6P: glucose-6-phosphate, G-3P: glyceraldehyde-3-phosphate, DHIV: 2,3-dihydroxyisovalerate, KIV: α-ketoisovalerate.
4. Discussion
Expression in S. cerevisiae of a set of heterologous enzymes that, theoretically, should be able to form a catabolic isobutanol pathway, resulted in low isobutanol yields (0.018±0.003 mol/mol glucose). The specific rate of isobutanol production by the engineered strain was too low to meet the cellular maintenance energy requirement and, consequently, did not support anaerobic growth. These results were similar to those obtained in previous academic studies on metabolic engineering of S. cerevisiae. A systematic mass balancing approach revealed massive accumulation of pathway intermediates and related metabolites.
The observation that, in micro-aerobic cultures, the yield of isobutyrate (0.065±0.005 mol/mol glucose) exceeded that of isobutanol is consistent with a previously reported limitation at the isobutyraldehyde branch-point (Park et al., 2014). Production of diacetyl, acetoin and 2,3-butanediol, the latter reaching a considerably high yield of 0.649±0.067 mol/mol glucose in aerobic cultures, identified a previously unreported ‘overflow’ at the level of acetolactate. This result indicates that the feedback-insensitive bacterial acetolactate synthase (coilvNM13, coilvB) was fully functional in the engineered strain, but that a significant limitation occurred downstream of acetolactate. Analysis of metabolic fluxes in micro-aerobic cultures indicated that production of KIV was significantly slower than that of DHIV. In the engineered strain, conversion of DHIV to KIV was catalysed by the dihydroxyacid dehydratase IlvD. Prokaryotic and eukaryotic dihydroxyacid dehydratases contain iron–sulphur (4Fe–4S) clusters and require iron–sulphur cluster biogenesis and assembly mechanisms for in vivo activity (Flint et al., 1993, Lill, 2009, Muhlenhoff et al., 2011, Rouault and Tong, 2005). In S. cerevisiae, iron–sulphur cluster biogenesis and assembly into mature proteins occurs predominantly in the mitochondrial matrix (Schilke et al., 1999), the location of the native yeast dihydroxyacid dehydratase Ilv3. Iron–sulphur cluster assembly can also occur in the yeast cytosol (Carlsen et al., 2013, Kozak et al., 2014a, Waks and Silver, 2009), but has a much lower capacity than the mitochondrial system (Brat et al., 2012, Sharma et al., 2010). Limitation of the in vivo activity of IlvD by biogenesis and assembly of its 4Fe-4S cluster is entirely consistent with low rate of KIV production observed in strain IME307. Moreover, it has also proven to be difficult to express other pathways that rely on cytosolic iron–sulphur cluster assembly in the yeast cytosol (Benisch and Boles, 2014, Carlsen et al., 2013).
However, we cannot exclude as well other secondary bottlenecks in the pathway that might require special attention in the future. Under the fermentation conditions used in the study, (oxygen limited to microaerobic), the main alcohol dehydrogenase expressed should be ADH1 (Knijnenburg et al., 2009). However, Adh1 has a non-optimal conversion kinetics of isobutyraldehyde. Overexpresion of the native Adh2 alcohol dehydrogenase which exhibits a five-fold higher rate than Adh1 for isobutyraldehyde has been shown to have a positive impact on isobutanol formation (Brat et al., 2012). Although significant the isobutanol improvement resulting from the overexpression of Adh2, or other alcohol dehydrogenases e.g. Adh6, Adh7, AdhA) (Brat et al., 2012, Kondo et al., 2012, Avalos et al., 2013, Matsuda et al., 2013) remained limited suggesting that this step while contributing to the overall flux did not represent the main controlling step of the pathway.
The extracellular accumulation of acetolactate, DHIV and KIV indicate the presence of export mechanisms for these pathway intermediates in the yeast plasma membrane. Consistent with the multi-genic nature of the transport of other carboxylic acids in S. cerevisiae (de Kok et al., 2012), screening of single deletion mutants failed to identify a unique acetolactate transporter (Dundon et al., 2011b). Even in the absence of kinetic limitations in the isobutanol pathway, export of its intermediates might interfere with efficient performance in S. cerevisiae. In S. cerevisiae, export of carboxylic acids remains a poorly understood subject, as exemplified by the fact that even export of simple organic acids such as acetic acid and lactic acid remain incompletely understood (Casal et al., 2008, Paiva et al., 2004, van Maris et al., 2004b). Identification and inactivation of transporters for pathway intermediates may therefore be relevant for further development of isobutanol-producing yeast strains.
A series of patent applications related to cytosolic iron–sulphur cluster availability (Dundon et al., 2011a), reducing extracellular accumulation of metabolites (Buelter et al., 2012, Dundon et al., 2011b), and improving the enzyme kinetics of the isobutanol pathway (Li et al., 2010, Liao et al., 2013, Porter-Scheinman et al., 2014) indicates that industrial researchers have, in all likelihood, already made substantial progress in addressing several of the issues indicated above. This notwithstanding, the present study helps to interpret the outcome of earlier academic studies and underlines the importance of a systematic, mass-balancing based approach in metabolic engineering studies.
Expression of coilvB, coilvNM13 (C. glutamicum), coilvC6E6 (E. coli), and coilvD (L. lactis) in S. cerevisiae strains harbouring individual deletions in the native valine biosynthesis pathway restored branched-chain amino acid prototrophy. Although originally designed to merely test the in vivo functionality of the heterologous genes used to assemble the isobutanol pathway, these experiments yielded new insights into branched-chain amino acid metabolism in S. cerevisiae. Firstly, cytosolic expression of the complete pathway led to a significant increase of intra- and extracellular valine concentrations. To our knowledge, this is the first demonstration that valine production in S. cerevisiae can be increased by bypassing the regulatory mechanisms of its native biosynthesis pathway. Secondly, the complementation of branched-chain amino acid auxotrophs indicates that either (i) the native gene deletion is complemented by its heterologous counter-part, implying that intermediates of the branched-chain amino acid biosynthesis pathway(s) can cross the mitochondrial membrane, and/or (ii) the complete cytosolic pathway is active and able to cytosolically produce valine, leucine and isoleucine. The engineered strains described in this study offer a unique experimental platform for introduction of additional mutations to explore trafficking of precursors, intermediates, and products of the branched-chain amino acid biosynthesis pathway between yeast cytosol and mitochondria.
Acknowledgement
This work was performed within the BE-Basic R&D Program (FS6.003) (http://www.be-basic.org/), which was granted an FES subsidy from the Dutch Ministry of Economic Affairs, Agriculture and Innovation (EL&I). The authors thank Vito Meulenberg, Edwin Janssens, Wandena Mahabier, Angela ten Pierick, Cor Ras, Anisha Goel, Marijke Luttik and Erik de Hulster for their assistance on this project.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.meteno.2016.01.002.
Appendix A. Supplementary material
Supplementary material
References
- Altiokka M.R., Citak A. Kinetics study of esterification of acetic acid with isobutanol in the presence of amberlite catalyst. Appl. Catal. , A. 2003;239:141–148. [Google Scholar]
- Avalos J.L., Fink G.R., Stephanopoulos G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nat. Biotechnol. 2013;31:335–341. doi: 10.1038/nbt.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian S., Liu X., Meyerowitz J.T., Snow C.D., Chen M.M.Y., Arnold F.H. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab. Eng. 2011;13:345–352. doi: 10.1016/j.ymben.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Benisch F., Boles E. The bacterial Entner-Doudoroff pathway does not replace glycolysis in Saccharomyces cerevisiae due to the lack of activity of iron–sulfur cluster enzyme 6-phosphogluconate dehydratase. J. Biotechnol. 2014;171:45–55. doi: 10.1016/j.jbiotec.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Boender L.G.M., de Hulster E.A.F., van Maris A.J.A., Daran-Lapujade P.A.S., Pronk J.T. Quantitative Physiology of Saccharomyces cerevisiae at Near-Zero Specific Growth Rates. Appl. Environ. Microbiol. 2009;75:5607–5614. doi: 10.1128/AEM.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat D., Boles E. Isobutanol production from D-xylose by recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13:241–244. doi: 10.1111/1567-1364.12028. [DOI] [PubMed] [Google Scholar]
- Brat D., Weber C., Lorenzen W., Bode H.B., Boles E. Cytosolic re-localization and optimization of valine synthesis and catabolism enables increased isobutanol production with the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels. 2012;5:65. doi: 10.1186/1754-6834-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelter, T., Hawkins, A., Porter-Scheinman, S. et al., 2012. Reduced by-product accumulation for improved production of isobutanol US8133715 B2.
- Carlsen S., Ajikumar P.K., Formenti L.R., Zhou K., Phon T.H., Nielsen M.L., Lantz A.E., Kielland-Brandt M.C., Stephanopoulos G. Heterologous expression and characterization of bacterial 2-C-methyl-D-erythritol-4-phosphate pathway in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013;97:5753–5769. doi: 10.1007/s00253-013-4877-y. [DOI] [PubMed] [Google Scholar]
- Casal M., Paiva S., Queiros O., Soares-Silva I. Transport of carboxylic acids in yeasts. FEMS Microb. Rev. 2008;32:974–994. doi: 10.1111/j.1574-6976.2008.00128.x. [DOI] [PubMed] [Google Scholar]
- Caspi R., Foerster H., Fulcher C.A. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2008;36:623–631. doi: 10.1093/nar/gkm900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Nielsen K.F., Borodina I., Kielland-Brandt M.C., Karhumaa K. Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol. Biofuels. 2011;4:21. doi: 10.1186/1754-6834-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes C., Mockel B., Eggeling L., Sahm H. Cloning, organization and functional analysis of ilvA, ilvB and ilvC genes from Corynebacterium glutamicum. Gene. 1992;112:113–116. doi: 10.1016/0378-1119(92)90311-c. [DOI] [PubMed] [Google Scholar]
- Cullin C., BaudinBaillieu A., Guillemet E., OzierKalogeropoulos O. Functional analysis of YCL09C: evidence for a role as the regulatory subunit of acetolactate synthase. Yeast. 1996;12:1511–1518. doi: 10.1002/(sici)1097-0061(199612)12:15<1511::aid-yea41>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dauner M., Sauer U. GC–MS analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol. Prog. 2000;16:642–649. doi: 10.1021/bp000058h. [DOI] [PubMed] [Google Scholar]
- de Kok S., Nijkamp J.F., Oud B., Roque F.C., de Ridder D., Daran J.M., Pronk J.T., van Maris A.J.A. Laboratory evolution of new lactate transporter genes in a jen1Δ mutant of Saccharomyces cerevisiae and their identification as ADY2 alleles by whole-genome resequencing and transcriptome analysis. FEMS Yeast Res. 2012;12:359–374. doi: 10.1111/j.1567-1364.2012.00787.x. [DOI] [PubMed] [Google Scholar]
- Dickinson J.R., Harrison S.J., Hewlins M.J.E. An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:25751–25756. doi: 10.1074/jbc.273.40.25751. [DOI] [PubMed] [Google Scholar]
- Droste P., Noh K., Wiechert W. Omix - a visualization tool for metabolic networks with highest usability and customizability in focus. Chem. Ing. Tech. 2013;85:849–862. [Google Scholar]
- Dundon, C.A., Aristidou, A., Hawkins, A., Lies, D., 2011a. Albert L.H. Methods of increasing dihydroxy acid dehydratase activity to improve production of fuels, chemicals, and amino acids. US8017376 B2.
- Dundon, C.A., Smith, C., Nahreini, P., Thevelein, J. Saerens, S., 2011b. Isobutanol production using yeasts with modified transporter expression. WO2011153144 A1.
- Ehrlich F. Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Berichte der deutschen chemischen Gesellschaft. 1907;40:1027–1047. [Google Scholar]
- Ehsani M., Fernandez M.R., Biosca J.A., Julien A., Dequin S. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009;75:3196–3205. doi: 10.1128/AEM.02157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisakova V., Patek M., Holatko J., Nesvera J.N., Leyval D., Goergen J.L., Delaunay S. Feedback-resistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2005;71:207–213. doi: 10.1128/AEM.71.1.207-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K.D., Kötter P. Yeast genetic strain and plasmid collections. Methods Microbiol. 2007;36:629–666. [Google Scholar]
- Flint D.H., Smykrandall E., Tuminello J.F., Draczynskalusiak B., Brown O.R. The inactivation of dihydroxy-acid dehydratase in Escherichia coli treated with hyperbaric oxygen occurs because of the destruction of its Fe-S cluster, but the enzyme remains in the cell in a form that can be reactivated. J. Biol. Chem. 1993;268:25547–25552. [PubMed] [Google Scholar]
- Generoso W.C., Schadeweg V., Oreb M., Boles E. Metabolic engineering of Saccharomyces cerevisiae for production of butanol isomers. Curr. Opin. Biotechnol. 2015;33:1–7. doi: 10.1016/j.copbio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Goldstein A.L., McCusker J.H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Grote A., Hiller K., Scheer M., Munch R., Nortemann B., Hempel D.C., Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:526–531. doi: 10.1093/nar/gki376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood L.A., Daran J.M., van Maris A.J.A., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., Kunji E.R.S., Martinou J.C. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Holmberg S., Petersen J.G.L. Regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 1988;13:207–217. doi: 10.1007/BF00387766. [DOI] [PubMed] [Google Scholar]
- Ida K., Ishii J., Matsuda F., Kondo T., Kondo A. Eliminating the isoleucine biosynthetic pathway to reduce competitive carbon outflow during isobutanol production by Saccharomyces cerevisiae. Microb. Cell Fact. 2015;14:62. doi: 10.1186/s12934-015-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilika I.T.R.I. Gevo produces isobutanol, hydro-carbons, and jet fuel from cellulosic biomass. Fuel Cells Bull. 2010:11–12. [Google Scholar]
- Jones E.W., Fink G.R. Regulation of amino acid and nucleotide biosynthesis in yeast. Cold Spring Harbor Monogr. Arch. 1982;11:181–299. [Google Scholar]
- Kingsbury J.M., McCusker J.H. Cytocidal amino acid starvation of Saccharomyces cerevisiae and Candida albicans acetolactate synthase (ilv2 delta) mutants is influenced by the carbon source and rapamycin. Microbiology-SGM. 2010;156:929–939. doi: 10.1099/mic.0.034348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt S., Saez-Rodriguez J., Gilles E.D. Structural and functional analysis of cellular networks with CellNetAnalyzer. BMC Syst. Biol. 2007;1:2–8. doi: 10.1186/1752-0509-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijnenburg T.A., Daran J.M., van den Broek M.A., Daran-Lapujade P.A., de Winde J.H., Pronk J.T., Reinders M.J., Wessels L.F. Combinatorial effects of environmental parameters on transcriptional regulation in Saccharomyces cerevisiae: a quantitative analysis of a compendium of chemostat-based transcriptome data. BMC Genomics. 2009;10:53. doi: 10.1186/1471-2164-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej R., Scheib J. Bio-isobutanol: the next-generation biofuel. Hydrocarb. Process. 2012;91 79-79. [Google Scholar]
- Kondo T., Tezuka H., Ishii J., Matsuda F., Ogino C., Kondo A. Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae. J. Biotechnol. 2012;159:32–37. doi: 10.1016/j.jbiotec.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kozak B.U., van Rossum H.M., Benjamin K.R., Wu L., Daran J.M., Pronk J.T., van Maris A.J. Replacement of the Saccharomyces cerevisiae acetyl-CoA synthetases by alternative pathways for cytosolic acetyl-CoA synthesis. Metab. Eng. 2014;21:46–59. doi: 10.1016/j.ymben.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Kozak B.U., van Rossum H.M., Luttik M.A., Akeroyd M., Benjamin K.R., Wu L., de V.S., Daran J.M., Pronk J.T., van Maris A.J. Engineering acetyl coenzyme A supply: functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. mBio. 2014;5:8–14. doi: 10.1128/mBio.01696-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers N.G., Solis-Escalante D., Bosman L., van den Broek M., Pronk J.T., Daran J.M., Daran-Lapujade P. A versatile, efficient strategy for assembly of multi-fragment expression vectors in Saccharomyces cerevisiae using 60 bp synthetic recombination sequences. Microb. Cell Fact. 2013;12 doi: 10.1186/1475-2859-12-47. 47-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Nelson, M.J., Liao, D.I., O'keefe, D.P., 2010. Ketol-acid reductoisomerase using NADH EP2222841 A2.
- Liao, D.I., Nelson, M., Bramucci, M.G., 2013. Bramucci Fermentive production of isobutanol using highly active ketol-acid reductoisomerase enzymes. EP2543721 2013 A1.
- Lill R. Function and biogenesis of iron–sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- Ljungdahl P.O., Daignan-Fornier B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics. 2012;190:885–929. doi: 10.1534/genetics.111.133306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttik M.A., Vuralhan Z., Suir E., Braus G.H., Pronk J.T., Daran J.M. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact. Metab. Eng. 2008;10:141–153. doi: 10.1016/j.ymben.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Mans R., van Rossum H.M., Wijsman M., Backx A., Kuijpers N.G.A., van den Broek M., Daran-Lapujade P., Pronk J.T., van Maris A.J.A., Daran J.M.G. CRISPR/Cas9: a molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15:004. doi: 10.1093/femsyr/fov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Ishii J., Kondo T., Ida K., Tezuka H., Kondo A. Increased isobutanol production in Saccharomyces cerevisiae by eliminating competing pathways and resolving cofactor imbalance. Microb. Cell Fact. 2013;12:119. doi: 10.1186/1475-2859-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne N., van Maris A.J.A., Pronk J.T., Daran J.M. Comparative assessment of native and heterologous 2-oxo acid decarboxylases for application in isobutanol production by Saccharomyces cerevisiae. Biotechnol. Biofuels. 2015;8:204. doi: 10.1186/s13068-015-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff U., Richter N., Pines O., Pierik A.J., Lill R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe–4S] proteins. J. Biol. Chem. 2011;286:41205–41216. doi: 10.1074/jbc.M111.296152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Nijkamp J.F., van den Broek M.A., Datema E. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb. Cell. Fact. 2012;11:36. doi: 10.1186/1475-2859-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oud B., Flores C.L., Gancedo C., Zhang X.Y., Trueheart J., Daran J.M., Pronk J.T., van Maris A.J.A. An internal deletion in MTH1 enables growth on glucose of pyruvate-decarboxylase negative, non-fermentative Saccharomyces cerevisiae. Microb. Cell Fact. 2012;11:131. doi: 10.1186/1475-2859-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva S., Devaux F., Barbosa S., Jacq C., Casal M. Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast. 2004;21:201–210. doi: 10.1002/yea.1056. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Agrimi G., Blanco E. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim. Biophys. Acta. 2006;1757:1249–1262. doi: 10.1016/j.bbabio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Pang S.S., Duggleby R.G. Expression, purification, characterization, and reconstitution of the large-and small subunits of yeast acetohydroxyacid synthase. Biochemistry. 1999;38:5222–5231. doi: 10.1021/bi983013m. [DOI] [PubMed] [Google Scholar]
- Park S.H., Kim S., Hahn J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of isobutanol and 3-methyl-1-butanol. Appl. Microbiol. Biotechnol. 2014;98:9139–9147. doi: 10.1007/s00253-014-6081-0. [DOI] [PubMed] [Google Scholar]
- Peters, M.W., Taylor, J.D., Jenni, M., Manzer, L.E. Henton, D.E., 2010. Integrated process to selectively convert renewable isobutanol to p-xylene US20110087000 A1.
- Porter-Scheinman, S., Urano, J. Meinhold, P., 2014. Acetolactate synthases for improved metabolite production. WO2014039060 A1.
- Pronk J.T. Auxotrophic yeast strains in fundamental and applied research. Appl. Environ. Microbiol. 2002;68:2095–2100. doi: 10.1128/AEM.68.5.2095-2100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T.A., Tong W.H. Iron–sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- Ryan E.D., Kohlhaw G.B. Subcellular-localization of isoleucine-valine biosynthetic enzymes in yeast. J. Bacteriol. 1974;120:631–637. doi: 10.1128/jb.120.2.631-637.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C., 2015. Success and challenges in the commercial production of isobutanol and its hydrocarbon derivatives. Copenhagen Bioscience Conference: cell factories and biosustainability 7, pp. 41–42.
- Schilke B., Voisine C., Beinert H., Craig E. Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:10206–10211. doi: 10.1073/pnas.96.18.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.K., Pallesen L.J., Spang R.J., Walden W.E. Cytosolic iron–sulfur cluster assembly (CIA) system: Factors, mechanism, and relevance to cellular iron regulation. J. Biol. Chem. 2010;285:26745–26751. doi: 10.1074/jbc.R110.122218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit B.A., Vlieg J.E.T.V., Engels W.J.M., Meijer L., Wouters J.T.M., Smit G. Identification, cloning, and characterization of a Lactococcus lactis branched-chain alpha-keto acid decarboxylase involved in flavor formation. Appl. Environ. Microbiol. 2005;71:303–311. doi: 10.1128/AEM.71.1.303-311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen H., Ronkainen P. Mechanism of diacetyl formation in yeast fermentation. Nature. 1968;220:792–793. doi: 10.1038/220792a0. [DOI] [PubMed] [Google Scholar]
- Urano, J., Dundon, C.A., Meinhold, P. et al., 2012. Cytosolic isobutanol pathway localization for the production of isobutanol EP2464736 A1.
- van Maris A.J.A., Geertman J.M.A., Vermeulen A., Groothuizen M.K., Winkler A.A., Piper M.D.W., van Dijken J.P., Pronk J.T. Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C-2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast. Appl. Environ. Microbiol. 2004;70:159–166. doi: 10.1128/AEM.70.1.159-166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maris A.J.A., Winkler A.A., Porro D., van Dijken J.P., Pronk J.T. Homofermentative lactate production cannot sustain anaerobic growth of engineered Saccharomyces cerevisiae: Possible consequence of energy-dependent lactate export. Appl. Environ. Microbiol. 2004;70:2898–2905. doi: 10.1128/AEM.70.5.2898-2905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco J.A., Cansado J., Pena M.C., Kawakami T., Laborda J., Notario V. Cloning of the dihydroxyacid dehydratase encoding gene (ILV3) from Saccharomyces cerevisiae. Gene. 1993;137:179–185. doi: 10.1016/0378-1119(93)90004-m. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Postma E., Scheffers W.A., van Dijken J.P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Postma E., Scheffers W.A., Vandijken J.P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 1990;136:395–403. doi: 10.1099/00221287-136-3-395. [DOI] [PubMed] [Google Scholar]
- Vuralhan Z., Luttik M.A.H., Tai S.L. Physiological characterization of the ARO10-dependent, broad-substrate-specificity 2-oxo acid decarboxylase activity of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005;71:3276–3284. doi: 10.1128/AEM.71.6.3276-3284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks Z., Silver P.A. Engineering a synthetic dual-organism system for hydrogen production. Appl. Environ. Microbiol. 2009;75:1867–1875. doi: 10.1128/AEM.02009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Farwick A., Benisch F., Brat D., Dietz H., Subtil T., Boles E. Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl. Microbiol. Biotechnol. 2010;87:1303–1315. doi: 10.1007/s00253-010-2707-z. [DOI] [PubMed] [Google Scholar]
- Wettling, T., Hirsch, S., Brym, M., Weis, M., 2013. Process for preparing higher molecular weight polyisobutylene US20130217847 A1.
- Zelenayatroitskaya O., Perlman P.S., Butow R.A. An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial-DNA stability. EMBO J. 1995;14:3268–3276. doi: 10.1002/j.1460-2075.1995.tb07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material