Abstract
Introduction
Frailty and muscle wasting, a component of frailty, are common in advanced stage chronic kidney disease (CKD). Whether frailty is associated with low urinary creatinine excretion (UCrE) as a measure of muscle mass in this population is unknown. Furthermore, reference values of UCrE are lacking. We first defined low UCrE and studied correlates of low UCrE, and subsequently studied cross-sectional associations of frailty with low UCrE in patients with advanced CKD.
Methods
A total of 2748 healthy individuals of the general population-based PREVEND study were included to define low UCrE (UCrE indexed for height, below the age- and sex-specific 5th percentile of the distribution). Frailty was defined using a modification of the Fried frailty phenotype. In a CKD population that included 320 and 967 participants of the PREPARE-2 and NECOSAD studies, respectively, cross-sectional associations of self-reported frailty, the individual components that define self-reported frailty, and frailty-associated variables with low UCrE were evaluated using multivariate logistic and linear regression models.
Results
Low UCrE was found in 38% of the CKD patients. A lower glomerular filtration rate was strongly associated with low UCrE. Self-reported frailty (adjusted odds ratio: 2.19; 95% confidence interval: 1.28−3.77) and the individual components were associated with low UCrE, independent of comorbidities. The frailty-associated variables hemoglobin and albumin were inversely associated with low UCrE, and parathyroid hormone was positively associated with low UCrE.
Discussion
Lower kidney function is a strong correlate of low UCrE and self-reported frailty, and the individual frailty components are associated with low UCrE as well, independent of comorbidities.
Keywords: chronic kidney disease, creatinine, frailty, muscle
Urinary creatinine excretion (UCrE), measured by 24-hour urine sampling, is an established marker of muscle mass in individuals at steady state.1, 2, 3, 4, 5, 6, 7 Low UCrE has been recognized as a predictor of mortality and adverse health outcomes in patients with stage 3 to 5 chronic kidney disease (CKD) and various other populations.7, 8, 9, 10, 11, 12, 13 However, the link between low muscle mass and adverse health outcomes remains unclear. Explanations include that low UCrE reflects worse muscle health, or is related to chronic low-grade inflammation, insulin resistance, or protein caloric malnutrition. We hypothesize that frailty is related to low UCrE because low muscle mass is an important component of frailty, and especially of the physical frailty definition.14 Frailty is common in patients with advanced CKD and has been associated with earlier need for dialysis initiation, lower quality of life, and increased mortality risk.15, 16, 17, 18, 19, 20 CKD has been hypothesized as an accelerator of decline of physical function that leads to frailty.21, 22, 23 However, whether frailty is associated with a low UCrE in advanced CKD has not yet been studied. Furthermore, reference values of UCrE are lacking. Therefore, we first aimed to determine low UCrE by using the UCrE distribution of a healthy population and subsequently evaluate correlates of low UCrE. Second, we aimed to evaluate associations of self-reported frailty, the individual components that define self-reported frailty, and frailty-associated variables with low UCrE in a cohort of patients with advanced CKD.
Materials and Methods
Study Design and Population
To define low UCrE values, we included as a healthy population a subsample representative of the general population (n = 3432) of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study. This prospective, population-based cohort study investigated the natural course of urinary albumin excretion and its relation to renal and cardiovascular disease. Detailed information on the design of the PREVEND study has been published previously.24 In summary, all inhabitants of the city of Groningen aged 28 to 75 years were sent a questionnaire and a vial to collect a first-morning urine sample. We excluded pregnant women and subjects with diabetes mellitus type 1 from the 40,856 respondents, and 2 cohorts were formed based on urinary albumin concentration. From these 2 cohorts, a subsample of 3432 subjects was derived that was representative of the general population. From this subsample, we excluded participants with no UCrE available, no serum creatinine and/or height available, those with comorbid conditions, or those aged younger than 25 years, which left 2892 participants for the present study. Participants with missing UCrE values did not differ significantly from participants for whom UCrE values were available. The PREVEND study was approved by the institutional review board, and all participants gave written informed consent.
The CKD population included participants of the multicenter observational PREdialysis PAtients REcords-study (PREPARE-2) and NEtherlands COoperative Study on the Adequacy of Dialysis (NECOSAD) studies. PREPARE-2 included 502 patients with stage 4 CKD aged 18 years or older who were treated by a nephrologist and had recently been referred to a specialized predialysis outpatient clinic. All patients had to be suitable for renal replacement therapy. Patients with chronic transplantation dysfunction were excluded from the study if the transplant was within the previous year. NECOSAD included 2051 patients with stage 5 CKD who were starting dialysis. To be eligible for inclusion in NECOSAD, adult patients (age 18 years or older) had to start with dialysis as their first renal replacement therapy. No other inclusion or exclusion criteria were applied. The institutional review boards of all participating hospitals approved the studies. All patients gave written informed consent. Detailed information on the design of the PREPARE-2 and NECOSAD studies has been published previously.25, 26
For this present study, we included 340 and 1055 participants of PREPARE-2 and NECOSAD respectively, aged between 25 years or older and 85 years and younger, for whom height and UCrE were available, and UCrE was collected before dialysis initiation (NECOSAD). In PREPARE-2, patients with missing UCrE values had a higher estimated glomerular filtration rate (eGFR), according to the Modification of Diet in Renal Disease Study equation, compared with patients for whom UCrE values were available (16.2 ml/min/1.73 m2 vs. 13.9 ml/min/1.73 m2, respectively; P = 0.03), a lower median albumin (39 g/L vs. 42 g/L, respectively; P < 0.001), and were less likely to have low physical performance (53% vs. 65%; P = 0.03). In NECOSAD, patients with missing UCrE values were slightly older (median age 64.4 years vs. 61.9 years; P = 0.004), had lower hemoglobin values (median 6.3 mmol/L vs. 6.4 mmol/L; P = 0.02), higher eGFR Modification of Diet in Renal Disease Study values (median 7.0 ml/min/1.73 m2 vs. 6.7 ml/min/1.73 m2; P = 0.02), and lower albumin values (median 35 g/L vs. 37 g/L; P < 0.001).
Frailty
Self-reported frailty was defined similarly to a frequently used modification of Fried’s criteria for frailty developed by Woods et al.27 and Johansen et al.27, 28, 29, 30, 31 Physical weakness and slowness was defined as a score <75 on the physical functioning scale of the Short Form of Health Survey-36. Exhaustion was defined as a score <55 on the vitality scale of the Short Form of Health Survey-36. A body mass index (BMI) <18.5 kg/m2 was used as a substitute for unintentional weight loss.19 Because underweight is a more accurate description of this modified criterion, the unintentional weight loss criterion is used as “underweight” hereafter. Physical inactivity was defined as the combination of self-reported moderate or extreme walking problems, with moderate or extreme usual activities limitations according the EuroQol 5-dimensional (EQ-5D) questionnaire.32 A total of 5 points was possible, with 2 points for low physical functioning and 1 point for each of the other criteria. Patients scoring ≥3 were defined as frail according to the literature.14, 27, 30 For NECOSAD patients, the Short Form of Health Survey-36 and EQ-5D questionnaire had to be completed before dialysis initiation or within 7 days from the start of dialysis. In an additional analysis, we studied the association of prefrailty (a self-reported frailty score of 1 or 2) with low UCrE. Patients in whom all frailty data were available were slightly different from those patients in whom not all frailty data were available. Therefore, patients in whom frailty was available had higher mean levels of UCrE (9.6 mmol/24 hours vs. 8.4 mmol/24 hours; P < 0.001), a higher BMI (26.4 kg/m2 vs. 25.2 kg/m2; P < 0.001), and higher levels of GFR (12.5 ml/min vs. 9.5 ml/min; P < 0.001), hemoglobin (7.5 mmol/L vs. 6.6 mmol/L; P < 0.001), and albumin (40 g/L vs. 37 g/L; P < 0.001).
Frailty-Associated Variables
Frailty-associated variables included cigarette smoking, albumin, parathyroid hormone, hemoglobin, protein-energy wasting according to the Subjective Global Assessment total score, and the Charlson Comorbidity Index. The Charlson Comorbidity Index was divided into 3 tertiles: 1: 0 to 2; 2: 3 to 5; and 3: 6 to 10.
Laboratory and Other Measurements
Standard laboratory techniques were used in the different centers participating in the PREPARE-2, NECOSAD, and PREVEND studies. GFR was calculated as the mean of urea and creatinine clearance, measured from 24-hour urine collections. The abbreviated Modification of Diet in Renal Disease Study equation was used to measure eGFR.
Educational levels were categorized according to the International Standard Classification of Education as bachelor, master or doctorate graduate (level 1), postsecondary or nontertiary or short-cycle tertiary education (level 2), upper secondary education (level 3), lower secondary education (level 4), and primary or less than primary education (level 5).33 Malignancy (n = 82) was defined as a history of (83%) or active (treated or untreated) malignancy (17%). The skin tumors squamous cell carcinoma and basal cell carcinoma were excluded for the definition of malignancy.
Statistical Analyses
Differences between patients with low UCrE versus normal range UCrE were tested for statistical significance using Student’s t-test, Mann-Whitney test, or χ2 test, as appropriate.
Low UCrE was defined stratified by sex, then indexed by height (UCrE/height); we subsequently calculated the 5th and 95th percentiles per 5-year age category. These values were plotted, and a third-order polynomial regression line was chosen, because this model yielded the highest R2 values. UCrE was indexed by height, because muscle mass is highly dependent on body size. All height-indexed UCrE values in patients with CKD that were below the 5th percentile of the healthy population were defined as low.
Logistic regression was used to identify correlates of low UCrE, presented in (i) crude analyses; (ii) analyses adjusted for age, race, sex, and height; and (iii) analyses with all variables added in 1 model. Furthermore, logistic regression was used to evaluate the associations of the frailty variables with low UCrE. These models are presented as (i) crude, and then adjusted for (ii) comorbidities, and (iii) GFR, using both the linear and quadratic function of GFR to allow for nonlinear associations. Adjustment for GFR was performed to model the effect of kidney function on both frailty and low UCrE. A subsidiary multivariate linear regression analysis was performed with UCrE treated as a continuous variable.
Subjects with UCrE values that were biologically implausible (UCrE <3.09 or >30.9 mmol/day) were excluded.34 Furthermore, patients with the 5% greatest differences between measured UCrE and calculated UCrE were excluded. For this purpose, we calculated the estimated UCrE by multiplying creatinine clearance (according to the Cockroft-Gault formula) with plasma creatinine.
Missing values of variables that were used for adjustment were imputed with standard multiple imputation techniques using 10 repetitions. Information on chronic lung disease and malignancy was missing in 31% of cases due to availability in the NECOSAD study only. Information on urea clearance was missing in 42% of cases. The multiple imputation model included the characteristics described in Table 1.
Table 1.
Patient characteristics according urinary creatinine excretion level
| Characteristics | All CKD patients N = 1287 |
Low UCrE 38% |
Normal UCrE 62% |
|---|---|---|---|
| UCrE in men (mmol/24 h) | 9.4 (7.6−11.4) | 7.5 (6.3−8.5) | 11.0a (9.7−12.4) |
| UCrE in women (mmol/24 h) | 6.9 (5.7−8.4) | 5.2 (4.7−6.1) | 8.0a (6.8−9.2) |
| Demographics | |||
| Age (yr) | 63.1 (51.9−72.4) | 62.5 (51.6−70.4) | 64.2b (52−74.1) |
| Men (%) | 63 | 67 | 60c |
| Non-Caucasian race (%) | 8 | 9 | 7 |
| Primary kidney disease (%) | |||
| Glomerulonephritis | 13 | 10 | 15 |
| Diabetes Mellitus | 15 | 18 | 14 |
| Renal vascular disease | 19 | 18 | 18 |
| Other | 53 | 53 | 53 |
| Educational level (%) | |||
| Level 1 | 5 | 5 | 6 |
| Level 2 | 9 | 9 | 9 |
| Level 3 | 18 | 19 | 17 |
| Level 4 | 42 | 43 | 42 |
| Level 5 | 26 | 25 | 26 |
| Smoking (%) | 27 | 32 | 24b |
| Anthropometry | |||
| Body mass index (kg/m2) | 24.8 (22.5−27.9) | 23.6 (21.5−26.1) | 25.7a (23.3−28.7) |
| Length (cm) | 171.3 ± 9.7 | 171.4 ± 9.3 | 171.3 ± 9.9 |
| Weight (kg) | 74.0 (65−84) | 70.7 (62−79) | 76.9a (67−87) |
| Comorbidities (%) | |||
| Myocardial infarction | 13 | 15 | 12 |
| Heart failure | 12 | 15 | 10c |
| DM | 23 | 26 | 22 |
| Peripheral vascular disease | 16 | 18 | 14 |
| CVA | 10 | 9 | 9 |
| Malignancy | 9 | 10 | 9 |
| Chronic lung disease | 7 | 9 | 6 |
| Charlson Comorbidity Index | |||
| Class 1 | 32 | 34 | 33 |
| Class 2 | 32 | 30 | 32 |
| Class 3 | 36 | 36 | 35 |
| Laboratory results | |||
| eGFR (ml/min/1.73 m2) | 8.0 (5.8−11.4) | 7.1 (5.5−10.0) | 8.3a (6.1−12.5) |
| GFR (ml/min) | 9.5 (7.3−12.3) | 7.4 (5.6−9.2) | 11.2a (8.9−14.0) |
| Hemoglobin (mmol/L) | 6.8 ± 1.1 | 6.5 ± 1.1 | 7.0 ± 1.2a |
| Albumin (g/L) | 38 (34−42) | 36 (31−40) | 39a (35−43) |
| PTH (pmol/L) | 17.8 (10−28.1) | 18.3 (8.3−46.9) | 16.9 (10.0−27.1) |
| SGA total score (%) | |||
| 1−5 = severe to moderate PEW | 9 | 17 | 9 |
| 6−7 = normal nutritional status | 91 | 83 | 92 |
CVA, cerebrovascular accident; DM, diabetes mellitus; GFR, glomerular filtration rate; PEW, protein-energy wasting; PTH, parathyroid hormone; SGA, subjective global assessment of nutritional status; UCrE, urinary creatinine excretion.
Data are given as mean ± SD or median (interquartile range).
Charlson Comorbidity Index: class 3 indicating the highest comorbidity burden.
Educational level: level 1 = university, level 5 = primary school or less.
P < 0.001; bP < 0.01; cP < 0.05, cases versus noncases.
Several sensitivity analyses were performed. First, we repeated the analyses without excluding subjects with UCrE values <3.09 or >30.9 mmol/day. Second, we repeated the analyses in complete cases. Third, we tested potential interactions of the frailty variables with the original study cohort. Subsequently, we repeated the analyses in the PREPARE-2 and NECOSAD cohorts separately.
A P value < 0.05 was considered statistically significant. All analyses were performed in SPSS (version 22.0; IBM, Armonk, New York).
Results
Study Participants
The healthy control group included 2748 PREVEND participants, after excluding subjects with UCrE values that were biologically implausible (UCrE <3.09 or >30.9 mmol/day) and excluding patients with the 5% highest differences between measured UCrE and calculated UCrE. The CKD population finally included 320 PREPARE-2 and 967 NECOSAD participants, after applying the same exclusion methods as the healthy cohort to exclude possibly incorrectly measured UCrE values. Patient characteristics according to study population are shown in Supplementary Table 1.
Definition of Low UCrE
The regression equation of the 5th percentile of UCrE values was modeled as follows:
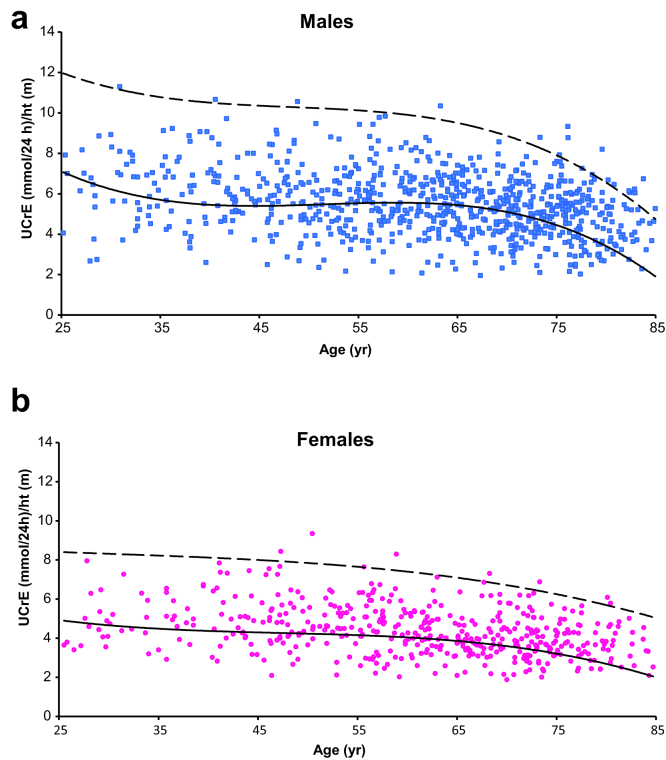
The fit of the regression model and the fits of the other models that were tested are shown in Supplementary Table 2, and the fit of the regression model is further visualized in Supplementary Figures 1a and 1b. Of the CKD patients, 38% had a low UCrE value according to the 5th percentile of the healthy population (Figures 1a and 1b). Of the patients with low UCrE, 88% originated from the NECOSAD study. Patients with low UCrE had a lower BMI, lower albumin, hemoglobin, and eGFR levels, compared with patients with a normal UCrE (Table 1).
Figure 1.
Low urinary creatinine excretion in (a) male and (b) female patients with advanced CKD. Dashed line represents the 95th percentile, and the solid line represents the 5th percentile of urinary creatinine excretion (UCrE) values in a healthy population.
Potential Correlates of Low UCrE
Crude odds of low UCrE were significantly higher in men, smokers, patients with heart failure, patients with peripheral vascular disease, and patients with lower GFR (Table 2). Excluding patients with peripheral vascular disease defined as an amputation (n = 14) yielded similar results (model 3: odds ratio [OR]: 1.88; 95% confidence interval [CI]: 1.13−3.12; P = 0.01). Remarkably, patients in the oldest age quintile showed significant lower odds of low UCrE compared with the youngest age quintile (OR: 0.31; 95% CI: 0.17−0.57; P < 0.001). A low GFR was the strongest correlate of low UCrE in model 3. Compared with patients with glomerulonephritis (GN) as a primary cause of renal disease, patients with other primary causes of renal disease (e.g., diabetes mellitus, hypertension) were more likely to have low UCrE.
Table 2.
Potential correlates of low urinary creatinine excretion
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (yr) | |||||||||
| <48.4 (ref) | |||||||||
| 48.5−59.3 | 1.26 | 0.88−1.79 | 0.21 | 1.24 | 0.87−1.77 | 0.24 | 1.20 | 0.73−1.97 | 0.48 |
| 59.4−67.35 | 1.43 | 1.002−2.03 | 0.05 | 1.33 | 0.93−1.91 | 0.12 | 1.40 | 0.84−2.34 | 0.20 |
| 67.36−74.07 | 1.30 | 0.91−1.86 | 0.14 | 1.23 | 0.85−1.76 | 0.27 | 0.88 | 0.51−1.51 | 0.63 |
| ≥74.1 | 0.50 | 0.34−0.73 | <0.001 | 0.45 | 0.30−0.67 | <0.001 | 0.31 | 0.17−0.57 | <0.001 |
| Male gender | 1.31 | 1.03−1.66 | 0.03 | 1.66 | 1.21−2.27 | 0.002 | 2.07 | 1.32−3.25 | 0.002 |
| Race | |||||||||
| Caucasian (ref) | |||||||||
| Non−Caucasian | 1.24 | 0.83−1.86 | 0.30 | 1.07 | 0.70−1.64 | 0.75 | 0.97 | 0.52−1.80 | 0.92 |
| Educational level | |||||||||
| Level 1 | |||||||||
| Level 2 | 0.96 | 0.53−1.74 | 0.89 | 0.80 | 0.43−1.50 | 0.48 | 0.88 | 0.40−1.93 | 0.75 |
| Level 3 | 1.07 | 0.67−1.73 | 0.77 | 0.90 | 0.54−1.50 | 0.69 | 1.06 | 0.56−2.01 | 0.86 |
| Level 4 | 1.17 | 0.80−1.71 | 0.41 | 1.07 | 0.71−1.61 | 0.76 | 1.67 | 0.98−2.83 | 0.06 |
| Level 5 | 1.05 | 0.77−1.42 | 0.78 | 0.95 | 0.70−1.32 | 0.77 | 1.00 | 0.66−1.51 | 0.99 |
| Primary kidney disease | |||||||||
| Glomerulonephritis (ref) | |||||||||
| DM | 2.16 | 1.39−3.35 | 0.001 | 2.30 | 1.46−3.62 | <0.001 | 3.02 | 1.28−7.09 | 0.01 |
| Renovascular disease | 1.58 | 1.04−2.41 | 0.03 | 1.77 | 1.14−2.75 | 0.01 | 2.27 | 1.22−4.24 | 0.01 |
| Other | 1.56 | 1.08−2.26 | 0.02 | 1.75 | 1.20−2.55 | 0.004 | 1.94 | 1.18−3.21 | 0.009 |
| Smoking | 1.38 | 1.06−1.78 | 0.02 | 1.33 | 1.02−1.74 | 0.04 | 1.36 | 0.95−1.96 | 0.10 |
| GFR quartiles (ml/min) | |||||||||
| 1st (1.6−5.3) | 25.8 | 16.0−41.8 | <0.001 | 33.2 | 20.0−55.1 | <0.001 | 41.4 | 22.5−76.2 | <0.001 |
| 2nd (5.3−7.2) | 6.15 | 3.95−9.55 | <0.001 | 7.13 | 4.50−11.3 | <0.001 | 9.03 | 5.21−15.7 | <0.001 |
| 3rd (7.2−9.6) | 2.26 | 1.36−3.78 | 0.002 | 2.29 | 1.35−3.87 | 0.002 | 2.35 | 1.26−4.38 | 0.008 |
| 4th (9.6−32.4) (ref) | |||||||||
| Myocardial infarction | 1.33 | 0.94−1.88 | 0.10 | 1.29 | 0.90−1.85 | 0.17 | 1.34 | 0.79−2.29 | 0.28 |
| Heart failure | 1.47 | 1.04−2.08 | 0.03 | 1.63 | 1.13−2.37 | 0.009 | 1.29 | 0.74−2.25 | 0.36 |
| DM | 1.25 | 0.95−1.64 | 0.11 | 1.28 | 0.96−1.70 | 0.09 | 1.14 | 0.59−2.21 | 0.70 |
| Peripheral vascular disease | 1.37 | 1.00−1.87 | 0.05 | 1.45 | 1.04−2.01 | 0.03 | 1.73 | 1.06−2.83 | 0.03 |
| CVA | 0.90 | 0.61−1.34 | 0.61 | 0.90 | 0.60−1.35 | 0.61 | 1.06 | 0.61−1.85 | 0.84 |
| Malignancy | 1.04 | 0.67−1.62 | 0.86 | 1.09 | 0.68−1.75 | 0.72 | 1.11 | 0.60−2.07 | 0.73 |
| Chronic lung disease | 1.47 | 0.85−2.54 | 0.17 | 1.48 | 0.85−2.58 | 0.17 | 2.06 | 1.03−4.14 | 0.04 |
CI, confidence interval; CVA, cerebrovascular accident; DM, diabetes mellitus; GFR, glomerular filtration rate; OR, odds ratio.
The dependent variable in the analyses is low urinary creatinine excretion.
Model 1 = crude; Model 2 = adjusted for age, race, sex, and height; Model 3 = all variables added in 1 model.
Educational level: level 1 = university; level 5 = primary school or less.
Self-Reported Frailty and Low UCrE
All the individual items of the self-reported frailty definition were available for 353 patients, 56% of whom were categorized as frail. Of the individual frailty components, physical inactivity was present in 42%, exhaustion and/or fatigue in 55%, low physical functioning in 66%, and underweight in 3% of the patients.
Self-reported frailty, and all the individual components that define self-reported frailty, were significantly associated with low UCrE (Table 3). Of the individual components, underweight yielded the highest odds of low UCrE (crude OR: 3.75; 95% CI: 1.76−7.98; P = 0.001). Adjustment for comorbidities in model 2 did not change the results. Additional adjustment for GFR in model 3 attenuated most associations, except for albumin and the individual component underweight. Similar results were obtained when UCrE was modeled as a continuous outcome (Supplementary Table 3). An additional analysis showed that prefrailty was associated with low UCrE with similar odds in models 1 and 2.
Table 3.
Associations of frailty, the individual components, and frailty-associated variables with low urinary creatinine excretion
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Frailty (n = 353) | 2.11 | 1.25−3.54 | 0.005 | 2.19 | 1.28−3.77 | 0.005 | 1.65 | 0.86−3.15 | 0.13 |
| Individual frailty components | |||||||||
| Poor physical performance (n = 364) | 1.36 | 1.04−1.79 | 0.03 | 1.35 | 1.02−1.79 | 0.04 | 1.31 | 0.93−1.84 | 0.12 |
| Exhaustion/fatigue (n = 371) | 2.37 | 1.42−3.96 | 0.001 | 2.40 | 1.41−4.08 | 0.001 | 1.79 | 0.96−3.35 | 0.07 |
| Underweight (n = 1281) | 3.75 | 1.76−7.98 | 0.001 | 3.86 | 1.80−8.28 | 0.001 | 4.26 | 1.74−10.4 | 0.002 |
| Physical inactivity (n = 376) | 1.72 | 1.06−2.78 | 0.03 | 1.79 | 1.08−2.97 | 0.02 | 1.42 | 0.77−2.59 | 0.26 |
| Frailty−associated variables | |||||||||
| Hemoglobin (mmol/L) (n = 927) | 0.70 | 0.62−0.79 | <0.001 | 0.70 | 0.62−0.79 | <0.001 | 1.12 | 0.95−1.31 | 0.18 |
| Albumin (g/L) (n = 1182) | 0.92 | 0.91−0.94 | <0.001 | 0.93 | 0.91−0.95 | <0.001 | 0.97 | 0.95−0.998 | 0.03 |
| PTH (per 10 pmol/L) (n = 251) | 1.14 | 1.00−1.29 | 0.05 | 1.16 | 1.02−1.33 | 0.03 | 1.04 | 0.91−1.20 | 0.57 |
| Charlson Comorbidity Index (n = 905) | |||||||||
| Class 1 (ref) | |||||||||
| Class 2 | 1.11 | 0.80−1.55 | 0.53 | 0.99 | 0.79−1.39 | 0.94 | 0.92 | 0.61−1.37 | 0.67 |
| Class 3 | 1.25 | 0.91−1.71 | 0.28 | 0.75 | 0.47−1.20 | 0.23 | 0.64 | 0.36−1.11 | 0.11 |
| Moderate to severe PEW (n = 260) | 2.22 | 0.90−5.47 | 0.08 | 2.22 | 0.90−5.65 | 0.10 | 1.43 | 0.48−4.28 | 0.53 |
CI, confidence interval; OR, odds ratio; PEW, protein-energy wasting according the Subjective Global Assessment of nutritional status; PTH, parathyroid hormone.
The dependent variable in the analyses is low urinary creatinine excretion.
Model 1 = crude; Model 2 = model 1 + adjusted for heart failure, diabetes mellitus, myocardial infarction , peripheral vascular disease, cerebrovascular accident, malignancy, and chronic lung disease; Model 3 = model 2 + glomerular filtration rate. Charlson Comorbidity Index: class 3 indicating the highest comorbidity burden.
Sensitivity Analyses
First, including subjects with UCrE values <3.09 or >30.9 mmol/day yielded similar results for the analysis of potential correlates of low UCrE, and for the analysis of self-reported frailty and low UCrE. Second, the results remained essentially similar when repeating the analyses with complete cases. Third, no significant interaction was found between the original study cohort and 1 of the frailty variables. Repeating the analyses in the PREPARE-2 and NECOSAD cohorts separately yielded essentially similar results, but did not reach significance in the NECOSAD cohort due to lower power because the frailty variables were available more often in patients in PREPARE-2 (n = 243) than in patients in NECOSAD (n = 110) (Supplementary Tables 4–7).
Discussion
This study showed that self-reported frailty, all the individual components that define self-reported frailty, and the frailty-associated variables hemoglobin, albumin, and parathyroid hormone were associated with low UCrE, independent of comorbidities. Furthermore, we found that low UCrE was present in more than one-third of patients with advanced CKD, and was strongly related to a lower kidney function level.
To the best of our knowledge this was the first study to investigate the associations of self-reported frailty with low UCrE as a measure of low muscle mass, which showed that self-reported frailty is associated with low UCrE. Although frailty has not been related to low UCrE yet, frailty has been associated with other measures of muscle mass. In a population of older community-dwelling adults, a high calf circumference was associated with a lower level of frailty, better muscle strength, and better performance on the Short Physical Performance Battery.35 Associations of frailty with lower quadriceps muscle area or with intracellular water as a measure of muscle mass have been reported in the hemodialysis population.36, 37
Low UCrE was prevalent in 38% of the patients with advanced CKD, and the prevalence was higher in patients with lower GFR values, which suggested an effect of CKD on muscle mass. This finding was in accordance with 2 previous studies that found lower UCrE per increasing CKD stage,8 and a decrease in UCrE with declining kidney function over time, independent of nutritional factors.38 Furthermore, a lower kidney function was associated with muscle atrophy, reduced walking speed, and more rapid declines in lower extremity strength over time.39 Studies that used other methods to measure muscle mass found the same association of impaired kidney function and low muscle mass.7, 21, 40, 41 Low UCrE might be a reflection of not only muscle mass, but also of low muscle function. Wilson et al. showed a lower creatinine generation rate per kilogram of skeletal muscle mass, measured as a fat-free mass by bioelectrical impedance analysis in those with lower GFR.7 This lower creatinine generation rate might be explained by altered muscle metabolism in advanced CKD that leads to lower UCrE and poorer muscle function. This hypothesis was favored by the recent study by Marcus et al., which showed that poor physical function in hemodialysis patients was not explained by low muscle mass or comorbid conditions.42
Theoretically, an alternative explanation could exist why UCrE is lower in later stage CKD. The lower UCrE could to some extent be explained by an increase in extrarenal clearance of creatinine.43, 44 Extrarenal clearance of creatinine was investigated in only 2 studies, which included a maximum of 10 patients each and reported a wide range of extrarenal clearance.45, 46 Most importantly, they did not measure muscle mass simultaneously, nor did they investigate the association between extrarenal clearance of creatinine and kidney function. Therefore, the degree of extrarenal clearance in relation to impaired kidney function is unknown.
The mechanisms that cause loss of muscle mass in CKD are incompletely understood. Muscle loss occurs either because of increased muscle protein breakdown or decreased muscle protein synthesis or a combination of both. Reduced muscle protein synthesis has been found in CKD.47 Increased muscle protein breakdown is a result of increased catabolic processes, which can be the result of several CKD-associated conditions such as chronic inflammation, uremia, acidosis, decline of insulin-like growth factor 1, alterations in vitamin D, and protein-energy wasting.22, 48, 49 Low physical activity, which is common in patients who start dialysis,50 induces loss of muscle mass as well. However, these mechanisms that cause loss of muscle mass in CKD are beyond the scope of this present study.
Remarkably, the associations of underweight and albumin with low UCrE were independent of GFR. Similarly, Tynkevich et al. described a strong association of a BMI <18.5 kg/m2 with low UCrE independently of measured GFR in patients with advanced CKD.38 These findings suggested a strong direct effect of underweight on muscle mass, not via chronic kidney disease per se, whereas the associations of other physical frailty components such as poor physical performance, exhaustion, and physical inactivity with low UCrE might be caused by reduced GFR, and the complications of CKD (e.g., metabolic acidosis, inflammation) more directly. Therefore, our hypothesis is that a reduced GFR leads to muscle wasting, both directly, and indirectly via weakness, slowness, physical inactivity, and exhaustion. Furthermore, the inflammation and other complications of advanced CKD might also contribute to loss of muscle mass.
Concerning the potential correlates of low UCrE, a remarkably lower risk of a low UCrE in patients with CKD aged 74 years and older was found. This lower risk might be explained by selection bias of a relatively healthier older population that was admitted to initiate dialysis therapy. Nephrologists might have been more cautious to refer elderly CKD patients in a poor physical condition to specialized predialysis outpatient clinics (PREPARE-2) or to initiate dialysis (NECOSAD) compared with younger CKD patients. Furthermore, a remarkable finding was a higher odds of low UCrE in men compared with women. There are several explanations. First, relatively more frail male patients might have been selected to initiate dialysis therapy compared with female patients. Second, CKD might have a larger impact on muscle mass in male patients compared with female patients. Non-GN causes of renal failure were significantly associated with low UCrE compared to GN. This finding might be explained by a higher comorbidity burden in the non-GN patients (e.g., hypertension, diabetes and cardiovascular diseases) and the fact that these comorbidities would result in a higher prevalence of frailty and low UCrE.31
This study used a self-reported definition of frailty, which was used in previous studies, except for the definition of low physical activity.27, 28, 29, 30, 31 This self-reported frailty definition was similar to a frequently used modification of the Fried frailty phenotype, which is based on 5 physical criteria. We modified the definition of low physical activity by using items of the EQ-5D, which yielded a similar percentage of patients who were physically inactive (42%) compared with other (advanced) CKD populations (36%−54%).17, 18, 19, 30, 51 Johansen et al. compared physical functioning according to the self-reported frailty definition with gait speed and grip strength in hemodialysis patients. Self-reported frailty identified nearly all patients with objective measured frailty with a sensitivity of 90%.20
In the sensitivity analysis, we found nonsignificant associations of self-reported frailty and the individual frailty components when NECOSAD was analyzed separately. However, the effect measures were similar in both separate cohorts, and an interaction term of the original cohort with the several independent frailty variables did not reach significance. Therefore, we argue that the associations of frailty with low UCrE were not cohort-dependent.
The strengths of this study included that this was the first study that used a large general population-based cohort as a control group to define low UCrE values, adjusted for height, age, and sex. Second, this was the first study that evaluated associations of low UCrE with self-reported frailty. UCrE values were available in a large cohort of well-phenotyped patients with advanced CKD. Furthermore, in the absence of measured GFR, we used the mean of creatinine and urea clearance, which is the best estimate of true GFR that is clinically available in patients with advanced renal failure.52
Our study should be interpreted in the light of several limitations. First, using creatinine excretion from 24-hour urine collections as a measure of muscle mass might include collection errors. However, in all 3 studies, participants received thorough instructions, and potential invalid collections were excluded. Furthermore, we checked for possible invalid urine collections in sensitivity analyses and found similar results. Second, the GFR values that we used were related to UCrE, because both were calculated from creatinine in 24-hour urinary samples. However, using the mean of urea and creatinine clearance for GFR reduced this effect. Third, the shape of the UCrE reference curve was extrapolated in the older age values because the healthy cohort included younger individuals compared with the patients with advanced CKD in this study. Future studies should check whether the UCrE reference curve is still accurate in elderly patients (i.e., older than 75 years) with advanced CKD. Fourth, because of the cross-sectional design, any causal interpretation regarding the direction of the associations between low UCrE and self-reported frailty could be considered only very carefully. Furthermore, low UCrE was defined in a mainly Caucasian population, which limited generalizability. In contrast, this homogeneity of race might have led to the elimination of potential complicating factors when deriving thresholds for low UCrE, making the data easier to understand. Finally, we used the EQ-5D as a substitute for the frailty criterion of physical inactivity and thereby presumed physical inactivity to be present indirectly based on patients’ report of physical disability and/or limitation. However, because the Minnesota Leisure Time Activity questionnaire, which was used by Fried et al. to define the physical inactivity criterion, is frequently not available in large cohorts, researchers have mostly used other definitions. The physical inactivity criterion is probably the most complicated to define and translate.53
This study provided an equation to define low UCrE values for epidemiological research and clinical practice for patients with advanced CKD. Although not commonly used in the United States, collection of 24-hour urine samples is routinely performed in Europe in clinical care and is accepted as a valuable procedure to assess kidney function and to estimate dietary compliance to sodium (and protein) restricted diets. In clinical practice, with 24-hour urine samples available, low UCrE might be used to identify patients with frailty and higher risk of adverse health outcomes, in whom interventions could be initiated to improve physical performance. Numerous studies demonstrated that exercise has beneficial effects on physical performance and quality of life in patients with various stages of CKD.54, 55, 56, 57 These studies implied that exercise may reverse certain aspects of frailty in CKD patients. Whether low UCrE has an association with outcomes (e.g. mortality), independent of BMI and GFR in advanced CKD patients needs to be investigated and is beyond the scope of the present study.
In conclusion, lower kidney function is a strong correlate of low UCrE, and self-reported frailty, and the individual frailty components are associated with low UCrE as well, independent of comorbidities.
Disclosures
All the authors declared no competing interests.
Acknowledgments
We are grateful to the patients and staff of all the participating hospitals of the NECOSAD, PREPARE-2, and PREVEND studies.
Footnotes
Figure S1. Urinary creatinine excretion (UCrE)/height values of healthy population versus age, with the regression equations of the 5th and 95th percentiles superimposed. (a) Males (blue graph), (b) females (purple graph).
Table S1. Patient characteristics according original study cohort.
Table S2. Fit of the third-order polynomial regression model and fits of the other tested models.
Table S3. Linear regression models of the associations of self-reported frailty and the individual components, and frailty-associated variables with urinary creatinine excretion (continuously).
Table S4. Sensitivity analysis: PREPARE-2 only: potential correlates of low urinary creatinine excretion.
Table S5. Sensitivity analysis: NECOSAD only: potential correlates of low urinary creatinine excretion.
Table S6. Sensitivity analysis: PREPARE-2 only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.
Table S7. Sensitivity analysis: NECOSAD only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Author Contributions
Conceived and/or designed the work that led to the submission (all authors); acquired data (FWD, SJLB, RTG) or played an important role in interpreting the results (HAPB, HN, FWD, CAJMG, RTG), drafted the article (HAPB), revised the article (HAPB, HN, FWD, SJLB, CAJMG, RTG), and approved the final version (all authors).
Supplementary Material
Urinary creatinine excretion (UCrE)/height values of healthy population versus age, with the regression equations of the 5th and 95th percentiles superimposed. (a) Males (blue graph), (b) females (purple graph).
Patient characteristics according original study cohort.
Fit of the third-order polynomial regression model and fits of the other tested models.
Linear regression models of the associations of self-reported frailty and the individual components, and frailty-associated variables with urinary creatinine excretion (continuously).
Sensitivity analysis: PREPARE-2 only: potential correlates of low urinary creatinine excretion.
Sensitivity analysis: NECOSAD only: potential correlates of low urinary creatinine excretion.
Sensitivity analysis: PREPARE-2 only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.
Sensitivity analysis: NECOSAD only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.
References
- 1.Heymsfield S.B., Arteaga C., McManus C. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 2.Proctor D.N., O'Brien P.C., Atkinson E.J., Nair K.S. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol. 1999;277:E489–E495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z.M., Gallagher D., Nelson M.E. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63:863–869. doi: 10.1093/ajcn/63.6.863. [DOI] [PubMed] [Google Scholar]
- 4.Welle S., Thornton C., Totterman S., Forbes G. Utility of creatinine excretion in body-composition studies of healthy men and women older than 60 y. Am J Clin Nutr. 1996;63:151–156. doi: 10.1093/ajcn/63.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Keshaviah P.R., Nolph K.D., Moore H.L. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol. 1994;4:1475–1485. doi: 10.1681/ASN.V471475. [DOI] [PubMed] [Google Scholar]
- 6.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 7.Wilson F.P., Xie D., Anderson A.H. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9:2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Micco L., Quinn R.R., Ronksley P.E. Urine creatinine excretion and clinical outcomes in CKD. Clin J Am Soc Nephrol. 2013;8:1877–1883. doi: 10.2215/CJN.01350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix J.H., de Boer I.H., Wassel C.L. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121:1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oterdoom L.H., Gansevoort R.T., Schouten J.P. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207:534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 11.ter Maaten J.M., Damman K., Hillege H.L. Creatinine excretion rate, a marker of muscle mass, is related to clinical outcome in patients with chronic systolic heart failure. Clin Res Cardiol. 2014;103:976–983. doi: 10.1007/s00392-014-0738-7. [DOI] [PubMed] [Google Scholar]
- 12.Sinkeler S.J., Kwakernaak A.J., Bakker S.J. Creatinine excretion rate and mortality in type 2 diabetes and nephropathy. Diabetes Care. 2013;36:1489–1494. doi: 10.2337/dc12-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tynkevich E., Flamant M., Haymann J.P. Urinary creatinine excretion, measured glomerular filtration rate and CKD outcomes. Nephrol Dial Transplant. 2015;30:1386–1394. doi: 10.1093/ndt/gfv047. [DOI] [PubMed] [Google Scholar]
- 14.Fried L.P., Tangen C.M., Walston J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple L.S., Katz R., Rifkin D.E. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol. 2013;8:2091–2099. doi: 10.2215/CJN.02870313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reese P.P., Cappola A.R., Shults J. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38:307–315. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roshanravan B., Khatri M., Robinson-Cohen C. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak M.G., Stehman-Breen C., Fried L.F. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm-Leen E.R., Hall Y.N., K Tamura M, Chertow G.M. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671.e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen K.L., Dalrymple L.S., Delgado C. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2014;64:600–607. doi: 10.1053/j.ajkd.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried L.F., Boudreau R., Lee J.S. Kidney function as a predictor of loss of lean mass in older adults: health, aging and body composition study. J Am Geriatr Soc. 2007;55:1578–1584. doi: 10.1111/j.1532-5415.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X.H., Mitch W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–516. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand S., Johansen K.L., Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69:315–322. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambers Heerspink H.J., Brantsma A.H., de Zeeuw D. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168:897–905. doi: 10.1093/aje/kwn209. [DOI] [PubMed] [Google Scholar]
- 25.Suttorp M.M., Hoekstra T., Mittelman M. Effect of erythropoiesis-stimulating agents on blood pressure in pre-dialysis patients. PLoS One. 2013;8:e84848. doi: 10.1371/journal.pone.0084848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jager D.J., Halbesma N., Krediet R.T. Is the decline of renal function different before and after the start of dialysis? Nephrol Dial Transplant. 2013;28:698–705. doi: 10.1093/ndt/gfs578. [DOI] [PubMed] [Google Scholar]
- 27.Woods N.F., LaCroix A.Z., Gray S.L. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 28.Delgado C., Shieh S., Grimes B. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol. 2015;42:134–140. doi: 10.1159/000439000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao Y., Dalrymple L., Chertow G.M. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansen K.L., Chertow G.M., Jin C., Kutner N.G. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 31.Johansen K.L., Dalrymple L.S., Glidden D. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11:626–632. doi: 10.2215/CJN.03710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szende A., Oppe M., Devlin N. Springer; Dordrecht, The Netherlands: 2007. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. [Google Scholar]
- 33.UNESCO Institute for Statistics: International Standard Classification of Education, ISCED 2011. UNESCO; Paris: 2011. [Google Scholar]
- 34.Ix J.H., Wassel C.L., Stevens L.A. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landi F., Onder G., Russo A. Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr. 2014;33:539–544. doi: 10.1016/j.clnu.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Delgado C., Doyle J.W., Johansen K.L. Association of frailty with body composition among patients on hemodialysis. J Ren Nutr. 2013;23:356–362. doi: 10.1053/j.jrn.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansen K.L., Dalrymple L.S., Delgado C. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. 2014;25:381–389. doi: 10.1681/ASN.2013040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tynkevich E., Flamant M., Haymann J.P. Decrease in urinary creatinine excretion in early stage chronic kidney disease. PLoS One. 2014;9:e111949. doi: 10.1371/journal.pone.0111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roshanravan B., Patel K.V., Robinson-Cohen C. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis. 2015;65:737–747. doi: 10.1053/j.ajkd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster B.J., Kalkwarf H.J., Shults J. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foley R.N., Wang C., Ishani A. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 42.Marcus R.L., LaStayo P.C., Ikizler T.A. Low physical function in maintenance hemodialysis patients is independent of muscle mass and comorbidity. J Ren Nutr. 2015;25:371–375. doi: 10.1053/j.jrn.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman R. Creatinine excretion in renal failure. Proc Soc Exp Biol Med. 1954;85:446–448. doi: 10.3181/00379727-85-20912. [DOI] [PubMed] [Google Scholar]
- 44.Mitch W.E., Walser M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron. 1978;21:248–254. doi: 10.1159/000181400. [DOI] [PubMed] [Google Scholar]
- 45.Mitch W.E., Collier V.U., Walser M. Creatinine metabolism in chronic renal failure. Clin Sci (Lond) 1980;58:327–335. doi: 10.1042/cs0580327. [DOI] [PubMed] [Google Scholar]
- 46.Jones J.D., Burnett P.C. Creatinine metabolism in humans with decreased renal function: creatinine deficit. Clin Chem. 1974;20:1204–1212. [PubMed] [Google Scholar]
- 47.Adey D., Kumar R., McCarthy J.T., Nair K.S. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278:E219–E225. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- 48.Fahal I.H. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29:1655–1665. doi: 10.1093/ndt/gft070. [DOI] [PubMed] [Google Scholar]
- 49.Kim J.C., Kalantar-Zadeh K., Kopple J.D. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24:337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 50.Johansen K.L., Chertow G.M., Kutner N.G. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Painter P., Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodial Int. 2013;17:41–49. doi: 10.1111/j.1542-4758.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 52.Lubowitz H., Slatopolsky E., Shankel S. Glomerular filtration rate. Determination in patients with chronic renal disease. JAMA. 1967;199:252–256. doi: 10.1001/jama.199.4.252. [DOI] [PubMed] [Google Scholar]
- 53.Eckel S.P., Bandeen-Roche K., Chaves P.H. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. 2011;23:209–216. doi: 10.1007/bf03324962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smart N., Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 2011;16:626–632. doi: 10.1111/j.1440-1797.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 55.Castaneda C., Gordon P.L., Uhlin K.L. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135:965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 56.Castaneda C., Gordon P.L., Parker R.C. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis. 2004;43:607–616. doi: 10.1053/j.ajkd.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Manfredini F., Mallamaci F., D'Arrigo G. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28:1259–1268. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Urinary creatinine excretion (UCrE)/height values of healthy population versus age, with the regression equations of the 5th and 95th percentiles superimposed. (a) Males (blue graph), (b) females (purple graph).
Patient characteristics according original study cohort.
Fit of the third-order polynomial regression model and fits of the other tested models.
Linear regression models of the associations of self-reported frailty and the individual components, and frailty-associated variables with urinary creatinine excretion (continuously).
Sensitivity analysis: PREPARE-2 only: potential correlates of low urinary creatinine excretion.
Sensitivity analysis: NECOSAD only: potential correlates of low urinary creatinine excretion.
Sensitivity analysis: PREPARE-2 only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.
Sensitivity analysis: NECOSAD only: associations of self-reported frailty and the individual components, and frailty-associated variables with low urinary creatinine excretion.