Abstract
Introduction
Obesity-related glomerulopathy is an established secondary glomerular disease that may occur in obese individuals with a body mass index (BMI) of ≥30 kg/m2. However, patients with moderate obesity (BMI ≤ 30 kg/m2) may also develop this disease.
Methods
A total of 20 patients with grade 1 obesity (25 ≤ BMI < 30 kg/m2) with persistent proteinuria, without evidence of other renal diseases, were analyzed retrospectively. These patients were compared with 20 patients with grade 2 or higher obesity (BMI ≥ 30 kg/m2) with persistent proteinuria. Biopsies of 31 kidney transplant donors as healthy controls were used to compare histologic parameters.
Results
Similar to the grade 2 or higher obesity group, the grade 1 obesity group had a male predominance (85%) and showed a high incidence of hypertension (80%). Urinary protein excretion and renal outcome parameters were comparable between the groups. Patients with grade 1 obesity showed typical histologic features of obesity-related glomerulopathy: low glomerular density with glomerulomegaly. The glomerular density and mean glomerular volume in the grade 1 group, the grade 2 or higher group, and the kidney transplant donors with grade 1 obesity were 1.6 ± 0.8 versus 1.4 ± 0.6 versus 3.0 ± 1.1 (per mm2) and 6.1 ± 2.1 versus 6.4 ± 1.6 versus 2.9 ± 0.8 (×106 μm3), respectively.
Discussion
A glomerulopathy similar to obesity-related glomerulopathy can occur in moderately obese individuals. Renal factor(s), such as low glomerular density, may thus underlie susceptibility to this disease entity as well as BMI.
Keywords: body mass index, glomerular density, hyperfiltration, obesity, renal biopsy
Obesity-related glomerulopathy (ORG) is an established disease entity that occurs in obese individuals. Kambham et al.1 reported an increased incidence of ORG among patients subjected to kidney biopsy in the United States. In that study, the number of ORG cases increased from 0.2% in 1986–1990 to 2.0% in 1996–2000. Likewise, reports from Spain, China, and Japan have suggested that the incidence of ORG is increasing, likely due to the increasing rates of obesity in the general population.2, 3, 4
ORG is defined as a proteinuric renal disease in patients with a body mass index (BMI) of ≥30 kg/m2 and without clinical and histopathologic evidence of other renal diseases.1, 2, 4 The typical clinical features of ORG include moderate or massive amounts of proteinuria without a decrease in serum levels of albumin.1, 2, 3, 4 Histopathologically, glomerulomegaly, and focal segmental glomerulosclerosis (FSGS) are characteristic findings,1, 2, 4 likely reflecting intraglomerular hyperfiltration in these patients.5, 6, 7 Moreover, the glomerular density (glomerular number per renal cortical area of a needle biopsy) is extremely low in patients with ORG.8 Such an uneven glomerular density distribution in patients with ORG is unlike that in biopsies of kidney transplant donors and patients with other primary glomerular diseases. In addition, an autopsy analysis of the glomerular density in nondiseased kidneys suggested that such a low glomerular density is present only in patients with ORG, and is rare in individuals with a BMI ≥30 kg/m2 in the absence of renal disease. Despite the marked glomerulomegaly in patients with ORG, the glomerular volume in kidneys of obese individuals without renal disease is only slightly greater. These results suggest that a low glomerular density associated with glomerulomegaly is a characteristic renal histologic finding in patients with ORG, and that this difference in histopathologic measures is not simply related to BMI.
In a survey of the Japanese population, moderate obesity (25 ≤ BMI < 30 kg/m2) was closely related to an increased incidence of various obesity-related diseases, and so this BMI category was defined as grade 1 obesity.9 Recent epidemiologic studies suggest that a substantial number of patients have a disease state similar to ORG, including those with grade 1 obesity.10, 11, 12 Currently, however, little is known regarding the renal complication(s) that can occur in patients in this BMI category. In the current study, we explored the clinicopathologic characteristics of a proteinuric renal disease in moderately obese subjects in whom renal biopsy was performed.
Methods
Patients
This study included patients who underwent renal biopsies of native kidneys at Jikei Hospital, Tokyo, from 1999 to 2008. The exclusion criteria are shown in Table 1. Patients with renal manifestations, including renal impairment or proteinuria, before becoming obese and those who showed acute-onset nephrotic syndrome were excluded. Impaired glucose tolerance alone, as defined as an HbA1c ≥ 6.5% at the time of biopsy, was not a criterion for exclusion. However, patients with a history of impaired glucose tolerance for >5 years or those with extrarenal diabetic complications, including retinopathy and neuropathy, were excluded from this study. Glomerular injuries in patients with obesity were defined morphologically as obesity-associated glomerulomegaly with or without FSGS lesions, as reported previously.1, 2, 3, 4, 8 Patients whose renal biopsies showed clinical and histologic evidence of other primary or secondary renal diseases, including diabetic nephropathy, were excluded. Increased glomerular basement membrane thickness alone was not a criterion for exclusion, because obese patients can exhibit an increased glomerular basement membrane thickness in the absence of diabetes.13, 14 Likewise, the presence of hypertension was not an exclusion criterion. The biopsy specimens of some obese hypertensive patients had moderate-to-severe vascular lesions, which were accompanied by collapsed glomeruli. Patients with such histologic features were diagnosed with hypertensive nephrosclerosis instead of glomerulopathy associated with obesity, and were excluded from this study. Patients with at least 2 years of follow-up data were included in this study.
Table 1.
The exclusion criteria
Patients who were excluded from the study
|
Not excluded
|
GBM, glomerular basement membrane.
Definitions
According to the criteria proposed by the Japan Society for the Study of Obesity, grade 1 obesity was defined as 25 ≤ BMI < 30 kg/m2, grade 2 as 30 ≤ BMI < 35 kg/m2, grade 3 as 35 ≤ BMI < 40 kg/m2, and grade 4 as a BMI ≥ 40 kg/m2.15 The biopsies of 20 patients with grade 1 obesity (group 1) and 20 patients with grade 2 or higher obesity (group 2; 16 with grade 2 and 4 with grade 3) were recruited from the renal biopsy archive during the study period. Renal biopsies of kidney transplant donors without apparent chronic kidney diseases were used for comparisons of histologic findings. Of these kidney transplant donors, 21 cases had a BMI of < 25 kg/m2 and 10 had a grade 1 obesity. The estimated glomerular filtration rate (eGFR) was calculated using a modified 3-variable equation for the GFR of Japanese individuals:16 eGFR = 194 × age–0.287 × sCr–1.094 (×0.739 if female), where sCr is the serum level of creatinine. Hypertension was defined as a systolic blood pressure > 140 mm Hg and/or diastolic blood pressure > 90 mm Hg, or use of antihypertensive medications. Subjects using antihypertensive medications, such as angiotensin blockers, for the purpose of renoprotection despite a normal blood pressure were considered to be normotensive.
Pathologic Analysis
Renal specimens were obtained by percutaneous needle biopsy. Renal biopsies of kidney transplant donors were performed under direct vision using a needle biopsy gun. Renal biopsy specimens obtained 1 hour after renal transplantation were used for the analyses. An 18-gauge biopsy needle was used in all cases. The tissues were embedded in paraffin, cut into 3- to 4-μm sections and stained with hematoxylin and eosin, periodic acid-Schiff, Masson’s trichrome, and periodic acid methenamine silver. All biopsy specimens were subjected to immunohistochemical and electron microscopy analyses. The area of interstitial fibrosis and/or tubular atrophy was evaluated semiquantitatively according to the proportion of cortical area involvement. The grade of arterial lesions was scored according to the most severe lesion within the specimen, and intimal thickening was scored by comparing the thickness of the intima with that of the media in the same vessel segment. We defined the severity of arterial lesions lacking intimal thickening as grade 0, those with ≤50% intimal thickening as grade 1, and those with >50% as grade 2. The grade of arteriolar lesions was evaluated based on the proportion of arterioles exhibiting hyalinosis as follows: grade 0, <5% lesions; grade 1, 5% to 25% lesions; and grade 2, >25% lesions. The percentage of glomeruli affected by segmental or global sclerosis was assessed. The variants of FSGS were determined for each glomerulus according to a classification previously reported, including tip variant, collapsing variant, cellular variant, perihilar variant, and not otherwise specified.17 The glomerular density was determined by calculating the number of glomeruli per total renal cortical area, which was measured using a computerized image analyzer (Leica IM500, Leica Microsystems, Wetzlar, Germany). The measurement of glomerular density is strongly influenced by the degree of global sclerosis. Therefore, 2 definitions of glomerular density were applied: the number of glomeruli that were not globally sclerotic per total renal cortical area and the number of glomeruli (including globally sclerotic glomeruli) per total renal cortical area. The glomerular area was defined as the area described by the outer capillary loops of the tuft using a computerized image analyzer (Leica IM500, Leica Microsystems). The mean glomerular area (GA) was calculated by averaging the areas of all glomeruli. The mean glomerular volume (GV) was calculated from the measured GA: GV = (GA)3/2 × β/d, where β is a dimensionless shape coefficient (β = 1.38 for spheres) and d is a size distribution coefficient used to adjust for variation in glomerular size.18 The analysis used d = 1.01, as in previous reports.19
Statistical Analysis
Continuous variables are expressed as means ± SD. The variables were assessed for normality both visually (normal probability plot) and by inferential statistics (Shapiro-Wilk W and Kolmogorov-Smirnov tests). Continuous variables were compared by the t-test or the Wilcoxon rank-sum test, as appropriate. Categorical variables were expressed as percentages and compared by the χ2 test. Values of P < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the SPSS software package (SPSS, Chicago, IL).
Results
Clinical Characteristics at the Time of Biopsy
The clinical characteristics at the time of biopsy of patients with glomerulopathy associated with grade 1 and grade 2 or higher obesity are shown in Table 2. Compared with group 2, those in group 1 tended to be older. There was a male predominance in both groups, with 80% in group 1 having hypertension. The serum levels of creatinines and the mean eGFR values were comparable between the groups. The mean serum levels of albumin were comparable between the groups, and no patients in either group had low concentrations. The mean serum concentrations of uric acid, total cholesterol, triglyceride, and HbA1c were comparable between the groups.
Table 2.
Comparison of clinical characteristics at the time of biopsy
| Variables | Grade 1 obesity, n = 20 | Grade 2 or higher obesity, n = 20 | P value, grade 1 versus grade 2 or higher |
|---|---|---|---|
| Age | 52 ± 11 | 45 ± 14 | 0.073 |
| Gender (%male) | 85 | 80 | 0.677 |
| BMI (kg/m2) | 26.9 ± 1.3 | 32.0 ± 2.4 | – |
| Hypertension (%) | 80 | 70 | 0.465 |
| Serum creatinine (mg/dl) | 1.1 ± 0.5 | 1.2 ± 0.7 | 0.812 |
| eGFR (ml/min per 1.73 m2) | 59 ± 20 | 64 ± 31 | 0.545 |
| Urinary protein excretion (g/d) | 2.1 ± 1.5 | 2.0 ± 1.8 | 0.800 |
| Serum albumin (g/dl) | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.924 |
| Serum uric acid (mg/dl) | 7.3 ± 1.6 | 7.5 ± 1.8 | 0.698 |
| Serum total cholesterol (mg/dl) | 237 ± 35 | 216 ± 37 | 0.073 |
| Serum triglyceride (mg/dl) | 282 ± 205 | 223 ± 122 | 0.273 |
| Hemoglobin A1c (%) | 5.9 ± 0.5 | 5.9 ± 0.5 | 0.676 |
BMI; body mass index, eGFR; estimated glomerular filtration rate.
Renal Outcomes
Renal outcome parameters were compared between the patients with grade 1 and those with grade 2 or higher obesity (Table 3). The average follow-up duration was 6.2 years in both groups. During the follow-up period, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were used by 18 (90%) of the patients in group 1 and 17 (85%) in group 2. No patients in either group received corticosteroids or immunosuppressive reagents. The slopes of renal function decline during the observation period (ΔeGFR), and the frequencies of patients with a 30% decrease in eGFR at the last observation, a 50% decrease in eGFR at the last observation, and those who developed end-stage renal disease were not significantly different between the groups.
Table 3.
Comparison of renal outcomea
| Variables | Grade 1 obesity, n = 20 | Grade 2 or higher obesity, n = 20 | P value |
|---|---|---|---|
| Duration of follow-up (yr) | 6.2 ± 3.0 | 6.2 ± 2.8 | 0.944 |
| Use of ACE-I/ARB (%) | 90 | 85 | 0.633 |
| Use of corticosteroids or immunosuppressants (%) | 0 | 0 | – |
| ΔeGFR (%/yr) | 7.4 ± 9.2 | 5.8 ± 7.1 | 0.450 |
| Patients with a 30% decrease in eGFR at the last observation (%) | 50 | 40 | 0.525 |
| Patients with a 50% decrease in eGFR at the last observation or those reached ESRD (%) | 25 | 15 | 0.212 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB; angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease.
Patients with >2-yr follow-up were included in the analyses.
Histopathologic Findings
The histopathologic findings are shown in Table 4. Patients in group 1 had mild-to-moderate levels of chronic renal injuries, including interstitial fibrosis and/or tubular atrophy, global glomerulosclerosis, and FSGS. Among the renal biopsy specimens from these patients, 21 glomeruli from 10 patients showed FSGS lesions, 6 (29%) of which were perihilar variants and the other 15 (71%) were of the not-otherwise-specified variant. These features were similar to those in group 2. Typical FSGS lesions showing cellular, collapsing, or tip variants were not identified in either group. Regarding vascular lesions, the mean grades of arterial lesions and arteriole lesions were comparable.
Table 4.
Comparison of histopathologic findings
| Variables | Grade 1 obesity, n = 20 | Grade 2 or higher obesity, n = 20 | P valuea | Kidney donor (BMI < 25), n = 21 | Kidney donor grade 1 obesity, n = 10 | P valueb |
|---|---|---|---|---|---|---|
| Interstitial fibrosis and/or tubular atrophy (%) | 28 ± 21 | 27 ± 20 | 0.921 | 5 ± 4 | 9 ± 8 | 0.003 |
| Glomeruli affected by global glomerulosclerosis (%) | 22 ± 17 | 25 ± 20 | 0.715 | 5 ± 7 | 4 ± 6 | <0.001 |
| Patients with FSGS (%) | 50 | 45 | 0.752 | 0 | 0 | <0.001 |
| Glomeruli affected by FSGS (%) | 6.2 ± 8.1 | 5.2 ± 7.6 | 0.704 | 0 | 0 | 0.003 |
| FSGS variant | ||||||
| Glomeruli with perihilar variant (%) | 1.5 ± 2.8 | 0.6 ± 1.5 | 0.198 | 0 | 0 | – |
| Glomeruli with NOS variant (%) | 4.5 ± 6.3 | 4.6 ± 6.9 | 0.968 | 0 | 0 | – |
| Glomeruli with cellular variant (%) | 0 | 0 | – | 0 | 0 | – |
| Glomeruli with collapsing variant (%) | 0 | 0 | – | 0 | 0 | – |
| Glomeruli with tip variant (%) | 0 | 0 | – | 0 | 0 | – |
| Arterial lesions (grade) | 0.8 ± 0.7 | 0.8 ± 0.8 | 1.000 | NA | NA | – |
| Arteriole lesions (grade) | 0.7 ± 0.7 | 0.6 ± 0.7 | 0.657 | NA | NA | – |
| Nonsclerosed glomerular number/total cortical area (per mm2) | 1.6 ± 0.8 | 1.4 ± 0.6 | 0.373 | 3.3 ± 1.1 | 3.0 ± 1.1 | 0.003 |
| Total glomerular number/total cortical area (per mm2) | 2.3 ± 1.4 | 1.9 ± 0.7 | 0.221 | 3.4 ± 1.2 | 3.1 ± 1.1 | 0.117 |
| Mean glomerular volume (×106 μm3) | 6.1 ± 2.1 | 6.4 ± 1.6 | 0.582 | 2.4 ± 0.8 | 2.9 ± 0.8 | <0.001 |
FSGS, focal segmental glomerulosclerosis; NA, not assessed; NOS, not otherwise specified.
Grade 1 obesity versus grade 2 or higher obesity.
Grade 1 obesity versus kidney donor with grade 1 obesity.
The mean glomerular density and volume values were compared between the groups. The glomerular densities in both groups were considerably lower than that of kidney transplant donors with or without obesity. The low glomerular density in these obese patients remained evident when globally sclerotic glomeruli were included in the calculation of glomerular density. In contrast, the glomerular volume of these obese patients was markedly larger than that of the kidney transplant donors with or without obesity. The glomerular density and volume did not differ significantly between the 2 obesity groups. In addition, they did not differ significantly between the kidney transplant donors with and without obesity.
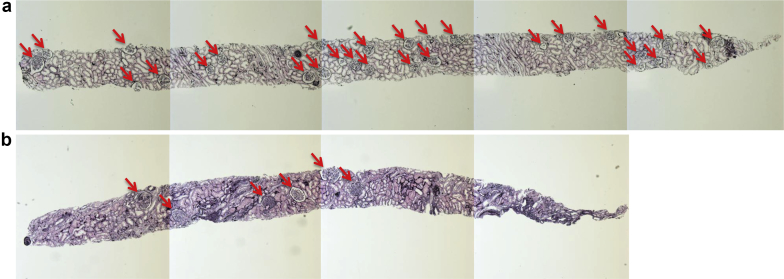
Figure 1 shows the representative renal biopsy findings in a case of kidney transplant donor (a) and a patient with glomerulopathy associated with grade 1 obesity (b). They are observed to be quite different with regard to the density and the size of the glomeruli.
Figure 1.
Representative renal biopsy findings in light microscopy. The representative renal biopsy findings in (a) a case of a kidney transplant donor (36-year-old normotensive man with the estimated glomerular filtration rate [eGFR] of 109 ml/min per 1.73 m2 and the body mass index [BMI] of 24.8 kg/m2) and (b) a patient with grade 1 obesity (42-year-old hypertensive woman with the eGFR of 81 ml/min per 1.73 m2 and the BMI of 25.5 kg/m2) are shown. The arrows indicate nonsclerotic glomeruli. Periodic acid methenamine silver stain (original magnification ×50).
Discussion
We explored the clinicopathologic characteristics of a proteinuric glomerulopathy found in individuals with grade 1 obesity (25 ≤ BMI < 30 kg/m2). Our results showed that the clinical characteristics of proteinuric glomerulopathy in such patients were similar to those in patients with grade 2 or higher obesity. In addition, during an average of 6.2 years of follow-up, the renal outcome parameters, including the slope of renal function decline and the incidence of progression, did not differ significantly between the groups. Furthermore, the 2 groups exhibited similar histopathologic findings. Notably, group 1 (grade 1 obesity) had typical histologic characteristics of patients with ORG with a BMI value of ≥30 kg/m2, that is, low glomerular density with glomerulomegaly.
The glomerular density was extremely low in proteinuric patients in both groups. Interestingly, such an uneven distribution of glomerular density in these obese patients was in sharp contrast to the widely distributed glomerular density in biopsies of kidney transplant donors with or without obesity, suggesting that the difference was not simply related to BMI. In addition, the low glomerular density in these obese patients is not likely to be due to glomerular injury, as indicated by the difference in glomerular density between the obese patients and kidney transplant donors, even when globally sclerotic glomeruli were included in the calculation of glomerular density. In addition, the glomerular density in these obese patients was significantly lower than that in patients with IgA nephropathy or membranous nephropathy who had chronic renal injuries of similar severity.20, 21 Finally, hypertension may be associated with the low glomerular density in obesity-associated glomerulopathy, as a previous autopsy study showed a relationship between essential hypertension and kidneys with a small number of nephrons.22 In fact, 80% of our patients in group 1 already had hypertension at the time of biopsy. However, the glomerular density did not differ between normotensive grade 1 obesity patients and hypertensive grade 1 obesity patients in the current series (data not shown), suggesting that hypertension is unlikely to be the only factor associated with the low glomerular density in these patients.
Although the origin of glomerulomegaly in patients with ORG is incompletely understood, several plausible explanations for this typical morphologic feature of ORG have been suggested. These include increased sympathetic nerve activity and/or systemic blood pressure, intraglomerular hypertension mediated by an increase in salt reabsorption and vasodilation of the afferent arterioles, and an increase in the number of glomerular capillaries due to angiogenesis-promoting factors.7, 23, 24, 25 In addition to these obesity-associated hemodynamic and humoral changes, a reduced total number of nephrons may lead to glomerular enlargement in patients with ORG. Recent autopsy studies have demonstrated a considerably greater variability in the nephron number in the general population than had been suspected.26, 27, 28 In addition, the number of nephrons is correlated with birth weight, and low birth weight, which is associated with an inappropriate intrauterine environment, is postulated to be a risk factor for hypertension and progression of renal diseases in later life.29, 30 Therefore, glomerular hyperfiltration may be markedly greater in individuals born with a substantially reduced number of nephrons and who then develop obesity. Together with the current results, these findings support the possibility that, in addition to obesity, renal factor(s) such as a reduced number of total nephrons may underlie the susceptibility to renal injuries associated with obesity.31 This notion is fully consistent with the fact that the absolute risk of progressive renal deterioration in an obese individual is low and only a minority of obese individuals actually develop glomerular injury. However, whether the glomerular density in a renal biopsy specimen represents the total number of nephrons in the kidney is unclear, because data on the birth weight and total cortical volume of the kidney were not available. Therefore, the finding of a low glomerular density does not demonstrate conclusively that the subjects had a small number of glomeruli. Determining the origin of the low glomerular density requires further investigation.
A significant proportion of the FSGS lesions in our patients in each group were of the perihilar variant. Other FSGS lesions found in these patients were of the not-otherwise-specified variant; the other FSGS variants (the cellular, collapsing, and tip variants) were not identified in this study. These results are consistent with previous reports that FSGS lesions in patients with ORG are mainly of the perihilar or not-otherwise-specified variant, and other variants are identified only rarely.1 However, the possibility remains that primary FSGS occurred in the presence of obesity in our patients, as the cause of idiopathic FSGS is unclear and idiopathic FSGS is diagnosed by the exclusion of FSGS of secondary causes.17
In conclusion, our findings suggest that the glomerulopathy in individuals with grade 1 obesity without other known renal diseases is similar to that in individuals with grade 2 or higher obesity in terms of the clinicopathologic features. Therefore, a glomerulopathy similar to ORG can occur in moderately obese subjects. Thus, renal factor(s) other than BMI may underlie the susceptibility to this disease entity.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Parts of this study were presented at the American Society of Nephrology Kidney Week 2014, November 2014, Philadelphia, PA. This study was supported by a Grant-in-Aid for Scientific Research (C) (NT).
References
- 1.Kambham N., Markowiz G.S., Valeri A.M. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 2.Praga M., Hernandez E., Morales E. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 3.Chen H.M., Li S.J., Chen H.P. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 4.Tsuboi N., Koike K., Hirano K. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol. 2013;17:379–385. doi: 10.1007/s10157-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 5.Chagnac A., Weinstein T., Korzets A. Glomerular hemodynamics in severe obesity. Am J Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 6.Chagnac A., Weinstein T., Herman M. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboi N., Utsunomiya Y., Hosoya T. Obesity-related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28(suppl 4):iv108–v113. doi: 10.1093/ndt/gft258. [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi N., Utsunomiya Y., Kanzaki G. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7:735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 9.Examination Committee of Criteria for “Obesity Disease” in Japan. Japan Society for the Study of Obesity New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 10.Iseki K., Ikemiya Y., Kinjo K. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 11.Fox C.S., Larson M.G., Leip E.P. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 12.Gelber R.P., Kurth T., Kausz A.T. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Goumenous D.S., Kawar B., El Nahas M. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24:3732–3738. doi: 10.1093/ndt/gfp329. [DOI] [PubMed] [Google Scholar]
- 14.Kato S., Nazneen A., Nakashima Y. Pathological influence of obesity on renal structural changes in chronic kidney disease. Clin Exp Nephrol. 2009;13:332–340. doi: 10.1007/s10157-009-0169-3. [DOI] [PubMed] [Google Scholar]
- 15.Japan Society for the Study of Obesity New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S., Imai E., Horio M., Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.D'Agati V.D. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 18.Weibel ER. Stereological Methods: Practical Methods of Biological Morphometry, Vol. 1. London: Academic Press; 1979: 44–45, 131–134.
- 19.Fulladosa X., Moreso F., Narva’ez J.A. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003;14:2662–2668. doi: 10.1097/01.asn.0000088025.33462.b0. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi N., Kawamura T., Koike K. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuboi N., Kawamura T., Miyazaki Y. Low glomerular density is a risk factor for progression in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2011;26:3555–3560. doi: 10.1093/ndt/gfr399. [DOI] [PubMed] [Google Scholar]
- 22.Keller G., Zimmer G., Mall G. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 23.Wahba I.M., Mak R.H. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 24.Griffin K.A., Kramer H., Bidani A.K. Adverse renal consequence of obesity. Am J Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y., Liu Z., Xiang Z. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 26.Nyengaard J.R., Bendtsen T.F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 27.Mañalich R., Reyes L., Herrera M. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 28.Hughson M., Farris A.B., III, Douglas-Denton R. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;83:S32–S37. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 29.Zanndi-Nejad K., Luyckx V.A., Brenner B.M. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:1–7. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 30.Ritz E., Amann K., Koleganova N., Benz K. Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol. 2011;7:137–144. doi: 10.1038/nrneph.2011.1. [DOI] [PubMed] [Google Scholar]
- 31.Praga M. Synergy of low nephron number and obesity: new focus on hyperfiltration nephropathy. Nephrol Dial Transplant. 2005;20:2594–2597. doi: 10.1093/ndt/gfi201. [DOI] [PubMed] [Google Scholar]