Abstract
Introduction
Chronic kidney disease (CKD) is often associated with poor hypertension control and treatment resistance, but whether CKD modifies the effect of hypertension control on outcomes is unknown.
Methods
We studied 10-year mortality and cardiovascular events according to hypertension control status and CKD (glomerular filtration rate <60 ml/min/1.73m2) in 4262 community-dwelling individuals (40% men) more than 65 years of age.
Results
At baseline, 19% had CKD, and 31.2% had controlled hypertension on ≤3 antihypertensive drugs, 62.3% uncontrolled hypertension ≥140/90 mm Hg on ≤2 drugs, and 6.5% apparent treatment-resistant hypertension (aTRH) ≥140/90 mm Hg with ≥3 drugs or use of ≥4 drugs regardless of level. There were 1115 deaths (305 total cardiovascular deaths) and 274 incident nonfatal or fatal strokes or coronary events. Compared to the reference group (controlled hypertension and no CKD), participants without CKD and with uncontrolled hypertension or aTRH had adjusted hazard ratios for all-cause mortality of 0.86 (0.74−1.01) and 1.09 (0.82−1.46), and those with CKD and controlled or uncontrolled hypertension, or aTRH, of 1.33 (1.06−1.68), 1.14 (0.93−1.39), and 1.34 (0.98−1.85), respectively. Participants with aTRH and CKD had a risk of coronary death more than 3 times higher than that of the reference group; participants with aTHR, with or without CKD, had a risk of stroke more than twice as high, and those with CKD but controlled hypertension a 2 times higher risk for cardiovascular deaths from other causes.
Discussion
CKD does not appear to amplify the risk of stroke and coronary events associated with aTRH in this older population. The reasons for excess cardiovascular mortality from other causes associated with controlled hypertension require further study.
Keywords: cardiovascular, chronic kidney disease, hypertension
Uncontrolled hypertension is common in patients with chronic kidney disease (CKD), including a substantial number who have treatment-resistant hypertension.1, 2, 3, 4, 5 Apparent treatment-resistant hypertension (aTRH), defined as uncontrolled blood pressure (BP), that is, ≥140/90 mm Hg while treated with 3 different antihypertensive drug classes or using 4 or more drug classes, regardless of BP level, is observed in 20% to 40% of people with CKD and treated for hypertension.5, 6, 7, 8, 9 Uncontrolled hypertension and aTRH have been associated with higher risks of all-cause mortality and major cardiovascular events in both the general population10, 11, 12 and in CKD cohorts.9, 13, 14 The optimal level of BP control, however, is still debated, particularly for the older population and for individuals with CKD. Target recommendations have recently risen from <130/80 mm 0/80 to <140/90 for CKD patients and up to <150/90 in the older population.15, 16
A recent meta-analysis of randomized clinical trials showed clear effects of intensive treatment to lower BP on combined major cardiovascular events, but less consistent findings for all-cause mortality and heart failure.17 The recent Systolic Blood Pressure Intervention Trial (SPRINT), however, reported a significant 25% risk reduction in major cardiovascular events and 27% reduction in all-cause mortality, with intensive versus standard BP control (systolic BP of <120 vs. <140 mm Hg) in adults with hypertension but without diabetes.18 Notably, the beneficial role of such strict BP control was seen in middle-aged to elderly people, including those 75 years and older. Neither the meta-analysis nor SPRINT, however, demonstrated that intensive BP control significantly affects outcomes in patients with CKD, although there was a 27% risk reduction for mortality of borderline significance in SPRINT. These results are consistent with observational studies showing no advantage and even higher mortality risk associated with achieving BP control of <130/90 mm Hg in CKD patients,19, 20, 21 particularly those on dialysis,19, 22, 23 and in community-dwelling frail elderly people treated with multiple antihypertensive agents.24 These observations may call into question the recommendation for strict BP control in elderly individuals with CKD. Nevertheless, insufficient data are available about whether CKD modifies the prognosis of uncontrolled and treatment-resistant hypertension in this population.
To test our hypothesis that CKD might modify the relation between hypertension control and outcomes in older populations, we studied the interaction between CKD and uncontrolled hypertension or aTRH in their relations to all-cause mortality and major cardiovascular events among hypertension-treated elderly participants in the population-based Three-City Study.
Materials and Methods
Study Design and Participants
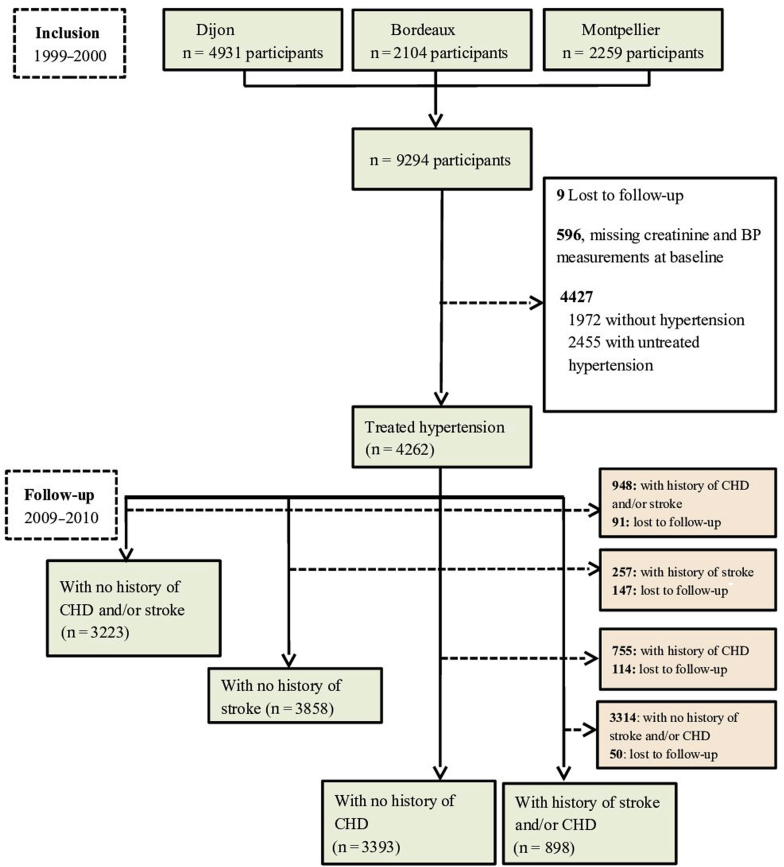
The Three-City Study is a population-based prospective cohort that included 9294 noninstitutionalized individuals aged 65 years or older who were randomly selected from electoral rolls of 3 French cities from March 1999 through March 2001: Bordeaux (2104), Dijon (4931), and Montpellier (2259). Details of the study protocol have been published elsewhere.25 Both BP and kidney function were measured at baseline in 8689 participants, 4262 of whom were then being treated for arterial hypertension (Figure 1).
Figure 1.
Flowchart of the study participants. BP, blood pressure; CHD, coronary heart disease.
The institutional review committee of Kremlin-Bicêtre University Hospital approved the study protocol, and all participants provided written informed consent.
Assessment of Hypertension Control
Blood pressure was measured twice (5 minutes separated the 2 measurements), most often at the participant’s home (61%), after at least 5 minutes at rest in a seated position by trained nurses using a validated digital electronic sphygmomanometer with an appropriately sized cuff on the right arm (OMRON M4; OMRON Corp., Kyoto, Japan).26 The mean of these 2 BP measurements was used in the analyses.
Hypertension was defined as controlled if the mean systolic and diastolic BP were <140 mm Hg and <90 mm Hg, respectively, for participants taking 1 to 3 antihypertensive drug classes (cHT), and as uncontrolled, but nonresistant, if it was ≥140 mm Hg and/or ≥90 mm Hg with 2 drugs (ucHT); apparent treatment-resistant hypertension (aTRH) was defined as BP of ≥140 mm Hg and/or ≥90 mm Hg in participants receiving ≥3 antihypertensive drug classes or ≥ 4, regardless of BP level.27, 28 In sensitivity analyses, we defined aTRH including the use of diuretics as a criterion as follows: BP of ≥140/90 mm Hg in participants receiving ≥3 antihypertensive drug classes, 1 of them a diuretic, or ≥4, regardless of BP level; consequently, the definition for uncontrolled hypertension changed for BP of ≥140/90 mm Hg with 2 drugs or with 3 drugs excluding a diuretic, whereas that for controlled hypertension remained unchanged.27
Study Outcomes
We studied both all-cause and cardiovascular mortality, overall and by cause: stroke, coronary heart disease, other cardiovascular causes (including heart failure, strict sudden death, myocardiopathy, unlocalized aneurysm, and other cardiovascular deaths) as well as incident fatal and nonfatal stroke and coronary events. In addition, we investigated risk for recurrent strokes and coronary events among participants with a history of these diseases at baseline. All participants were actively followed up to assess 10-year mortality, and only 9 individuals were lost to follow-up. An adjudication committee analyzed and confirmed the causes of death based on all available clinical information collected from hospitalization reports and interviews with the participant's family physician or specialists, nursing home staff (for participants who entered in nursing home during follow-up), or proxy.29 Detailed definitions of the study endpoints have been published elsewhere.25 Two adjudication committees, one for coronary events and another for strokes, validated coding of myocardial infarction, sudden death, and stroke and classified each event according to the International Classification of Diseases—10th Edition (ICD-10).25 Coronary events included definite hospitalized angina, definite myocardial infarction, definite cardiovascular death, coronary balloon dilatation, and coronary artery bypass. Stroke was considered when a new focal neurological deficit of sudden or rapid onset was diagnosed and attributable to a cerebrovascular event that persisted for more than 24 hours. If a participant had multiple cardiovascular events during follow-up, the date of the first event was used in the statistical analyses.25
Assessment of Chronic Kidney Disease
Serum creatinine was measured in a single laboratory with the Jaffé assay and further standardized to the isotope dilution mass spectrometry (IDMS) traceable enzymatic creatinine assay, as described elsewhere.30 We calculated the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation without correcting for race, which was unavailable.30 CKD was defined as an eGFR of <60 ml/min per 1.73 m2.31
Other Data Collection
Trained staff administered standardized questionnaires and performed clinical examinations at baseline. Sociodemographic data, smoking status, and history of cardiovascular diseases (CVD) (coronary heart disease, myocardial infarction, heart failure, stroke, peripheral artery disease, artery surgery, or angioplasty) were recorded. Body mass index (BMI) was calculated and categorized (<25, 25−30, ≥30 kg/m2). We used the World Health Organization's Anatomical Therapeutic chemical classification system to code drugs. All three centers collected blood at baseline. Hypercholesterolemia was defined as use of statins or fasting serum cholesterol of ≥6.2 mmol/l. Diabetes was defined as the current use of antidiabetic drugs and/or fasting serum glucose of ≥7.2 mmol/l, or nonfasting serum glucose of ≥11 mmol/l.
Statistical Analyses
We classified participants into 6 groups combining 2 categories of CKD status based on eGFR < or 60 ml/min per 1.73 m2 and the following 3 categories of hypertension control status: controlled hypertension, uncontrolled hypertension, and aTRH. Participants with controlled hypertension and no CKD formed the reference group for the other 5 groups.
We first compared participants' baseline characteristics among the 6 groups as defined above, with a nonparametric Wilcoxon test, analysis of variance, or a χ2 test, as appropriate. Second, we estimated crude rates of all-cause and cardiovascular mortality, as well as of fatal or nonfatal stroke and coronary heart disease, and other cardiovascular deaths for each group (Figure 1). Follow-up of participants for all outcomes started from the date of interview with BP measurements, which took place between 1999 and 2001, and then participants were examined 5 times over a 10-year period. Participants who did not develop the events of interest at the time point in 2009 to 2010 were censored at this date. For all-cause mortality, participants lost to follow-up (n = 9) were censored at their last follow-up date. As we used a cause-specific Cox regression model, participants who did not develop the outcomes of interest (e.g., stroke or coronary heart disease, or cardiovascular death other than stroke and coronary heart disease) at the time point in 2009 to 2010 were also censored at this time for the study of these outcomes. Those who had no follow-up date for stroke or coronary heart disease were considered lost to follow-up for this event. The administrative censoring date was the date of first event or the date of last visit for that event, or 2009 to 2010 time point. We estimated the hazard ratios (HRs) and 95% confidence intervals (95% CIs) of mortality and cardiovascular outcomes associated with exposure (combined hypertension control status and CKD). In all models, we adjusted for established cardiovascular risk factors including sex, diabetes, body mass index, history of cardiovascular disease, smoking status, hypercholesterolemia, and education level. Third, we estimated incidence rates and adjusted HRs (95% CIs) for fatal or nonfatal stroke or coronary heart diseases associated with hypertension control status and CKD in 3223 participants without a history of either of these diseases at baseline (Figure 1). Similarly, we estimated crude incidence rates (using a person-years method) and adjusted HRs (95% CIs) for first occurrence of nonfatal or fatal stroke and coronary heart diseases separately in 3858 and 3393 participants without a previous stroke or coronary heart disease, respectively. Finally, we estimated incidence rates and adjusted HRs (95% CIs) for recurrent stroke or coronary heart disease, together and separately, in 898 participants with a history of either stroke or coronary heart disease (Figure 1). All HRs were adjusted for sex, diabetes, body mass index, smoking status, hypercholesterolemia, and education level.
In all Cox proportional hazards regression models, we used age at baseline as a time-scale to better control for the confounding effect of age on studied outcomes in this longitudinal study comprising elderly participants.32, 33 We formally tested the interaction between CKD and hypertension control status in their relations with all studied outcomes by using Cox models including hypertension control status and CKD separately, as well as an interaction term. Similarly, we also tested interactions between the variable of interest (combined CKD and hypertension control status) and diabetes, sex, history of CVD, and body mass index (BMI) in relation to the outcomes studied. The proportional hazards assumption was tested for all models using the Schoenfeld residuals method. All analyses were conducted with SAS software 9.4 (SAS Institute, Cary, NC); all probabilities were 2-tailed, and a P value of ≤0.05 was considered as statistically significant.
Results
Participant Characteristics
At baseline, the mean age of the 4262 study participants treated for hypertension was 75.1 ± 5.6 years; 40% were men and 60% were women. Overall, 31.2% had controlled hypertension, 62.3% uncontrolled hypertension, and 6.5% aTRH; 19.1% had CKD. In those with CKD, 57.0% had uncontrolled hypertension and 11.8% aTRH, compared to 63.6% and 5.2%, respectively, of those without CKD. Participants with CKD or uncontrolled hypertension or aTRH were on average older, more often men, current or former smokers, or obese, and more often had diabetes, hypercholesterolemia, and a history of CVD than their counterparts with controlled hypertension and no CKD (Table 1). The maximum follow-up was 10 years irrespective of the outcome of interest. Median systolic and diastolic BPs were of the same order of magnitude in participants with and without CKD for those with controlled or uncontrolled hypertension, but systolic BP was higher in participants with aTRH and CKD. The percentage of participants on more than 4 antihypertensive drug classes was twice as high in those with compared to those without CKD.
Table 1.
Participant baseline characteristics according to CKD and hypertension control status
| Baseline characteristic | Without CKD (n = 3450) |
With CKD (n = 812) |
P valuea | ||||
|---|---|---|---|---|---|---|---|
| cHT | ucHT | aTRH | cHT | ucHT | aTRH | ||
| n = 1077 | n = 2194 | n = 179 | n = 253 | n = 463 | n = 96 | ||
| Age, yrc | 74.4 ± 5.3 | 74.6 ± 5.4 | 75.1 ± 5.6 | 76.9 ± 5.8 | 77.7 ± 5.8 | 77.9 ± 6.1 | <0.001 |
| Men | 31.8 | 43.6 | 46.9 | 32.8 | 37.4 | 44.8 | <0.001 |
| Low education level | 20.7 | 20.8 | 24.6 | 17.8 | 24.6 | 16.7 | 0.170 |
| Current or former smoker | 34.3 | 40.2 | 43.6 | 37.9 | 37.8 | 43.8 | 0.016 |
| BMI ≥30 kg/m2 | 17.2 | 17.5 | 29.1 | 17.4 | 17.1 | 27.1 | <0.001 |
| Hypercholesterolemia | 57.4 | 57.2 | 56.4 | 67.6 | 58.3 | 62.5 | 0.047 |
| Diabetesb | 11.0 | 14.3 | 31.3 | 9.5 | 10.8 | 29.2 | <0.001 |
| History of CVD | 13.2 | 10.5 | 24.6 | 18.2 | 12.1 | 32.3 | <0.001 |
| eGFR, ml/min/1.73m2c | 79.4 ± 13.2 | 79.0 ± 12.7 | 79.8 ± 14.6 | 50.3 ± 8.2 | 50.8 ± 7.9 | 48.1 ± 8.7 | <0.001 |
| Blood pressure, mm Hg | |||||||
| SBP, median (IQR) | 129 (121–135) | 157 (148–170) | 159 (149–175) | 130 (122–135) | 157 (148–172) | 167 (151–179) | |
| DBP, median (IQR) | 75 (69–80) | 87 (80–94) | 86 (80–93) | 73 (67–80) | 86 (79–93) | 85 (79–91) | |
| No. of drugs, median (IQR)d | 5 (4–7) | 5 (3–7) | 7 (6–9) | 6 (4–8) | 5 (4–7) | 7 (6–9) | |
| Antihypertensive drugs | |||||||
| 1 class | 66.5 | 68.5 | 50.2 | 57.0 | |||
| 2 classes | 28.1 | 31.5 | 37.9 | 42.9 | |||
| 3 classes | 5.4 | 89.9 | 11.8 | 79.2 | |||
| ≥4 classes | 9.1 | 20.8 | |||||
All values are percentages if not indicated otherwise.
aTRH, apparent treatment-resistant hypertension (defined as systolic and diastolic blood pressure ≥140/90 while taking ≥3 antihypertensive drugs or number of antihypertensive drugs ≥4); BMI, body mass index; CKD, chronic kidney disease (defined as estimated glomerular filtration rate <60 ml/min/1.73 m2); cHT, controlled hypertension (defined as systolic and diastolic blood pressure <140/90 mm Hg while taking 1−3 antihypertensive drugs); CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate calculated with the Modification of Diet for Renal Diseases equation; IQR, interquartile range; SPB, systolic blood pressure; ucHT, uncontrolled hypertension (defined as systolic and diastolic blood pressure ≥140/90 while taking 1 or 2 antihypertensive drugs).
P value for global comparison of baseline characteristics of participants according to hypertension control and CKD status.
Diabetes defined as use of antidiabetic medication or fasting glycemia ≥7.2 mmol/l or nonfasting glycemia ≥11 mmol/l.
Values are mean ± SD.
No. of drugs refers to total number of drug classes.
All-Cause and Cardiovascular Mortality According to CKD and Hypertension Control Status
Over a median (interquartile range) follow-up of 8.8 (7.6−9.4) years, 9 participants were lost to follow-up for mortality, and 1115 deaths were reported, 305 of them from cardiovascular causes: 38 from stroke, 79 from coronary heart disease, and 188 from other cardiovascular causes. Compared with the reference group with controlled hypertension and no CKD, participants with uncontrolled hypertension or aTRH and no CKD did not have a higher risk of all-cause or cardiovascular mortality after multivariable adjustment (Table 2). In participants with CKD, controlled hypertension was associated with a significantly higher risk of all-cause and cardiovascular mortality than in the reference group, mainly from causes other than stroke and coronary heart disease (73% from heart failure), whereas aTRH was associated with a significantly higher risk of mortality from coronary heart disease (Table 2). Uncontrolled hypertension in participants with CKD was not associated with higher mortality than in the reference group. Because of the small number of stroke deaths (7 among the 812 participants with CKD, and only 1 among the 96 with aTRH), we were unable to estimate HRs for this outcome. Interactions between CKD and hypertension control status in relation to all-cause or stroke and coronary heart disease mortality were not statistically significant, but the interaction with the relation to cardiovascular mortality from other causes did approach statistical significance (P for interaction = 0.07). No significant interaction was found with sex, BMI, diabetes, or history of CVD in the relations that we studied. The inclusion of diuretic use as a criterion in the definition of aTRH yielded similar results (Supplementary Table S1).
Table 2.
Crude mortality rates and adjusted hazard ratios for all-cause and cardiovascular deaths according to CKD and hypertension control status
| Mortality | n | Person-years | Events | Crude IR [95% CI] per 1000 person-years | Adjusted HR [95% CI]a | P for interactionb |
|---|---|---|---|---|---|---|
| All-cause | 0.95 | |||||
| All participants | 4262 | 33,904 | 1115 | 32.9 [31.0–34.9] | ||
| Without CKD | ||||||
| cHT (Ref) | 1077 | 8778 | 260 | 29.6 [26.2–33.4] | 1 | |
| ucHT | 2194 | 17,758 | 480 | 27.0 [24.7–29.5] | 0.86 [0.74–1.01] | |
| aTRH | 179 | 1368 | 59 | 43.1 [33.1–55.2] | 1.09 [0.82–1.46] | |
| With CKD | ||||||
| cHT | 253 | 1867 | 100 | 53.5 [43.8–64.8] | 1.33 [1.06–1.68] | |
| ucHT | 463 | 3476 | 170 | 48.9 [42.0–56.7] | 1.14 [0.93–1.39] | |
| aTRH | 96 | 656 | 46 | 70.1 [51.9–92.6] | 1.34 [0.98–1.85] | |
| All cardiovascularc | 0.68 | |||||
| All participants | 4262 | 33,904 | 305 | 9.0 [8.0–10.0] | ||
| Without CKD | ||||||
| cHT (Ref) | 1077 | 8778 | 68 | 7.7 [6.1–9.8] | 1 | |
| ucHT | 2194 | 17,758 | 119 | 6.7 [5.6–8.0] | 0.82 [0.60–1.11] | |
| aTRH | 179 | 1368 | 21 | 15.4 [9.8–23] | 1.34 [0.81–2.23] | |
| With CKD | ||||||
| cHT | 253 | 1867 | 33 | 17.7 [12.4–24.5] | 1.63 [1.07–2.48] | |
| ucHT | 463 | 3476 | 49 | 14.1 [10.6–18.5] | 1.28 [0.87–1.87] | |
| aTRH | 96 | 656 | 15 | 22.8 [13.3–36.7] | 1.56 [0.88–2.77] | |
| Coronary heart disease | 0.21 | |||||
| All participants | 4262 | 33,904 | 79 | 2.3 [1.9–2.9] | ||
| Without CKD | ||||||
| cHT (Ref) | 1077 | 8778 | 15 | 1.7 [1.0–2.7] | 1 | |
| ucHT | 2194 | 17,758 | 36 | 2.0 [1.4–2.8] | 1.01 [0.54–1.88] | |
| aTRH | 179 | 1368 | 3 | 2.2 [0.6–5.9] | 0.71 [0.2–2.53] | |
| With CKD | ||||||
| cHT | 253 | 1867 | 5 | 2.7 [1.0–5.9] | 1.10 [0.40–3.07] | |
| ucHT | 463 | 3476 | 12 | 3.5 [1.9–5.8] | 1.36 [0.62–2.99] | |
| aTRH | 96 | 656 | 8 | 12.2 [5.7–23.0] | 3.27 [1.34–7.99] | |
| Cardiovascular death other than stroke or coronary heart diseased | 0.07 | |||||
| All participants | 4262 | 33,904 | 188 | 5.5 [4.8–6.4] | ||
| Without CKD | ||||||
| cHT (Ref) | 1077 | 8778 | 44 | 5.0 [3.7–6.7] | 1 | |
| ucHT | 2194 | 17,758 | 63 | 3.5 [2.8–4.5] | 0.70 [0.47–1.04] | |
| aTRH | 179 | 1368 | 16 | 11.7 [7.0–18.5] | 1.69 [0.93–3.08] | |
| With CKD | ||||||
| cHT | 253 | 1867 | 26 | 13.9 [9.3–20.1] | 1.94 [1.18–3.18] | |
| ucHT | 463 | 3476 | 33 | 9.5 [6.7–13.2] | 1.37 [0.86–2.19] | |
| aTRH | 96 | 656 | 6 | 9.1 [3.8–18.8] | 1.02 [0.43–2.42] |
aTRH, apparent treatment-resistant hypertension (systolic and/or diastolic blood pressure ≥140 and/or ≥90 while taking ≥3 antihypertensive drugs or number of antihypertensive drugs ≥4); CHD, coronary heart diseases; cHT, controlled hypertension; CKD, chronic kidney disease (defined as estimated glomerular filtration rate <60 ml/min/1.73m2; cHT: systolic and diastolic blood pressure <140/90 mm Hg while taking 1−3 antihypertensive drugs); HR, hazard ratio; IR, incidence rate; ucHT, uncontrolled hypertension (defined as systolic and/or diastolic blood pressure ≥140 and/or ≥90 while taking 1 or 2 antihypertensive drugs).
All models were adjusted for center, sex, diabetes (defined as use of antidiabetic medication or fasting glycemia ≥7.2 mmol/L or nonfasting glycemia ≥11 mmol/l), history of cardiovascular events, body mass index, hypercholesterolemia, smoking status, and education level.
All interaction between hypertension control status and chronic kidney disease.
All cardiovascular mortality included deaths from stroke, coronary heart disease, strict sudden death, heart failure, and other cardiovascular deaths.
Cardiovascular deaths other than stroke or coronary heart disease included heart failure, strict sudden death, myocardiopathy, unlocalized aneurysm, and other cardiovascular deaths.
Incident Stroke and Coronary Events According to CKD and Hypertension Control Status
For combined nonfatal and fatal strokes or coronary heart disease, the median follow-up (interquartile range) was 8.4 (5.4−9.2) years, 8.5 (5.6–9.2) for nonfatal and fatal strokes, and 8.4 (5.5–9.2) for nonfatal and fatal coronary heart diseases. A total of 169 participants had no follow-up date for nonfatal and fatal stroke, 155 for nonfatal and fatal coronary heart disease, and 141 for both. Because of the exclusion of prevalent cases at baseline, the number of participants lost to follow-up for these events varies. During follow-up, 178 incident fatal or nonfatal strokes and 225 coronary events were reported; 349 participants had 1 or both events. Cause-specific Cox regression models showed that, compared with the reference group, participants with uncontrolled hypertension and no CKD had a 50% higher risk of stroke, and those with aTRH had a risk more than 2 times higher, whether or not they had CKD (Table 3). There was no significant interaction with CKD in the association between hypertension control status and risk of stroke. Neither aTRH nor uncontrolled hypertension was significantly associated with a higher risk of coronary events, with or without CKD. In the sensitivity analysis, the use of the diuretic drug criterion in the definition of aTRH tended to weaken the HR estimates for stroke (Supplementary Table S2).
Table 3.
Crude incidence rates and adjusted hazard ratios for first fatal and nonfatal stroke or coronary heart disease according to CKD and hypertension control status
| Baseline characteristic | n | Person-years | Events | Crude IR [95% CI] per 1000 person-years | Adjusted HR [95% CI]a | P for interactionb |
|---|---|---|---|---|---|---|
| Stroke or coronary heart diseasec | 0.74 | |||||
| All participants | 3223 | 23,245 | 349 | 15 [13.5–16.7] | ||
| Without CKD | ||||||
| cHT | 786 | 5872 | 75 | 12.8 [10.1–15.9] | 1 | |
| ucHT | 1745 | 12,650 | 194 | 15.3 [13.3–17.6] | 1.01 [0.77–1.32] | |
| aTRH | 113 | 762 | 20 | 26.2 [16.5–39.7] | 1.50 [0.90–2.47] | |
| With CKD | ||||||
| cHT | 168 | 1184 | 15 | 12.7 [7.4–20.4] | 0.91 [0.52–1.59] | |
| ucHT | 356 | 2405 | 38 | 15.8 [11.4–21.4] | 0.95 [0.64–1.42] | |
| aTRH | 55 | 370 | 7 | 18.9 [8.4–37.1] | 0.98 [0.45–2.15] | |
| Stroke | 0.87 | |||||
| All participants | 3858 | 28,284 | 178 | 6.3 [5.4–7.3] | ||
| Without CKD | ||||||
| cHT | 970 | 7354 | 31 | 4.2 [2.9–5.9] | 1 | |
| ucHT | 2034 | 15,071 | 103 | 6.8 [5.6–8.3] | 1.51 [1.00–2.28] | |
| aTRH | 157 | 1088 | 12 | 11.0 [6–18.7] | 2.33 [1.18–4.61] | |
| With CKD | ||||||
| cHT | 219 | 1535 | 8 | 5.2 [2.5–9.8] | 1.08 [0.49–2.35] | |
| ucHT | 404 | 2750 | 18 | 6.5 [4.0–10.1] | 1.27 [0.70–2.29] | |
| aTRH | 74 | 485 | 6 | 12.4 [5.1–25.5] | 2.12 [0.87–5.17] | |
| Coronary heart disease | 0.68 | |||||
| All participants | 3393 | 24,588 | 225 | 9.2 [8.0–10.4] | ||
| Without CKD | ||||||
| cHT | 829 | 6305 | 50 | 8.1 [6.0–10.5] | 1 | |
| ucHT | 1821 | 13,449 | 121 | 9.1 [7.6–10.8] | 0.92 [0.65–1.28] | |
| aTRH | 124 | 861 | 12 | 14.0 [7.7–23.8] | 1.14 [0.59–2.17] | |
| With CKD | ||||||
| cHT | 175 | 1263 | 10 | 8.0 [4.1–14.2] | 0.94 [0.47–1.86] | |
| ucHT | 380 | 2625 | 24 | 9.3 [6.1–13.6] | 0.89 [0.54–1.47] | |
| aTRH | 64 | 403 | 8 | 19.9 [9.4–37.5] | 1.69 [0.79–3.62] |
aTRH, apparent treatment-resistant hypertension (defined as systolic and/or diastolic blood pressure ≥140 and/or ≥90 while taking ≥3 antihypertensive drugs or number of antihypertensive drugs ≥4); cHT, controlled hypertension (defined as systolic and diastolic blood pressure <140/90 mm Hg while taking 1−3 antihypertensive drugs); CKD, chronic kidney disease (defined as estimated glomerular filtration rate < 60 mL/min/1.73m2); HR, hazard ratio; IR, incidence rate; ucHT, uncontrolled hypertension (defined as systolic and/or diastolic blood pressure ≥140 and/or ≥90 while taking 1 or 2 antihypertensive drugs).
cCombined incident fatal and nonfatal stroke or coronary heart disease: defined as the first occurrence of stroke or coronary heart disease, whichever occurred first. (Participants with history of stroke and/or coronary heart disease, stroke, or coronary heart disease were excluded when estimating the risk for combined fatal and nonfatal incident stroke or coronary heart disease, incident stroke, and incident coronary heart disease respectively).
All models were adjusted for center, sex, diabetes, history of cardiovascular events, body mass index, hypercholesterolemia, smoking status and education level.
All interaction between hypertension control status and chronic kidney disease.
Combined incident fatal and nonfatal stroke or coronary heart disease: defined as the first occurrence of stroke or coronary heart disease, whichever occurred first. (Participants with history of stroke and/or coronary heart disease, stroke, or coronary heart disease were excluded when estimating the risk for combined fatal and nonfatal incident stroke or coronary heart disease, incident stroke, and incident coronary heart disease respectively).
Recurrent Stroke and Coronary Events According to CKD and Hypertension Control Status
For combined recurrent strokes or coronary heart diseases, the median follow-up (interquartile range) was 6.4 (3.5–8.8) years, 6.1 (3.5–8.6) for recurrent strokes, and 6.8 (3.6–8.9) for recurrent coronary heart diseases. Participants with CKD and uncontrolled hypertension or aTRH had a risk of combined recurrent stroke or coronary event (i.e., any second stroke or coronary event) that was approximately twice as high as that in the reference group, mostly due to coronary heart disease, whereas their counterparts without CKD had no such excess risk (Table 4). The interaction between CKD and hypertension control status, however, was not statistically significant. As above, the sensitivity analysis tended to weaken the HR estimates (Supplementary Table S3).
Table 4.
Crude incidence rates and adjusted hazard ratios for recurrent fatal or nonfatal stroke or coronary heart disease according to CKD and hypertension control status
| Baseline characteristics | Number | Person-years | Events | Crude IR [95% CI] (per 1000 person-years) | Adjusted HR [95% CI]a | P for interactionb |
|---|---|---|---|---|---|---|
| Stroke or coronary heart diseasec | 0.10 | |||||
| All participants | 898 | 5373 | 204 | 38.0 [33.0–43.5] | ||
| Without CKD | ||||||
| cHT | 245 | 1554 | 50 | 32.2 [24.2–42.1] | 1 | |
| ucHT | 404 | 2492 | 94 | 37.7 [30.7–45.9] | 1.08 [0.76–1.53] | |
| aTRH | 59 | 381 | 8 | 21.0 [9.9–39.6] | 0.60 [0.28–1.29] | |
| With CKD | ||||||
| cHT | 66 | 352 | 13 | 36.9 [20.7–61.3] | 1.10 [0.59–2.06] | |
| ucHT | 89 | 434 | 25 | 57.6 [38.2–83.6] | 1.70 [1.04–2.80] | |
| aTRH | 35 | 159 | 14 | 87.8 [50.3–143.3] | 2.15 [1.16–3.98] | |
| Stroke | ||||||
| All participants | 235 | 1381 | 32 | 23.2 [16.1–32.3] | ||
| Without CKD | ||||||
| cHT | 59 | 377 | 6 | 15.9 [6.6–32.8] | 1 | |
| ucHT | 106 | 636 | 16 | 25.2 [15.0–39.9] | Not estimated | |
| aTRH | 14 | 90 | 1 | 11.1 [1.0–51.8] | Not estimated | |
| With CKD | ||||||
| cHT | 10 | 49 | 0 | Not estimated | Not estimated | |
| ucHT | 34 | 165 | 7 | 42.3 [18.9–83.1] | Not estimated | |
| aTRH | 12 | 64 | 2 | 31.0 [6.2–99.4] | Not estimated | |
| Coronary heart disease | 0.13 | |||||
| All participants | 714 | 4379 | 143 | 32.7 [27.6–38.3] | ||
| Without CKD | ||||||
| cHT | 198 | 1270 | 38 | 29.9 [21.5–40.6] | 1 | |
| ucHT | 322 | 2063 | 64 | 31 [24.1–39.3] | 1.00 [0.66–1.52] | |
| aTRH | 48 | 310 | 4 | 12.9 [4.3–30.7] | 0.40 [0.14–1.15] | |
| With CKD | ||||||
| cHT | 59 | 322 | 12 | 37.3 [20.4–63.1] | 1.25 [0.64–2.43] | |
| ucHT | 61 | 299 | 16 | 53.4 [31.8–84.7] | 1.98 [1.08–3.64] | |
| aTRH | 26 | 114 | 9 | 78.5 [38.8–143.2] | 2.04 [0.95–4.37] |
aTRH, apparent treatment-resistant hypertension (defined as systolic and/or diastolic blood pressure ≥140 and/or ≥90 while taking ≥3 antihypertensive drugs or number of antihypertensive drugs ≥4); cHT, controlled hypertension (defined as systolic and diastolic blood pressure <140/90 mm Hg while taking 1−3 antihypertensive drugs); CKD, chronic kidney disease (defined as estimated glomerular filtration rate <60 mL/min/1.73m2); HR, hazard ratio; IR, incidence rate; ucHT, uncontrolled hypertension (systolic and/or diastolic blood pressure ≥140 and /or ≥90 while taking 1 or 2 antihypertensive drugs).
cCombined fatal and nonfatal stroke and CHD: defined as the recurrence of either a stroke or CHD.
All models were adjusted for center, sex, diabetes, body mass index, hypercholesterolemia, smoking status, and education level.
All interactions between hypertension control status and chronic kidney disease.
Combined fatal and nonfatal stroke and CHD: defined as the recurrence of either a stroke or CHD.
Discussion
In this community-dwelling elderly population, participants with CKD had an overall higher risk of all-cause and cardiovascular mortality, regardless of their hypertension control status. As expected, both aTRH and uncontrolled hypertension were associated with higher risks of incident stroke, apparently unmodified by CKD; participants with both CKD and aTRH also had significantly higher risks of coronary death and of combined recurrent stroke or coronary events. The most intriguing finding was the excess cardiovascular mortality risk from other causes, mainly heart failure, observed in participants with CKD and well-controlled hypertension.
The findings of our study are difficult to compare with those of others, which have usually included younger participants, have not systematically assessed CKD as a potential effect modifier in the relation of aTRH with outcomes, and have sometimes used BP values of ≥130/80 mm Hg rather than ≥140/90 to define poor BP control in individuals with CKD. It was nevertheless somewhat surprising to find that aTRH in these elderly participants was not significantly associated with a higher risk of all-cause mortality. This finding differs from some,10, 11, 34 although not all,12, 35 non-CKD cohort studies that have compared all-cause mortality between individuals with and without aTRH after adjusting for CKD. In the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT), aTRH did not significantly increase all-cause mortality in participants older than 65 years.34 The somewhat higher HR for all-cause mortality associated with aTRH in our study in participants with CKD compared with the reference group (with controlled hypertension and no CKD) is probably due to the well-established higher risk conferred by CKD,36 which we have previously reported for this population,30 rather than to aTRH. Among participants with CKD, those with aTRH were not at higher risk than those with controlled hypertension, in contrast to findings from 2 other CKD cohorts.9, 13
Our findings regarding cardiovascular risk are consistent with previous studies for several points but differ for others. First, we observed a risk of mortality from coronary heart disease associated with aTRH in participants with CKD, but not in those without CKD, and no significantly higher rate of coronary heart disease events. Several studies have shown higher rates of coronary heart disease events associated with aTRH, both before and after adjustment for CKD.11, 12, 37 Discrepancies in findings about coronary heart disease events between these studies and ours may be due to a lack of power in our study. It may also result, however, from the impact of CKD per se on mortality rather than on the incidence of coronary heart disease in relation to aTRH. Support for this hypothesis comes from repeated findings that the concomitant presence of CKD increases mortality risk in a variety of chronic diseases.38, 39
Second, participants with aTRH had a risk of incident stroke that was more than twice as high as that in the reference group, with HRs of similar magnitude with or without CKD. This was true for either aTRH definition that we used, although study power was stronger without diuretic use as a criterion. Participants with uncontrolled hypertension were also at higher risk for stroke, although the association was statistically significant only in those without CKD. These findings confirm the well-established role of hypertension in the occurrence of stroke.12, 34, 35 In addition, we showed that CKD appears to influence the recurrence of stroke and coronary heart disease.
One of the most striking observations of our study is that participants with CKD and controlled hypertension were at significantly higher risk for cardiovascular mortality from other causes than those with controlled hypertension without CKD. In ALLHAT, patients with aTRH were at higher risk for fatal, hospitalized, or treated nonhospitalized heart failure.34 Although unexpected, this finding is in line with some studies showing an adverse effect of excessive BP reduction,24 demonstrated by the J-shaped association between BP and mortality risk in patients with CKD, whether or not they were on dialysis.20, 40, 41 In apparent contrast, SPRINT, which compared the effect of tight systolic BP control to a level of <120 mm Hg versus standard BP control (<140 mm Hg) in hypertensive patients over a median follow-up of 3.3-years, showed significant risk reductions for cardiovascular outcomes and all-cause mortality, even in the subgroup of patients older than 75 years.18 It also showed a significant reduction in the risk of heart failure. Nonetheless, this trial did not include patients with difficult-to-control hypertension, and there were important adverse events in the intervention arm, especially severe hypotension, syncope, and acute kidney injury, which do not preclude a worse long-term prognosis for these patients.42 Moreover, SPRINT failed to demonstrate that intensive treatment that lowered BP to its target ranges had any effect on outcomes in patients with CKD. In our study, heart failure was classified as the primary cause of death for three-fourths of the participants who died of other cardiovascular diseases. It is possible that a lower BP level at baseline reflects the heart's compromised capacity to maintain an adequate ejection fraction and thus makes it easier to achieve BP control. The frequency of heart failure due to nonischemic heart disease increases as CKD progresses. Coronary atherosclerosis is the most common cause of death in the general population, but not among patients with CKD. In this population, decreased cardiac perfusion of various causes and diffuse interstitial myocardial fibrosis with increased oxygen diffusion distance are the major causes of congestive heart failure, arrhythmia, and sudden cardiac death.43, 44 Moreover, other factors such as concomitant diabetes, electrolyte shifts, divalent ion abnormalities, sympathetic overactivity, impaired baroreflex effectiveness, inflammation, infection, and inappropriate drug use also contribute to the higher risk of mortality in patients with CKD.43, 44, 45, 46 Thus, we cannot exclude the possibility that participants with CKD and controlled hypertension at baseline had low BP due to incipient heart failure.
Major strengths of this study include its large sample size and the low number of participants lost to follow-up for mortality. In addition, standardized BP was measured by trained nurses during in-home examination in the majority of participants, which lessens the risk of white-coat hypertension. Home BP monitoring has been shown to be superior to office BP in predicting target organ damage and all-cause mortality47 and functional decline in elderly individuals.48 Moreover, creatinine measurements were taken in a single laboratory with further calibration to the IDMS reference method, and long-term follow-up and adjudication of cardiovascular events. Of note, we used the MDRD equation with which eGFR was normally distributed, in contrast to the CKD-EPI equation in this elderly population. Previous analyses showed that both equations provided similar prevalence estimates of CKD8 and similar hazard ratios of all-cause and cardiovascular mortality associated with CKD.30
Our study also has limitations. First, we included only elderly participants, precluding extrapolation to other age groups. Second, as treatment adherence and ambulatory BP data were not available, true treatment-resistant hypertension may have been misclassified with pseudo-resistance in a number of cases. Third, albuminuria was not available at baseline and therefore could not be adjusted for in the multivariable analyses or used in the definition of CKD. Fourth, we may have lacked statistical power in some of the associations studied, particularly for stroke events. There was also a significant loss to follow-up for stroke and coronary events, with the ensuing potential selection bias. Finally, the observational design of the study makes causal interpretation impossible.
In conclusion, we found that community-dwelling elderly individuals with both CKD and aTRH were at higher risk for coronary heart disease mortality and incident stroke. In this study, the presence of CKD did not appear to amplify the risks for the occurrence of these 2 disease entities associated with aTRH (P value for interaction not statistically significant), but we cannot rule out that it may enhance the lethality of coronary heart disease (P for interaction = 0.07). Using either definition of aTRH, with or without the diuretic use criteria, did not alter the main conclusion of this study. Adequate monitoring of kidney function and appropriate titration of antihypertensive medication with decreasing GFR may help to decrease aTRH and thereby impede its harmful prognosis. The reasons that older people with CKD and controlled hypertension might be at higher risk for CV mortality from heart failure require further study. Randomized controlled studies are needed to assess whether or not strict BP control, compared to less strict control, is beneficial in elderly people, and whether or not mild-to-moderate CKD is an additional aggravating condition.
Disclosure
CH received lecture fees from a commercial sponsor in November 2014. TBD received consulting and lectures fees during 2014-2015 from Amgen, FMC, and Kirin. BS received consulting fees from MSD and grants from Amgen, Baxter, FMC, Lilly, MSD, and Otsuka for a research project. All the other authors declared no competing interests.
Acknowledgments
The Three-City Study is conducted under a partnership agreement between the Institut National de la Santé and la Recherche Médicale (INSERM), the Victor Segalen Bordeaux II University, and Sanofi-Aventis. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The Fondation Plan Alzheimer partly funded the follow-up of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN, Institut de la Longévité, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, and Ministry of Research–INSERM Programme “Cohortes et collections de données biologiques”. The study also received a grant from the Agence Nationale de la Recherche (ANR). The CKD ancillary study at the 4-year follow-up was funded by the French-speaking Society of Nephrology. No donors played a role in the design and the conduct of the study.
Jean Kaboré is supported by a research grant from the French Ministry of Research.
We thank Jo-Ann Cahn for editing the English version.
Footnotes
Table S1. Crude mortality rates and adjusted hazard ratios for all-cause and cardiovascular deaths according to chronic kidney disease and hypertension control status
Table S2. Crude incidence rates and adjusted hazard ratios for stroke and coronary heart disease according to chronic kidney disease and hypertension control status
Table S3. Crude incidence rates and adjusted hazard ratios for recurrent stroke and coronary heart disease according to chronic kidney disease and hypertension control status
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
Crude mortality rates and adjusted hazard ratios for all-cause and cardiovascular deaths according to chronic kidney disease and hypertension control status
Crude incidence rates and adjusted hazard ratios for stroke and coronary heart disease according to chronic kidney disease and hypertension control status
Crude incidence rates and adjusted hazard ratios for recurrent stroke and coronary heart disease according to chronic kidney disease and hypertension control status
References
- 1.Muntner P., Anderson A., Charleston J. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai G., Zheng Y., Sun X., Chen X. Prevalence, awareness, treatment, and control of hypertension in elderly adults with chronic kidney disease: results from the survey of prevalence, awareness, and treatment rates in chronic kidney disease patients with hypertension in china. J Am Geriatr Soc. 2013;61:2160–2167. doi: 10.1111/jgs.12551. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R., Pappas M.K., Sinha A.D. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2015;27:1–9. doi: 10.1681/ASN.2015030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plantinga L.C., Miller E.R., Stevens L.A. Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension. 2009;54:47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossignol P., Massy Z.A., Azizi M. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015;386:1588–1598. doi: 10.1016/S0140-6736(15)00418-3. [DOI] [PubMed] [Google Scholar]
- 6.Persell S.D. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 7.Tanner R.M., Calhoun D.A., Bell E.K. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583–1590. doi: 10.2215/CJN.00550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaboré J., Metzger M., Helmer C. Kidney function decline and apparent treatment-resistant hypertension in the elderly. PLoS One. 2016;11:e0146056. doi: 10.1371/journal.pone.0146056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Beus E., Bots M.L., van Zuilen A.D. MASTERPLAN Study Group. Prevalence of apparent therapy-resistant hypertension and its effect on outcome in patients with chronic kidney disease. Hypertension. 2015;66:998–1005. doi: 10.1161/HYPERTENSIONAHA.115.05694. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty S.L., Powers J.D., Magid D.J. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irvin M.R., Booth J.N., Shimbo D. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–413. doi: 10.1016/j.jash.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung C.-Y., Wang K.-Y., Wu T.-J. Resistant hypertension, patient characteristics, and risk of stroke. PLoS One. 2014;9:e104362. doi: 10.1371/journal.pone.0104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Nicola L., Gabbai F.B., Agarwal R. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61:2461–2467. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G., Xie D., Chen H.-Y. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387–396. doi: 10.1161/HYPERTENSIONAHA.115.06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James P.A., Oparil S., Carter B.L. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 16.Chrysant S.G. The impact of SPRINT on the future treatment of hypertension: a mini review. Drugs Today (Barc) 2016;52:193–198. doi: 10.1358/dot.2016.52.3.2467721. [DOI] [PubMed] [Google Scholar]
- 17.Xie X., Atkins E., Lv J. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 18.SPRINT Research Group. Wright J.T., Williamson J.D. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zager P.G., Nikolic J., Brown R.H. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 20.Kovesdy C.P., Bleyer A.J., Molnar M.Z. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy C.P., Lu J.L., Molnar M.Z. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174:1–8. doi: 10.1001/jamainternmed.2014.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Port F.K., Hulbert-Shearon T.E., Wolfe R.A. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Lacson E., Lowrie E.G. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Benetos A., Labat C., Rossignol P. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents. JAMA Intern Med. 2015;175:989–995. doi: 10.1001/jamainternmed.2014.8012. [DOI] [PubMed] [Google Scholar]
- 25.3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 26.Brindel P., Hanon O., Dartigues J.-F. Prevalence, awareness, treatment, and control of hypertension in the elderly: the Three City Study. J Hypertens. 2006;24:51–58. doi: 10.1097/01.hjh.0000198028.84353.86. [DOI] [PubMed] [Google Scholar]
- 27.Chobanian A.V., Bakris G.L., Black H.R. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 28.Egan B.M., Zhao Y., Axon R.N. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alpérovitch A., Bertrand M., Jougla E. Do we really know the cause of death of the very old? Comparison between official mortality statistics and cohort study classification. Eur J Epidemiol. 2009;24:669–675. doi: 10.1007/s10654-009-9383-2. [DOI] [PubMed] [Google Scholar]
- 30.Stengel B., Metzger M., Froissart M. Epidemiology and prognostic significance of chronic kidney disease in the elderly—the Three-City prospective cohort study. Nephrol Dial Transplant. 2011;26:3286–3295. doi: 10.1093/ndt/gfr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey A.S., Coresh J., Greene T. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Lamarca R., Alonso J., Gómez G., Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 33.Cologne J., Hsu W.-L., Abbott R.D. Proportional hazards regression in epidemiologic follow-up studies. Epidemiology. 2012;23:565–573. doi: 10.1097/EDE.0b013e318253e418. [DOI] [PubMed] [Google Scholar]
- 34.Muntner P., Davis B.R., Cushman W.C. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2014;64:1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850. [DOI] [PubMed] [Google Scholar]
- 35.Kumbhani D.J., Steg P.G., Cannon C.P. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K., van der Velde M., Astor B.C. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal R., Brim N.J., Mahenthiran J. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 38.Mahmoodi B.K., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380:1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg J.A. Removing confounders from the relationship between mortality risk and systolic blood pressure at low and moderately increased systolic blood pressure. J Hypertens. 2003;21:49–56. doi: 10.1097/00004872-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:830–837. doi: 10.2215/CJN.06201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal R. Hypertension: rest before blood pressure measurement: a lesson from SPRINT. Nat Rev Nephrol. 2016;12:127–128. doi: 10.1038/nrneph.2016.2. [DOI] [PubMed] [Google Scholar]
- 43.Drüeke T.B., Massy Z.A. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723–735. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- 44.Shamseddin M.K., Parfrey P.S. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol. 2011;7:145–154. doi: 10.1038/nrneph.2010.191. [DOI] [PubMed] [Google Scholar]
- 45.Pecoits-Filho R., Bucharles S., Barberato S.H. Diastolic heart failure in dialysis patients: mechanisms, diagnostic approach, and treatment. Semin Dial. 2012;25:35–41. doi: 10.1111/j.1525-139X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 46.Breton G., Froissart M., Janus N. Inappropriate drug use and mortality in community-dwelling elderly with impaired kidney function—the Three-City population-based study. Nephrol Dial Transplant. 2011;26:2852–2859. doi: 10.1093/ndt/gfq827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stergiou G.S., Argyraki K.K., Moyssakis I. Home blood pressure is as reliable as ambulatory blood pressure in predicting target-organ damage in hypertension. Am J Hypertens. 2007;20:616–621. doi: 10.1016/j.amjhyper.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Nishinaga M., Takata J., Okumiya K. High morning home blood pressure is associated with a loss of functional independence in the community-dwelling elderly aged 75 years or older. Hypertens Res. 2005;28:657–663. doi: 10.1291/hypres.28.657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crude mortality rates and adjusted hazard ratios for all-cause and cardiovascular deaths according to chronic kidney disease and hypertension control status
Crude incidence rates and adjusted hazard ratios for stroke and coronary heart disease according to chronic kidney disease and hypertension control status
Crude incidence rates and adjusted hazard ratios for recurrent stroke and coronary heart disease according to chronic kidney disease and hypertension control status