Abstract
In addition to the classic and well-established “feedback control” of potassium balance, increasing investigative attention has focused on a novel and not widely recognized complementary regulatory paradigm for maintaining potassium homeostasis—the “feed-forward control” of potassium balance. This regulatory mechanism, initially defined in rumen, has recently been validated in normal human subjects. Studies are being conducted to determine the location for this putative potassium sensor and to evaluate potential signals, which might increase renal potassium excretion. Awareness of this more updated integrative control mechanism for potassium homeostasis is ever more relevant today, when the medical community is increasingly focused on the challenges of managing the hyperkalemia provoked by renin–angiotensin–aldosterone system inhibitors (RAASis). Recent studies have demonstrated a wide gap between RAASi prescribing guidelines and real-world experience and have highlighted that this gap is thought to be attributable in great part to hyperkalemia. Consequently we require a greater knowledge of the complexities of the regulatory mechanisms subserving potassium homeostasis. Sodium polystyrene sulfonate has long been the mainstay for treating hyperkalemia, but its administration is fraught with challenges related to patient discomfort and colonic necrosis. The current and imminent availability of newer potassium binders with better tolerability and more predictive dose–response potassium removal should enhance the management of hyperkalemia. Consequently it is essential to better understand the intricacies of mammalian colonic K+ handling. We discuss colonic transport of K+ and review evidence for potassium (BK) channels being responsible for increased stool K+ in patients with diseases such as ulcerative colitis.
Keywords: chronic kidney disease, potassium
The integrated mechanisms controlling the maintenance of potassium homeostasis are well established and are defined by the classic “feedback control” of potassium balance. In recent years, increasing investigative attention has focused on novel physiological paradigms that increase the complexity but also the precision of homeostasis. In this Review we will briefly consider the classic and well-established “feedback control” of potassium and then consider subsequent investigations that inform on an intriguing and not widely recognized complementary paradigm—the “feed-forward” control of potassium balance. Awareness of this more updated integrative control mechanism for potassium homeostasis is ever more relevant today at a time when the medical community is increasingly focused on the challenges of managing the hyperkalemia provoked by renin–angiotensin–aldosterone system inhibitors (RAASis).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11
It is well established that RAASis confer substantive benefits, such as reducing cardiovascular events and retarding progression of renal disease in several disease states, including congestive heart failure, chronic kidney disease, and diabetes.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Regretfully, treatment with RAASis can be complicated by hyperkalemia, which is a frequent side effect of RAASi therapy.1, 2, 3, 4, 9, 10, 11 Indeed, the problem will only become increasingly prominent and frequent, because hyperkalemia will remain an issue with newly introduced drugs such as neprolysin inhibitors (LCZ-696)—as reported in the PARADIGM Heart Failure Study.24 Furthermore, the wide gap between RAASi prescribing guidelines and reality is widely thought to be attributable to hyperkalemia.1, 2, 3, 4, 9, 10, 11 As a consequence, we require a greater knowledge of the complexities of the regulatory mechanisms subserving potassium homeostasis. Finally, with the imminent availability of newer polymer resins to control hyperkalemia,2, 4, 5, 6, 7, 9 it is essential to understand how these newer drug agents may interact with the new resin binders. We require effective and safe treatments to control hyperkalemia, which in turn will facilitate treatment with optimal recommended doses of RAASis. Such a treatment paradigm should preclude unwanted down-titration and discontinuation of these life-saving medications, which have recently been documented to positively impact cardiorenal outcomes.3, 9, 10, 11
Normal Potassium Balance and Renal Potassium Excretion
In order to establish the context and foundation for considering newer, albeit poorly recognized adaptive mechanisms for subserving potassium homeostasis, we will first summarize key concepts of steady-state potassium handling. Normal persons who consume a typical Western diet ingest approximately 70 to 80 mmol of potassium per day.25, 26 The intestine absorbs virtually all of the ingested potassium and delivers it to the liver for processing by means of the hepatoportal circulation. In normal circumstances, minimal amounts of potassium are excreted in the feces.
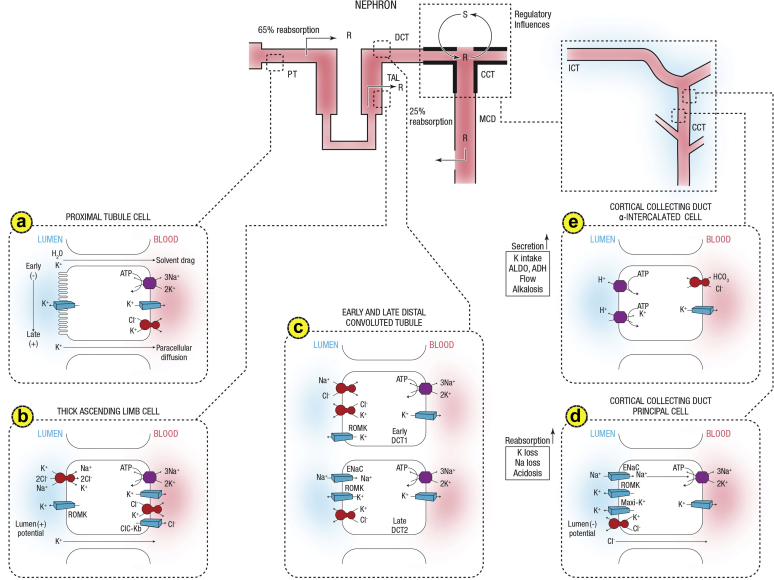
The principal defense against chronic potassium imbalances is renal potassium excretion, which depends on free filtration at the glomerulus, extensive proximal tubule reabsorption, and a highly regulated secretory process in the distal convoluted tubule and segments of the collecting duct in the cortex and outer medulla (the cortical collecting duct and the outer medullary collecting duct, respectively). Principal cells, which constitute approximately 75% of collecting duct cells, mediate sodium reabsorption and potassium secretion and also constitute targets for angiotensin II,27 aldosterone, mineralocorticoid receptor antagonists, and potassium-sparing diuretics (Figure 1).
Figure 1.
Summary of potassium transport along the nephron. Following filtration, potassium is extensively reabsorbed along the proximal tubule and the loop of Henle. Potassium is secreted along the initial and cortical collecting tubules. Net secretion can be replaced by net reabsorption in states of potassium depletion. Also shown are the 2 cell types lining the distal tubule and cortical collecting duct. (a) A cell model for K+ transport in the proximal tubule. K+ reabsorption in the proximal tubule primarily occurs through the paracellular pathway. Active Na+ reabsorption drives net fluid reabsorption across the proximal tubule, which, in turn, drives K+ reabsorption through a solvent drag mechanism. As fluid flows down the proximal tubule, the luminal voltage shifts from slightly negative to slightly positive. The shift in transepithelial voltage provides an additional driving force favoring K+ diffusion through the low-resistance paracellular pathway. Experimental studies suggest that there may be a small component of transcellular K+ transport; however, the significance of this pathway is not known. K+ uptake through the Na+-K+-ATPase pump can exit the basolateral membrane through a conductive pathway or coupled to Cl–. An apically located K+ channel functions to stabilize the cell negative potential, particularly in the setting of Na+-coupled cotransport of glucose and amino acids, which has a depolarizing effect on cell voltage. (b) A cell model for K+ transport in the thick ascending limb of Henle. K+ reabsorption occurs by both paracellular and transcellular mechanisms. The basolateral Na+-K+-ATPase pump maintains intracellular Na+ at a low level, thus providing a favorable gradient to drive the apically located Na+-K+-2Cl– cotransporter (an example of secondary active transport). The apically located renal outer medullary K+ (ROMK) channel provides a pathway for K+ to recycle from cell to lumen, and ensures an adequate supply of K+ to sustain Na+-K+-2Cl– cotransport. This movement through ROMK creates a lumen-positive voltage, providing a driving force for passive K+ reabsorption through the paracellular pathway. Some of the K+ entering the cell through the cotransporter exits the cell across the basolateral membrane, accounting for transcellular K+ reabsorption. K+ can exit the cell through a conductive pathway or in cotransport with Cl–. ClC-Kb is the primary pathway for Cl– efflux across the basolateral membrane. (c) A cell model for K+ transport in the distal convoluted tubule (DCT). In the early DCT, luminal Na+ uptake is mediated by the apically located thiazide-sensitive Na+-Cl– cotransporter. The transporter is energized by the basolateral Na+-K+-ATPase, which maintains low intracellular Na+ concentration, thus providing a favorable gradient for Na+ entry into the cell through secondary active transport. The cotransporter is abundantly expressed in the DCT1 but progressively declines along the DCT2. ROMK is expressed throughout the DCT and into the cortical collecting duct. Expression of the epithelial Na+ channel (ENaC), which mediates amiloride-sensitive Na+ absorption, begins in the DCT2 and is robustly expressed throughout the downstream connecting tubule and cortical collecting duct. The DCT2 is the beginning of the aldosterone-sensitive distal nephron as identified by the presence of both the mineralocorticoid receptor and the enzyme 11β-hydroxysteroid dehydrogenase II. This enzyme maintains the mineralocorticoid receptor free to only bind aldosterone by metabolizing cortisol to cortisone, which has no affinity for the receptor. Electrogenic-mediated K+ transport begins in the DCT2 with the combined presence of ROMK, ENaC, and aldosterone sensitivity. Electroneutral K+-Cl– cotransport is present in the DCT and collecting duct. Conditions that promote a low luminal Cl– concentration increase K+ secretion through this mechanism, which occurs with delivery of poorly reabsorbable anions, such as sulfate, phosphate, or bicarbonate. (d) The cell that is responsible for K+ secretion in the initial collecting duct and the cortical collecting duct is the principal cell. This cell possesses a basolateral Na+-K+-ATPase that is responsible for the active transport of K+ from the blood into the cell. The resultant high cell K+ concentration provides a favorable diffusion gradient for movement of K+ from the cell into the lumen. In addition to establishing a high intracellular K+ concentration, the activity of this pump lowers intracellular Na+ concentration, thereby maintaining a favorable diffusion gradient for movement of Na+ from the lumen into the cell. The movements of both Na+ and K+ across the apical membrane occur through well-defined Na+ and K+ channels. (e) Reabsorption of HCO3 in the distal nephron is mediated by apical H+ secretion by the α-intercalated cell. Two transporters secrete H+, a vacuolar H+-ATPase and an H+-K+-ATPase. The H+-K-ATPase uses the energy derived from ATP hydrolysis to secrete H+ into the lumen and reabsorb K+ in an electroneutral fashion. The activity of the H+-K+-ATPase increases in K+ depletion and thus provides a mechanism by which K+ depletion enhances both collecting duct H+ secretion and K+ absorption.33 ADH, antidiuretic hormone; ALDO, aldosterone; ASDN, aldosterone-sensitive distal nephron; ATPase, adenosine triphosphatase; CCT, cortical collecting tubule; Cl, chloride; ClC-Kb, chloride channel Kb; DCT, distal convoluted tubule; ENaC, epithelial sodium channel; HCO3, bicarbonate; ICT, initial connecting tubule; MCD, medullary collecting duct; PT, proximal tubule; R, reabsorption; ROMK, renal outer medullary potassium; S, secretion; TAL, thick ascending limb. Courtesy of Murray Epstein.
K+ Transport in the Proximal Tubule
K+ reabsorption in the proximal tubule primarily occurs through the paracellular pathway. Active Na+ reabsorption drives net fluid reabsorption across the proximal tubule, which, in turn, promotes K+ reabsorption by a solvent drag mechanism. As fluid flows down the proximal tubule, the luminal voltage shifts from slightly negative to slightly positive. The shift in transepithelial voltage provides an additional driving force favoring K+ diffusion through the low-resistance paracellular pathway. K+ uptake through the Na+-K+-ATPase pump can exit the basolateral membrane through a conductive pathway or coupled to Cl−. An apically located K+ channel functions to stabilize the cell negative potential, particularly in the setting of Na+-coupled cotransport of glucose and amino acids, which has a depolarizing effect on cell voltage.
K+ Transport in the Thick Ascending Limb of Henle
K+ reabsorption occurs by both paracellular and transcellular mechanisms. The basolateral Na+-K+-ATPase pump maintains intracellular Na+ low, thereby producing a favorable gradient to drive the apically located Na+-K+-2Cl− cotransporter (an example of secondary active transport). The apically located renal outer medullary K+ (ROMK) channel provides a pathway for K+ to recycle from cell to lumen, and ensures an adequate supply of K+ to sustain Na+-K+-2Cl− cotransport. This movement through ROMK creates a lumen-positive voltage, providing a driving force for passive K+ reabsorption through the paracellular pathway. Some of the K+ entering the cell through the cotransporter exits the cell across the basolateral membrane, accounting for transcellular K+ reabsorption. K+ can exit the cell through a conductive pathway or in cotransport with Cl−. ClC-Kb is the primary pathway for Cl− efflux across the basolateral membrane.
K+ Transport in the Distal Convoluted Tubule
In the early distal convoluted tubule (DCT), luminal Na+ uptake is mediated by the apically located thiazide-sensitive Na+-Cl− cotransporter. This transporter is energized by the basolateral Na+-K+-ATPase, which maintains intracellular Na+ concentration low, thereby providing a favorable gradient for Na+ entry into the cell through secondary active transport. Whereas the cotransporter is abundantly expressed in the DCT1, it declines progressively along the DCT2.
ROMK is expressed throughout the DCT and into the cortical collecting duct. Expression of the epithelial Na+ channel (ENaC), which mediates amiloride-sensitive Na+ absorption, begins in the DCT2 and is expressed throughout the downstream connecting tubule and cortical collecting duct.
The DCT2 is the beginning of the aldosterone-sensitive distal nephron (ASDN) as identified by the presence of both the mineralocorticoid receptor and the enzyme 11β-hydroxysteroid dehydrogenase II. Importantly, this enzyme maintains the mineralocorticoid receptor free to only bind aldosterone by metabolizing cortisol to cortisone; the latter has no affinity for the receptor. Electrogenic-mediated K+ transport begins in the DCT2 with the combined presence of ROMK, ENaC, and aldosterone sensitivity. Electroneutral K+-Cl− cotransport is present in the DCT and collecting duct. Conditions that cause a low luminal Cl− concentration increase K+ secretion through this mechanism, which occurs with delivery of poorly reabsorbable anions, such as sulfate, phosphate, or bicarbonate.
Principal cells exploit the electrochemical gradient established by sodium entry into the cell through a sodium channel at the luminal membrane (the molecular target of amiloride) and the basolateral membrane Na-K-ATPase to drive potassium secretion through 2 classes of luminal membrane potassium channels.28 One of these, the renal outer medullary potassium (also termed ROMK) channels, secrete potassium under normal tubular fluid flow conditions and are inserted into or internalized from the luminal membrane, depending on the demand for potassium secretion. The other class of potassium channels is the so-called “big” conductance channels (known as BK channels), which are relatively inactive under normal conditions but exhibit increased activity during high tubular flow or high-potassium conditions.28 The factors that regulate principal cell potassium secretion include previous potassium intake; intracellular potassium level; sodium delivery to the cells; urine flow rate; and hormones, such as aldosterone and catecholamines.29 In contrast, the other collecting duct cell type, intercalated cells, mediate acid–base transport but upregulate expression of luminal H-K-ATPases during potassium depletion to enhance potassium reabsorption25, 30 (Figure 1).
To summarize, the kidney excretes sufficient milliequivalents of potassium to maintain total body homeostasis. Although the proximal nephron reabsorbs the bulk of the potassium filtered at the level of the glomerulus, it is a distal site, the collecting duct, that ultimately fine-tunes potassium excretion, thereby determining the final amount of potassium excreted in the urine. Consequently the collecting duct is the major site that responds to increased potassium intake, and in turn is subject to several regulatory influences.
Feedback Control of Potassium Balance
The classic feedback control of potassium is an exemplar of a homeostatic system that uses the consequence, or output, of a process to “feed back” and regulate the process itself. The feedback control of potassium is defined by the following stepwise cascade: In response to a high-potassium meal that includes glucose, pancreatic insulin secretion activates skeletal muscle and liver Na-K-ATPase, which moves potassium (Na/K exchange) from the plasma to the intracellular fluid of these cells. This mechanism minimizes the postprandial increase in plasma potassium concentration.31 With muscle activity, potassium is released into the plasma and filtered at the glomerulus. In order to maintain balance, the amount of potassium consumed in the meal (minus the small amount lost in the feces) is excreted into the urine.
When an increase in potassium consumption increases plasma potassium concentration sufficiently, it triggers aldosterone synthesis and release from the adrenals, which stimulates the activity and synthesis of Na-K-ATPase and luminal potassium channels in collecting duct principal cells to secrete the excess potassium32 (Figure 1). Aldosterone also enhances potassium excretion in the distal colon.33 This latter function can be extremely important in the adaptation that occurs when renal function is compromised.
Conversely, if potassium intake is very low or urinary potassium excretory losses are excessive, plasma potassium concentration decreases and the feedback regulation is invoked, redistributing potassium from intracellular fluid to plasma thereby minimizing hypokalemia. Concomitantly skeletal muscle becomes insulin-resistant to potassium (but not glucose) uptake even before plasma potassium concentration decreases, which acts to blunt the shift of potassium from plasma into the cell.34
After hypokalemia ensues, the expression of skeletal muscle Na-K-ATPase α2 isoform decreases, which facilitates a net potassium “leak” from intracellular fluid to the plasma.35 The low plasma potassium concentration suppresses adrenal aldosterone release; as a result the kidney can reclaim essentially all but about 1% of the filtered potassium (Figure 1). This renal potassium conservation involves downregulation of potassium secretion by means of the ROMK channels in cortical collecting duct principal cells. In conditions of potassium depletion, potassium reabsorption can occur in the collecting duct. This appears to be mediated by upregulation in the apically located H-K-ATPase on intercalated cells.30
What is of great interest is the realization that this intricate feedback mechanism for potassium regulation is not the sole mechanism for compensatory renal potassium excretion. Rather there is a complementary regulatory mechanism, a “feed-forward” control of potassium regulation, which acts in a complementary manner to subserve potassium homeostasis.
“Feed-Forward” Control of Potassium Balance
Although feedback loops are commonly recognized as regulators of biological systems, “feed-forward” loops of various sorts are also important biologic regulators. “Feed-forward” control refers to a pathway in a homeostatic system that responds to a signal in the environment in a predetermined manner, without responding to how the system subsequently reacts (that is, without responding to feedback). A widely recognized example of “feed-forward” control is the conditioned salivation of Pavlov’s dogs in anticipation of food.36 The Pavlovian conditioned salivary reflex is an example of a “feed-forward” regulatory loop in that a stimulus temporally associated with food (input) triggers salivation (output) in advance of presentation of food.36
Another intriguing example that has recently been proposed is closed “feed-forward” loops in which outputs that upregulate inputs also occur, leading to self-sustaining loops as in the case of a variety of intracrines.37 Intracrine action permits the development of an active form of differentiation by which intracrines can spread from 1 cell to a target cell, upregulate intracrine action in that cell, and thereby establish a self-sustaining intracrine loop in that cell—a loop that will persist even when the extracellular intracrine is removed. An example is the recent observations that support the formulation of a “feed-forward” mineralocorticoid receptor–intracrine renin–angiotensin system interaction irrespective of whether aldosterone or another moiety activates the mineralocorticoid receptor in diverse disease states.37
With this theoretical construct as context, we will now consider the formulation of another recent example of “feed-forward” control that is highly relevant and of great immediacy—the “feed-forward” control of potassium. By analogy, a similar “feed-forward” control mechanism is involved in potassium homeostasis. In describing this regulatory loop, a caveat is in order. It is a truism that any mechanism for homeostatic regulation cannot be called “feedback” or “feed-forward” until the details of regulation have been delineated. In the case of the feedback system for K+ regulation, which has been described above, these details are well defined. As will be apparent in the following sections, the details of the “feed-forward” regulatory mechanism have not been fully defined; neither the locus nor the molecular nature of the receptors nor even the signals between the kidney and the gastrointestinal (GI) tract have been elucidated. Consequently, for the purposes of our discussion in describing the phenomenology, we will refer to this homeostatic mechanism as “feed-forward” (with quotes), fully conceding that the mechanistic details await elucidation.
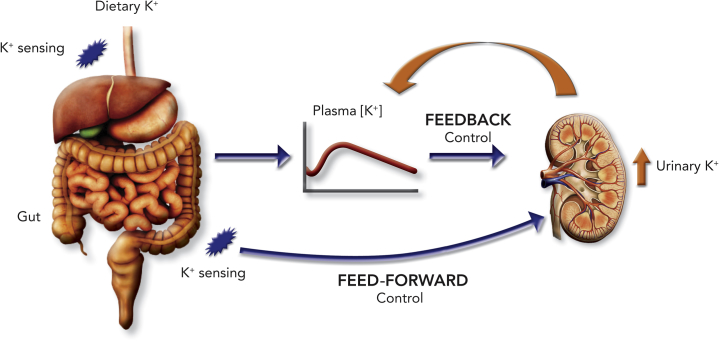
The “feed-forward” control mechanism subserving potassium homeostasis posits that even minor changes in dietary potassium intake, which are insufficient to alter plasma concentrations of either potassium38 or aldosterone,39 and consequently insufficient to activate feedback control, are capable of evoking rapid changes in renal potassium excretion through “feed-forward” mechanisms (Figure 2).
Figure 2.
A schematic depicting the complementary roles of the classic feedback and “feed-forward” control mechanisms for maintaining potassium homeostasis. An increase in plasma potassium evokes an array of responses that promote a kaliuresis. In contrast, the “feed-forward” control mechanism is engaged when dietary potassium is sensed by K+ sensors in the gastrointestinal tract in the absence of perceptible changes in plasma potassium. Courtesy of Murray Epstein.
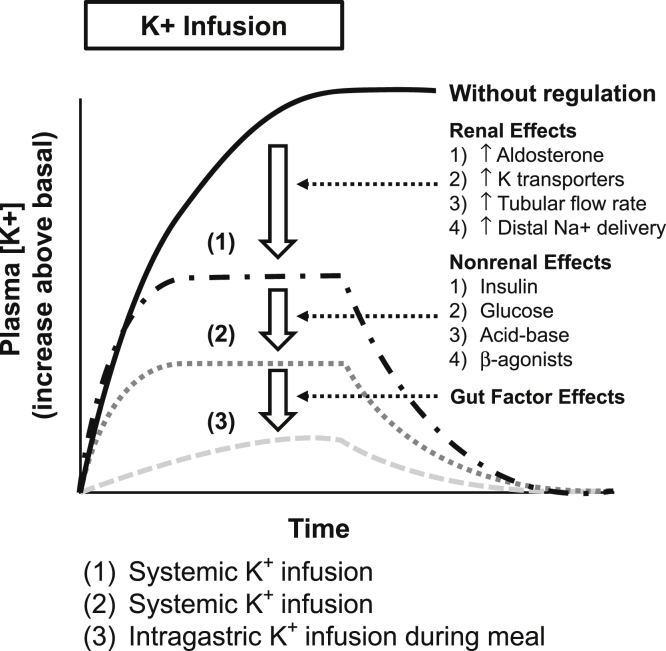
Thirty years ago, Rabinowitz and associates40 conducted a serious of elegant experiments in sheep, which demonstrated that potassium intake in food or potassium placed into the rumen (sheep stomach) was associated with a large and significant increase in urinary potassium excretion. Although others41, 42 had earlier demonstrated that feeding was associated with an increase in urinary potassium excretion, Rabinowitz et al.40 designed a series of 13 experiments to explore the known factors that regulated urinary potassium excretion to determine which of them might contribute to this effect. They showed that the increase in urinary potassium excretion was not related to an increase in serum potassium or glomerular filtration rate and thus the increase in urinary potassium excretion was not a consequence of an increase in filtered potassium, but rather tubular potassium excretion. They demonstrated that aldosterone was not responsible for this by either (i) showing there was no change in plasma aldosterone concentration, (ii) infusing aldosterone and showing it did not alter the effect, or (iii) giving an early aldosterone antagonist (potassium canrenoate), which also did not alter the effect. Similarly, when urine flow rate or sodium excretion was altered or urine pH was altered, the effect persisted. They concluded as follows: “The efferent factors involved in this regulation remain to be determined. They do not appear to be aldosterone, urine flow, sodium excretion, or acid/base status, nor do changes in plasma potassium appear to be necessary or sufficient to produce the changes in potassium excretion associated with meal intake or fasting.” They stated “that there was a correlation between changes in rumen fluid potassium concentration and renal potassium excretion.” They concluded that their studies were “compatible with the presence of receptors located at some point prior to the systemic circulation, which sense enteric potassium levels and influence renal potassium excretion” (Figure 3).
Figure 3.
Summary of the integrated roles of the kidney, extrarenal mechanisms, and gastrointestinal effectors in modulating potassium homeostasis. It demonstrates that the undamped increase in plasma K+ in response to potassium administration is progressively attenuated by the adaptive responses by the kidney, by a hierarchy of nonrenal mechanisms including participation by insulin and glucose, and by gastrointestinal mechanisms evoked by gastrointestinal potassium sensors.33 Courtesy of Murray Epstein.
Adapted with permission from Youn JH. Gut sensing of potassium intake and its role in potassium homeostasis. Semin Nephrol. 2013 May;33:248–256. Copyright © 2013, Elsevier Inc.
Numerous studies have subsequently been conducted (i) to confirm these findings in additional species, including humans, (ii) to try to determine the location for this putative potassium sensor, and (iii) to evaluate potential signals that might increase renal potassium excretion.
Studies in Different Species to Confirm That an Effect of Potassium Administration Into the GI Tract Directly Leads to an Increase in Renal Potassium Excretion
Lee et al.,43 Oh et al.,44 and Morita et al.45 conducted studies in anesthetized rats with findings consistent with those of Rabinowitz et al.40 Calo et al.38 found qualitatively similar findings in humans. In these human studies, during a water diuresis, intake of potassium led to an increase in urinary potassium excretion within 20 minutes, at a time when neither plasma potassium nor aldosterone had increased.
Recently Preston et al.46 conducted studies to further delineate this GI–renal kaliuretic signaling axis in 32 normal subjects in a clinical research unit while on a 20-mmol sodium and 60-mmol potassium diet. The serum potassium concentration, potassium excretion, aldosterone, and insulin were measured following either a 35-mmol oral potassium load, a potassium- and sodium-deficient complex meal, or a potassium-deficient complex meal plus 35 mmol potassium. This experimental design facilitated determination of the component effects on potassium handling of the meal and potassium load separately. The meal plus potassium test was repeated following aldosterone blockade achieved with eplerenone treatment in order to specifically evaluate the role of aldosterone. In response to the potassium-deficient meal plus 35 mmol potassium, the serum potassium did not increase but the hourly mean potassium excretion increased sharply. This kaliuresis persisted following aldosterone blockade with eplerenone, thereby suggesting independence from aldosterone. The authors concluded that this experiment further substantiated the existence of a GI–renal kaliuretic signaling axis in humans that is capable of mediating potassium excretion independent of changes in the serum potassium concentration and aldosterone.
Studies Designed to Determine the Location for the GI Potassium Sensor
The original studies by Rabinowitz and colleagues39, 40 involved direct administration of potassium into the rumens of sheep. Morita and colleagues45, 47 suggested the sensor might be in the hepatoportal area, whereas Lee et al.43 indicated that the stomach and not the portal circulation was more important. In Morita’s studies, intraportal infusion of potassium led to a greater renal potassium excretion than did an i.v. infusion. Cutting the periarterial hepatic nervous plexus diminished the increase in urinary potassium excretion. In addition, potassium infused directly into the hepatoportal circulation stimulated hepatic afferent nerve activity and increased urinary potassium excretion without changes in plasma potassium.
Studies Designed to Determine the Potential Signal(s) That Might Increase Renal Potassium Excretion
Oh et al.44 evaluated a number of GI hormones to test whether they might be responsible for the increase in renal potassium excretion. They evaluated guanylin, uroguanylin, glucagon-like peptide, and extraintestinal hormones such as arginine vasopressin, α- and γ-melanocyte-stimulating hormone, and aldosterone. Their data do not support a role for these hormones in this phenomenon, leading them to suggest that there might be “previously unknown humoral factors that stimulate renal K+ excretion during dietary K+ intake.”
Although not a GI hormone, the renal kallikrein–kinin system is activated by high-K+ diet or acute K+ loading leading to an increase in urinary tissue kallikrein.48 El Moghrabi et al.49 showed, in mice, that renal tissue kallikrein can lead to an increase in renal potassium excretion by both activating the epithelial Na channel to increase K+ excretion by principal cells and inhibiting H-K-ATPase in intercalated cells to decrease K+ reabsorption. Renal K+ excretion increased in concert with tissue kallikrein excretion following a single meal. They further showed that in tissue kallikrein knockout mice there was a greater increase in serum potassium following a meal, suggesting that tissue kallikrein plays a role in renal potassium excretion. Thus, although renal tissue kallikrein is not a GI-derived signal, it could play a role in this phenomenon at the level of the kidney.
The studies of Sorensen et al.50 may be relevant in further considering mechanisms that are operative at the level of the kidney. They studied mice and found that an acute intragastric potassium load led to rapid dephosphorylation of the renal sodium chloride cotransporter (NCC), which was associated, as would be predicted, with an increase in sodium and potassium excretion. Sorensen et al.50 showed that this effect was independent of aldosterone.
Although glucagon is also a circulating hormone that acts on the kidney to increase electrolyte excretion, including potassium, it is unlikely to play a role. Glucagon acts at the level of the proximal tubule, and its potential role as a mediator of the kaliuresis invoked by an oral potassium load has not been delineated.
Physiological Observations of Sodium That May Serve as an Analogous Template for K+
Several lines of experimental evidence, focusing on sodium homeostasis, constitute a template for the concept that a “feed-forward” mechanism with sensors in the GI area, the hepatosplanchnic area, or both areas participates in potassium homeostasis. Sensing the amount of ingested sodium is 1 mechanism by which sodium balance is regulated. In a seminal paper published almost 40 years ago, Carey51 reported that the rapidity of renal sodium excretion in response to an administered sodium load differs depending on the route of administration; an oral sodium load is excreted more rapidly than an identical sodium load administered intravenously. Neural mechanisms52 and gut hormones (e.g., uroguanylin [Guca2b], cholecystokinin) have been proposed to mediate the natriuresis of an oral sodium load.
The natriuresis following ingestion of a certain amount of sodium may be due to the enterokine gastrin, which is secreted by G cells in the stomach and duodenum and released into the circulation.53, 54 Gastrin is taken up by renal cortical tubules to a greater extent than the other enterokines released after a meal.55 Gastrin then acts on its receptor, the cholecystokinin B receptor (CCKBR), expressed in several nephron segments54, 56 to alter sodium transport.
Additional Determinants of Renal Potassium Excretion
Circadian Rhythm of Potassium Excretion
The presence of circadian rhythms that characterize renal function and excretion has been documented for over 60 years.57, 58 Kidney parameters with such rhythms include glomerular filtration rate, renal plasma flow, and tubular reabsorption and secretion for most of the major urinary electrolytes. Of interest for the focus of this Review is the demonstration of a circadian rhythm that characterizes renal potassium excretion in humans, with a peak in the middle of the day.59 This pattern is independent of activity, posture, and dietary intake; this circadian rhythm persists for days in individuals, and it is isolated from most external cues.
Originally this pattern of renal potassium excretion was thought to be driven by factors mainly external to the kidney. Recent studies, however, have demonstrated rhythms within the tubule, which would account for many of these changes. Steele et al.60 suggested that transtubular potassium gradients could be the driving force for the cycles in potassium excretion. Zuber et al.61 demonstrated rhythmicity in transcripts from cells of the rat distal nephron (distal convoluted tubule/connecting tubule and cortical collecting duct) in potassium channels such as ROMK1, KCNK1, and KCNJ10. Their finding of ROMK1 cycling by about 30% at the mRNA level might explain part of the circadian variation observed in renal potassium excretion. Finally, since aldosterone is acknowledged to play a critical role in renal potassium excretion, the demonstration of a circadian rhythm in aldosterone secretion62 and its consequent effects at the level of the tubule63 allow for the possibility that cycles in renal potassium excretion could be controlled both by cycles outside the kidney such as aldosterone and by cycles within the kidney tubules themselves.
Posture and Potassium Excretion
Several studies conducted 60 years ago found little or no effect of posture on renal potassium excretion.64 “Posture had comparatively little prolonged effect on absolute potassium excretion when allowance was made for diurnal rhythmic variations.”65 “Change of posture usually produced less disturbance of the diurnal rhythm in potassium excretion, and changes in potassium excretion (urinary K+) were usually small in comparison with those in sodium output.”66 In summary, posture can exert a considerable effect on urine flow rate and sodium excretion, but the available data suggest that changes in potassium excretion are relatively small.
GI Potassium Absorption and Excretion
As indicated above, stool potassium usually averages about 10% of ingested potassium. Thus, on a standard Western diet with approximately 80 mEq of potassium, this would regularly lead to approximately 8 mEq of potassium in the stool.25, 26 In normal individuals, the large fraction of ingested potassium is absorbed by the small intestine, and the contribution of the normal colon to net potassium absorption or secretion is small.67 Potassium transport in the duodenum, jejunum, ileum, and colon has been characterized as passive absorption, although the colon also demonstrates passive secretion.53, 67 Colonic potassium secretion exists in mammals68 and has been shown to play a role in potassium homeostasis in patients on dialysis.69 Administration of mineralocorticoids leads to small but significant increases in stool potassium excretion.67, 70 Factors that might lead to an increase in stool potassium have been reviewed,71 and are briefly summarized.
The Role for the Liver in Subserving Potassium Homeostasis
Recently Halperin’s group72 proposed an engaging new formulation suggesting a pivotal role for the liver in subserving potassium homeostasis. They suggested that lactate augmented potassium uptake by the liver. Studies were conducted in normal rats compared with rats with acute hyperkalemia. There was a significant fall in plasma K+ in normal rats and an even larger fall in plasma K+ in both models of acute hyperkalemia when the plasma l-lactate rose, caused by a shift of K+ from cells, or by a positive K+ balance. A rise in the plasma l-lactate in portal venous blood led to a fall in the plasma K+, and insulin was permissive. Interestingly, studies conducted to delineate the time course for changes in the plasma K+ in normal rats disclosed that plasma K+ fell significantly to its nadir within the first 15 minutes, and it remained close to this level for the next 30 minutes.
The investigators suggested that absorption of glucose by the Na+-linked glucose transporter permits enterocytes to produce enough adenosine diphosphate to augment aerobic glycolysis, raising the plasma l-lactate in the portal vein, thereby preventing postprandial hyperkalemia. Overall, the integrative physiology of this proposed regulatory process provides a rationale for having SLGT-1 in enterocytes—the release of l-lactic acid into the portal vein—and proposes a central role for the liver to minimize the risk of having a large rise in the plasma K+ delivered to the heart following the ingestion of K+.
Colonic Potassium Handling
Relatively recent studies in rodents, over the past 2 decades, have further defined the intricacies of mammalian colonic K+ handling.71 One important basic aspect of K+ transport in the colon is its segmental difference. Under normal conditions, the proximal colon performs net K+ secretion while net K+ absorption is observed in the distal colon. The proximal colon does not absorb K+ actively. Active K+ absorption in the distal colon occurs via the transcellular route and involves a primary active K+ entry step across the apical membrane. In the mammalian distal colon this active transport is conducted by the non-gastric H-K-ATPase localized in surface cells of the distal colon. Mice with this H-K-ATPase knocked out maintain a normal serum K+ when fed a normal diet, but become hypokalemic on a low-K+ diet. Thus, this H-K-ATPase plays a critical role in colonic K+ absorption.
Net K+ secretion is found in both the proximal colon and distal colon in animals fed a high-K+ diet. Present evidence suggests that net K+ secretion is carried out by K+ channels. Although there are several potential K+ channels that could perform this function, BK channels seem to be the only functionally significant K+ secretory ion channel in the apical membrane of the distal colon.71 The role of the colon in clinically modulating K+ excretion is not adequately recognized. Early studies in end-stage renal disease found that the colon was responsible for a small but significant increase in K+ excretion.73 Over a number of years these findings have been confirmed74, 75 and in recent times have been attributed to an increase in BK channel activity.76 This being the case, it might seem attractive to try to develop pharmacologic agents that could be used to stimulate BK potassium channel activity. If such an agent were to exist, it could have clinical utility in settings of hyperkalemia, particularly where oral intake might be limited and thus potassium binding agents might not be useful.
In patients without renal disease, large-volume diarrhea has historically been attributed to Cl secretion with Na following the Cl, and little K+ is found in these stools.77 van Dinter et al., including John Fordtran, reported on a well-studied woman with secretory diarrhea in the setting of colonic pseudo-obstruction in which K+ secretion seemed to be responsible for the diarrhea with stool K+ averaging 154 mEq/l and serum K+ frequently being below 3.5 mEq/l.78 In that same report they indicated that they were aware of 3 other patients with similar findings. Following this report, the group in Leeds, Sandle's group, reported a patient with secretory diarrhea following hemorrhagic shock79 with high levels of K+ in the stool and histologic evidence of “massive over-expression of BK channel protein in colonic crypts.” In a subsequent review this same group80 reported evidence for potassium (BK) channels being responsible for increased stool K+ in patients with ulcerative colitis, colonic pseudo-obstruction, laxative abuse with bisacodyl, and renal failure. Thus, they suggest that in all these settings increased K+ excretion in the stool, including up to levels of over 150 mEq/l, could all be mediated by increased BK channel activity.
As mentioned earlier, normal individuals on a normal diet absorb virtually all ingested K+, and most of this ingested K+ is excreted in the urine. Importantly, in the setting of chronic kidney disease, and in particular chronic dialysis patients, colonic K+ secretion is greatly enhanced and becomes an important accessory K+ excretory pathway.69 The imminent clinical availability of 2 new polymer potassium binders, patiromer and sodium zirconium cyclosilicate, that modulate their effects in large part in the colon has further emphasized the importance of increasing our understanding of the physiology of colonic potassium handling.
Potassium Binding in the GI Tract
The Evolving Role of Potassium-Binding Resins in Hyperkalemia
Because of the widely documented array of complications of hyperkalemia, determination of hyperkalemia constitutes an “action item” for the clinician. Most clinicians feel compelled to respond vigorously to hyperkalemia. As detailed in several recent reviews, hyperkalemia may be an immediately life-threatening condition.81, 82, 83, 84 Severe hyperkalemia can cause ventricular fibrillation or asystole, unless extracellular potassium is reduced.81 Even relatively mild (5.5–6.0 mEq/l) hyperkalemia is associated with an increased risk of mortality within the next 24 hours, even without electrocardiogram changes.81 Because of these well-recognized associations, clinicians feel compelled to intervene and respond vigorously to hyperkalemia.
As detailed earlier, the majority of potassium is renally excreted, but about 5% to 10% is secreted in the colon. Because potassium is regularly excreted in the stool and since that process could potentially be augmented by cation exchange resins, such treatment could lead to an increase in stool potassium excretion. Treatment with exchange resins has been used in the management of clinical hyperkalemia for over 60 years. The most commonly used resin to date has been sodium polystyrene sulfonate (SPS) (Kayexalate). However, the utility and safety of SPS in the treatment of hyperkalemia is currently being challenged. Several investigators have questioned the effectiveness of SPS in lowering serum potassium levels.85
As of the writing of this article (November 2015), several of the agents that we currently use for the treatment of acute hyperkalemia are unproven with respect to efficacy as judged by currently mandated criteria—but it is reasonable to aver that the majority clinical consensus is that they do work.86
SPS potassium-binding resins (Kayexalate, Kionex, and others), with and without an associated cathartic (usually sorbitol), have been in use for almost 60 years and are approved by the US Food and Drug Administration (FDA) for the treatment of hyperkalemia. In September 2009, the FDA recommended against the “concomitant use of sorbitol” with Kayexalate powder because of associated cases of colonic necrosis and other severe GI side effects.87 However, this warning did not apply to premixed SPS in 33% sorbitol for oral use (various manufacturers), the only SPS resin that is currently available in many hospitals.
SPS potassium-binding resins work by increasing colonic potassium excretion. In hyperkalemic patients, oral SPS mixed in water significantly decreases serum potassium within 24 hours. Colonic necrosis associated with SPS and sorbitol is most commonly seen in patients who have received enemas in the setting of recent abdominal surgery, bowel injury, or intestinal dysfunction. The agent most likely associated with colonic necrosis is 70% sorbitol, and animal data support that etiology. There are very few data to suggest that oral SPS given with 33% sorbitol (in the premixed form) or SPS powder in water orally or as an enema causes colonic necrosis.
In 2010, Sterns et al.,85 in an editorial review, concluded that SPS resins are “largely unproven and potentially harmful,” especially when administered with sorbitol, and that clinicians should “exhaust other alternatives.” Some investigators objected to the condemnation and stated that these conclusions are immoderate.86, 88 When indicated, and in patients who have no contraindication to their use, SPS resins are effective and reasonably safe.
In light of these considerations, what might constitute the “therapeutic niche” for SPS? Watson et al.86 have appropriately highlighted an important role for SPS in the treatment of acute hyperkalemia under dire and challenging conditions after a natural or man-made disaster.89, 90 It may be used for trauma-associated hyperkalemia and as prophylaxis in dialysis-dependent patients who are not able to get to a dialysis unit.89, 90 Everyday examples that the readers can readily relate to include the recent mid-Atlantic blizzard of 5 to 6 February 2010, commonly referred to as Snowmageddon or Snowpocalypse, a category 3 (“major”) nor’easter and severe weather event, when many chronic dialysis patients were unable to be transported to their dialysis units. Additional examples include the aftermath of Hurricane Katrina and after the Haitian earthquake.91 In such situations where dialysis availability is limited, SPS may be the only option for potassium removal in hyperkalemic patients, especially chronic dialysis patients.
We wish to stand back from this ongoing controversy and share a few thoughts and recommendations. Unrelated to the controversy of SPS’s efficacy, and how frequently colonic necrosis may supervene, it is readily apparent that the administration of SPS is fraught with additional challenges related to its administration and patient discomfort. These considerations set the stage for the imminent availability of the newer efficacious and “patient-friendly” potassium binders—patiromer and sodium zirconium cyclosilicate (ZS-9).
Patiromer was approved by the FDA for the treatment of hyperkalemia on 21 October 2015. ZS-9 is currently in the late stages of clinical development and is expected to be approved and in clinical use shortly. These agents may offer advantages over existing approaches to hyperkalemia treatment. Both patiromer and ZS-9 act to remove potassium by exchanging cations (calcium for patiromer and sodium for ZS-9) for potassium in the distal colon, binding potassium, and increasing its fecal excretion.4, 5, 6, 7 Patiromer is an organic, non-absorbed polymer that increases fecal potassium excretion by exchanging potassium for calcium primarily in the distal colon. It is a free-flowing, insoluble powder of small (∼100 μm) spherical beads with low viscosity.6, 7 ZS-9 is an inorganic polymer, which selectively attracts potassium ions to its negatively charged crystalline lattice structure and exchanges them for sodium and hydrogen. It is formulated as a free-flowing, insoluble powder that is not absorbed systemically.92
As of this writing, multiple clinical trials have demonstrated the safety and efficacy of both binders in diverse disease states including congestive heart failure, chronic kidney disease, diabetic nephropathy, and, in a preliminary study, resistant hypertension.93 The reader is referred to the article by Weir in Kidney International Supplements94 for a detailed summary and analysis of these clinical trials.
In summary, it appears that there will be 2 new products available in the near future to facilitate the management of hyperkalemia in patients with chronic and recurrent hyperkalemia. Patiromer (Valtessa) was approved by the FDA for a broad indication of “treatment of hyperkalemia.” ZS-9 is in the latest stages of clinical development but not yet approved. These agents may offer to serve as “enablers” facilitating treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists in optimal recommended doses, obviating down-titration and discontinuation of these life-saving treatments.
Conclusions
The integrated mechanisms controlling the maintenance of potassium homeostasis are well established and are defined by the classic “feedback control” of potassium balance. This “feedback control” of potassium homeostasis has long constituted the platform for defining therapeutic interventions for the management of hyperkalemia. This relatively straightforward theoretical construct has recently been amplified by a wide array of physiological studies. In recent years, increasing investigative attention has focused on novel physiological paradigms that increase the complexity but also the precision of homeostasis—the “feed-forward” control of potassium balance. The elegant studies of Rabinowitz and associates over 3 decades ago have very recently been confirmed in human subjects by the well-defined studies of Preston and associates. Subsequent physiological studies have been conducted in an effort to determine both the location for the GI potassium sensor and the potential signal(s), which might increase renal potassium excretion. Concomitant studies in animal models have further defined the intricacies of mammalian colonic K+ handling. In concert these investigative efforts have increased both the complexity but also the precision of homeostasis, with a resultant enhanced investigative interest in potassium.
These new insights are relevant to the future design of clinical trials delineating renal potassium handling. As an example, the insights regarding “feed-forward” control of potassium mandate that the conditions of study, especially attention to standardization of the fasting or fed state of subjects, are a requisite for the trial design. A recent study has demonstrated that imposition of rigorous dietary potassium intake serves to reduce variability in serum potassium levels.95
Finally, the demonstration of massive overexpression of BK channel protein in “colonic crypts,” and evidence for potassium (BK) channels being responsible for increased stool K+ in some patients with diseases such as ulcerative colitis, introduce interesting therapeutic speculations. As an example, it may be attractive to attempt to develop pharmacologic agents that could be used to stimulate colonic BK potassium channel activity. If such an agent were to exist, it could have clinical utility in settings of hyperkalemia, particularly where oral intake might be limited and thus potassium binding agents might not be useful. The next few years should be rewarding concerning the wide array of physiological and clinical investigations of potassium homeostasis based on the recent insights and discoveries detailed in this Review.
Disclosure
ME is a consultant for Relypsa Inc. No funding was obtained to support this article.
Footnotes
This Review is an extensively expanded and updated version of an earlier review by the authors published in Kidney International Supplements 2016;6:7–15.
References
- 1.Epstein M. Hyperkalemia as a constraint to therapy with combination renin-angiotensin system blockade: the elephant in the room. J Clin Hypertens (Greenwich) 2009;11:55–60. doi: 10.1111/j.1751-7176.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitt B., Anker S.D., Bushinsky D.A. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein M., Reaven N.L., Funk S.E. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(suppl):S212–S220. [PubMed] [Google Scholar]
- 4.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 5.Packham D.K., Rasmussen H.S., Lavin P. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 6.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 7.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease. The AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 8.Edner M., Benson L., Dahlström U. Association between renin–angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36:2318–2326. doi: 10.1093/eurheartj/ehv268. [DOI] [PubMed] [Google Scholar]
- 9.Rassi A.N., Cavender M.A., Fonarow G.C. Temporal trends and predictors in the use of aldosterone antagonists post-acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. doi: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni A.P., Anker S.D., Dahlström U., Heart Failure Association of the ESC Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 11.Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol. 2015;3:993–1003. doi: 10.1016/S2213-8587(15)00289-2. [DOI] [PubMed] [Google Scholar]
- 12.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 13.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B., Zannad F., Remme W.J., Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B., Remme W., Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F., McMurray J.J.V., Krum H. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 17.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Available at: http://www2.kidney.org/professionals/KDOQI/guidelines_bp/guide_11.htm. Accessed June 11, 2015. [PubMed]
- 19.National Institute for Health and Care Excellence. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care. Available at: http://www.nice.org.uk/guidance/cg182. Accessed June 12, 2015. [PubMed]
- 20.American Diabetes Association Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg R., Yusuf S., Collaborative Group on ACE Inhibitor Trials Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 22.Maschio G., Alberti D., Janin G., The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 23.GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 24.McMurray J.J., Packer M., Desai A.S., PARADIGM-HF Investigators and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 25.Palmer B.F. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott P., Dyer A., Stamler R. The INTERSALT study: results for 24 hour sodium and potassium, by age and sex. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:323–330. [PubMed] [Google Scholar]
- 27.Subramanya A.R., Ellison D.H. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansom S.C., Welling P.A. Two channels for one job. Kidney Int. 2007;72:529–530. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 29.Field M.J., Stanton B.A., Giebisch G.H. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBose T.D., Codina J., Burges A. Regulation of H/K ATPase expression in kidney. Am J Physiol. 1995;269:F500–F507. doi: 10.1152/ajprenal.1995.269.4.F500. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo R.A., Felig P., Ferrannini E. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 32.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 33.Giebisch G., Krapf R., Wagner C. Renal and extrarenal regulation of potassium. Kidney Int. 2007;72:397–410. doi: 10.1038/sj.ki.5002288. [DOI] [PubMed] [Google Scholar]
- 34.McDonough A.A., Youn J.H. Role of muscle in regulating extracellular potassium. Semin Nephrol. 2005;25:335–342. doi: 10.1016/j.semnephrol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 35.McDonough A.A., Thompson C.B., Youn J.H. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 36.Babilonia E., Domjan M., Cusato B. Pavlovian “feed-forward” mechanisms in the control of social behavior. Behav Brain Sci. 2000;23:235–249. doi: 10.1017/s0140525x00002430. [DOI] [PubMed] [Google Scholar]
- 37.Re R.N. A mechanism for mineralocorticoid participation in renal disease and heart failure. J Am Soc Hypertens. 2015;9:586–591. doi: 10.1016/j.jash.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Calo L., Borsatti A., Favaro S. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron. 1995;69:253–258. doi: 10.1159/000188466. [DOI] [PubMed] [Google Scholar]
- 39.Rabinowitz L., Denham S.C., Gunther R.A. Aldosterone and postprandial renal excretion of sodium and potassium in sheep. Am J Physiol. 1977;233:F213–F216. doi: 10.1152/ajprenal.1977.233.3.F213. [DOI] [PubMed] [Google Scholar]
- 40.Rabinowitz L., Green D.M., Sarason R.L. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol. 1988;254:R357–R380. doi: 10.1152/ajpregu.1988.254.2.R357. [DOI] [PubMed] [Google Scholar]
- 41.Dewhurst L.K., Harrison F.A., Keynes R.D. Renal excretion of potassium in the sheep. J Physiol Lond. 1968;195:609–621. doi: 10.1113/jphysiol.1968.sp008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarelius I.H., Greenway R.M. Rhythmic fluctuations in the urine composition of sheep. Pflugers Arch. 1975;355:243–259. doi: 10.1007/BF00583687. [DOI] [PubMed] [Google Scholar]
- 43.Lee F.N., Oh G., McDonough A.A. Evidence for gut factor in K homeostasis. Am J Physiol Renal Physiol. 2007;293:F541–F547. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 44.Oh K.S., Oh Y.T., Kim S.W. Gut sensing of dietary K intake increases renal K excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R421–R429. doi: 10.1152/ajpregu.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita H., Fujiki N., Miyahara T. Hepatoportal bumetanide-sensitive K sensor mechanism controls urinary K excretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1134–R1139. doi: 10.1152/ajpregu.2000.278.5.R1134. [DOI] [PubMed] [Google Scholar]
- 46.Preston R.A., Afshartous D., Rodco R. Evidence for a gastrointestinal-renal kaliuretic signaling axis in humans. Kidney Int. 2015;88:1383–1391. doi: 10.1038/ki.2015.243. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya Y., Nakashima S., Banno Y. Effect of high NaCl or high KCl diet on hepatic Na and K receptor sensitivity and NKCC1 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R591–R596. doi: 10.1152/ajpregu.00559.2003. [DOI] [PubMed] [Google Scholar]
- 48.Vio C.P., Figueroa C.D. Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int. 1987;31:1327–1334. doi: 10.1038/ki.1987.146. [DOI] [PubMed] [Google Scholar]
- 49.El Moghrabi S., Houillier P., Picard N. Tissue kallikrein permits early renal adaptation to potassium load. Proc Natl Acad Sci U S A. 2010;107:13526–13531. doi: 10.1073/pnas.0913070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorensen M.V., Grossmann S., Roesinger M. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 51.Carey R.M. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res. 1978;43:19–23. doi: 10.1161/01.res.43.1.19. [DOI] [PubMed] [Google Scholar]
- 52.Furness J.B., Rivera L.R., Cho H.J. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 53.Michell A.R., Debnam E.S., Unwin R.J. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., Asico L.D., Zheng S. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62:927–933. doi: 10.1161/HYPERTENSIONAHA.113.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melis M., Krenning E.P., Bernard B.F. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: species and gender differences. Nucl Med Biol. 2007;34:633–641. doi: 10.1016/j.nucmedbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 56.von Schrenck T., Ahrens M., de Weerth A. CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney Int. 2000;58:995–1003. doi: 10.1046/j.1523-1755.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 57.Gumz M.L., Rabinowitz L., Wing C.S. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills J.N., Stanbury S.W. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117:22–37. [PMC free article] [PubMed] [Google Scholar]
- 59.Moore Ede M.C., Brennan M.F., Ball M.R. Circadian variation of intercompartmental potassium fluxes in man. J Appl Physiol. 1975;38:163–170. doi: 10.1152/jappl.1975.38.1.163. [DOI] [PubMed] [Google Scholar]
- 60.Steele A., deVeber H., Quaggin S.E. What is responsible for the diurnal variation in potassium excretion? Am J Physiol. 1994;267:R554–R560. doi: 10.1152/ajpregu.1994.267.2.R554. [DOI] [PubMed] [Google Scholar]
- 61.Zuber A.M., Centeno G., Pradervand S. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doi M., Takahashi Y., Komatsu R. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2009;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 63.Susa K., Sohara E., Isobe K. WNK-OSR1/SPAK-NCC signal cascade had circadian rhythm dependent on aldosterone. Biochem Biophys Res Commun. 2012;427:743–747. doi: 10.1016/j.bbrc.2012.09.130. [DOI] [PubMed] [Google Scholar]
- 64.Viar W.N., Olover B.B., Eisenberg S. The effect of posture and of compression of the effect on excretion of electrolytes and glomerular filtration: further studies. Circulation. 1951;111:105–115. doi: 10.1161/01.cir.3.1.105. [DOI] [PubMed] [Google Scholar]
- 65.Thomas S. Some effects of change of posture on water and electrolyte excretion of the human kidney. J Physiol. 1957;139:337–352. doi: 10.1113/jphysiol.1957.sp005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas S. Effect of change of posture on diurnal renal excretion rhythm. J Physiol. 1959;148:489–506. doi: 10.1113/jphysiol.1959.sp006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal R., Afzalpurkar R., Fordtran J.S. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107:548–571. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 68.Kliger A.S., Binder H.J., Sastl C. Demonstration of active potassium transport in the mammalian colon. J Clin Invest. 1981;67:1189–1196. doi: 10.1172/JCI110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandle G.I., Gaiger E., Tapster S. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci. 1987;73:247–252. doi: 10.1042/cs0730247. [DOI] [PubMed] [Google Scholar]
- 70.Relman A.S., Schwartz W.B. The effect of DOCA on electrolyte balance in normal man and its relation to sodium chloride intake. Yale J Biol Med. 1952;24:540–558. [PMC free article] [PubMed] [Google Scholar]
- 71.Sorensen M.V., Matos J.E., Praetorius H.A. Colonic potassium handling. Pflugers Arch. 2010;459:645–656. doi: 10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 72.Cheema-Dhadli S., Chong C.K., Kamel K.S. An acute infusion of lactic acid lowers the concentration of potassium in arterial plasma by inducing a shift of potassium into cells of the liver in fed rats. Nephron Physiol. 2012;120:7–15. doi: 10.1159/000336321. [DOI] [PubMed] [Google Scholar]
- 73.Hayes C.P., McLeod M.D., Robinson R.R. An extrarenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- 74.Wilson D.R., Ing T.S., Metcalfe-Gibson A. The chemical composition of faeces in uraemia, as revealed by in vivo faecal dialysis. Clin Sci. 1968;35:197–209. [PubMed] [Google Scholar]
- 75.Sandle G.I., Gaiger E., Tapster S. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci. 1986;71:393–401. doi: 10.1042/cs0710393. [DOI] [PubMed] [Google Scholar]
- 76.Mathialahan T., Maclennan K.A., Sandle L.N. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 77.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Dinter T.G., Fuerst F.C., Richardson C.T. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology. 2005;129:1268–1273. doi: 10.1053/j.gastro.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 79.Simon M., Duong J.P., Mallet V. Over-expression of colonic K channels associated with severe potassium secretory diarrhea after haemorrhagic shock. Nephrol Dial Transplant. 2008;23:3350–3352. doi: 10.1093/ndt/gfn411. [DOI] [PubMed] [Google Scholar]
- 80.Sandle G.I., Hunter M. Apical potassium (BK) channels and enhanced potassium secretion in human colon. Q J Med. 2010;103:85–89. doi: 10.1093/qjmed/hcp159. [DOI] [PubMed] [Google Scholar]
- 81.Kovesdy C.P. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 82.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drawz P.E., Babineau D.C., Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60:310–315. doi: 10.1111/j.1532-5415.2011.03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarafidis P.A., Blacklock R., Wood E. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol. 2012;7:1234–1241. doi: 10.2215/CJN.01150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sterns R.H., Rojas M., Bernstein P. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 86.Watson M., Abbott K.C., Yuan C.M. Damned if you do, damned if you don't: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5:1723–1726. doi: 10.2215/CJN.03700410. [DOI] [PubMed] [Google Scholar]
- 87.US Food and Drug Administration. Kayexalate (sodium polystyrene sulfonate) powder. Safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER). 2009. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm186845.htm. Accessed September 15, 2015.
- 88.Watson M.A., Baker T.P., Nguyen A. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60:409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 89.Kopp J.B., Ball L.K., Cohen A. Kidney patient care in disasters: lessons from the hurricanes and earthquake of 2005. Clin J Am Soc Nephrol. 2007;2:814–824. doi: 10.2215/CJN.03481006. [DOI] [PubMed] [Google Scholar]
- 90.Miller A.C., Arquilla B. Chronic diseases and natural hazards: impact of disasters on diabetic, renal and cardiac patients. Prehosp Disaster Med. 2008;23:185–194. doi: 10.1017/s1049023x00005835. [DOI] [PubMed] [Google Scholar]
- 91.Amundson D., Dadekian G., Etienne M. Practicing internal medicine onboard the USNS COMFORT in the aftermath of the Haitian earthquake. Ann Intern Med. 2010;152:733–737. doi: 10.7326/0003-4819-152-11-201006010-00215. [DOI] [PubMed] [Google Scholar]
- 92.Stavros F., Yang A., Leon A. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9:e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Epstein M., Mayo M., Garza D. Patiromer controls hyperkalemia in resistant hypertensive patients on RAASi, with diabetic kidney disease. Circulation. 2015;132(suppl 3):A14271. [Google Scholar]
- 94.Weir M.R. Current and future treatment options for managing hyperkalemia. Kidney Int Suppl. 2016;6:29–34. doi: 10.1016/j.kisu.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bushinsky DA, Mayo M, Garza D, et al. Wide range in variation in serum potassium in hyperkalemic patients with CKD, response to a fixed 60 mEq potassium diet. Paper presented at: 2015 American Society of Nephrology Kidney Week. November 3–8, 2015; San Diego, CA.