Introduction
Malignancy-associated vasculitis represents only 0.4% to 4.2% of all vasculitis cases1, 2, 3 and consists mostly of cancer-associated cutaneous leukocytoclastic vasculitis.2, 4, 5, 6 Malignancy-associated systemic vasculitic syndromes are very rare and mostly associated with hematological malignancies. Here we report on the case of a 64-year-old woman in whom perinuclear autoantibody-associated neutrophilic cytoplasmic antigen (ANCA)-associated vasculitis (AAV) was diagnosed with a concurrent adenocarcinoma of the gastroesophageal junction. Resection of the tumor resulted in a partial improvement of her symptoms. Subsequent treatment with rituximab resulted in a complete and durable remission of the AAV-associated signs and symptoms. After more than 1 year of follow-up there were no signs of recurrence of her malignancy or AAV relapse. This is the first report on the beneficial effect of rituximab on malignancy-associated vasculitis.
Case Presentation
A 64-year-old woman was admitted to the hospital with a 2-month history of anorexia, intermittent fever, fatigue, nightly cough, staggered myalgia, and paresthesia in her fingers. She had a medical history of arterial hypertension, hypercholesterolemia, hyperthyroidism, and left-sided Bell’s palsy with complete recuperation. The findings of physical examination at time of presentation were unremarkable except for those of the neurological examination, which showed symmetric superficial and deep sensory disturbances distal in the upper and lower limbs, with overpowered extension and spreading of the fingers, hip flexion, knee flexion, and foot extension (Medical Research Council scale for muscle power grade 3). Reflexes were all present and rather hyperactive, but without clonus. Electromyography demonstrated an axonal sensorimotor peripheral neuropathy. Laboratory findings showed signs of inflammation (minimal leukocytosis; elevated C-reactive protein level; a normochromic normocytic anemia with iron deficiency; and an acute kidney injury stage 3 [failure] according to the risk, injury, loss, and end-stage classification) (Table 1). Urinalysis showed microscopic, dysmorphic hematuria and limited proteinuria. Renal ultrasonography showed no abnormalities. Perinuclear ANCAs (1/1280) were positive against myeloperoxidase with a titer higher than 134 U/ml (<3.5 = negative, >5 = positive). The results of testing for anti–glomerular basement membrane antibodies were negative. A kidney biopsy was performed and showed glomerulonephritis with vasculitis and crescents (Figure 1a and b) with negative immunohistochemical stains confirming the diagnosis of perinuclear ANCA-associated pauci-immune vasculitis. Because of anorexia and fatigue, positron emission tomography–computed tomography was performed with high tracer uptake at the gastroesophageal junction and the right upper lobe of the lung. Thoracic computed tomography showed ground glass opacities suggestive of an inflammatory or infectious pulmonary disease. Gastroscopy showed a polypoid lesion with a maximal diameter of 2.5 cm at the gastroesophageal junction (Figure 1c). Histopathological examination revealed a moderately differentiated adenocarcinoma with mucinous differentiation that was staged pT2N1M0.
Table 1.
Laboratory findings
| Indicator | At diagnosis | 6 wk after esophagectomy | 6 mo after esophagectomy | 1 yr after esophagectomy | Reference value |
|---|---|---|---|---|---|
| Creatinine level (mg/dl) | 2.88 | 2.34 | 1.02 | 1.30 | 0.51–0.95 |
| eGFR (ml/min per 1.73 m2) | 17 | 22 | 59 | 43 | (CKD-EPI) |
| u-RBC (per μl) | Macroscopic hematuria | 18 | 6 | Negativea | ≤25 |
| Proteinuria (g/g creatinine) | 1.76 | 0.90 | 0 .47 | 0.10 | ≤ 0.17 |
| p-ANCAs (U/ml) | 1 of 1280 | 1 of 320 | 1 of 80 | Undetectable |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; mo, month(s); p-ANCAs, perinuclear antibodies against cytoplasmic antigens; wk, week(s); yr, year(s).
Negative: <5 rbc/μl.
Figure 1.
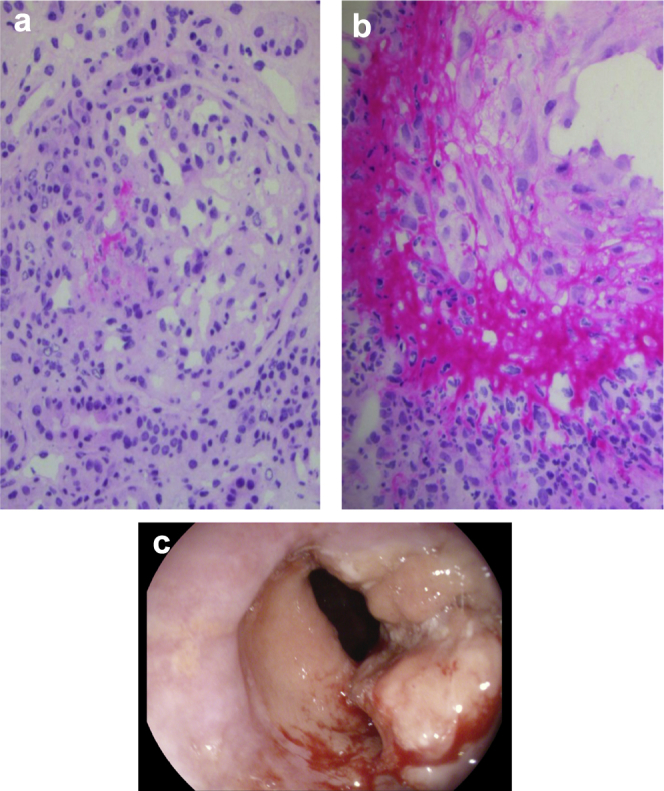
(a) Glomerulonephritis with crescents and vasculitis. Recent segmental necrosis of the capillary loops, followed by crescent formation. (b) Acute vasculitis: medium-sized artery showing endothelial swelling, fibrinoid necrosis, and a mixed inflammatory cell infiltrate composed of neutrophils and lymphocytes. (c) Polypoid lesion with a maximal diameter of 2.5 cm at the gastroesophageal junction that is suggestive of an adenocarcinoma at the gastroesophageal junction.
The patient's kidney function deteriorated progressively, and she required intermittent hemodialysis 20 days after hospital admission. The patient subsequently underwent a curative partial esophagectomy. There was a marked improvement of her condition following the operation: attenuation of paresthesia and better gait, improvement of kidney function with no further need for dialysis, and a decrease in ANCA titers (1/320) after 6 weeks. Because of incomplete neurological and renal response, corticosteroids and rituximab, 375 mg/m2, weekly were administered, starting 6 weeks postoperatively according to the Rituximab in ANCA-Associated Vasculitis protocol. We opted for this protocol because high-dose steroid monotherapy was judged to not be appropriate as steroids are known to impede wound healing after surgery (recent esophagectomy) and because of the risk for development of steroid myopathy (which would have further impeded this patient’s ability to walk).
Treatment with rituximab led to further recovery of kidney function 6 and 12 months after esophagectomy and further diminishing of proteinuria and microscopic hematuria 1 year after esophagectomy (Table 1). Also clinically, the sensorimotor polyneuropathy recovered markedly after the initiation of rituximab; there was a progressive improvement in gait and a further increase in muscle strength. Nevertheless, paresthesia persisted in the lower limbs with a numb feeling in both legs. A positron emission tomography–computed tomography scan at 6 months' follow-up showed an increased metabolism at the neoesophagus that was suggestive of inflammatory changes, which was confirmed by esophagogastroscopy. A follow-up positron emission tomography–computed tomography scan after 1 year showed no evidence of malignancy or vasculitis.
Discussion
In general, AAV is idiopathic and appears to involve genetically determined HLA specificities that allow the autoimmune response to develop.7 The association of AAV with solid tumors has been sporadically reported.8, 9, 10, 11, 12, 13 To the best of our knowledge, only 5 cases of paraneoplastic ANCA vasculitis and gastrointestinal cancers have been described before.11, 13, 14, 15, 16 Resolution of vasculitis following effective treatment of the putatively linked malignancy, and recurrence of vasculitis heralding tumor recurrence or progression, would provide strong evidence for vasculitis being a true paraneoplastic syndrome.17 Of interest, only a few case reports describe the response of paraneoplastic rapidly progressive glomerulonephritis to treatment. Edgar et al. reported a patient who experienced a remission of renal disease after surgical resection in combination with prednisone plus cyclophosphamide.10 In contrast, Navarro et al. reported on a patient with AAV and a concurrent undifferentiated adenocarcinoma in whom treatment with prednisone and cyclophosphamide resulted in remission of renal disease while there was progression of the malignancy.18 Hosoya et al. reported a case of ANCA-associated interstitial nephritis that resolved after resection of a well-differentiated gastric adenocarcinoma.15 Finally, Abe et al. reported a 74-year-old female patient with an myeloperoxidase-ANCA pauci-immune glomerulonephritis with concurrent carcinomas of the stomach and duodenum.16 Resection of the tumors resulted in improvement of renal function, disappearance of microscopic hematuria, and a decrease in myeloperoxidase-ANCA levels. However, proteinuria, elevated C-reactive protein level, and cylindruria improved only following additional treatment with prednisolone.16 In our case the patient had an esophageal cancer, for which she underwent a curative partial esophagectomy, with partial improvement of her condition afterward.
So our and other case reports suggest that AAV can be paraneoplastic.19 The data linking cancer with the occurrence of AAV are unequivocal. In a British study, the frequency of preceding or concomitant cancer was 6-fold higher in the AAV group.9 In a German study, no difference in prevalence of malignancy was found between patients with granulomatosis with polyangiitis (GPA) and patients with rheumatoid arthritis,8 although the risk for a diagnosis of cancer 3 months before or after diagnosis was substantially increased in patients with GPA (odds ratio = 18.00; 95% confidence interval: 2.30–140.67).8 In a Swedish study, bladder carcinoma (preceding the diagnosis of GPA at a median of 1.5 years) was twice as common in the cohort at the time of diagnosis of vasculitis compared with in the general population (relative risk = 2.1; 95% confidence interval: 0.6–3.6).20 In contrast, in a Danish study the prevalence of cancer at any site before the diagnosis of vasculitis was not significantly increased in the GPA cohort (odds ratio = 1.4, 95% confidence interval: 0.9–2.2).21 Furthermore, the incidence of malignancies in the first years after a diagnosis of vasculitis was not significantly increased among patients with GPA.22
For idiopathic severe AAV, cyclophosphamide and glucocorticoids or rituximab and glucocorticoids have been the cornerstone of remission induction therapy.23 The Rituximab in ANCA-Associated Vasculitis trial demonstrated that rituximab therapy was not inferior to daily cyclophosphamide treatment for induction of remission in severe AAV and may be superior in relapsing disease.24 Because of the major surgery and concurrent malignancy, we initially avoided the use of steroids and cyclophosphamide in our patient. Because of only partial resolution of vasculitis-associated signs and symptoms after surgery, rituximab in combination with corticosteroids were administered 6 weeks postoperatively. This led to further clinical and biochemical recovery and after more than 1 year of follow-up there is no evidence of recurrence of her malignancy and there are no signs of vasculitic disease activity. Our report is the first to demonstrate that rituximab can be safely administered to a patient with cancer-associated AAV with only an incomplete response after treatment of the underlying malignancy.
Conclusion
AAV could be paraneoplastic in nature, with remission obtained after curative treatment of the underlying malignancy. Here we have reported on a case of myeloperoxidase-associated vasculitis in the context of an esophageal cancer with substantial improvement following resection of the malignancy. Rituximab was administered because of incomplete response and resulted in further renal and neurological improvement. There was no recurrence of vasculitis or malignancy after more than 1 year of follow-up.
Disclosures
All the authors declared no competing interests. No funding was obtained to support this article.
References
- 1.Garcia-Porrua C., Gonzalez-Gay M.A. Cutaneous vasculitis as a paraneoplastic syndrome in adults. Arthritis Rheum. 1998;41:1133–1135. doi: 10.1002/1529-0131(199806)41:6<1133::AID-ART23>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Hutson T.E., Hoffman G.S. Temporal concurrence of vasculitis and cancer: a report of 12 cases. Arthritis Care Res. 2000;13:417–423. doi: 10.1002/1529-0131(200012)13:6<417::aid-art13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Blanco R., Martinez-Taboada V.M., Valverde V. Cutaneous vasculitis in children and adults. Associated diseases and etiologic factors in 303 patients. Medicine (Baltimore) 1998;77:403–418. doi: 10.1097/00005792-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Fain O., Hamidou M., Cacoub P. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57:1473–1480. doi: 10.1002/art.23085. [DOI] [PubMed] [Google Scholar]
- 5.Kurzrock R., Cohen P.R., Markowitz A. Clinical manifestations of vasculitis in patients with solid tumors. A case report and review of the literature. Arch Intern Med. 1994;154:334–340. [PubMed] [Google Scholar]
- 6.Sanchez-Guerrero J., Gutierrez-Urena S., Vidaller A. Vasculitis as a paraneoplastic syndrome. Report of 11 cases and review of the literature. J Rheumatol. 1990;17:1458–1462. [PubMed] [Google Scholar]
- 7.Lyons P.A., Rayner T.F., Trivedi S. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsis E., Reinhold-Keller E., Steindorf K. Wegener's granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999;42:751–756. doi: 10.1002/1529-0131(199904)42:4<751::AID-ANR19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst T., Savage C.O., Gordon C. Malignancy is increased in ANCA-associated vasculitis. Rheumatology (Oxford) 2004;43:1532–1535. doi: 10.1093/rheumatology/keh374. [DOI] [PubMed] [Google Scholar]
- 10.Edgar J.D., Rooney D.P., McNamee P. An association between ANCA positive renal disease and malignancy. Clin Nephrol. 1993;40:22–25. [PubMed] [Google Scholar]
- 11.Hruby Z., Bronowicz A., Rabczynski J. A case of severe anti-neutrophil cytoplasmic antibody (ANCA)-positive crescentic glomerulonephritis and asymptomatic gastric cancer. Int Urol Nephrol. 1994;26:579–586. doi: 10.1007/BF02767663. [DOI] [PubMed] [Google Scholar]
- 12.Baschinsky D.Y., Baker P.B., Niemann T.H. Pauci-immune ANCA-positive crescentic glomerulonephritis associated with metastatic adenocarcinoma of the lung. Am J Kidney Dis. 2000;36:E24. doi: 10.1053/ajkd.2000.17727. [DOI] [PubMed] [Google Scholar]
- 13.Diez-Porres L., Rios-Blanco J.J., Robles-Marhuenda A. ANCA-associated vasculitis as paraneoplastic syndrome with colon cancer: a case report. Lupus. 2005;14:632–634. doi: 10.1191/0961203305lu2153cr. [DOI] [PubMed] [Google Scholar]
- 14.Yedidag A., Zikos D, B Esophageal carcinoma presenting with nephrotic syndrome: association with anti-neutrophil cytoplasmic antibody. Am J Gastroenterol. 1997;92:326–328. [PubMed] [Google Scholar]
- 15.Hosoya Y., Minota S., Lefor A. Resolution of anti-neutrophil cytoplasmic antibody-associated vasculitis after resection of gastric cancer. Mod Rheumatol. 2010;20:102–105. doi: 10.1007/s10165-009-0238-1. [DOI] [PubMed] [Google Scholar]
- 16.Abe H., Momose S., Takeuchi T. Microscopic polyangitis complicating double carcinoma of the stomach and duodenum: improvement after the resection of these carcinomas. Rheumatol Int. 2011;31:105–108. doi: 10.1007/s00296-009-1162-6. [DOI] [PubMed] [Google Scholar]
- 17.Solans-Laque R., Bosch-Gil J.A., Perez-Bocanegra C. Paraneoplastic vasculitis in patients with solid tumors: report of 15 cases. J Rheumatol. 2008;35:294–304. [PubMed] [Google Scholar]
- 18.Navarro J.F., Quereda C., Rivera M. Anti-neutrophil cytoplasmic antibody-associated paraneoplastic vasculitis. Postgrad Med J. 1994;70:373–375. doi: 10.1136/pgmj.70.823.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien Y.H., Lai L.W. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7:85–95. doi: 10.1038/nrneph.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight A., Askling J., Granath F. Urinary bladder cancer in Wegener's granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis. 2004;63:1307–1311. doi: 10.1136/ard.2003.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faurschou M., Mellemkjaer L., Sorensen I.J. Cancer preceding Wegener's granulomatosis: a case-control study. Rheumatology (Oxford) 2009;48:421–424. doi: 10.1093/rheumatology/kep009. [DOI] [PubMed] [Google Scholar]
- 22.Faurschou M., Sorensen I.J., Mellemkjaer L. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35:100–105. [PubMed] [Google Scholar]
- 23.Miller A., Chan M., Wiik A. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol. 2010;160:143–160. doi: 10.1111/j.1365-2249.2009.04078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone J.H., Merkel P.A., Spiera R. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]


