Abstract
Introduction
The pharmacokinetics, pharmacodynamics, and safety and tolerability profile of etelcalcetide (ONO-5163/AMG 416), a novel, i.v., long-acting calcium-sensing receptor agonist, were studied in Japanese hemodialysis patients with secondary hyperparathyroidism.
Methods
This multicenter, randomized, double-blind, placebo-controlled, parallel-group study consisted of a single dose part and a multiple dose (3 times weekly for 4 weeks) part. Major inclusion criteria were hemodialysis for at least 90 days, serum intact parathyroid hormone (iPTH) ≥ 300 pg/ml, and serum albumin-corrected Ca (cCa) ≥ 9.0 mg/dl. There were 3 single-dose cohorts (n = 6 each) randomized 2:1 to 5, 10, or 20 mg etelcalcetide or placebo, and 2 multiple-dose cohorts (n = 11 each) randomized 8:3 to 2.5 or 5 mg etelcalcetide or placebo.
Results
Etelcalcetide plasma concentration decreased rapidly after i.v. administration, generally remained stable from 24 hours postdose to the next dialysis, and then decreased by dialysis. Etelcalcetide exposure increased dose proportionally. Etelcalcetide plasma predialysis concentration reached almost steady state at week 4. A single dose of etelcalcetide dose-dependently reduced serum iPTH in 30 minutes, and the reduction reached a plateau at 1 hour that lasted until 8 hours. The percent change from baseline serum iPTH thereafter showed a trend to gradually decrease; it was still −30% or greater on day 3. Similar results were obtained at the last injection (days 27–29) of the multiple dose. The effect of the multiple dose was sustained during the interdialytic period. Etelcalcetide decreased serum cCa in a more gradual but dose-dependent and sustained manner.
Discussion
Etelcalcetide dose-dependently reduced serum iPTH and serum cCa. Moreover, the effect was sustained in the interdialytic period.
Keywords: calcium-sensing receptor agonist (calcimimetics), clinical trial, etelcalcetide (ONO-5163/AMG 416), secondary hyperparathyroidism
Secondary hyperparathyroidism (SHPT) is a complex disorder associated with chronic kidney disease (CKD), in which impaired mineral homeostasis and reduced synthesis of calcitriol, the active form of vitamin D, leads to excessive parathyroid hormone (PTH) levels.1, 2, 3 Elevated PTH levels and impaired mineral metabolism are linked to a variety of pathological changes, including parathyroid hyperplasia,1, 2, 3 metabolic bone disease,1, 2, 3 vascular calcification,4 and left ventricular hypertrophy.5 These pathological changes increase the risk of cardiovascular events, the leading cause of morbidity and mortality in these patients.6 Thus, the importance of appropriate management of SHPT is underscored by various clinical practice guidelines.7, 8
Active vitamin D analogues and cinacalcet are major treatment options for SHPT.7, 8 Although active vitamin D analogues inhibit PTH secretion, these analogues also stimulate intestinal calcium transport and bone resorption, thereby increasing the risk of hypercalcemia, leaving many patients with elevated PTH levels.9 Cinacalcet, a calcium-sensing receptor (CaSR) agonist, is recommended for such patients. Cinacalcet reduces PTH secretion by binding to CaSR located on parathyroid chief cells, without increasing extracellular calcium.10, 11 Numerous clinical trials have demonstrated that cinacalcet reduces not only PTH levels, but also serum calcium and phosphorus (P).12 Despite the beneficial clinical properties of cinacalcet, use of the drug has been limited by gastrointestinal adverse events (AEs), including nausea and vomiting, leading to poor compliance.12, 13, 14 Thus, there is a need for other calcimimetic agents with good efficacy and better tolerability that promote increased compliance.
Etelcalcetide (ONO-5163/AMG 416) is a novel peptide consisting of 7 D-amino acids, is administered i.v., is an agonist of CaSR,15 and has a long action in hemodialysis patients with limited or no kidney function because renal clearance is the main elimination pathway in healthy individuals.16 Etelcalcetide lowers the threshold for CaSR activation in response to extracellular calcium, and directly activates CaSR itself in the absence or presence of extracellular calcium as well as cinacalcet.17 Phase I and II studies demonstrated that etelcalcetide quickly reduced serum intact PTH (iPTH) and serum albumin-corrected calcium (cCa) in a dose-dependent manner in non-Japanese hemodialysis patients with SHPT.18, 19, 20 The effects were sustained during the interdialytic period when the drug was administered 3 times weekly for 4 weeks. Their studies supported clinical efficacy, demonstrating sustained etelcalcetide plasma concentrations throughout the study. In addition, the desirable timing to evaluate serum PTH and Ca for etelcalcetide may be different from that for cinacalcet, considering the pharmacokinetics of each.
The present study was conducted to evaluate the pharmacokinetics, pharmacodynamics including the time-course changes and daily fluctuations, safety, and tolerability of etelcalcetide in Japanese hemodialysis patients with SHPT. The possible treatment of less severe SHPT with different doses of etelcalcetide was also evaluated, because Japanese patients have lower baseline iPTH compared to non-Japanese patients.21
Materials and Methods
Study Patients
This was a randomized, double-blind, placebo-controlled, parallel-group study. The study population comprised Japanese patients ≥20 years of age in medically stable condition with CKD who had been treated with hemodialysis 3 times a week for at least 90 days. The primary inclusion criteria were mean serum iPTH ≥ 300 pg/ml and serum cCa ≥ 9.0 mg/dl at screening, and calcium concentrations of dialysate stable (≥2.25 mEq/l) for at least 2 weeks prior to screening.
The major exclusion criteria were as follows: primary hyperparathyroidism; parathyroidectomy within 90 days or parathyroid intervention within 28 days before the start of screening; symptomatic angina pectoris or chronic heart failure; history of myocardial infarction within 180 days of the first dose of study drug; uncontrollable diabetes; and bisphosphonate therapy within 24 weeks of the first dose of study drug.
Informed consent was obtained from all participants included in the study. All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (Institutional Review Board approval number ONO-5163-02) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was registered as JapicCTI-121978.
Study Design
This study comprised 2 parts: a single-dose study at 1 study center, and a multiple-dose study at 16 centers. Doses of 5, 10, and 20 mg (S1, S2, and S3) and of 2.5 and 5 mg (M1 and M2) were selected for the single-dose and multiple-dose parts, respectively, based on the following considerations: (i) a single i.v. injection at 5 to 60 mg was tolerable in non-Japanese SHPT patients18; (ii) 3-times-weekly 5- and 10-mg doses for 4 weeks were tolerable in non-Japanese patients19; (iii) a single injection of 5 mg was tolerable in healthy Japanese subjects with pharmacokinetics similar to those of non-Japanese subjects (unpublished data); and (iv) a possibility that a lower dose might benefit patients with lower baseline iPTH.
The patients were randomly assigned via a centralized randomization system to receive either etelcalcetide or placebo. For the single-dose part, 6 patients each were enrolled in 3 cohorts and randomized 2:1 to receive etelcalcetide or placebo (Supplementary Figure S1). The first cohort (S1) received a 5-mg i.v. injection of etelcalcetide via the venous blood line after dialysis. After all patients in cohort S1 or S2 met the safety criteria, the cohort S2 or S3 was started. The safety criteria included the following: serum cCa ≥ 7.5 mg/dl; no occurrence of hypocalcemia requiring treatment; no occurrence of corrected QT interval (QTc) prolongation on 12-lead electrocardiograms (ECGs); iPTH ≥ 60 pg/ml; QTc by the Fridericia formula ≤ 450 ms; and no occurrence of moderate- or higher-grade AEs that were not determined to be unrelated to the study drug.
For the multiple-dose part, 11 patients each were enrolled in 2 cohorts and randomized 8:3 to receive etelcalcetide or placebo (Supplementary Figure S1). After the safety criteria were met for cohort S1, the first cohort (M1) received a 2.5-mg i.v. injection of etelcalcetide 3 times weekly for 4 weeks. The second cohort (M2) followed the same treatment schedule with 5 mg after the safety criteria were met for cohorts S3 and M1.
Bisphosphonates were prohibited from 24 weeks before the start of screening to the final visit. Calcitonin, estrogens, synthetic estrogens, selective estrogen receptor modulators, and cinacalcet hydrochloride were prohibited 14 days prior to iPTH determination at screening and from 28 days before the first dose to the final visit. Phosphate binders, active vitamin D derivatives, and calcium formulations were allowed if the dosage and administration schedules were unchanged from that at 14 days and 7 days before screening, respectively.
Biochemical Determinations
All samples were analyzed by SRL Medisearch Inc. (Tokyo, Japan) for laboratory tests and determination of serum iPTH, cCa, P, bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase−5b (TRACP-5b), and fibroblast growth factor 23 (FGF23).
Antibodies Against Etelcalcetide
Antibodies against etelcalcetide were determined by Eurofins Pharma Bioanalytics Service US Inc. (St. Charles, MO) using a validated enzyme-linked immunosorbent assay (performed between 2013 and 2014).
Determination of Plasma Etelcalcetide Concentration
The plasma concentration of etelcalcetide was determined by Quintiles BioSciences, Inc. (Ithaca, NY) using liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS).
Safety and Tolerability
The safety and tolerability profiles of etelcalcetide were assessed in terms of AEs, vital signs, clinical laboratory measurements, and antietelcalcetide antibodies. Vital signs were monitored at each institution, and 12-lead ECGs were monitored and analyzed by Suzuken Co. Ltd (Nagoya, Japan).
Statistical Analyses
There was no hypothesis to be verified a priori in this study. Therefore, the planned sample sizes were determined based on practical considerations. All pharmacodynamic and safety analyses were performed in a descriptive manner, and no statistical inferences were made in this study. The pharmacokinetic analysis set included all patients who received etelcalcetide and from whom plasma etelcalcetide concentration data were obtained. The maximum plasma concentration (Cmax) and area under the plasma concentration−time curve (AUC) from time 0 to the last time point with quantifiable concentration before the next hemodialysis (AUClast) were calculated for each subject using a noncompartmental analysis method. Descriptive statistics were calculated for both pharmacokinetic parameters.
Results
Single-Dose Study
Patients
Demographic and clinical characteristics of patients are summarized in Table 1. The mean serum iPTH at screening was 414.0 ± 396.5, 338.3 ± 34.2, 516.6 ± 224.1, and 392.3 ± 91.6 pg/ml for the placebo (S1+S2+S3) and etelcalcetide (S1, S2, and S3) groups, respectively.
Table 1.
Demographic and clinical characteristics of patients in single-dose study
| Groups |
Placebo (S1+S2+S3) | Cohort S1 |
Cohort S2 |
Cohort S3 |
|
|---|---|---|---|---|---|
| Dose | 5 mg/person | 10 mg/person | 20 mg/person | ||
| No. of patients, n | 6 | 4 | 4 | 4 | |
| Sex | Male | 3 (50.0%) | 4 (100.0%) | 3 (75.0%) | 3 (75.0%) |
| Female | 3 (50.0%) | 0 (0.0%) | 1 (25.0%) | 1 (25.0%) | |
| Age (yr) | Mean ± SD | 58.2 ± 17.4 | 54.0 ± 11.1 | 60.5 ± 18.4 | 55.3 ± 4.9 |
| Median | 59.5 | 54.0 | 62.0 | 57.5 | |
| Range | 35–79 | 41–67 | 40–78 | 48–58 | |
| Body weight (kg) (postdialysis) | Mean ± SD | 64.28 ± 9.51 | 84.65 ± 26.30 | 62.95 ± 6.34 | 64.78 ± 16.65 |
| Median | 62.05 | 74.60 | 64.30 | 63.80 | |
| Range | 53.0–79.3 | 66.4–123.0 | 54.5–68.7 | 46.0–85.5 | |
| BMI (kg/m2) | Mean ± SD | 24.69 ± 5.05 | 28.71 ± 7.36 | 23.56 ± 2.20 | 24.13 ± 4.79 |
| Median | 22.39 | 26.80 | 22.64 | 23.19 | |
| Range | 19.8–32.6 | 22.4–38.8 | 22.1–26.8 | 20.2–29.9 | |
| Duration of dialysis (yr) | Mean ± SD | 6.400 ± 6.253 | 5.250 ± 3.304 | 8.875 ± 8.910 | 8.250 ± 3.202 |
| Median | 5.500 | 4.000 | 7.500 | 7.000 | |
| Range | 0.27–17.00 | 3.00–10.00 | 0.50–20.00 | 6.00–13.00 | |
| Vitamin D treatment | Yes | 5 (83.3%) | 3 (75.0%) | 4 (100.0%) | 4 (100.0%) |
| No | 1 (16.7%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | |
| Phosphate binder treatment | Yes | 6 (100.0%) | 4 (100.0%) | 3 (75.0%) | 4 (100.0%) |
| No | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | |
| Serum iPTH (pg/ml) | Mean ± SD | 414.0 ± 61.3 | 338.3 ± 34.2 | 516.6 ± 224.1 | 392.3 ± 91.6 |
| Median | 396.5 | 328.5 | 460.5 | 393.0 | |
| Range | 342–499 | 309–387 | 321–825 | 309–474 | |
| Serum cCa (mg/dl) | Mean ± SD | 9.52 ± 0.38 | 9.55 ± 0.44 | 10.08 ± 0.56 | 9.83 ± 0.30 |
| Median | 9.45 | 9.65 | 10.10 | 9.80 | |
| Range | 9.1–10.2 | 9.0–9.9 | 9.4–10.7 | 9.5–10.2 | |
| Serum P (mg/dl) | Mean ± SD | 4.82 ± 1.35 | 5.88 ± 1.04 | 5.48 ± 2.01 | 5.53 ± 0.54 |
| Median | 5.40 | 6.20 | 6.00 | 5.50 | |
| Range | 2.4–6.1 | 4.4–6.7 | 2.6–7.3 | 4.9–6.2 | |
BMI, body mass index; cCa, albumin-corrected Ca; iPTH, serum intact parathyroid hormone; P, phosphorus; S, single-dose.
Pharmacokinetics
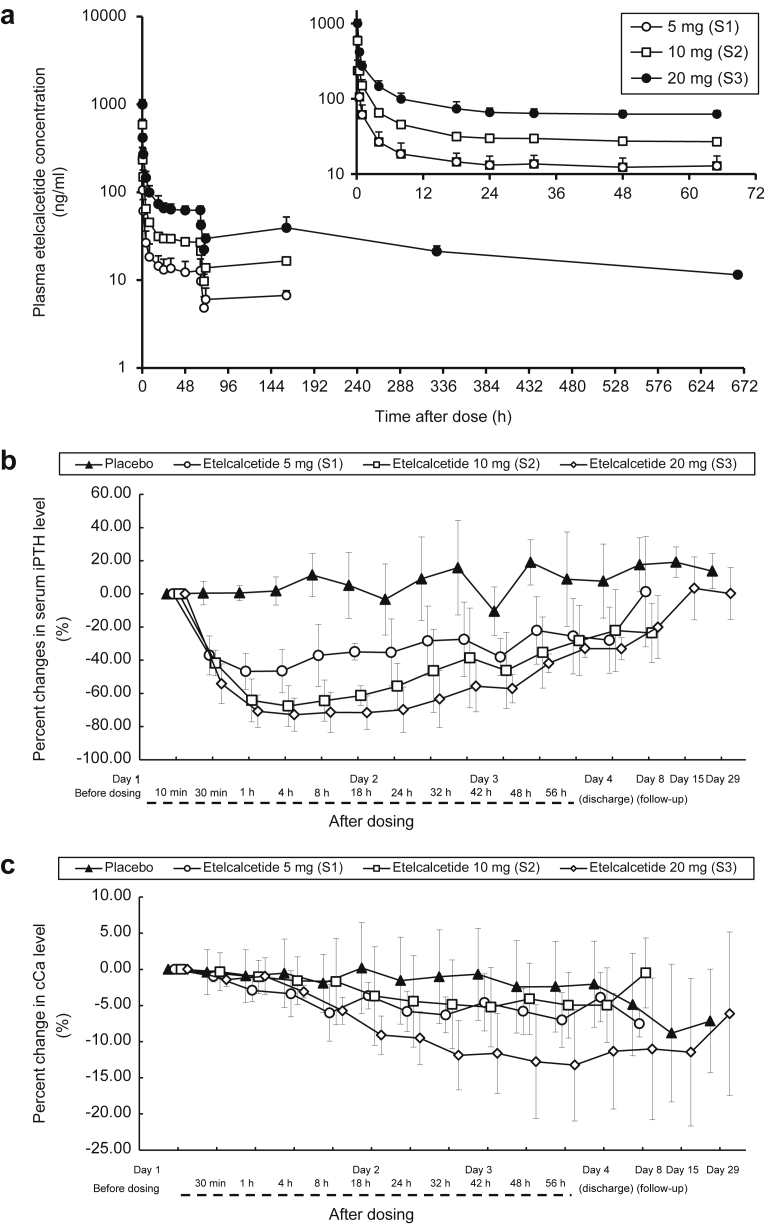
The time course of plasma etelcalcetide concentrations after a single dose is shown in Figure 1a. Injected etelcalcetide quickly decreased with time, but remained fairly constant from 24 h to the next dialysis. AUClast and Cmax increased dose-proportionally (Table 2).
Figure 1.
Single-dose study. Etelcalcetide was injected via the venous blood line after dialysis in hemodialysis patients with secondary hyperparathyroidism (SHPT). (a) Mean (+SD) plasma concentration of etelcalcetide following single-dose i.v. injection. (Inset) First 65 hours of the plasma concentration curve. (b) Percent changes in serum intact parathyroid hormone (iPTH) after single-dose i.v. injection. Data are expressed as mean ± SD. (c) Percent changes in serum albumin-corrected calcium (cCa) after single-dose i.v. injection. Data are expressed as mean ± SD.
Table 2.
Pharmacokinetic parameters (summary statistics) in single-dose study
| Cohort (dosage level) | n | AUClast (ng·h/ml) |
Cmax (ng/ml) |
|---|---|---|---|
| Mean ± SD | |||
| S1 (5 mg) | 4 | 1110 ± 360 | 236 ± 89 |
| S2 (10 mg) | 4 | 2550 ± 110 | 589 ± 88 |
| S3 (20 mg) | 4 | 5460 ± 680 | 999 ± 135 |
AUC last, area under the curve from time 0 to last time point with quantifiable concentration before the next hemodialysis; S, single-dose.
Pharmacodynamics
Serum iPTH was quickly reduced in 30 minutes after a single dose, and a plateau was reached at 1 hour in the etelcalcetide groups (Figure 1b). The effect was sustained until day 4, and the percent change from the baseline was approximately −30% with 20 mg at 56 hours. The effect was also dose dependent, with a mean maximum change from the baseline of −16.21% ± 8.51% for placebo, −50.43% ± 12.63% for 5 mg, −69.26% ± 11.35% for 10 mg, and 74.47% ± 11.12% for 20 mg. On the other hand, serum cCa did not change appreciably for 8 hours, then gradually decreased after 18 hours, and reached a plateau at 56 hours in the etelcalcetide groups (Figure 1c). The mean maximum change from the baseline was −4.13% ± 5.41% for placebo, −7.26% ± 3.48% for 5 mg, −6.79% ± 5.86% for 10 mg, and −14.26% ± 6.01% for 20 mg. No patients developed hypocalcemia with cCa < 7.5 mg/dl in both groups. Serum P followed a similar time course in the placebo and etelcalcetide groups until 4 hours after drug administration. From 4 hours to day 4, serum P remained lower in the etelcalcetide groups compared with the placebo group.
Safety and Tolerability
No deaths or serious adverse events (AEs) were reported during the study. AEs were reported in 1 patient (25.0%) each in the etelcalcetide 5-mg group (abnormal activated partial thromboplastin time [APTT] and abnormal prothrombin) and 10-mg group (APTT) group, and in 2 patients (33.3%) in the placebo group (atrial flutter in 1 patient, and fatigue, peripheral pain, and dermatitis in the other). With the exception of atrial flutter in the placebo group, all AEs were considered unrelated to treatment. No antietelcalcetide antibodies were detected during the study period.
Multiple-Dose Study
Patients
Demographic and clinical characteristics of patients are summarized in Table 3. The mean serum iPTH at screening was 636.7 ± 183.0, 695.0 ± 345.7, and 453.5 ± 127.5 pg/ml for the placebo (M1+M2), etelcalcetide M1, and M2 group, respectively.
Table 3.
Demographic and clinical characteristics of patients in multiple-dose study
| Groups |
Placebo (M1+M2) | Cohort M1 |
Cohort M2 |
|
|---|---|---|---|---|
| Dose | 2.5 mg/person | 5 mg/person | ||
| No. of patients, n | 6 | 8 | 8 | |
| Sex | Male | 2 (33.3%) | 4 (50.0%) | 3 (37.5%) |
| Female | 4 (66.7%) | 4 (50.0%) | 5 (62.5%) | |
| Age (yr) | Mean ± SD | 54.2 ± 16.9 | 64.4 ± 9.4 | 54.0 ± 14.6 |
| Median | 54.0 | 61.5 | 53.5 | |
| Range | 30–74 | 54–81 | 37–83 | |
| Body weight (kg) (postdialysis) | Mean ± SD | 52.13 ± 6.56 | 48.05 ± 11.47 | 63.88 ± 18.52 |
| Median | 48.80 | 46.25 | 63.90 | |
| Range | 46.3–60.5 | 33.2–67.4 | 38.9–88.4 | |
| BMI (kg/m2) | Mean ± SD | 21.16 ± 1.21 | 19.63 ± 2.83 | 25.16 ± 6.09 |
| Median | 20.99 | 18.74 | 23.69 | |
| Range | 19.6–22.9 | 16.5–24.2 | 17.0–35.9 | |
| Duration of dialysis (yr) | Mean ± SD | 8.2 ± 5.6 | 13.1 ± 6.2 | 9.6 ± 6.0 |
| Median | 8.0 | 11.0 | 10.0 | |
| Range | 1–17 | 7–24 | 2–21 | |
| Active vitamin D treatment | Yes | 4 (66.7%) | 7 (87.5%) | 7 (87.5%) |
| No | 2 (33.3%) | 1 (12.5%) | 1 (12.5%) | |
| Phosphate binder treatment | Yes | 5 (83.3%) | 8 (100.0%) | 8 (100.0%) |
| No | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | |
| Serum iPTH (pg/ml) | Mean ± SD | 636.7 ± 183.0 | 695.0 ± 345.7 | 453.5 ± 127.5 |
| Median | 655.5 | 556.5 | 415.0 | |
| Range | 350–813 | 408–1420 | 341–726 | |
| Serum cCa (mg/dl) | Mean ± SD | 9.62 ± 0.79 | 9.80 ± 0.50 | 10.20 ±0.79 |
| Median | 9.25 | 9.75 | 10.05 | |
| Range | 9.0–10.9 | 9.2–10.7 | 9.4–11.8 | |
| Serum P (mg/dl) | Mean ± SD | 4.95 ± 0.80 | 5.48 ± 1.35 | 5.36 ± 1.22 |
| Median | 4.95 | 5.35 | 5.60 | |
| Range | 4.0–5.8 | 3.9–7.5 | 3.3–6.9 | |
| Serum BAP (μg/l)a | Mean ± SD | 27.90 ± 7.26 | 35.58 ± 40.78 | 17.30 ± 9.68 |
| Median | 27.55 | 21.85 | 15.70 | |
| Range | 20.2–40.2 | 10.8–135.0 | 8.0–37.3 | |
| Serum TRACP-5b (mU/dl)a | Mean ± SD | 821.0 ± 178.1 | 697.6 ± 355.8 | 548.5 ± 297.7 |
| Median | 838.5 | 572.0 | 488.0 | |
| Range | 504–1050 | 328–1390 | 188–1090 | |
| Serum FGF23 (pg/ml)a (logarithm) | Mean ± SD | 7.81 ± 1.53 | 8.66 ± 2.31 | 9.54 ± 1.64 |
| Median | 7.96 | 9.64 | 9.67 | |
| Range | 5.7–10.2 | 5.5–11.0 | 6.6–11.8 | |
BAP, bone alkaline phosphatase; BMI, body mass index; cCa, albumin-corrected Ca; FGF23, fibroblast growth factor−23; iPTH, serum intact parathyroid hormone; M, multiple-dose; P, phosphorus; TRACP-5b, tartrate-resistant acid phosphatase−5b.
Data obtained for baseline measurements.
Pharmacokinetics
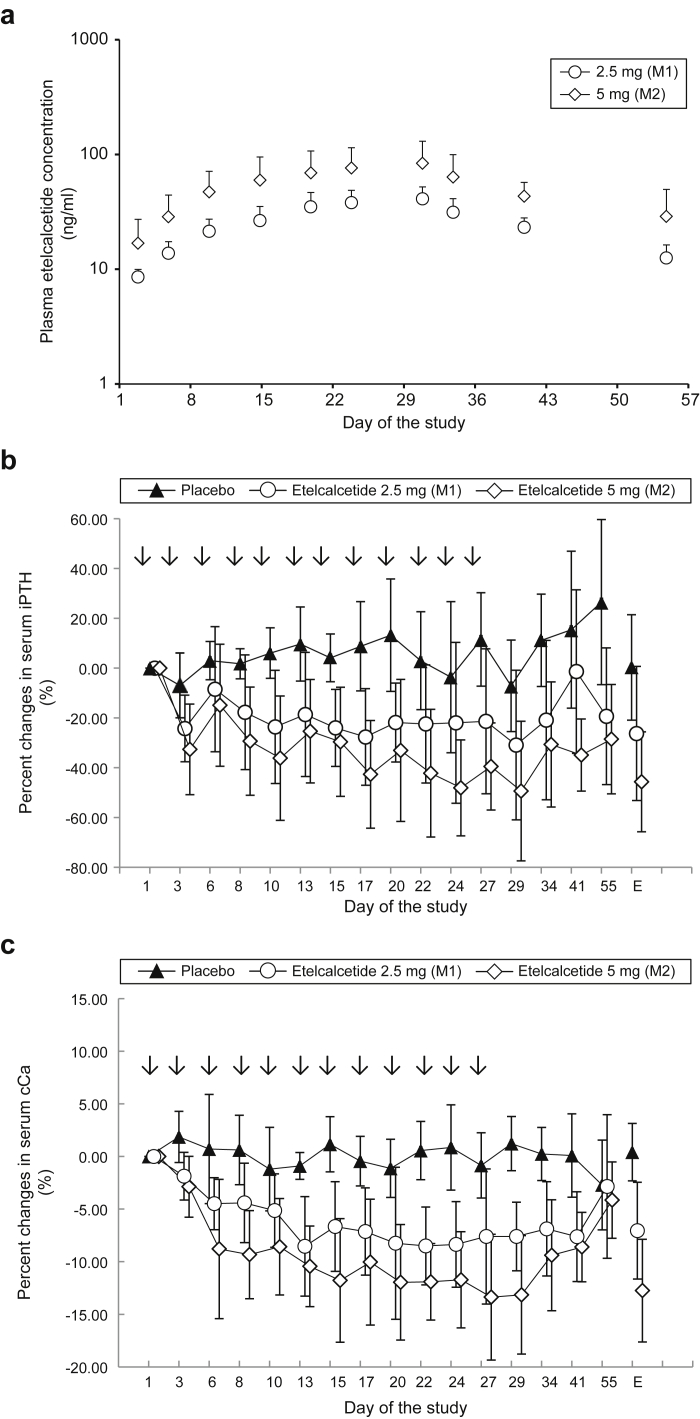
Etelcalcetide plasma concentration reached a plateau by 4 weeks (Figure 2a). AUClast and Cmax at week 4 were up to 3.05 and 1.18 times higher than those after the initial dose, respectively. AUClast and Cmax increased dose-dependently (Table 4).
Figure 2.
Multiple-dose study. Etelcalcetide was injected via the venous blood line after dialysis on days 1, 3, 6, 8, 10, 13, 15, 17, 20, 22, 24, and 27 (arrows in b and c) in hemodialysis patients with secondary hyperparathyroidism (SHPT). Blood samples were obtained before each dialysis. (a) Mean (+SD) plasma concentration of etelcalcetide following multiple-dose i.v. injections. (b) Percent changes in serum intact parathyroid hormone (iPTH) during the study period (multiple-dose i.v. injections and follow-up). iPTH levels over the study period (from day 1 to day 55). Data are expressed as mean ± SD. E denotes at the end of the study (day 55) or at withdrawal from the study. (c) Percent changes in serum albumin-corrected calcium (cCa) during the study period (multiple-dose i.v. injections and follow-up). cCa levels over the study period (from day 1 to day 55). Data are expressed as means ± SD. E denotes at the end of the study (day 55) or at withdrawal from the study.
Table 4.
Pharmacokinetic parameters (summary statistics) in multiple-dose study
| Cohort (dosage level) | n | Evaluation date | AUClast (ng·h/ml) |
Cmax (ng/ml) |
|---|---|---|---|---|
| Mean ± SD | ||||
| M1 (2.5 mg) | 8 | Day 1a | 600 ± 133 | 151 ± 56 |
| 7 | Day 27b | 1830 ± 380 | 168 ± 24 | |
| 7 | Day 27/day 1 | 3.05 (2.91–3.19)c | 1.18 (0.86–1.62)c | |
| M2 (5 mg) | 8 | Day 1 | 1200 ± 560 | 315 ± 89 |
| 8 | Day 27 | 3860 ± 1840 | 356 ± 143 | |
| 8 | Day 27/day 1 | 3.20 (2.87–3.57)c | 1.09 (0.90–1.32)c | |
AUClast, area under the curve from time 0 to last time point with quantifiable concentration before the next hemodialysis; M, multiple-dose.
First administration day of multiple-dose part of study.
Last administration day of multiple-dose part of study.
Geometric mean (95% confidence interval).
Pharmacodynamics
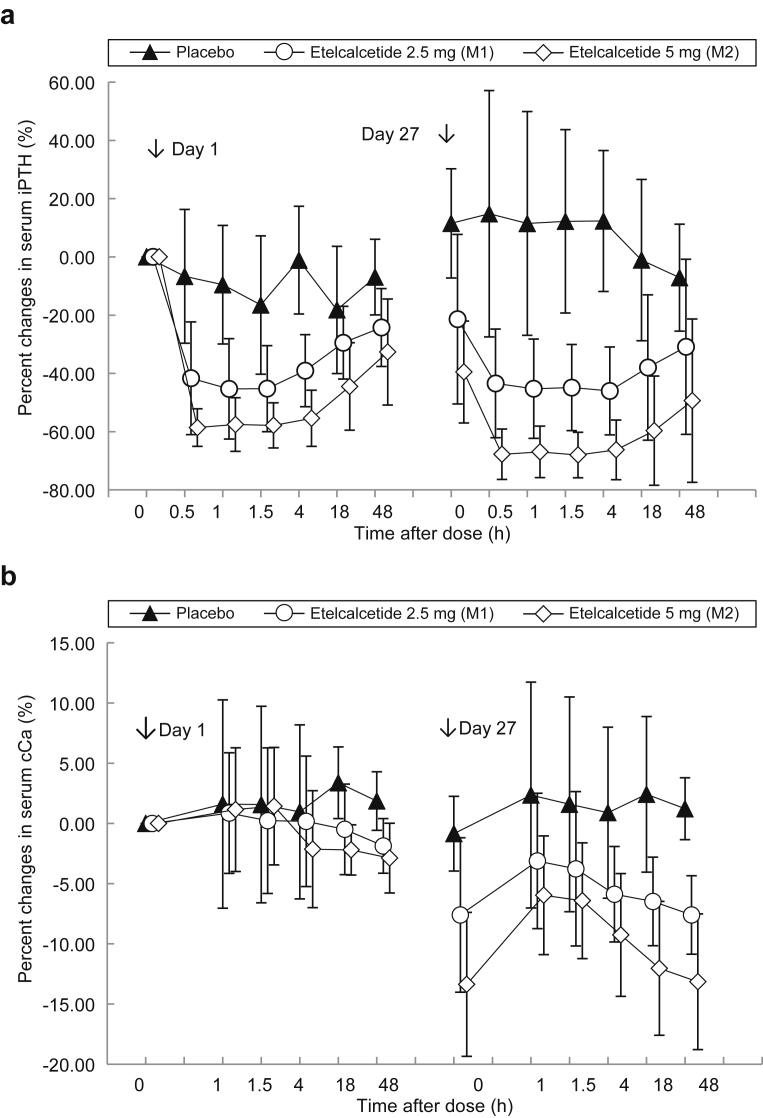
Etelcalcetide injections resulted in marked dose-dependent reductions in serum iPTH 30 minutes later (−6.68% ± 22.97% for placebo, −41.65% ± 19.36% for 2.5 mg, and −58.60% ± 6.47% for 5 mg) and stayed at the same level up to 4 hours, remaining −24.25% ± 13.36% for 2.5 mg and −32.64% ± 18.20% for 5 mg lower than baseline on day 3 compared with placebo at −6.94% ± 13.00%. Similar results were obtained at the last injection (day 27 to day 29) in the etelcalcetide groups (Figure 3a). The reductions in serum iPTH were sustained throughout the study period, with a percent change from the baseline of −26.25% ± 26.94% for 2.5 mg, −45.65% ± 20.06% for 5 mg, and 0.25% ± 21.17% for placebo at the end of the study or at study withdrawal (Figure 2b). Etelcalcetide also dose-dependently reduced serum cCa from day 2 through day 41 (Figure 2c). The mean percent change from the baseline at the end of the study or at withdrawal was 0.41% ± 2.73% for placebo, −7.05% ± 4.60% for 2.5 mg, and −12.75% ± 4.88% for 5 mg. No patients experienced hypocalcemia with serum cCa < 7.5 mg/dl in both groups. The mean percent changes from the baseline in serum P were greater in the etelcalcetide groups compared with the placebo group. At the end of the treatment or withdrawal from the study, the percent changes from the baseline were −5.75% ± 11.71% for placebo, −14.68% ± 13.38% for 2.5 mg, and −13.79% ± 18.18% for 5 mg.
Figure 3.
Percent changes in serum intact parathyroid hormone (iPTH) and albumin-corrected calcium (cCa) during the study period (multiple-dose i.v. injections and follow-up). Etelcalcetide was injected via the venous blood line after dialysis on days 1, 3, 6, 8, 10, 13, 15, 17, 20, 22, 24, and 27 in hemodialysis patients with secondary hyperparathyroidism (SHPT). Blood samples were obtained before each dialysis. Data are expressed as mean ± SD. (a) iPTH levels following drug administration (from day 1 to day 3, and from day 27 to day 29). (b) cCa levels following drug administration (from day 1 to day 3, and from day 27 to day 29).
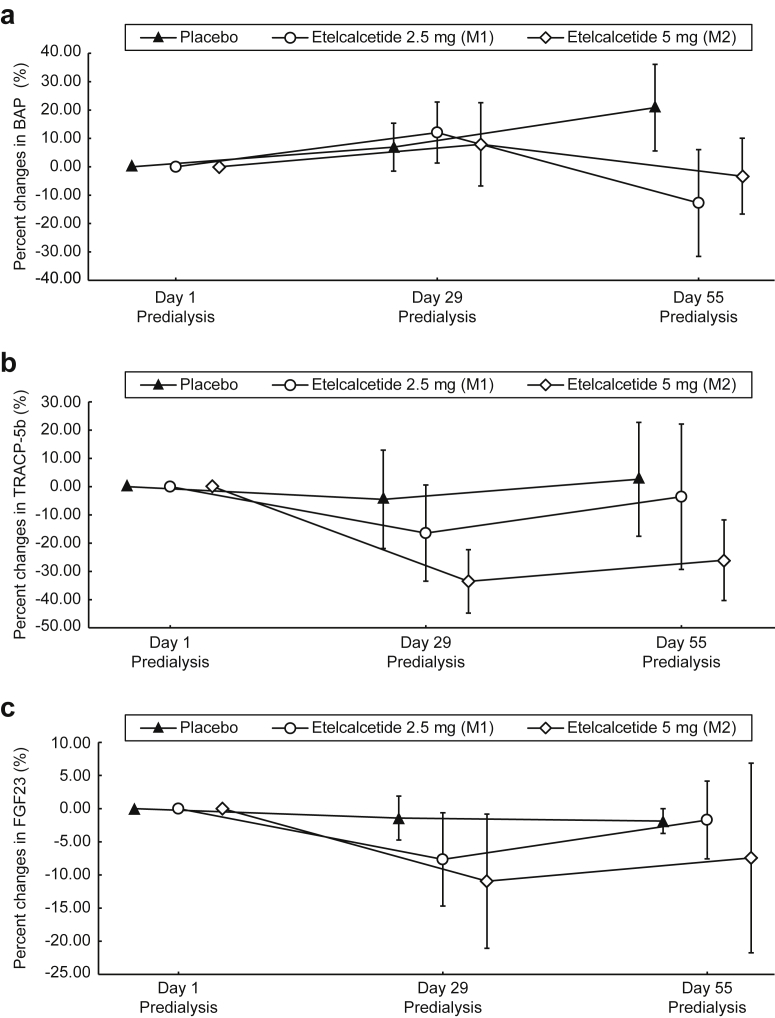
Serum BAP, TRACP-5b, and FGF23 levels did not change in the placebo group throughout the study period (Figure 4a−c). Concentrations of BAP before hemodialysis also remained unchanged on day 29 in the etelcalcetide groups, but showed trends toward decreases on day 55. On the other hand, for TRACP-5b, a trend toward decreases in the etelcalcetide groups on day 29 and a return to baseline on day 55 was shown. Etelcalcetide tended to decrease FGF23 on day 29, showing a trend toward a return to baseline levels on day 55.
Figure 4.
Percent changes in bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase−5b (TRACP-5b), and fibroblast growth factor 23 (FGF23) serum levels during the study period (multiple-dose i.v. injection and follow-up). Etelcalcetide was injected via the venous blood line after dialysis on days 1, 3, 6, 8, 10, 13, 15, 17, 20, 22, 24, and 27 in hemodialysis patients with secondary hyperparathyroidism (SHPT). Data are expressed as mean ± SD. (a) BAP; (b) TRACP-5b; (c) FGF23.
Safety and Tolerability
AEs were reported in 7 patients (87.5%) in the etelcalcetide 2.5-mg group (atrial fibrillation, bradycardia, cardiopulmonary arrest, supraventricular extrasystoles, paroxysmal tachycardia, ventricular extrasystoles, edema peripheral, APTT prolonged, increased blood creatine phosphokinase, prothrombin time prolonged, white blood cell count increased, neutrophil percentage increased, lymphocyte percentage decreased, and upper respiratory tract inflammation), 3 patients (37.5%) in the 5-mg group (vertigo positional, decrease in blood calcium, and hypotension), and 2 patients (33.3%) in the placebo group (blood creatine phosphokinase increased, blood potassium increased, and heat rash). For bradycardia and cardiopulmonary arrest, supraventricular extrasystoles and ventricular extrasystoles, paroxysmal tachycardia, and increased blood creatine phosphokinase, 1 patient each in the 2.5-mg group, and a decrease in blood calcium in the 5-mg group, the relationships to treatment were not ruled out. With the exception of cardiopulmonary arrest in the 2.5-mg group, most of these AEs were mild and disappeared during the study period. No deaths occurred during the study period. No antietelcalcetide antibodies were detected during the study period.
Discussion
The present study demonstrated that single- and multiple-dose i.v. injections of etelcalcetide resulted in dose-dependent and sustained reductions of serum iPTH and cCa in Japanese hemodialysis patients with SHPT as well as in a non-Japanese patient population.18, 19, 20
Compared with previous studies in non-Japanese patients, this study included patients with serum iPTH much lower than 500 pg/ml, which is the threshold for treatment with calcimimetics such as cinacalcet, according to the guidelines.7 The dosage levels in this study, the single dose at 5, 10, or 20 mg and the multiple dose at 2.5 or 5 mg 3 times weekly for 4 weeks, were also relatively lower compared with previous studies of a single dose at 5 to 60 mg and multiple doses at 5 to 10 mg 3 times weekly for 4 weeks.18, 19 There was a consideration to evaluate the possibility that a lower dose might benefit patients with less severe SHPT, thereby preventing disease progression and leading to better prognosis. This study clearly indicated that a multiple doses of 2.5 mg 3 times weekly were effective in reducing serum iPTH in Japanese patients with moderate to severe SHPT.
Etelcalcetide lowers PTH and is expected to affect bone metabolism and to lower Ca. It is important to clarify the relation between pharmacokinetics and pharmacodynamic parameters of etelcalcetide to appropriately assess its efficacy and safety. If unstable fluctuations of PTH and Ca are observed, the timing for assessment of efficacy and safety needs to be considered. Etelcalcetide plasma concentrations remained constant from 24 hours after administration to the next dialysis, and were increased with repeated administration. Accordingly, the mean AUClast following the day 27 injection was approximately 3 times higher than the AUClast following the day 1 injection, whereas the mean Cmax following both injections was approximately the same. In the present study, dialysis reduced etelcalcetide plasma concentrations to 30% to 40%. Based on the pharmacokinetics profile of etelcalcetide, a single dose resulted in sustained reductions of serum iPTH and cCa. Similarly, multiple doses for 4 weeks showed sustained reductions despite changes in bone metabolism biomarkers, such as BAP and TRACP-5b, and marked daily fluctuations of PTH and Ca did not occur.
Although cinacalcet has a short half-life and therefore induces diurnal fluctuation of serum iPTH, etelcalcetide may maintain a stable level of serum iPTH. In addition, oral administration of cinacalcet is often associated with gastrointestinal AEs, causing poor patient adherence to the therapy.12, 13, 14 To avoid these AEs, various timings of cinacalcet dosing, such as dosing after a meal or before sleep, have been attempted. However, changing the dose timing also affects measured values of serum iPTH and cCa, owing to diurnal fluctuation.22 To ascertain the effects and safety of cinacalcet, iPTH measurements are recommended to be performed before drug administration. Such a requirement might not be necessary for etelcalcetide, because etelcalcetide is administered via the venous blood line after dialysis and because diurnal changes in serum iPTH and cCa in etelcalcetide-treated patients are small.
In contrast to the quick reduction in serum iPTH, the effect of etelcalcetide on serum Ca appeared gradually and steadily. It is therefore possible to predict possible hypocalcemia in advance through careful observation. This is an advantage over cinacalcet, which reduces serum Ca 8 to 12 hours after oral administration, expected to be during sleep. Because the timing of blood sampling and measurement affects the evaluation of drug effects, serum Ca was suggested to be evaluated 8 to 12 hours after administration of cinacalcet.23
FGF23 serum level is a known factor that predicts CKD progression and prognosis.1, 2 In addition, high FGF23 is associated with mortality, left ventricular hypertrophy, and cardiovascular events independent of P, iPTH, and other clinical factors.5 Although trends toward decreases in FGF23 were shown with etelcalcetide, the amount of exposure was limited in the present study; further investigation with clinical doses will be necessary.
Good safety and tolerability profiles were demonstrated for etelcalcetide. In terms of serious AEs, cardiopulmonary arrest reported in the 2.5-mg group of the multiple-dose study, the relationship to treatment was not ruled out, although possible causes included patient background such as aortic valve stenosis/incompetence, and long-term dialysis history of 19 years as well as the increased fluid volume. The event promptly recovered with heart massage and pharmacotherapy. No other AE that led to drug discontinuation was reported except for this patient, and no serious AE was reported in the 5-mg group. Although AEs of nausea and vomiting were not reported, the amount of exposure was limited in the study; further investigation with clinical doses will be needed.
This is the first study of etelcalcetide in Japanese hemodialysis patients with SHPT. Study limitations include the small number of patients and the short period of dosing, as the patients had to be hospitalized for frequent and accurate blood collection timing to clarify the relationship between the pharmacokinetics of etelcalcetide and pharmacodynamics. Therefore, more definite quantitative data from a subsequent phase 2 or 3 study extending patient numbers and treatment duration are required to confirm these observations with statistical evaluation.
In conclusion, the present study clarified the relationship between the pharmacokinetics and pharmacodynamics of etelcalcetide, and demonstrated that etelcalcetide produced dose-dependent and sustained reductions in serum iPTH and cCa in Japanese hemodialysis patients with SHPT. The i.v. injection of etelcalcetide will provide a new alternative drug therapy offering increased compliance and benefits for SHPT in dialysis patients.
Disclosure
KY, MF, TS, TA, and TA report consultancies with Ono Pharmaceutical Co., Ltd. and Kyowa Hakko Kirin; AF, AY, and MO report employment with Ono Pharmaceutical Co., Ltd.
Acknowledgments
This work, including writing and editing services provided by ASCA Corporation, was funded by Ono Pharmaceutical Co., Ltd. The authors thank the Clinical Development Department, Ono Pharmaceutical Co., Ltd., for their assistance in preparing and writing this paper. The investigators were as follows: T. Iitsuka, Ibaraki Seinan Medical Center Hospital; H. Ogura, Kurosawa Hospital, Bishinkai Medical Corporation; Y. Kanamaru, Clinique Soigner; K. Oguchi, Ikegami General Hospital, Medical Corporation Showakai; T. Suzuki, Suzuki Urology Clinic; Y. Kanno, Kanno Dialysis & Vascular Access Clinic; H. Shimosaka, Tajimi Clinic, Hakuyoukai Medical Corporation; M. Horie, Daiyukai Daiichi Hospital; H. Kasuga, Nagoya Kyoritsu Hospital and Kaikoukai Central Clinic, Kaikoukai Medical Group; K. Harada, Kasaoka Daiichi Hospital; and S. Funakoshi, Nagasaki Jin Hospital, Japan.
Footnotes
Figure S1. Participant flow.
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
Participant flow.
References
- 1.Cunningham J., Locatelli F., Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 2.Fraser W.D. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 3.Drüeke T.B. The pathogenesis of parathyroid gland hyperplasia in chronic renal failure. Kidney Int. 1995;48:259–272. doi: 10.1038/ki.1995.292. [DOI] [PubMed] [Google Scholar]
- 4.Jean G., Bresson E., Terrat J.C. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 5.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganesh S.K., Stack A.G., Levin N.W. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CDK-MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 8.Guideline Working Group, Japanese Society for Dialysis Therapy Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12:514–525. doi: 10.1111/j.1744-9987.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Goodman W.G. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17:209–216. doi: 10.1111/j.0894-0959.2004.17308.x. [DOI] [PubMed] [Google Scholar]
- 10.Brown E.M., Gamba G., Riccardi D. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E.F., Heaton W.H., Miller M. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 12.Block G.A., Martin K.J., de Francisco A.L. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. New Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 13.Gincherman Y., Moloney K., McKee C. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int. 2010;14:68–72. doi: 10.1111/j.1542-4758.2009.00397.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa M., Yumita S., Akizawa T. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328–335. doi: 10.1093/ndt/gfm534. [DOI] [PubMed] [Google Scholar]
- 15.Walter S., Baruch A., Dong J. Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther. 2013;346:229–240. doi: 10.1124/jpet.113.204834. [DOI] [PubMed] [Google Scholar]
- 16.Shen J., Xiao J., Pickthorn K. A pharmacokinetic/pharmacodynamic model for AMG 416, a novel calcimimetic peptide, following a single intravenous dose in healthy subjects. J Clin Pharmacol. 2014;54:1125–1133. doi: 10.1002/jcph.314. [DOI] [PubMed] [Google Scholar]
- 17.Alexander S.T., Hunter T., Walter S. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol. 2015;88:853–865. doi: 10.1124/mol.115.098392. [DOI] [PubMed] [Google Scholar]
- 18.Martin K.J., Pickthorn K., Huang S. AMG 416 (velcalcetide) is a novel peptide for the treatment of secondary hyperparathyroidism in a single-dose study in hemodialysis patients. Kidney Int. 2014;85:191–197. doi: 10.1038/ki.2013.289. [DOI] [PubMed] [Google Scholar]
- 19.Bell G., Huang S., Martin K.J. A randomized, double-blind, phase 2 study evaluating the safety and efficacy of AMG 416 for the treatment of secondary hyperparathyroidism in hemodialysis patients. Curr Med Res Opin. 2015;31:943–952. doi: 10.1185/03007995.2015.1031731. [DOI] [PubMed] [Google Scholar]
- 20.Bushinsky D.A., Block G.A., Martin K.J. Treatment of secondary hyperparathyroidism: results of a phase 2 trial evaluating an intravenous peptide agonist of the calcium-sensing receptor. Am J Nephrol. 2015;42:379–388. doi: 10.1159/000442754. [DOI] [PubMed] [Google Scholar]
- 21.Tentori F., Wang M., Bieber B.A. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bover J., Ureña P., Ruiz-Garcia C. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161–174. doi: 10.2215/CJN.01760215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuno S., Ishimura E., Inaba M. Cinacalcet for mineral metabolism management: is it possible to achieve the goal set in the JSDT guideline? In: Akiba T., Akizawa T., editors. Hemodialysis Therapy IX. Igaku Tosho Shuppan, Japan; Tokyo: 2009. pp. 32–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Participant flow.