Abstract
Introduction
Ex vivo normothermic perfusion offers an alternative method of organ preservation, allowing donor kidneys to be reanimated and evaluated prior to transplantation. Beyond preservation, it can be used to characterize the immunological contribution of the donor kidney in isolation. Furthermore, it has the potential to be used as an immunomodulatory strategy to manipulate donor kidneys prior to transplantation.
Methods
Explanted porcine kidneys underwent 6 hours of perfusion. Sequential perfusate samples were collected and leukocytes characterized via flow cytometry. An inflammatory profile was generated via cytokine quantification. Cell-free DNA was also determined as markers of cell death.
Results
All kidneys functioned within normal parameters and met the criteria for transplantation at the end of perfusion. Throughout perfusion there were continuous increases in pro-inflammatory cytokines, including large concentrations of interferon-γ, suggesting that perfusion drives a significant inflammatory response. Increasing concentrations in cell-free DNA were also observed, suggesting cell death. During perfusion there was a marked cellular diapedesis of T cells, B cells, natural killer (NK) cells, and monocytes from the kidney into the circuit. Minor populations of granulocytes and macrophages were also detected.
Discussion
We demonstrate that ex vivo normothermic perfusion initiates an inflammatory cytokine storm and release of mitochondrial and genomic DNA. This is likely to be responsible for immune cell activation and mobilization into the circuit prior to transplantation. Interestingly this did not have an impact on renal function. These data therefore suggest that normothermic perfusion can be used to immunodeplete and to saturate the pro-inflammatory capacity of donor kidneys prior to transplantation.
Keywords: ex vivo normothermic perfusion, kidney transplantation, allorecognition, passenger leukocytes, immune migration
Kidney transplantation is severely limited by a shortage of suitable donor organs. This has led to prolonged waiting list times and subsequent high waiting list mortality rates. In an attempt to overcome this problem, there has been a drive to develop ex vivo normothermic perfusion (EVNP) as a tool to evaluate and to recondition expanded criteria donor kidneys, with marginal function, and kidneys donated after circulatory death. Aside from the survival benefit that EVNP has offered to patients by enabling the safe evaluation and transplantation of such organs, this technology may also represent a novel therapy to manipulate the donor leukocyte repertoire prior to transplantation.1, 2, 3
Although the importance of donor-derived leukocytes in the allorecognition and rejection process has been demonstrated previously, a detailed evaluation of the temporal kinetics of leukocyte migration from the kidney has not been possible prior to EVNP. To date we have a limited understanding of the natural history of passenger leukocyte transfer from the donor kidney to the recipient. Given that the kidney is equipped with a sophisticated localized immune system, such data are important. During homeostasis, the kidney remains in a regulatory state. However, following insult, a significant immune response can occur. In severe cases of injury and inflammation such as brain death, a robust immune response ensues. This includes local and eventually systemic inflammation manifesting in a local cytokine storm and mass cellular infiltrate in the donor.4, 5, 6 Using current strategies for transplantation, it is at this point that the inflamed donor kidney with global immune activation is implanted in the recipient. Direct presentation of alloantigens by donor antigen presenting cells to recipient T cells occurs rapidly following transplantation.7 The latter cells infiltrate the graft, and the rejection process is initiated. These early interactions are key to the long-term clinical outcome of the patient, and as such warrant detailed investigation.
This study was designed to determine the immune and inflammatory contribution of the donor kidney following reperfusion in isolation. For this purpose, we have used a porcine model of EVNP to replicate the response of the donor kidney to revascularization in the absence of any contribution from a recipient immune system.
In addition, we hypothesize that mobilization and removal of a proportion of passenger leukocytes using EVNP may reduce graft immunogenicity prior to transplantation, thereby reducing acute rejection and improving clinical outcome.8
Materials and Methods
Procurement of Donor Organs
Kidneys from 5 Landrace pigs with a mean weight of 80 kg were collected from a local abattoir. All pigs were culled under schedule 1 of the Home Office Scientific Act 1986 regulations. In brief, pigs were rendered unconscious via electrical stunning followed by exsanguination. Approximately 3L of blood was collected into a sterile receptacle containing 100 ml of normal saline solution supplemented with 40,000 iU of unfractionated heparin (Fannin, UK). The abdomen was opened using a midline incision, and the kidneys were exposed and excised. Kidneys were immediately placed on ice and dissected, allowing inspection for lacerations or cysts. If deemed acceptable, the renal artery and ureter were isolated and cannulated, and 20 ml of glyceryl trinitrate (Hameln Pharmaceuticals, Gloucestorshire, UK) was flushed through the renal artery. This was immediately followed by 1L 4°C Soltran (Soltran; Baxter Healthcare, Thetford, UK) supplemented with 10,000 iU of unfractionated heparin at a hydrostatic pressure of 100 mm Hg. Kidneys were then submerged in Soltran and placed on ice for a standardized static cold storage time of 2 hours.
Perfusion Circuit
The EVNP circuit consisted of an organ chamber with a porous platform on which the kidney was placed, allowing the renal outflow to drain into the reservoir. The perfusate was re-circulated using a centrifugal pump, passing through a membranous oxygenator attached to a heater−cooler system to maintain the organ at normothermia. Pressure and temperature probes were incorporated to maintain these parameters.
EVNP Procedure
EVNP was performed using an adaption of the currently published protocol.9
Priming
Prior to placing the kidney onto the EVNP circuit, the system was primed with perfusate to ensure that the circuit was de-aired. This consisted of 350 ml of Ringer’s solution supplemented with 5% bovine serum albumin (BSA). Approximately 500 ml of autologous, leukocyte-depleted (Haemonetics, UK) packed red blood cells were infused to achieve a target hematocrit of 20% to 25%. Following re-circulation, the perfusate was further supplemented with 13.2 mg of dexamethasone (Hameln Pharmaceuticals), 30 ml of 10% mannitol, 20 ml of 8.4% sodium bicarbonate and 4,000 iU of unfractionated heparin. A syringe driver containing 20 ml of Nutriflex supplemented with 14 ml of 8.4% sodium bicarbonate, 100 iU of actrapid insulin (Novo Nordisk, Denmark), and 25 ml of 15% glucose was infused into the circuit at a flow rate of 10 ml/h. A second syringe driver containing 18 ml of epoprostenol (Sandoz, Surrey, UK) and 42 ml of normal saline solution were also infused at an initial rate of 24 ml/h. Finally, a gas mixture of 95% oxygen and 5% carbon dioxide was supplied to the membrane oxygenator at a flow rate of approximately 0.5 L/min. A blood gas analyser was used to assess the pO2, pCO2, and electrolyte concentrations within the circuit, which were corrected to maintain physiological levels. The oxygen consumption was calculated from venous and arterial blood using the following equation: (pO2art – pO2ven) × flow / weight. Creatinine was added to the perfusate to achieve a circulating concentration of 1500 μmol/L.
Preparation for Perfusion
Once the perfusion circuit had been fully primed and de-aired and the blood gas variables were within physiological reference ranges, the kidneys were removed from ice. A baseline photograph was taken to provide a reference during perfusion so that any structural changes in the kidney could be monitored. Soltran residue was removed from the vasculature with 200 ml of 4°C Ringer’s solution. Finally, a needle core biopsy was obtained, and the kidney was weighed to provide the baseline parameters.
Perfusion
Following preparation, the kidneys were connected to the perfusion circuit via the renal artery cannula. A pressure probe was attached to the arterial arm of the circuit to allow the pressure of perfusate entering the artery to be measured and controlled. The initial flow rate was reduced to approximately 0.05 L/min to allow adequate de-airing of the kidney prior to attachment and to prevent endothelial damage due to hemodynamic sheer stress. The flow rate was then gradually increased until the arterial pressure reached a mean of 55 mm Hg, and the observations were recorded. At the same time, the ureter cannula was placed into a measuring cylinder to collect and record the urine output. The arterial pressure was increased by 5 mm Hg every 5 minutes until the target pressure of 75 mm Hg was achieved 20 minutes after reperfusion. At this time, the infusion of epoprostenol was started. Observations and blood gas analysis were routinely collected, and any changes in the physiology were corrected. Urine output was regularly monitored and replaced with Ringer’s solution to maintain normal physiology and circuit volume. The circulating perfusate temperature was set to 38°C ± 1°C to rapidly re-warm the kidney to physiological temperatures, allowing kidney function to be restored and cellular metabolism to be re-established. The organ chamber was covered to maintain this temperature and to retain the humidity throughout the perfusion period. If there was a drop in functional parameters, therapeutic interventions were made, in line with clinical practice, so as to maintain tissue viability and normal physiology. The rate of epoprostenol infusion was increased in 1.6-ml/h increments for every 10% drop in renal blood flow to a maximum of 34.8 ml/h. If the response was inadequate despite the maximum infusion, or if urine output dropped below 10 ml/h, then a bolus of either 10% mannitol or Ringer’s solution supplemented with 5% bovine serum albumin was added. All interventions did not exceed doses used clinically, and responses were closely monitored to ensure that irreversible renal failure had not occurred. In all cases, kidneys were perfused for 6 hours.
Sample Collection During EVNP
Perfusate
Prior to initiation of EVNP, 15 ml of perfusate was collected to provide a baseline sample. Additional samples were collected at 15 and 30 minutes, followed by 30-minute intervals up to 3 hours of perfusion when samples were collected hourly. At baseline, 1 hour, and 6 hours, 100 μl of perfusate was analyzed by flow cytometry. All perfusate samples were centrifuged at 2000 g for 5 minutes, and 1-ml aliquots of plasma and whole blood were stored at −80°C.
Urine
Following 15 minutes of perfusion, 5 ml of urine was collected. Additional samples were collected at 30-minute intervals up to 3 hours of perfusion, when samples were collected hourly. Samples were aliquoted and stored at −80°C.
DNA Extraction
DNA was extracted from plasma samples of the perfusate using the QIAamp DNA Mini and Blood Mini kit, according to the manufacturer’s protocol (Qiagen, Manchester, UK).
Quantitative Polymerase Chain Reaction
All primers used for quantitative polymerase chain reaction were designed using the Primer Express Software v3.0.1 (Life Technologies, Paisley, UK) and their homology assessed using BLAST. Primers (Sigma Aldrich, Dorset, UK) were adjusted to 50nM using nuclease-free water (Ambion, Thermo Fisher Scientific, Waltham, MA, USA). All quantitative polymerase chain reaction was performed using a QuantStudio 12K Flex system (Life Technologies, Paisley, UK) with Power SYBR green PCR master mix (Life Technologies, Paisley, UK).
Flow Cytometry
Immunophenotyping of the perfusate samples was performed on a BD LSR II flow cytometer (Becton Dickinson, Oxford, UK). Leukocytes were identified and gated as CD45+ and their viability assessed using an efluor 506 viability dye (ebioscience, San Diego, CA). Following this, a panel of antibodies was utilized to characterize T helper cells (CD3ε+CD4α+), cytotoxic T cells (CD3ε+CD8α+), double-positive T cells (CD3ε+CD4α+CD8α+), double-negative T cells (CD3ε+CD4α-CD8α−), γδ T cells (γδ+), B cells (CD3ε-CD21+), classical monocytes (CD14+CD163−), nonclassical monocytes (CD14+CD163+), immature neutrophils (6D10+2B2−), mature neutrophils (6D10+2B2+), mature eosinophils/basophils (6D10-2B2+), and natural killer cells (CD335+). Cells were treated with red blood cell lysing solution (BD Biosciences, UK), washed, and re-suspended in 0.3 ml of staining buffer. A 20-μl quantity of e123count beads (eBioscience, CA, USA) were added, and samples were analysed for 3 minutes. All gating strategies and analysis were performed using FlowJo version 10.0.6.
Luminex Analysis
The concentration of cytokines and chemokines within the perfusate was assessed using plasma samples collected at baseline, 60 minutes, and 360 minutes. A commercially available porcine 13-plex magnetic bead panel (Merck Millipore, Billerica, MA, USA) was used, following the manufacturer’s protocol. The plate was read using a Bio-Plex 200 system (Bio Rad, Hertfordshire, UK).
Statistical Analysis
All statistical analysis was carried out using IBM SPSS software version 22. Data normality was determined by assessing mean, SD, skewness, and kurtosis. Formal evaluation was performed using the Shapiro−Wilk test. Changes in renal hamodynamics and biochemistry, cell number, and cytokine and DNA concentrations were analyzed using the general linear model or the Friedman’s 2-way analysis of variance by ranks depending on the distribution of the data. For comparisons between RBF at different time points, a paired t test or Wilcoxon signed rank test was used. Data were considered significantly different if a P value of <0.05 was observed.
Results
Changes in Renal Hemodynamics and Oxygen Consumption During EVNP
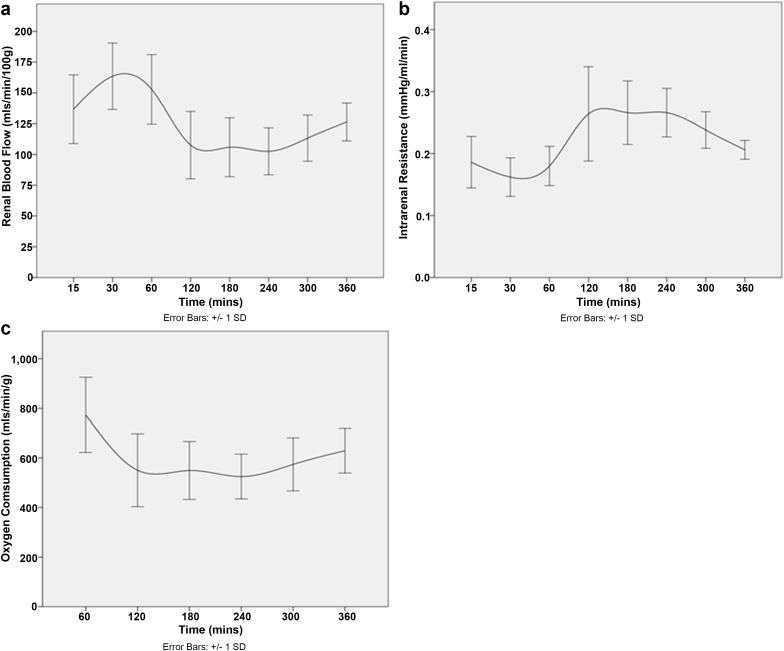
To assess the validity of the model and the viability of the kidneys, the functional parameters of the kidney were determined. Urine output, mean arterial pressure, and renal blood flow (RBF) were continually recorded. Intrarenal resistance was also determined by calculating the mean arterial pressure/RBF.
All kidneys were reperfused without any complications and appeared evenly perfused, with a significant increase in RBF between 0 and 30 minutes of perfusion from 29.7 ± 4.9 ml/min/100 g to 155.6 ± 30.4 ml/min/100 g (P = 0.043). A change in RBF was observed over the 6-hour perfusion (P < 0.001), declining after 60 minutes of perfusion, although the level remained within physiological parameters (Figure 1a). Following a short period of decrease, RBF stabilized with an increasing trend following 2 hours of perfusion. Intrarenal resistance also fluctuated during perfusion (P = 0.020) but did not exceed 0.3 mm Hg/ml/min and was inversely proportionate to the RBF (Figure 1b). The level of oxygen consumption observed throughout the perfusion remained high, peaking in the first hour. Following this, there was a slight decline that plateaued after 2 hours of perfusion and remained stable for the duration of the procedure (P = 0.006) (Figure 1c).
Figure 1.
Renal hemodynamics and oxygen consumption remain stable during perfusion. (a) Renal blood flow and (b) intrarenal resistance remained within acceptable ranges during perfusion. (c) Oxygen consumption was also maintained throughout the experiment.
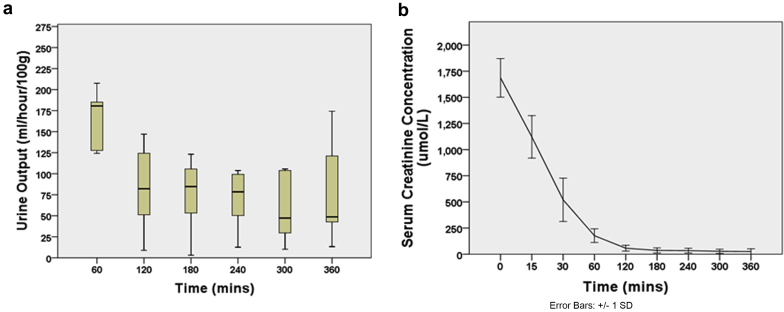
Urine Output and Creatinine Clearance During EVNP
All kidneys began producing urine immediately after being connected to the circuit (Figure 2a). The average urine production per hour was 92.03 ± 78.13 ml, with a mean total urine output of 530.04 ± 243.78 ml. The pattern of urine output changed during the perfusion period (P = 0.001), with a decline in urine output after the first hour of perfusion. The serum creatinine concentration in the circuit significantly decreased during the 6-hour perfusion (P < 0.001, Figure 2b).
Figure 2.
Tubular function is restored and retained over 6 hours of perfusion. (a) Urine production began immediately following revascularization, peaking in the first hour of perfusion. (b) Creatinine is continually removed from the circuit by the kidney and excreted in the urine.
Chemokine and Cytokine Concentrations Within the Perfusate
To characterize the inflammatory profile during perfusion, 13 cytokines and chemokines were assessed. Increasing concentrations of interferon (IFN)−γ (P = 0.021), interleukin (IL)−1α (P = 0.016), IL-1β (P = 0.010), IL-1RA (P = 0.009), IL-2 (P = 0.017), IL-6 (P < 0.001), C-X-C motif chemokine ligand 8 (CXCL-8) (P = 0.007), IL-10 (P = 0.007), and IL-18 (P < 0.001) were detected. This occurred immediately following initiation of perfusion and was maintained throughout the procedure. However granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, IL-12, and tumor necrosis factor (TNF)−α did not change from baseline, suggesting that EVNP drives a significant inflammatory response (Figure 3 a−d).
Figure 3.
Cytokine secretion increases over time during ex vivo perfusion. (a−d) Serial perfusate samples were analyzed by Luminex to detect a range of cytokines and chemokines. After approximately 60 minutes on the circuit, EVNP is associated with a rapid increase in the secretion of IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-6, CXCL-8, IL-10, and IL-18. The concentration of GM-CSF, IL-4, IL-12, and TNF-α remains unaffected. (CXCL, C-X-C motif chemokine ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.)
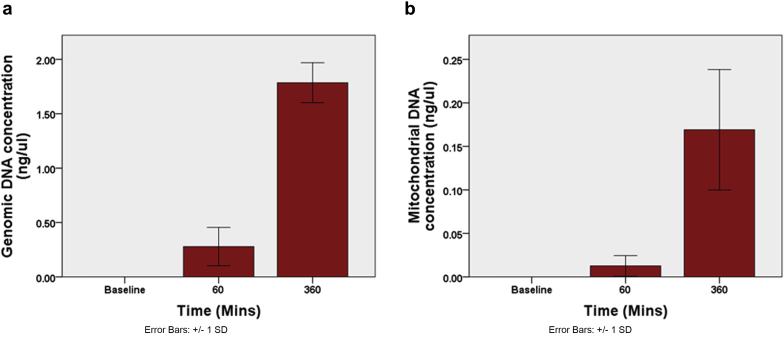
Concentration of Circulating Cell-Free Mitochondrial and Genomic DNA
To determine whether EVNP induces cell death during perfusion, circulating cell-free mitochondrial and genomic DNA were quantified. An increase in the concentration of both cell-free genomic and mitochondrial DNA was observed (P < 0.001 and P = 0.008, respectively), suggesting that EVNP causes a degree of cellular injury upon reperfusion (Figure 4 a and b).
Figure 4.
Ex vivo normothermic perfusion is associated with increasing concentrations of (a) cell-free genomic DNA and (b) mitochondrial DNA. Cell-free mitochondrial and genomic DNA concentrations from plasma samples were quantified using quantitative polymerase chain reaction. Increasing concentrations are detected during perfusion, suggesting cellular injury occurs.
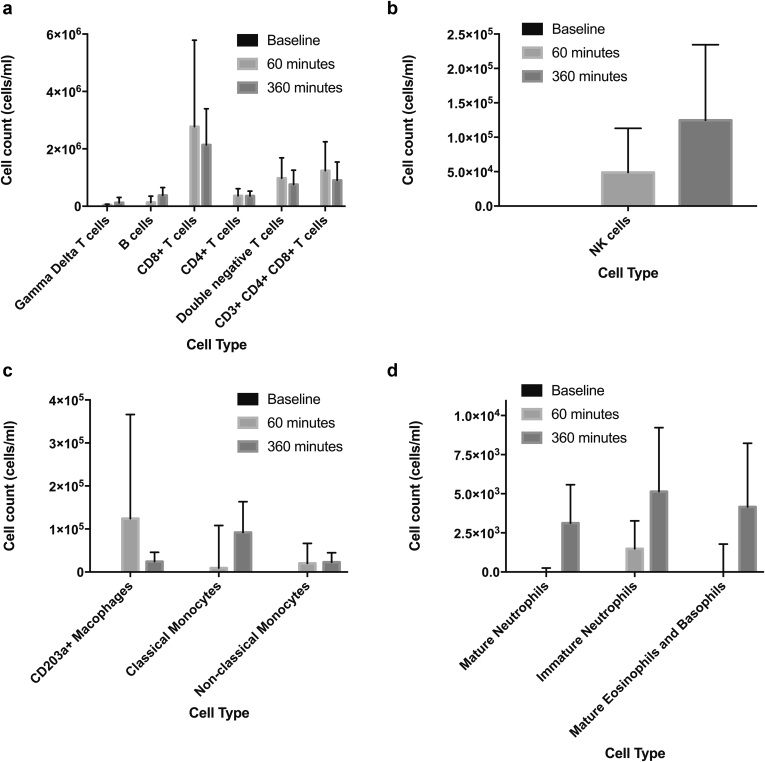
Cellular Efflux From the Donor Kidney Into the Perfusate
The immune cell efflux from the donor kidney into the EVNP circuit was characterized to assess the contribution of passenger leukocytes from the donor kidney following transplantation (Figure 5 a−d). Major populations of donor T cells including helper, cytotoxic double-negative, and CD4+CD8+ T cells were identified within the first 60 minutes of perfusion and increased throughout the procedure (P = 0.015, P = 0.022, P = 0.012, and P = 0.021, respectively). A large population of B cells was also detected, with the greatest number being identified following 6 hours of perfusion (P = 0.041).
Figure 5.
Donor leukocytes migrate out of the kidney into the perfusate during ex vivo normothermic perfusion. Serial perfusate samples were analyzed by flow cytometry to identify migrating leukocytes. Large populations of T cells, B cells (a), and NK cells (b) were detected in increasing concentrations during the perfusion period. Populations of monocytes, macrophages (c), neutrophils and basophils and eosinophils (d) were also detected.
Of the granulocyte lineage, mature basophils and eosinophils migrated into the circuit with an increase in numbers during perfusion (P = 0.015). Equally, the number of mature and immature neutrophils increased (P = 0.026 and P = 0.038 respectively). Classical monocytes represented the major monocyte population, with nonclassical monocytes being less abundant. However, the size of both populations remained consistent during perfusion (P = 0.059 and P = 0.549 for classical and nonclassical repertoires, respectively). Minor populations of natural killer (NK) cells and macrophages were also detected, with NK cells observed to increase over time (P = 0.015 and P = 0.449, respectively).
Discussion
Normothermic EVNP provides unique insight into the immunological and inflammatory contribution of the donor kidney following revascularization. In this study, EVNP resulted in a significant inflammatory storm with surprisingly high concentrations of IL-6 (80,242 ± 15,950 pg/ml perfusate), CXCL-8 (49,980 ± 39 pg/ml perfusate), and IFN-γ (16,292 ± 26917 pg/ml perfusate) detectable at the end of perfusion. Other pro-inflammatory cytokines also increased throughout the 6-hour perfusion period, including IL-1β, IL-1RA, and IL-12. To a lesser extent, increases in IL-4, IL-10, IL-18, and TNF-α were detected, but did not exceed 500 pg/ml throughout perfusion. We believe that these data are of importance, as they provide insight into the potential contribution of the donor kidney to the inflammatory events that occur immediately following transplantation. For example, if the donor kidney drives a similar CXCL-8 response following revascularization, this will result in both donor and recipient cellular mobilization and an efflux of cells out of the donor kidney and into the recipient vasculature. Recipient immune cells expressing the CXCL-8 receptors CXCR1 and CXCR2 (including dendritic cells and T cells) will infiltrate the donor kidney via this CXCL-8−dependent chemokine gradient. Therefore the CXCL-8 response could contribute to the diapedesis of donor leukocytes to recipient lymph nodes, and the recruitment of recipient dendritic cells and T cells to the graft. As recipient T cells infiltrate the graft they encounter alloantigen presented directly via donor dendritic cells, which self-present donor leukocyte antigens or donor peptides. Infiltrating recipient dendritic cells also process and present donor peptides to recipient T cells, which collectively represent the hallmark of allorecognition and ultimately lead to permanent T-cell alloreactivity.10 IL-6 is an acute-phase cytokine that plays a central role in ischemia-reperfusion injury and contributes to graft rejection by orchestrating leukocyte recruitment, activation, and proliferation of a range of lymphocytes.11, 12 IL-6 pathway inhibitors have subsequently been used following transplantation and have shown promise in improving early graft survival. Given the profoundly high levels observed in our study, the donor kidney may represent a major source of IL-6 following transplantation and as such may represent a therapeutic target. We also observed high levels of IFN-γ, which is a major pro-inflammatory cytokine involved in allorecognition and alloreactivity. IFN-γ induces the up-regulation of class I major histocompatibility complex on graft epithelial and endothelial cells and drives recipient leukocyte infiltration of the donor kidney.13, 14 However, as EVNP is a closed system, IL-6, CXCL-8, and IFN-γ are released prior to transplantation and had no adverse effect on renal function during perfusion. Instead, the pro-inflammatory cytokine response observed may be responsible for the activation and mobilization of donor-derived leukocytes out of the donor kidney during perfusion. Furthermore, inducing the secretion of IL-6, CXCL-8, and IFN-γ may exhaust the pro-inflammatory response of the donor kidney prior to transplantation, and may also impede the ability of donor cells to respond in the posttransplantation setting. As such, the presence of these cytokines may be reduced following transplantation, thereby inhibiting their deleterious effects and promoting graft survival.
From the current study it is not possible to decipher the cytokine source; however, increasing concentrations of mitochondrial and genomic DNA were detected throughout perfusion. This is likely as a result of a loss of leukocyte viability within the circuit due to the interaction of these cells with the plastic circuit, as well as the inventible ischemia-reperfusion injury that ensues. The release of endogenous damage-associated molecular patterns such as extracellular DNA are associated with the initiation and maintenance of a pro-inflammatory response and immune activation.15 Although this may partly explain the increase in a range of cytokines, the EVNP circuit itself may influence the inflammatory response, as it consists of a modified cardiopulmonary bypass machine. It is well documented that patients placed on cardiopulmonary bypass during surgical procedures develop systemic inflammatory response syndrome (SIRS).16 This is proposed to be due to the interaction of leukocytes with an artificial surface, leading to cellular activation and resultant cytokine secretion.17 Interestingly, this does not appear to influence clinical outcome. Indeed, in our study, kidney function was maintained throughout perfusion despite increasing cytokine and extracellular DNA concentrations. All kidneys functioned within normal parameters, remained biochemically stable, and met the criteria for transplantation throughout perfusion.18 Urine production began immediately and continued for the duration of the perfusion, although a decline was observed after 1 hour. Following an ischemic insult and subsequent development of acute tubular necrosis, a polyuric phase is often observed that can then stabilize when tubular function is restored.19 We propose that the initial large volume of urine is reflective of acute tubular injury that stabilizes during reperfusion and adequate oxygenation. In corroboration of this, acid−base balance was maintained, suggesting that tubular function remained viable. Interestingly, an increase in lactate concentration was observed, which can be an indicator of regional ischemia and hypoperfusion. In a clinical setting, it is widely considered that a lactate concentration above 4 mmol/L is associated with poorer patient outcomes.20, 21 During this study, the mean lactate concentration did not exceed 2.5 mmol/L and was therefore unlikely to be representative of impaired kidney function. However, it is important to note that the kidney was exposed to only 2 hours of static cold storage to minimize the ischemic injury, thereby providing a clear indication of the inflammatory capacity of the donor organ. Prolonged ischemic times may influence the inflammatory profile described as a result of tissue injury.
Aside from cytokine secretion in the presence of good renal function, we also demonstrate that the kidney contains a large reservoir of leukocytes that rapidly mobilize following revascularization. Major populations of donor leukocytes were detected in the perfusate as early as 60 minutes postperfusion, suggesting that these cells are marginal and can extravasate from the tissue and enter the recipient circulation following transplantation. This includes large populations of T cells (4,154,908 ± 1,894,451 cells/ml perfusate) and B cells (376,098 ± 275,830 cells/ml perfusate) that have the capacity to drive inflammation, secrete pro-inflammatory cytokines (including IL-6, CXCL-8, and IFN-γ), and orchestrate alloantigen-specific immune responses. Ultimately this can lead to acute rejection and graft loss. It should be noted that within this T cell population, a proportion may be of regulatory phenotype, although this was not determined in this study. Removing these cells prior to transplantation may have a negative impact on clinical outcomes via a loss of tolerance induction. However, we propose that a significant proportion of these cells are unlikely, given the predominantly inflammatory environment. Significant numbers of NK cells were also observed within the perfusate (124,644 ± 109,934 cells/ml perfusate). NK cells have multiple functions and can secrete high levels of IL-6, CXCL-8, and IFN-γ upon stimulation. They are also central in the recruitment of cytotoxic T cells during inflammation, which are recruited into the kidney and contribute to tissue destruction and a loss of graft function. The diapedesis of major populations of cells with antigen-presenting capacity also occurred, including monocytes, macrophages, and B cells. These donor leukocytes are essential for direct presentation and can provide co-stimulatory signals to recipient T cells.22
A porcine model was used in this study because of the similarity in physiology and organ development to those of humans.23 The pigs used were a size similar to that of humans, and all pigs were the same breed and size. In addition, the mode of death was standardized to minimize data variation. However, it should be noted that using abattoir animals is not without limitations, as these animals are from an uncontrolled environment, although this is more reflective of a heterogeneous population.
Although the fate of the immune efflux cannot be determined in this study, we propose that the migration of billions of donor leukocytes in conjunction with the secretion of an IFN-γ, IL-6, and CXCL-8 storm would drive direct allorecognition and would orchestrate significant recipient immune activation following transplantation. Furthermore, a major proportion of these donor leukocytes are likely to home to lymph nodes and self-present donor antigens to naive recipient T cells.24 Removing this inflammatory burden prior to transplantation may therefore confer significant clinical benefit.8 Based on our findings, we now believe that immunomodulatory strategies to alter the donor immune environment prior to transplantation warrant development.
Disclosure
All the authors declared no competing interests.
References
- 1.Nicholson M.L., Hosgood S.A. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13:1246–1252. doi: 10.1111/ajt.12179. [DOI] [PubMed] [Google Scholar]
- 2.Hosgood S.A., Patel M., Nicholson M.L. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. 2013;182:153–160. doi: 10.1016/j.jss.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Hosgood S.A., Barlow A.D., Yates P.J. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res. 2011;171:283–290. doi: 10.1016/j.jss.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Takada M., Nadeau K.C., Hancock W.W. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533–1542. doi: 10.1097/00007890-199806270-00001. [DOI] [PubMed] [Google Scholar]
- 5.van der Hoeven J.A., Molema G., Ter Horst G.J. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64:1874–1882. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 6.Nijboer W.N., Schuurs T.A., van der Hoeven J.A. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation. 2004;78:978–986. doi: 10.1097/01.tp.0000135565.49535.60. [DOI] [PubMed] [Google Scholar]
- 7.Game D.S., Lechler R.I. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 8.Stone J.P., Critchley W.R., Major T. Altered immunogenicity of donor lungs via removal of passenger leukocytes using ex vivo lung perfusion. Am J Transplant. 2016;16:33–43. doi: 10.1111/ajt.13446. [DOI] [PubMed] [Google Scholar]
- 9.Patel M., Hosgood S., Nicholson M.L. The effects of arterial pressure during normothermic kidney perfusion. J Surg Res. 2014;191:463–468. doi: 10.1016/j.jss.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Giral-Classe M., Hourmant M., Cantarovich D. Delayed graft function of more than six days strongly decreases long-term survival of transplanted kidneys. Kidney Int. 1998;54:972–978. doi: 10.1046/j.1523-1755.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 11.Papanicolaou D.A., Wilder R.L., Manolagas S.C. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Waiser J., Budde K., Katalinic A. Interleukin-6 expression after renal transplantation. Nephrol Dial Transplant. 1997;12:753–759. doi: 10.1093/ndt/12.4.753. [DOI] [PubMed] [Google Scholar]
- 13.Anders H.-J., Muruve D.A. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 14.Goes N., Urmson J., Hobart M. The unique role of interferon-gamma in the regulation of MHC expression on arterial endothelium. Transplantation. 1996;62:1889–1894. doi: 10.1097/00007890-199612270-00036. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q., Raoof M., Chen Y. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paparella D., Yau T.M., Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardio-Thorac Surg. 2002;21:232–244. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Price R., Phiroz D. Systemic inflammatory response during cardiopulmonary bypass and strategies. J Extra-corp Technol. 2005;37:180–188. [PubMed] [Google Scholar]
- 18.Hosgood S.A., Barlow A.D., Dormer J. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J Translat Med. 2015;13:329. doi: 10.1186/s12967-015-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of acute kidney injury. Comp Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraut J.A., Madias N.E. Lactic Acidosis. N Engl J Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 21.Andersen L.W., Mackenhauer J., Roberts J.C. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rissoan M.C., Soumelis V., Kadowaki N. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 23.Giraud S., Favreau F., Chatauret N. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol. 2011;2011:532127. doi: 10.1155/2011/532127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creusot R.J., Yaghoubi S.S., Chang P. Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood. 2009;113:6638–6647. doi: 10.1182/blood-2009-02-204321. [DOI] [PMC free article] [PubMed] [Google Scholar]