Abstract
Paracoccidioidomycosis (PCM) is a fungal disease caused by Paracoccidioides spp., which can cause a systemic granulomatous infection with tegumentary and visceral involvement. Sarcoid-like skin lesions are uncommon and can be misdiagnosed due to similarities with other granulomatous diseases. We report a case of a women presenting with erythematous infiltrated plaques on her face that was treated for leprosy and rosacea with no response and was later diagnosed with PCM, presenting positive serology for Paracoccidioides lutzii.
Keywords: Paracoccidioidomycosis, Skin lesion, Sarcoid-like, Paracoccidioides lutzii
1. Introduction
Paracoccidioidomycosis (PCM) is the most frequent systemic endemic fungal infection in Latin America, especially in Brazil [1]. A large endemic area exists in southeast, midwest and south of the country and, currently, also in the western amazon region [2]. The disease is caused by Paracoccidioides spp., a group of thermally dimorphic fungi. Until recently, PCM was thought to be caused by the single species Paracoccidioides brasiliensis; however, based on phylogenetic differences, another species was recognized: Paracoccidioides lutzii [2].
The characteristics of the disease caused by P. lutzii, as well as its epidemiology, are still poorly known [2]. Infection occurs through inhalation of soil-born propagules, initially reaching lungs. Some patients develop asymptomatic infection, in other cases the fungus proliferates, then disseminating to other organs and systems by hematogenous and lymphatic pathways [1].
There are two main clinical forms: acute/subacute and chronic. The acute/subacute form is more common among children and young adults, presenting with disseminated lesions, hepatomegaly, splenomegaly and lymph node enlargement [1], [2]. The chronic form affects mainly adult males and is usually characterized by pulmonary involvement, with prolonged symptoms such as coughing and dyspnea, associated with mucosal and skin lesions [1]. This form is strongly related to the consumption of alcohol and tobacco [3].
Cutaneous involvement of PCM is generally represented by single or multiple ulcers, which classically present with granulomatous base and hemorrhagic dotting [4]. However, other patterns can be found, such as vegetative or ulcerovegetative lesions, papular lesions and infiltrated lesions [4]. The distinct cutaneous manifestations are related to different immunological responses in each individual [5]. When cutaneous presentation involves infiltrated lesions, it can be misinterpreted as other granulomatous diseases, such as sarcoidosis and tuberculoid leprosy, due to clinical and histopathological similarities among them [6].
2. Case
On April 2012, a 23 year-old white female from a rural area of Santa Maria de Jetibá, Espírito Santo state, Brazil, with no history of previous diseases and non-smoker, was seen due to the appearance of erythematous lesions on her forehead. Initially, she was diagnosed with leprosy in a different medical center and was treated with rifampicin, dapsone and clofazimine for a period of 2 years, without any response. On November 2014, she was treated for rosacea with metronidazole topical gel and oral doxiciclin for one year, also with no improvement. She had been previously submitted to skin biopsy, which showed a diffuse lymphohistiocytic inflammatory process with granulomas. Tuberculin skin test and sputum bacilloscopy were negative.
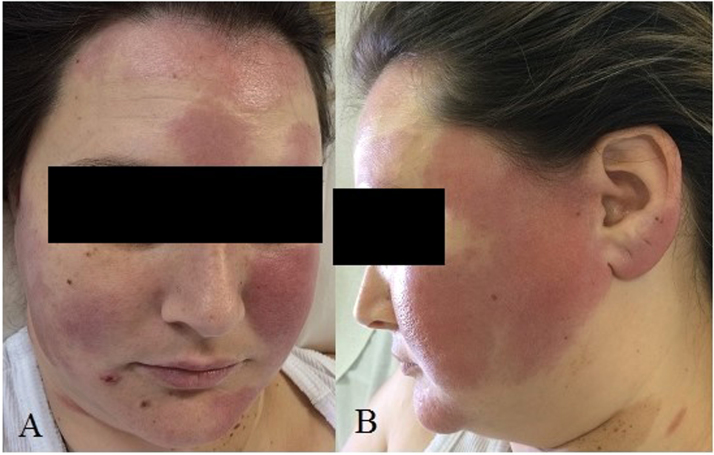
After November 2015, her lesions became larger and new ones emerged on different areas of her face. On April 14th, 2016 (day 0), 4 years after the first symptoms, she was admitted to Cassiano Antonio Moraes University Hospital (HUCAM/UFES) in Vitoria, Espírito Santo (ES), Brazil for investigation. Skin examination showed erythematous infiltrated plaques on her forehead, both of her cheeks, chin and left ear lobe and a small ulcer of about three millimeters next to right labial commissure (Fig. 1).
Fig. 1.
A) and B) Erythematous infiltrated plaques (day 0).
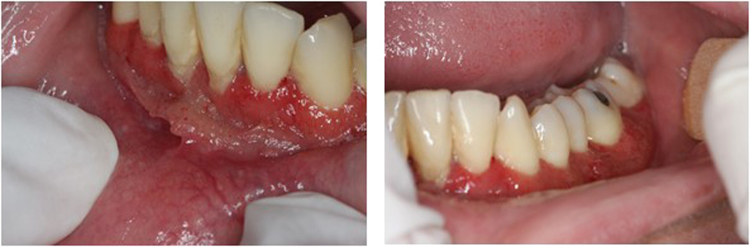
Oral examination revealed mulberry-like ulcers with hemorrhagic dots in gingiva (Fig. 2). Cervical palpation demonstrated enlarged posterior lymph nodes and submandibular lymph nodes, measuring between 1.5 and 2 cm. There were no signs of fever, hepatomegaly, splenomegaly or respiratory alterations. Pulmonary computed tomography showed residual calcified granulomas in the middle lobe and cervical computed tomography showed enlarged lymph nodes in jugular, submandibular, intraparotid and posterior chains.
Fig. 2.
Clinical aspect of oral paracoccidioidomycosis (PMC). Note gingival lesions granular and erythematous with punctate hemorrhage of the mulberry-like appearance.
The patient was submitted to fine needle aspiration of one of the enlarged lymph nodes (day 6), which showed yeast cells with multiple budding, consistent with Paracoccidioides spp. It was also performed direct microscopic examination of ulcer secretion (day 6), which demonstrated the same findings (Fig. 3). Double immunodiffusion tests using antigenic preparation from the standard strain P. brasiliensis B-339 generated negative results, whereas preparation using cell-free antigen (CFA) from a P. lutzii autochthonous strain (EPM 208) produced positivity reaction with not diluted serum.
Fig. 3.
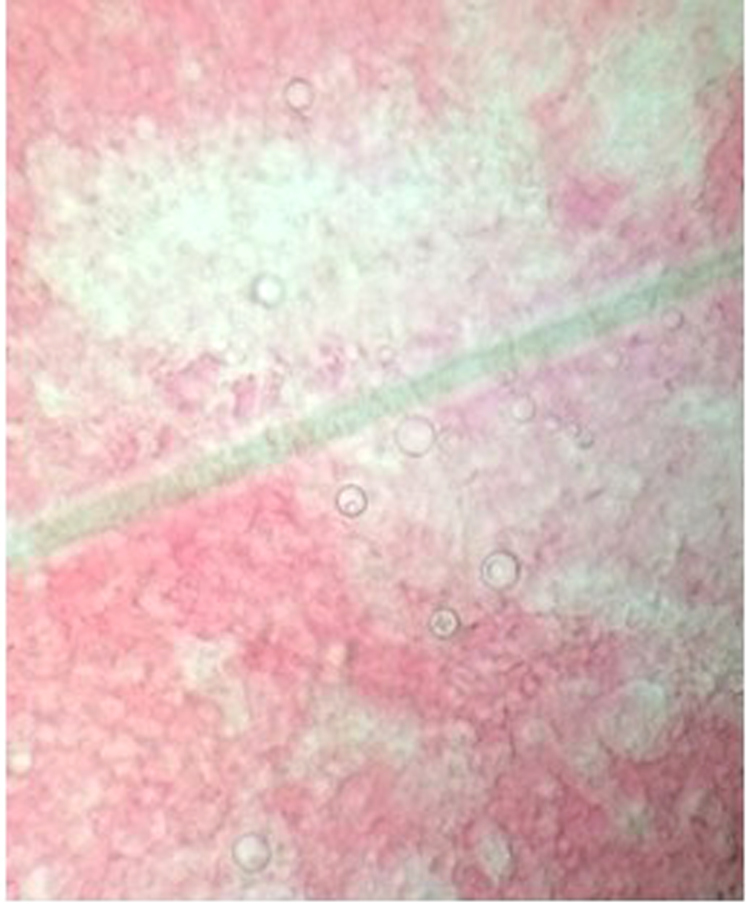
Yeast form seen at direct examination.
A diagnosis of sarcoid-like PCM was established and the patient started treatment with sulfamethoxazole/trimethoprim 800/160 mg three times a day (day 6). She remained in the improved state on the medical follow-up (Fig. 4).
Fig. 4.
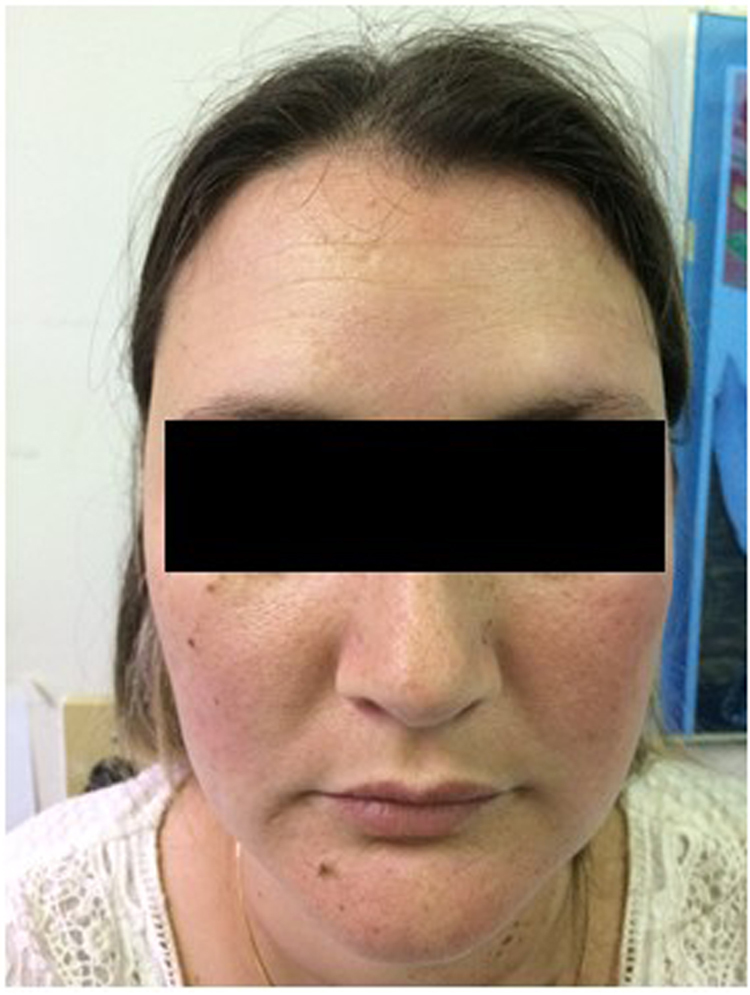
Improvement of skin lesions (day 210).
3. Discussion
Cutaneous lesions caused by Paracoccidioides spp. affect 30–54% of the patients [6]. They may originate from hematogenous dissemination of the fungus, contiguous preexistent lesions or, rarely, direct inoculation into the skin [4], [6]. The face is the most common site of skin lesions and ulcer or ulcerous-vegetative lesions are the main morphological pattern followed by the infiltrative one, although the sarcoid-type is unusual [5], [6]. This latter form differs from the others due to the presence of tuberculoid granulomas in the histopathological exam [5].
The pattern of lesions depends on many factors related to the fungus pathogenicity and immunologic response of the host [5]. Resistant patients usually stimulate cellular response of T helper 1 lymphocytes (Th1) leading to an organized tuberculoid granuloma, with rare fungi [5]. The fact that fungi may not be found in skin samples makes the diagnosis of PMC difficult. Furthermore, the serology may be negative or in low titers [5].
PCM in sarcoid form can simulate other granulomatous diseases, with emphasis on leprosy, which has already been described as a misdiagnosis in other case reports [5], [6], [7]. Leprosy (Hansen's disease) remains prevalent in Brazil, with the state of Espírito Santo being an endemic area for this disorder [8]. Thus, it is necessary to make differential diagnosis between these two diseases, especially in those patients coming from rural areas.
The age and gender of this patient normally would influence the pathogenesis due to hormonal protection in case of P. brasiliensis, due to the ability of estradiol to decrease or delay mycelium-to-yeast transformation. However, this did not happened in this case, since this ability may be lost in case of P. lutzii infection [9].
The criteria for a definitive diagnosis of PMC are based on visualization in clinical specimens by the direct examination or histopathology of characteristics cells with multiple buds in biological fluids or fungal isolation. However, culture is relatively difficult obtain [10], [11]. In this case report, as the direct examination made it possible to diagnose the disease, we believe that it was not necessary to cultivate the fungus. Moreover, it is a slow procedure. We opted to use of serological techniques as additional test since it is a good option for PMC diagnosis. The method of choice was Double immunodiffusion (DID). This test has high specificity and sensitivity, which may vary from 65% to 100% depending on the type of antigen used [10], [12], [13]. It has been demonstrated by DID that the serum from patients with PMC due P. brasiliensis does not recognized any antigen contained in cell-free preparations of P. lutzii [10]. In our case, positive result was obtained with not diluted serum.
It is also important consider that even though there is still not much information available about the differences between the disease caused by P. lutzii and P. brasiliensis. It is known that these two species show distinct patterns of host-parasite interaction and cell adhesion [9], which could reflect in rare forms of presentation, such as the one exposed by this case report.
Acknowledgements
We are grateful to hospital odontology and rheumatology team of the Cassiano Antonio Moraes University Hospital.
Acknowledgments
Conflict of interest
The other authors have no conflict of interest to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Fortes M.R., Kurokawa C.S., Marques S.A., Miot H.A., Marques M.E. Imunologia da paracoccidioidomicose. An. Bras. Dermatol. 2011;86(3):516–525. doi: 10.1590/s0365-05962011000300014. [DOI] [PubMed] [Google Scholar]
- 2.Martinez R. Epidemiology of paracoccidioidomycosis. Rev. Inst. Med. Trop. 2015;57(19):11–20. doi: 10.1590/S0036-46652015000700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguiar dos Santos W., Silva B.M., Passos E.D., Zandonade E., Falqueto A. Associação entre tabagismo e paracoccidioidomicose: um estudo de caso-controle no estado do Espírito Santo, Brasil. Cad. Saúde Pública. 2003;19(1):245–253. doi: 10.1590/s0102-311x2003000100027. [DOI] [PubMed] [Google Scholar]
- 4.Marques S.A., Cortez B.D., Lastória J.C., Camargo R.M., Marques M.E. Paracoccidioidomicose: freqüência, morfologia e patogênese de lesões tegumentares. An. Bras. Dermatol. 2007;82(4):411–417. [Google Scholar]
- 5.De Medeiros V.L.S., Arruda L. Sarcoid-like lesions in paracoccidioidomycosis: immunological factors. An. Bras. Dermatol. 2013;88(1):113–116. doi: 10.1590/S0365-05962013000100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marques S.A., Lastória J.C., Putinatti M.S., Camargo R.M., Marques M.E. Paracoccidioidomycosis: infiltrated, sarcoid-like cutaneous lesions misinterpreted as tuberculoid leprosy. Rev. Inst. Med. Trop. 2008;50:47–50. doi: 10.1590/s0036-46652008000100010. [DOI] [PubMed] [Google Scholar]
- 7.Muller S.F., Miranda M.F. Sarcoid-like paracoccidioidomycosis presenting with perineural granuloma. An. Bras. Dermatol. 2013;88(6):994–995. doi: 10.1590/abd1806-4841.20132309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampaio P.B., Madeira E.S., Diniz L., Noia E.L., Zandonade E. Spatial distribution of leprosy in areas of risk in Vitoria, State of Espirito Santo, Brazil, 2005 to 2009. Rev. Soc. Bras. Med. Trop. 2013;46(3):329–334. doi: 10.1590/0037-8682-0070-2012. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira H.C., Assato P.A., Marcos C.M., Scorzoni L., de Paula E., Silva A.C.A., Da Silva J.F. Paracoccidioides-host Interaction: an overview on recent advances in the paracoccidioidomycosis. Front. Microbiol. 2015;25(6):1319. doi: 10.3389/fmicb.2015.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gegembauer G., Araujo L.M., Pereira E.F., Rodrigues A.M., Paniago A.M.M., Hahn R.C. Serology of Paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2014;8(7):e2986. doi: 10.1371/journal.pntd.0002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Camargo Z.P., de Franco M.F. Current knowledge on pathogenesis and immunodiagnosis of paracoccidioidomycosis. Ver. Iberoam. Micol. 2000;17(2):41–48. [PubMed] [Google Scholar]
- 12.Mendes-Giannini M.J.S., Del Negro G.B., Siqueira A.M. CRC Press I; Boca Raton, Fla: 1994. Serodiagnosis Paracoccidioidomycosis; pp. 345–363. [Google Scholar]
- 13.da Silva J.F., de Oliveira H.C., Marcos C.M., Assato P.A., Fusco-Almeida A.M., Mendes-Giannini M.J.S. Advances and challenges in paracoccidioidomycosis serology caused by Paracoccidioidesspecies complex: an update. Diagn. Microbiol. Infect. Dis. 2016;84(1):87–94. doi: 10.1016/j.diagmicrobio.2015.06.004. [DOI] [PubMed] [Google Scholar]