Abstract
Hepatocellular carcinoma (HCC) is one of the most common and lethal cancers globally. With advances in therapy for chronic viral hepatitis, changing social circumstances, and increasing practice of HCC surveillance, the epidemiology of HCC is expected to change over time. We explored the temporal trends in HCC in Singapore, a multiethnic Asian country, over the last 3 decades. Patients with HCC were prospectively enrolled and stratified into two cohorts (C1, 1988‐2002; C2, 2003‐2016). Patient and tumor characteristics, management, and survival were compared between the two cohorts, and a survival census was performed on October 31, 2015. There were 1,401 patients, and the mean age at diagnosis of HCC for C1 and C2 was 60.1 and 63.5 years, respectively. Male patient preponderance decreased significantly, with the male to female ratio falling from 5.2:1 to 3.9:1 between C1 and C2. Hepatitis B, although still the predominant risk factor for HCC, showed a significant decline from C1 to C2 (76.5% to 68.2%), while the nonviral etiology increased significantly over the same period (14.4% versus 25.0%, respectively). Significantly more patients in C2 than C1 were diagnosed through surveillance (39.2% versus 11.3%, respectively) and had better physical performance (Eastern Cooperative Oncology Group 0, 62.1% versus 20.4%, respectively). While Child‐Pugh status was comparable, significantly more patients in C2 than C1 had early stage disease (Barcelona Clinic Liver Cancer 0‐A, 39.5% versus 7.4%, respectively), which translated into significantly higher median survival (18.6 months versus 3.8 months, respectively). Conclusion: Over the past 3 decades, hepatitis B‐related HCC has been decreasing while HCC due to nonviral etiology has been increasing significantly. Surveillance to diagnose early stage HCC is important in improving the outcome of HCC. (Hepatology Communications 2017;1:564–571)
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CHB
chronic hepatitis B
- ECOG
Eastern Cooperative Oncology Group
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- Ig
immunoglobulin
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RFA
radio frequency ablation
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. In 2012, HCC had an estimated global incidence of 782,000 cases, representing the fifth most common cancer in male individuals and ninth most common cancer in female individuals.1 More importantly, HCC is a lethal cancer as it is the second leading cause of cancer deaths globally and accounted for 746,000 deaths worldwide in 2012.1
While HCC has traditionally been recognized to have distinct geographic heterogeneity, the global pattern of HCC has undergone substantial changes over the last few decades.2, 3, 4 Increasing incidence of HCC has been observed in regions with traditionally low prevalence of HCC, such as North America and Europe, while the converse has been reported in high‐prevalence regions, such as China.2, 5, 6, 7 Nevertheless, the incidence rates in high‐prevalence regions remain much higher than those seen in the low‐prevalence regions. Changing patient characteristics, including exposure to changing HCC risk factors, dictate the temporal trends in epidemiology of HCC. Equally, improved understanding and advances in management of liver disease, cirrhosis, and HCC have had a positive impact on the outcome of HCC.8
Exploration of the epidemiological trends in HCC within Singapore provides a unique vantage point to scrutinize various factors at work. First, Singapore lies within Asia, which is home to 75% of all HCC cases worldwide.2 Second, the impact of chronic hepatitis B (CHB) as a cause for HCC has been declining due to the implementation of successful nationwide hepatitis B vaccination programs and use of antiviral therapies, as can be reflected in Singapore. Nationwide hepatitis B screening/vaccination programs have been implemented in Singapore since 1987, resulting in a declining hepatitis B seroprevalence.9 Third, with rapid development, urbanization, and changing lifestyles in Singapore, an increasing prevalence of obesity, diabetes mellitus, hypertension, and dyslipidemia has been reported.10 As these metabolic risk factors are commonly associated with nonalcoholic fatty liver disease (NAFLD), the prevalence of NAFLD would also be expected to be on the upsurge.11
In view of the temporal change in risk factors, we hypothesize that the decline of CHB as a risk factor for HCC may be offset by the emergence of NAFLD as a rising cause of HCC in Singapore. Indeed, in one of the Singaporean community‐based cross‐sectional studies, 40% of subjects were observed to have NAFLD.12 The aim of this study is to explore the temporal trends in HCC characteristics and epidemiology that have occurred over the last 3 decades. Appreciation of the epidemiological trends will permit clinicians to anticipate and adapt accordingly in terms of public health perspectives, resource allocation, and formulation of management guidelines for patients with HCC.
Materials and Methods
STUDY POPULATION
Patients were recruited from an ongoing HCC registry database that has been prospectively enrolling patients seen in our department who had been diagnosed with HCC since 1988. Our institution is the largest not‐for‐profit tertiary care teaching hospital in Singapore, consisting of 1,600 beds and over 30 clinical disciplines. As such, we are one of the major national referral centers for subspecialty care, and a considerable number of patients with HCC are seen in our institution. However, other hospitals in Singapore also treat HCC. Similarly, oncologists in our institution also manage patients with advanced HCC, but as the majority of these patients also have a background of liver cirrhosis, they would concurrently be under the care of our department. HCC was diagnosed based on conventional diagnostic criteria according to the time period.13, 14, 15 These criteria included histology and diagnostic radiology imaging techniques, such as hepatic angiography and positive lipiodol angiography, prior to 1990 and dynamic contrast‐enhanced radiology modalities thereafter.
STUDY DESIGN
A retrospective analysis of patients with HCC diagnosed between January 1, 1988, and April 30, 2016, was performed. Demographic and clinical data were collected. The etiology of HCC was defined as hepatitis B related if hepatitis B surface antigen (HBsAg) serology was positive, hepatitis C related if immunoglobulin (Ig)G antibody serology for hepatitis C was positive, and alcohol related if patients had a daily consumption of more than 60 g of alcohol for 10 years or more. Alcohol consumption was assessed by trained medical staff interviewers using a standardized questionnaire ascertaining the type of alcoholic beverage consumed, strength of alcoholic beverage (percentage alcohol), number of drinks, frequency of consumption (daily/weekly/monthly/hardly ever/never), and duration of alcoholic consumption. If deemed clinically indicated, additional tests, such as antinuclear antibody, serum ceruloplasmin, and anti‐liver antibodies, were performed to elucidate the underlying liver disease. Cases were considered cryptogenic HCC if viral hepatitis B/C serology was negative, there was no documented excessive alcohol intake, and other causes of chronic liver disease had been excluded. Child‐Pugh class and functional performance status by Eastern Cooperative Oncology Group (ECOG) grading were recorded as was the manner in which HCC was diagnosed, viz., while under routine surveillance or otherwise. Under our surveillance practices, patients received a regular ultrasound abdominal scan and serum alpha‐fetoprotein on a 6 monthly schedule or more frequently as deemed necessary by the patient's physician. Staging of HCC was assessed using the Barcelona Clinic Liver Cancer (BCLC) classification.16 Type of treatment provided for the HCC was categorized into surgical curative, local ablative, systemic chemotherapy, and best supportive care. Curative therapies were defined by surgical resection, liver transplantation, and radio frequency ablation (RFA) of HCC less than 3 cm. All the current standard of care treatment modalities of HCC were available in our hospital; hence, our hospital offers all possible standard of care treatment approaches to optimize patient outcomes. Details of patient survival were computed using death data from the Singapore National Registry of Births and Deaths. As the law mandates compulsory reporting to the National Registry of Births and Deaths only for deaths of Singapore citizens and as the survival census was performed on October 31, 2015, survival analysis was restricted to a cohort of 1,270 (90.6% of total cohort) patients who were Singapore citizens with HCC diagnosed before the census date of October 31, 2015. Clinical characteristics, treatment modalities, and survival outcomes were compared between two time periods: cohort 1 spanning 1988‐2002 (C1) and cohort 2 spanning 2003‐2016 (C2). The study was approved by the institutional review board of Singapore General Hospital.
STUDY ANALYSIS
Descriptive statistics were computed for all variables, including frequency with percentages (%) for categorical variables and mean with SD for continuous variables. Differences in demographic, clinical, and laboratory data were explored between patients in C1 and C2, using the Student t test and chi‐square testing for continuous and categorical variables, respectively. Survival analysis was performed using Kaplan‐Meier with log‐rank testing for significance differences between groups. All statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY). All P values were two‐sided with P < 0.05 considered statistically significant.
Results
There were 1,401 patients included in the study, of which 764 patients and 637 patients were in C1 and C2, respectively. Distribution of the etiology of HCC across the two time periods is illustrated in Table 1. Hepatitis B remained the dominant etiology of HCC in both eras; however, there was a significant decline in hepatitis B‐related HCC between C1 and C2. On the other hand, there was a significant increase in the number of patients with nonviral HCC from C1 to C2. The frequency of alcohol‐related HCC increased from 1.7% in C1 to 6.7% in C2, while that of cryptogenic HCC increased from 12.7% to 17.8%, respectively.
Table 1.
DISTRIBUTION OF ETIOLOGY AMONG PATIENTS WITH HCC
| Etiology | Overall (%) |
C1 n = 764 (%) |
C2 n = 637 (%) |
P Value |
|---|---|---|---|---|
| Hepatitis B | 1001 (72.7) | 572 (76.5) | 429 (68.2) | <0.001 |
| Hepatitis C | 56 (4.1) | 23 (3.1) | 33 (5.2) | 0.054 |
| Hepatitis B and C co‐infection | 55 (4.0) | 45 (6.0) | 10 (1.6) | <0.001 |
| Alcohol | 55 (4.0) | 13 (1.7) | 42 (6.7) | <0.001 |
| Cryptogenic | 207 (15.0) | 95 (12.7) | 112 (17.8) | 0.010 |
| Primary biliary cholangitis | 2 (0.15) | 0 | 2 (0.32) | ‐‐ |
| Autoimmune hepatitis | 1 (0.07) | 0 | 1 (0.16) | ‐‐ |
| Viral | 1112 (80.8) | 640 (85.6) | 472 (75.0) | <0.001 |
| Nonviral | 264 (19.2) | 108 (14.4) | 157 (25.0) |
The mean age at diagnosis of HCC was significantly older in C2 compared to C1 (63.5 versus 60.1 years, respectively; P < 0.001) (Table 2). Patients with hepatitis B‐related HCC were diagnosed at an older age in the later (C2) cohort compared to the earlier (C1) cohort, whereas the mean age for the other etiologies did not change (alcohol, cryptogenic) or had decreased (hepatitis C).
Table 2.
BASELINE PATIENT CHARACTERISTICS
| Characteristics | C1 (n = 764) | C2 (n = 637) | P Value | |
|---|---|---|---|---|
| Age at diagnosis (years) mean ± SD | Overall | 60.1 ± 13.0 | 63.5 ± 10.9 | <0.001 |
| Hep B | 58.0 ± 13.0 | 61.9 ± 10.9 | <0.001 | |
| Hep C | 67.0 ± 11.1 | 60.5 ± 10.4 | 0.018 | |
| Hep B and C co‐infection | 66.1 ± 10.0 | 65.8 ± 12.8 | 0.861 | |
| Alcohol | 62.6 ± 8.3 | 63.1 ± 8.9 | 0.897 | |
| Cryptogenic | 65.9 ± 11.8 | 69.2 ± 8.7 | 0.062 | |
| Viral | 58.9 ± 13.0 | 61.9 ± 10.9 | 0.001 | |
| Nonviral | 65.5 ± 11.4 | 67.7 ± 9.2 | 0.194 | |
|
Male (%) Male:female ratio |
641 (83.9) 5.2:1 |
508 (79.7) 3.9:1 |
0.044 | |
| Chinese (%) | 686 (89.8) | 561 (88.1) | 0.305 | |
| ECOG score | 0 | 150 (20.4) | 354 (62.1) | <0.001 |
| 1, 2 | 518 (70.3) | 173 (30.4) | <0.001 | |
| 3, 4 | 69 (9.4) | 43 (7.5) | 0.260 | |
| Child‐Pugh class | A | 339 (48.6) | 315 (52.9) | 0.123 |
| B | 254 (36.4) | 205 (34.4) | 0.457 | |
| C | 104 (14.9) | 75 (12.6) | 0.230 | |
| Child‐Pugh score | Median | 7.00 | 6.00 | 0.040 |
Interestingly, there was a slight but significant reduction in the proportion of male patients with HCC in C2 compared to C1 (79.7% versus 83.9%, respectively; P < 0.05). Patients with HCC in C2 had better physical performance status, with 62.1% having ECOG 0 compared to only 20.4% in C1. There was no significant difference in severity of liver disease between the two temporal cohorts as reflected by the similar distribution of Child‐Pugh class between C1 and C2.
The mode of diagnosis of HCC, HCC extent (BCLC stage), and treatment provided between C1 and C2 are described in Table 3. There was a significant increase in HCC diagnosed via surveillance in C2 compared to C1 (39.2% versus 11.3%, respectively; P < 0.001). Patients with HCC in C2 had better ECOG scores and BCLC stages. Correspondingly, significantly more patients in C2 were amenable to curative and loco‐ablative treatment modalities. This translated to a significantly improved median survival in C2 compared to C1 (18.6 versus 3.8 months, respectively; P < 0.001) (Fig. 1). When survival analysis in C2 was stratified according to treatment modality, patients who received curative therapies had a median survival of 60.2 months as opposed to a significantly lower median survival of 9.4 months (P < 0.001) for those who did not receive curative therapies. Further stratification of treatment modality into liver transplant, hepatic resection, RFA, loco‐ablative, and supportive care demonstrated median survivals of 73.5, 50.9, 18.8, 3.6, and 5.2 months, respectively. In C1, similar survival trends were also noted, with patients given curative therapies having significantly better median survival compared to noncurative modalities (70.1 versus 3.0 months, respectively; P < 0.001).
Table 3.
HCC CHARACTERISTICS
| C1 (n = 764) (%) | C2 (n = 637) (%) | P Value | |||
|---|---|---|---|---|---|
| Mode of diagnosis | Surveillance | 86 (11.3) | 250 (39.2) | <0.001 | |
| Nonsurveillance | 678 (88.7) | 387 (60.8) | |||
| BCLC | 0 | 8 (1.2) | 73 (13.1) | <0.001 | |
| A | 40 (6.2) | 147 (26.4) | <0.001 | ||
| B | 47 (7.3) | 114 (20.5) | <0.001 | ||
| C | 453 (70.3) | 156 (28.0) | <0.001 | ||
| D | 96 (14.9) | 67 (12.1) | 0.146 | ||
| Treatment | Curative | 91 (13.0) | 282 (61.4) | <0.001 | |
| Other therapy | Loco‐ablative | 83 (11.8) | 83 (18.1) | <0.003 | |
| Chemotherapy | 104 (14.8) | 30 (6.5) | <0.001 | ||
| Supportive | 424 (60.4) | 64 (13.9) | <0.001 | ||
Figure 1.
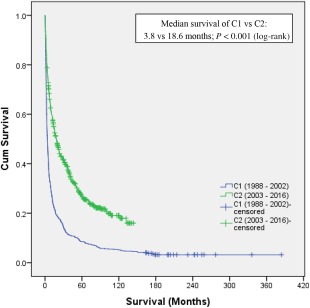
Kaplan‐Meier survival comparison between C1 and C2.
Discussion
Our study highlights several striking evolutionary changes of HCC in Singapore over the last 3 decades in both patient and tumor characteristics. In terms of etiology, the dominance of CHB is on the decline while heralding a trend of increasing nonviral‐related HCC. Hepatitis B remains endemic in many parts of Asia and strongly correlates to the risk of HCC development.17, 18 Fortunately, with the development and successful implementation of nationwide hepatitis B immunization programs, a significant decline in seroprevalence of HBsAg and CHB has been observed in many countries, including Singapore. Singapore began a nationwide hepatitis B immunization program in 1987, and this has now had an impact on the seroprevalence rates in the younger population where HBsAg prevalence dropped from 4.1% to 1.1% among young adults below 30 years of age.9 Along similar lines, a separate study conducted between 2008 to 2010 demonstrated that HBsAg prevalence was only 0.3% in subjects less than 17 years of age.19 Hence, a decrease in CHB‐related HCC rates can be expected with a successful reduction in CHB infection incidence. This was demonstrated in Taiwan, where a hepatitis B virus (HBV) immunization program started in 1984 was followed by a significant decline in HCC incidence among Taiwanese children.20 A subsequent follow‐up report of the same cohort 12 years later showed that the significant lowering of the HCC incidence rate had extended beyond childhood into adolescence.21 A separate explanation to account for the decline of CHB‐related HCC is the positive impact of HBV treatment. Both interferon and nucleotide analogue‐based regimes have been shown to reduce the risk of HCC.22, 23, 24, 25 In addition, long‐term therapy with nucleotide analogues has been associated with the regression of fibrosis and cirrhosis, thus negating one of the important pathways of oncogenesis.26
Juxtaposed against the decline of CHB‐related HCC is the emergence of nonviral‐related HCC. An upsurge of alcoholic‐related HCC was seen across the 2 eras, reflecting an increase in alcoholic liver disease. Evidence for this is suggested by the rising prevalence of frequent (from 4.5% to 7.5%), regular (from 2.9% to 3.1%), and binge drinking (from 5.1% to 10.0%) between 1992 and 2004, which in turn is attributed to the rapid economic transition and shift toward an evolving drinking culture seen in Singapore over the last few decades.27 Along similar lines, the incidence of cryptogenic HCC has also been increasing across the 2 eras. Cryptogenic HCC often represents “burnt out” nonalcoholic steatohepatitis (NASH), where a significant number of these patients have the clinical phenotype consistent with NASH, such as higher prevalence of metabolic risk factors.28, 29 Socioeconomic changes seen with rapid modernization, increasing affluence, and a shift to a more sedentary lifestyle and obesogenic dietary patterns predispose to NASH.11, 30 In our local context, the prevalence of NASH risk factors, such as obesity, increased from 6% in 1998 to 10.8% in 2010, while diabetes mellitus increased from 9% in 1998 to 11.3% in 2010, which intuitively would translate into an increasing prominence of NASH and consequently NASH‐related (i.e., cryptogenic) HCC.10 This can also be extrapolated to many other countries that are afflicted by the burgeoning obesity and diabetes epidemic.31, 32, 33
An interesting evolving patient characteristic is the “aging” of the patient with HCC; the mean age at which patients develop HCC has increased over time. This is consistent with current literature reported in both Western and Asian studies.34, 35, 36 In our study, this aging effect was observed only in patients with CHB‐related HCC. One postulation is that nucleotide analogue therapy slows rather than prevents hepatocarcinogenesis, particularly in the context of preexisting advanced fibrosis/cirrhosis.37, 38 Alternatively, increased use of hepatitis B vaccination and CHB treatment by the younger population has reduced the incidence of HCC in the younger age groups; as this does not benefit the patients with CHB from the prevaccination or treatment eras, the mean age of HCC development is shifted to an older age. This has been shown by Hung and colleagues39 in a study in which elderly patients (>65 years) with HCC comprised 49.1% of their cohort and upward trends of HCC incidence were observed only in elderly patients. Similarly, Seto et al.40 have recently shown that the decline in age‐adjusted HCC incidence in age groups <65 years old in Hong Kong is likely due to higher use of nucleoside analogues by the younger population. The clinical implication of this aging effect is that adequate resources and workflow must be tailored to screen, diagnose, and treat HCC in a more geriatric population.
Another interesting but less described trend is the evolving sex distribution. While male preponderance remains across the 2 eras, there was a significant decline in the proportion of male patients in the later era, such that the male to female ratio decreased from 5.2:1 to 3.9:1 across the 2 eras. The worldwide progressive sex disparity in HCC incidence is not well understood.2, 3 As there are significantly fewer patients with HBV‐related HCC in C2 compared to C1, one possible explanation for the greater reduction of HCC in the male compared to the female population in C2 is the greater impact of HBV treatment in male patients compared to female patients. It has been shown that due to differential sex hormone and androgen receptor activity, HBV replication is approximately twice as efficient in male mice, which is one reason for the sex discrepancy of HBV‐related HCC.41 We postulate that the advent of highly effective nucleotide/nucleoside analogue therapy for HBV in C2 has resulted in better viremic control of HBV overall and consequently reduced HBV‐related hepatocarcinogenesis. However, this may be more distinctly appreciated in male patients in the context of interaction between sex hormone/androgen receptor activity and reduced HBV viremia. This translated into a greater decrease in HBV‐related HCC seen in male patients compared to female patients in C2. As the majority of HCC cases are still HBV related in C2, this has in turn resulted in a significant fall in the male to female ratio of HCC in C2 compared to C1.
With regards to tumor characteristics over the last 3 decades, our study demonstrated that a significantly greater proportion of HCC cases was diagnosed via surveillance programs in the later era. Hence, as expected, patients in the later era also had better performance status (ECOG) and earlier tumor stage (BCLC). These translated to more patients in the later era being eligible for active HCC therapy, with a significant improvement in survival over the earlier era. These results are consistent with several previous studies exploring evolutionary temporal trends in HCC. The role of HCC surveillance in chronic liver disease has become well established over the years, with benefits reported in several studies.42, 43, 44 Similarly, a significant improvement in diagnostic modalities, such as dynamic computerized tomography, contrast‐enhanced magnetic resonance imaging, and ultrasound, has also played an important role in detecting HCC earlier.35 Intuitively, identifying asymptomatic patients with HCC earlier would translate to patients being diagnosed at initial stages of disease, a term coined stage migration. Certainly, this was evident in our study where there were more patients with HCC in early BCLC stages (0, A, and B) in the later era. This has a tremendous impact on eligibility for treatment, allowing more patients to receive curative and effective palliative therapy, such as surgical resections and local ablative treatment. As illustrated in our study, more patients with HCC underwent curative or loco‐ablative therapy with fewer patients receiving supportive care in the later era. Liver transplantation was considered a curative treatment modality but may not have had a significant impact on outcomes as our hospital liver transplant program was only established in 2004 and still remains a low‐volume center due to low organ supply. Undeniably, better surgical techniques, improved peri‐operative care, and the introduction of new effective treatment options, such as RFA and transhepatic arterial chemoembolization, have also contributed to improved outcomes. As a result, patient survival significantly increased in the later era, which is a reflection of the various factors mentioned above. Other studies have also observed similar trends in survival.34, 37, 45, 46 Despite demonstrating a significant improvement of survival over time, the median survival of 18.6 months in the later era still seems rather low. Golabi and colleagues47 looked at 2‐year survival rates of patients with HCC in the Surveillance, Epidemiology and End Results database between 2001 and 2009 and found that 75.4% of the patients had died within 2 years; further subanalysis showed mortality within 2 years for those who underwent liver transplant, hepatic resection, and nonsurgical treatment to be 29.1%, 43.7%, and 85.3%, respectively. It is likely that the high mortality rate of those patients in the nonsurgical treatment group greatly impacted the overall survival rates in their study. We found similar circumstances in our study, with the noncurative treatment group dramatically pushing our overall median survival down.
This study has several strengths. The data were extracted from a large and robust HCC registry that spanned 3 decades, allowing sufficient time to decipher evolutionary trends. Furthermore, our survival data were accurate and comprehensive because the survival census was based on our National Registry of Births and Deaths and the reporting of all deaths is mandated by law. Some limitations of our study include the possible subjective nature of assessment of alcohol consumption based on patient reporting, which we have tried to minimize with our standardized questionnaire. We also acknowledge the inability to retrospectively record some data, such as metabolic risk factors, in the patients enrolled early on and a possible biased patient population as we are a referral center for HCC and viral hepatitis in the country. Nevertheless, our study provides detailed insight into the temporal trends of HCC in Singapore, a country that has undergone rapid socioeconomic development over the time period of the study.
In conclusion, there have been significant changes in the epidemiology and management of HCC during the last 3 decades. These include changing etiologies, better use of surveillance programs to diagnose earlier stage HCC, and translating to improved survival. These data can be further used to forecast future trends and guide management strategies to reduce the incidence and disease burden of HCC.
Acknowledgment
We thank our colleagues from the Department of Gastroenterology and Hepatology, Singapore General Hospital, for allowing their patients to be enrolled and studied and staff from the National Registry of Diseases Office, Ministry of Health, Singapore, for assistance in providing data on the survival census. We thank Mr. Clement Lin for maintaining the HCC registry and for his help with the statistical analyses.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No.11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed July 25, 2016. [Google Scholar]
- 2. Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978‐2007. Int J Cancer 2016;139:1534‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goh GB, Chang PE, Tan CK. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol 2015;29:919‐928. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Ren JS, Shi JF, Li N, Wang YT, Qu C, et al. International trends in primary liver cancer incidence from 1973 to 2007. BMC Cancer 2015;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol 2016;34:1787‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao S, Yang WS, Bray F, Va P, Zhang W, Gao J, et al. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976‐2005. Eur J Epidemiol 2012;27:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor‐Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 2009;3:353‐367. [DOI] [PubMed] [Google Scholar]
- 9. Ang LW, Cutter J, James L, Goh KT. Seroepidemiology of hepatitis B virus infection among adults in Singapore: a 12‐year review. Vaccine. 2013;32:103‐110. [DOI] [PubMed] [Google Scholar]
- 10. Epidemiology and Disease Control Division, Ministry of Health, Singapore . National Health Survey 2010. https://www.moh.gov.sg/content/moh_web/home/statistics/Health_Facts_Singapore/Disease_Burden.html. Accessed August 2, 2016.
- 11. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 12. Goh GB, Kwan C, Lim SY, Venkatanarasimha NK, Abu‐Bakar R, Krishnamoorthy TL, et al. Perceptions of non‐alcoholic fatty liver disease ‐ an Asian community‐based study. Gastroenterol Rep (Oxf) 2016;4:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. [DOI] [PubMed] [Google Scholar]
- 15. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61‐74. [DOI] [PubMed] [Google Scholar]
- 17. Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al; Taiwan Community‐Based Cancer Screening Project Group . Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002;347:168‐174. [DOI] [PubMed] [Google Scholar]
- 18. Mori M, Hara M, Wada I, Hara T, Yamamoto K, Honda M, et al. Prospective study of hepatitis B and C viral infections, cigarette smoking, alcohol consumption, and other factors associated with hepatocellular carcinoma risk in Japan. Am J Epidemiol 2000;151:131‐139. [DOI] [PubMed] [Google Scholar]
- 19. Ang LW, Tey SH, Cutter J, James L, Goh KT. Seroprevalence of hepatitis B virus infection among children and adolescents in Singapore, 2008‐2010. J Med Virol 2013;85:583‐588. [DOI] [PubMed] [Google Scholar]
- 20. Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 1997;336:1855‐1859. [DOI] [PubMed] [Google Scholar]
- 21. Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20‐year follow‐up study. J Natl Cancer Inst 2009;101:1348‐1355. [DOI] [PubMed] [Google Scholar]
- 22. Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta‐analysis: the impact of oral anti‐viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther 2013;38:98‐106. [DOI] [PubMed] [Google Scholar]
- 23. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521‐1531. [DOI] [PubMed] [Google Scholar]
- 24. Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long‐term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology 1999;29:971‐975. [DOI] [PubMed] [Google Scholar]
- 25. Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 2015;62:956‐967. [DOI] [PubMed] [Google Scholar]
- 26. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5‐year open‐label follow‐up study. Lancet 2013;381:468‐475. [DOI] [PubMed] [Google Scholar]
- 27. Lim WY, Fong CW, Chan JM, Heng D, Bhalla V, Chew SK. Trends in alcohol consumption in Singapore 1992 2004. Alcohol Alcoh 2007;42:354‐361. [DOI] [PubMed] [Google Scholar]
- 28. Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134‐140. [DOI] [PubMed] [Google Scholar]
- 29. Page JM, Harrison SA. NASH and HCC. Clin Liv Dis 2009;13:631‐647. [DOI] [PubMed] [Google Scholar]
- 30. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia‐‐as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013;10:307‐318. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto E, Tokushige K. Hepatocellular carcinoma in non‐alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res 2012;42:1‐14. [DOI] [PubMed] [Google Scholar]
- 32. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 33. Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non‐B non‐C hepatocellular carcinoma in a hepatitis B‐endemic area. Digestion 2011;84 Suppl 1:17‐22. [DOI] [PubMed] [Google Scholar]
- 34. Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, et al. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol 2012;56:397‐405. [DOI] [PubMed] [Google Scholar]
- 35. Yim SY, Seo YS, Jung CH, Kim TH, Lee JM, Kim ES, et al. The management and prognosis of patients with hepatocellular carcinoma: what has changed in 20 years? Liver Int 2016;36:445‐453. [DOI] [PubMed] [Google Scholar]
- 36. Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Changes in the characteristics and survival rate of hepatocellular carcinoma from 1976 to 2000: analysis of 1365 patients in a single institution in Japan. Cancer 2004;100:2415‐2421. [DOI] [PubMed] [Google Scholar]
- 37. Bucci L, Garuti F, Lenzi B, Pecorelli A, Farinati F, Giannini EG, et al; Italian Liver Cancer (ITA.LI.CA) group . The evolutionary scenario of hepatocellular carcinoma in Italy: an update. Liver Int 2017;37:259‐270. [DOI] [PubMed] [Google Scholar]
- 38. Park YH, Kim BK, Kim JK, Kim HC, Kim DY, Park JY, et al. Long‐term outcomes of chronic hepatitis B virus infection in the era of antiviral therapy in Korea. J Gastroenterol Hepatol 2014;29:1005‐1011. [DOI] [PubMed] [Google Scholar]
- 39. Hung GY, Horng JL, Yen HJ, Lee CY, Lin LY. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J hepatol 2015;63:1390‐1396. [DOI] [PubMed] [Google Scholar]
- 40. Seto WK, Lau EH, Wu JT, Hung IF, Leung WK, Cheung KS, et al. Effects of nucleoside analogue prescription for hepatitis B on the incidence of liver cancer in Hong Kong: a territory-wide ecological study. Aliment Pharmacol Ther 2017;45:501‐509. [DOI] [PubMed] [Google Scholar]
- 41. Tian YE, Xie XU, Lin Y, Tan G, Zhong WU. Androgen receptor in hepatocarcinogenesis: recent developments and perspectives. Oncol Lett 2015;9:1983‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med 2008;121:119‐126. [DOI] [PubMed] [Google Scholar]
- 43. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000;31:330‐335. [DOI] [PubMed] [Google Scholar]
- 44. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20‐year experience. Ann Surg 2011;253:745‐758. [DOI] [PubMed] [Google Scholar]
- 46. Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Characteristics and prognosis of patients with hepatocellular carcinoma after the year 2000 in Japan. J Gastroenterol Hepatol 2011;26:1765‐1771. [DOI] [PubMed] [Google Scholar]
- 47. Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017;96:e5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


