Abstract
In contrast to patients with viral hepatitis, patients with nonalcoholic fatty liver disease (NAFLD) can progress to hepatocellular carcinoma during the initial stages of liver fibrosis. Development and implementation of noninvasive methods for diagnosis and progression prediction are important for effective NAFLD surveillance. Mac‐2 binding protein (Mac‐2bp) is a useful nonalcoholic steatohepatitis (NASH) diagnosis biomarker and a powerful prediction biomarker for NAFLD fibrosis stage. Wisteria floribunda agglutinin (WFA)‐positive Mac‐2bp (WFA+‐M2BP) is a novel serum fibrosis biomarker for chronic hepatitis C that has clinical validity. Mac‐2bp and WFA+‐M2BP are also clinical NAFLD biomarker candidates. We examined the efficacy of Mac‐2bp and WFA+‐M2BP for NAFLD assessment using patients with biopsy‐proven NAFLD (n = 510; NAFLD cohort) and subjects who received a health check‐up (n = 2,122; check‐up cohort). In the NAFLD cohort, we set the fibrosis predicting cutoff values as 1.80 (F1), 2.21 (F2), and 2.24 μg/mL (F3). In the subjects with fatty liver from the check‐up cohort (n = 1,291), the serum Mac‐2bp levels were >1.80 μg/mL in 38.6% of the subjects (n = 498), and >2.24 μg/mL in 24.6% of the subjects (n = 318). The NAFLD cohort results indicated that Mac‐2bp and WFA+‐M2BP were equally useful for NASH diagnosis. During the early stages of fibrosis (F1, F2), the increase in Mac‐2bp was statistically significant but WFA+‐M2BP did not increase. Logistic regression analysis revealed that Mac‐2bp was an independent determinant for the prediction of advanced fibrosis stage (≥F2), even when adjusted for WFA+‐M2BP. Immunohistochemical staining of Mac‐2bp revealed that hepatocytes strongly expressed Mac‐2bp in patients with NAFLD. Conclusion: Our results indicated that hepatocyte‐derived Mac‐2bp would be a useful single biomarker for NASH diagnosis and fibrosis stage prediction in patients with NAFLD. (Hepatology Communications 2017;1:780–791)
Abbreviations
- Alb
albumin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- BMI
body mass index
- CHC
chronic hepatitis type C
- COI
cutoff index
- FBS
fasting blood glucose
- FIB4
fibrosis‐4
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- IHC
immunohistochemical
- IRI
immunoreactive insulin
- Mac‐2bp
Mac‐2 binding protein
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity scoring
- NASH
nonalcoholic steatohepatitis
- NC
negative control
- NFS
NAFLD fibrosis score
- PC
positive control
- PNPLA3
patatin‐like phospholipase domain containing 3
- WFA
Wisteria floribunda agglutinin
- WFA+‐M2BP
WFA‐positive Mac‐2bp
Introduction
Nonalcoholic fatty liver disease (NAFLD) is among the most common causes of chronic liver disease worldwide and is a growing medical problem in industrialized countries.1 A wide spectrum of hepatic histologic changes occurs in patients with NAFLD. These changes range from the generally nonprogressive nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). During the clinical progression of NAFLD, assessment of liver fibrosis degree is important to predict further progression and to make therapeutic management decisions.2, 3 Hepatocellular carcinoma (HCC) incidence increases with the progression of liver fibrosis in chronic hepatitis type C (CHC) patients.4, 5, 6 In contrast with viral hepatitis, patients with NAFLD can progress to HCC during the early stages of liver fibrosis.7, 8, 9, 10 Therefore, diagnosis of NASH during the early stages is important during clinical follow‐up of the patient. Liver biopsy remains the gold standard for liver fibrosis assessment.11, 12 However, liver biopsy has significant limitations, including pain, risk of severe complications, sampling error,13 cost,14 and patient unwillingness to undergo invasive testing. Recently, Angulo et al.15 reported that liver fibrosis is independently associated with long‐term outcome in patients with NAFLD. Therefore, there is an urgent need for a reliable and noninvasive test that can be used to accurately assess the degree of liver fibrosis.
Novel noninvasive approaches, e.g., transient elastography (FibroScan, acoustic radiation force impulse, and magnetic resonance elastography), and various scoring systems can be used to measure the severity of fibrosis in patients with chronic liver disease.16, 17, 18, 19, 20, 21 However, transient elastography has reduced interobserver agreement in patients with early stage fibrosis, moderate to severe steatosis, or increased body mass index (BMI). Distinguishing early liver fibrosis (F1‐F2) from the nonfibrotic liver is difficult when transient elastography is used. Various scoring systems for measurement of liver fibrosis severity are available as noninvasive methods,19, 20, 21, 22, 23, 24, 25 but few individual biomarkers have clinical validity.
Recently, we found that serum Mac‐2 binding protein (Mac‐2bp) levels can be used to predict histologic severity of hepatic fibrosis in patients with NAFLD.26, 27 Mac‐2bp is a glycoprotein with seven potential N‐glycosylation sites.28, 29 Serum Mac‐2bp concentrations increase in patients with pancreatic, breast, and lung cancers, viral hepatitis, and autoimmune disease.28 Mac‐2bp is almost undetectable in normal liver but is easily detected in hepatocytes from CHC patients as liver fibrosis progresses.30 Cheung et al.31 used proteome analysis and found that serum Mac‐2bp is a potential marker of fibrosis progression in CHC patients. Wisteria floribunda agglutinin (WFA)‐positive Mac‐2bp (WFA+‐M2BP) is a novel serum fibrosis biomarker for CHC.32 This biomarker can distinguish the glycan structure of WFA‐detectable Mac‐2bp. It was developed using a glycan‐based immunoassay for the assessment of liver fibrosis severity in CHC patients.32 WFA+‐M2BP can also be used for the assessment of liver fibrosis stage in patients with NAFLD.33 Taken together, these findings indicate that serum Mac‐2bp and WFA+‐M2BP levels are useful liver fibrosis biomarkers. However, the validity of the use of these two biomarkers for NASH diagnosis and fibrosis stage prediction in patients with NAFLD is not fully understood.
The objective of this study was to examine the efficacy of Mac‐2bp and WFA+‐M2BP for the assessment of NAFLD. We investigated these biomarkers in patients with biopsy‐proven NAFLD (n = 510) and subjects who received a health check‐up (n = 2,122).
Materials and Methods
ETHICAL COMMITTEE APPROVAL
The research and informed consent protocols were approved for use in a multicenter study by the institutional review boards at Osaka University Hospital, Kochi Medical School Hospital, Hiroshima University Hospital, Osaka City University Hospital, Nara City Hospital, and aMs New Otani Clinic. Written informed consent was obtained from each subject at the time of liver biopsy or at the time of enrollment at each institute. The study was conducted in accordance with the Helsinki Declaration.
PATIENTS WITH BIOPSY‐PROVEN NAFLD
From 2002 to 2013, 510 patients (200 with NAFL and 310 with NASH) with biopsy‐confirmed NAFLD who were treated at one of five hepatology centers in Japan (Osaka University Hospital, Kochi Medical School Hospital, Hiroshima University Hospital, Osaka City University Hospital, and Nara City Hospital) were enrolled in the study.
Each patient with biopsy‐proven NAFLD had received a percutaneous liver needle biopsy. The liver samples were embedded in paraffin blocks following standard procedures and were stained with hematoxylin and eosin and Masson's trichrome stains. All biopsy specimens were centrally evaluated by two hepatic pathologists (Y.K. and H.F.) who were blinded to the clinical data. An adequate liver sample was defined as >1.5 cm in length or containing more than six portal tracts or both. Matteoni's classification was used to confirm the presence of NASH.34 Patients with NAFLD with ballooning hepatocytes (Matteoni type 3) and patients with NAFLD with liver fibrosis (Matteoni type 4) were assigned to the NASH cohort. Patients whose liver biopsy analysis revealed simple steatosis or steatosis with nonspecific inflammation were assigned to the NAFL cohort. NAFLD activity scoring (NAS) was used to assess and quantify each sample.35 The stages of steatosis (0‐3), lobular inflammation (0‐2), and hepatocellular ballooning (0‐2) were quantified. Individual fibrosis parameters were scored independently using the NASH Clinical Research Network scoring system.35 Advanced fibrosis was classified as a stage 2‐4 (F2‐F4) disease. The exclusion criteria included a history of other hepatic disease, a substance abuse‐induced hepatic disorder, and a history of alcohol abuse (>20 g of alcohol daily). In this study, we could measure both Mac‐2bp and WFA+‐M2BP in 116 of the 510 patients with NAFLD.
Mac‐2bp IMMUNOSTAINING
Normal liver tissue was obtained from the livers of patients with hepatic colorectal carcinoma metastasis who had surgery at Osaka University Hospital. The liver biopsy specimens from the patients with NAFLD and the normal liver tissues were prepared using the immunohistochemical (IHC) staining VECTASTAIN ABC kit (Funakoshi, Co., Ltd. Tokyo, Japan). Mouse anti‐human Mac‐2bp antibody (1:200; ab67353; Abcam, Cambridge, MA) was used for immunodetection. We classified the IHC liver histology into three classes according to IHC intensity (negative/weak/strong; 0/1/2) (Supporting Figs. S1 and S2).
SUBJECTS IN MEDICAL HEALTH CHECK‐UPS
A total of 2,122 (1,495 male; 627 female) of the 2,215 Japanese adult subjects (1,538 male; 677 female) who underwent a health check‐up at the aMs New Otani Clinic (Osaka, Japan) between 2008 to 2011 were initially recruited into the study. The exclusion criteria included a history of hepatic disease, such as CHC or concurrent active hepatitis B (seropositive for hepatitis B surface antigen), autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, hemochromatosis, α1‐antitrypsin deficiency, Wilson's disease, hepatic injury caused by substance abuse, or a current or previous history of daily consumption of >20 g alcohol. The diagnosis of fatty liver was based on the results of an abdominal ultrasound examination performed by trained technicians after exclusion of other etiologies of steatogenic liver disease. A fatty liver was defined as a liver parenchyma with an echogenicity greater than that of the kidney cortex, the presence of vascular blurring, and deep attenuation of the ultrasound signal.36, 37 Among the 2,122 subjects, 1,291 (1,022 male, 269 female) were diagnosed as having a fatty liver and 831 (473 male, 358 female) were diagnosed as not having a fatty liver, using abdominal ultrasound. Blood serum was collected from each subject during the health check‐up and was kept frozen at –80°C until analysis.
ANTHROPOMETRIC AND LABORATORY EVALUATION
Anthropometric variables (height and weight) were measured while each subject was in the standing position. BMI was calculated as weight (kg) divided by the square of height in meters (m2). Serum biochemical variables (aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ‐glutamyltransferase, alkaline phosphatase [ALP], choline esterase, total cholesterol, triglyceride, high‐density lipoprotein cholesterol, fasting blood glucose [FBS], immunoreactive insulin [IRI], albumin [Alb], ferritin, hyaluronic acid, and platelet count) were measured using a conventional automated blood analyzer. The fibrosis‐4 (FIB4) index (based on the age, serum AST and ALT levels, and platelet counts) was calculated for each of the subjects as reported (age × AST [U/L] / platelet count [×109/L] / √ALT [U/L]).24, 38 The NAFLD fibrosis score (NFS) was calculated for each of the subjects as reported (–1.675 + 0.037 × age [years] + 0.094 × BMI [kg/m2] + 1.13 × impaired fasting glycemia/diabetes mellitus [yes = 1, no = 0] + 0.99 × AST/ALT – 0.013 × platelet count (×109/L) – 0.66 × Alb [g/dL]).20 Impaired fasting glucose (IFG) was defined as FBS of 110–125 mg/dL. The presence of diabetes mellitus (DM) was defined as FBS ≥ 126 mg/dL, HbA1c (NGSP) ≥ 6.5 %, or treatment with anti‐diabetic drugs.
Mac‐2bp AND WFA+‐M2BP MEASUREMENT
We used an enzyme‐linked immunosorbent assay kit (code #27362; Immuno‐Biological Laboratory Co., Ltd., Fujioka, Japan) to measure serum Mac‐2bp.26 WFA+‐M2BP quantification was based on a lectin‐antibody sandwich immunoassay performed using a fully automatic immunoanalyzer (HISCL‐2000i; Sysmex Co., Hyogo, Japan).32 The measured values of WFA+‐M2BP conjugated to WFA were indexed with the obtained values using the following equation:
where [WFA+‐M2BP]sample was the WFA+‐M2BP count for the serum sample, PC was the positive control, and NC was the negative control. The PC was supplied as a calibration solution preliminarily standardized to yield a COI value of 1.0.39 Measurement of WFA+‐M2BP was supported by the Sysmex Co.
STATISTICAL ANALYSIS
Statistical analysis was performed using JMP Pro 12.0 software (SAS Institute Inc., Cary, NC). Results were expressed as mean ± SD values. Statistical analysis included descriptive statistics, analysis of variance, the Wilcoxon and Kruskal–Wallis tests, the Pearson test, and Spearman R correlation analysis. The diagnostic performance of each marker was assessed using receiver operating characteristic (ROC) curves. The probabilities of true positive (sensitivity) and true negative (specificity) results were determined for selected cutoff values, and the area under the ROC curve (AUROC) was calculated for each index. The Youden index was used to identify the optimal cutoff points. Multivariate logistic regression analyses were performed to identify parameters that significantly contributed to the estimation of liver fibrosis severity. Differences were considered to be statistically significant at P < 0.05.
Results
CHARACTERISTICS OF THE SUBJECTS
The results for the clinical and biochemical characteristics of the individuals included in the study population (i.e., patients with biopsy‐proven NAFLD [NAFLD cohort], subjects with health check‐ups [check‐up cohort]) are presented in Table 1. In the NAFLD cohort, the ratio of males was increased in the NASH patient group compared with the NAFL patient group. In the check‐up cohort, the ratio of males was greater in the fatty liver subject group [FL(+)] compared with the group of subjects without fatty liver [FL(–)]. Age and BMI were greater in the patients with NASH and in the FL(+) subjects in the NAFLD cohort and check‐up cohort, respectively. In the NAFLD cohort, the serum levels of AST, ALT, ALP, IRI, and ferritin were also higher in the patients with NASH compared with the patients with NAFL. The values of the FIB4 index and NFS also increased in patients with NASH. The values for Alb and platelet count were lower in the patients with NASH. In the check‐up cohort, the serum levels of AST, ALT, γ‐glutamyltransferase, ALP, choline esterase, total cholesterol, triglyceride, FBS, and Alb were higher in the FL(+) subjects than in the FL(–) subjects. The NFS was higher but the FIB4 index was lower in the FL(+) subjects than in the FL(–) subjects. Serum high‐density lipoprotein cholesterol levels were lower in the FL(+) subjects. In the NAFLD cohort, serum Mac‐2bp levels were significantly increased in the patients with NASH compared with the patients with NAFL (Table 1; Fig. 1A). In the check‐up cohort, both serum Mac‐2bp and WFA+‐M2BP levels were higher in the FL(+) subjects.
Table 1.
CLINICAL AND SEROLOGICAL CHARACTERISTICS OF THE SUBJECTS
| Variables | Biopsy‐Proven NAFLD Patients | Health Check‐Up Subjects | ||||
|---|---|---|---|---|---|---|
| NAFL | NASH | P Value* | Fatty Liver (–) | Fatty Liver (+) | P Value* | |
| Number | 200 | 310 | 831 | 1291 | ||
| Age (y) | 50.7 ± 10.3 | 52.8 ± 14.2 | <0.005 | 54.1 ± 8.7 | 54.6 ± 7.0 | <0.05 |
| Sex (M/F) | 69/131 | 141/169 | <0.0001 | 473/358 | 1022/269 | <0.0001 |
| BMI (kg/m2) | 26.0 ± 3.9 | 28.1 ± 5.1 | <0.0005 | 21.9 ± 2.7 | 26.0 ± 3.6 | <0.0001 |
| AST (U/L) | 42.0 ± 26.3 | 64.2 ± 44.1 | <0.0001 | 24.3 ± 14.2 | 31.1 ± 16.8 | <0.0001 |
| ALT (U/L) | 75.4 ± 49.8 | 98.0 ± 73.8 | <0.01 | 23.4 ± 23.0 | 41.1 ± 24.0 | <0.0001 |
| GGT (U/L) | 111.0 ± 143.1 | 88.5 ± 79.7 | N.S. | 50.5 ± 94.4 | 74.5 ± 94.2 | <0.0001 |
| ALP (U/L) | 238.3 ± 85.4 | 274.9 ± 125.8 | <0.05 | 200.9 ± 74.8 | 210.9 ± 58.0 | <0.0001 |
| CHE (U/L) | 368.4 ± 75.3 | 363.4 ± 89.1 | N.S. | 316.1 ± 71.7 | 376.9 ± 67.2 | <0.0001 |
| T‐Chol (mg/dL) | 193.2 ± 36.7 | 206.1 ± 36.0 | N.S. | 194.9 ± 30.8 | 208.7 ± 35.3 | <0.0001 |
| TG (mg/dL) | 136.2 ± 93.6 | 143.2 ± 64.4 | N.S. | 92.3 ± 80.3 | 155.4 ± 106.9 | <0.0001 |
| HDL‐C (mg/dL) | 49.2 ± 12.0 | 49.2 ± 15.2 | N.S. | 64.7 ± 14.3 | 54.3 ± 11.7 | <0.0001 |
| FBS (mg/dL) | 98.6 ± 15.1 | 106.1 ± 25.0 | N.S. | 108.3 ± 28.1 | 120.2 ± 32.1 | <0.0001 |
| IRI (mU/mL) | 10.1 ± 5.6 | 14.7 ± 9.1 | <0.05 | N.D. | N.D. | |
| Albumin (g/dL) | 4.57 ± 0.38 | 4.42 ± 0.45 | <0.05 | 4.24 ± 0.22 | 4.39 ± 0.22 | <0.0001 |
| Ferritin (ng/mL) | 179.2 ± 127.3 | 287.0 ± 285.7 | <0.005 | N.D. | N.D. | |
| Hyaluronic acid (ng/mL) | 37.4 ± 37.4 | 106.5 ± 206.6 | <0.05 | N.D. | N.D. | |
| Platelet count (× 104/μL) | 23.8 ± 5.6 | 22.1 ± 6.9 | N.S. | 21.7 ± 5.4 | 22.0 ± 5.1 | 0.07 |
| FIB4 index | 1.00 ± 0.53 | 1.97 ± 1.47 | <0.0001 | 1.39 ± 0.65 | 1.33 ± 0.70 | <0.005 |
| NFS | –2.40 ± 1.32 | –1.56 ± 1.56 | <0.0001 | –1.71 ± 1.11 | –1.53 ± 1.13 | <0.0005 |
| Mac‐2bp (μg/mL) | 1.46 ± 0.77 | 2.74 ± 1.52 | <0.0001 | 1.11 ± 0.69 | 1.81 ± 1.09 | <0.0001 |
| WFA+‐M2BP (COI) | N.D. | N.D. | 0.51 ± 0.36 | 0.60 ± 0.31 | <0.0001 | |
Data are presented as the mean ± SD. *P values correspond to the comparison between NAFL and NASH groups. Wilcoxon test for continuous factors and Pearson's chi‐square test for categorical factors were used.
Abbreviations: CHE, choline esterase; GGT, gamma glutamyltranspeptidase; HDL‐C, high‐density lipoprotein cholesterol; N.D., not determined; N.S., not significant; T‐Chol, total cholesterol; TG, triglyceride.
Figure 1.
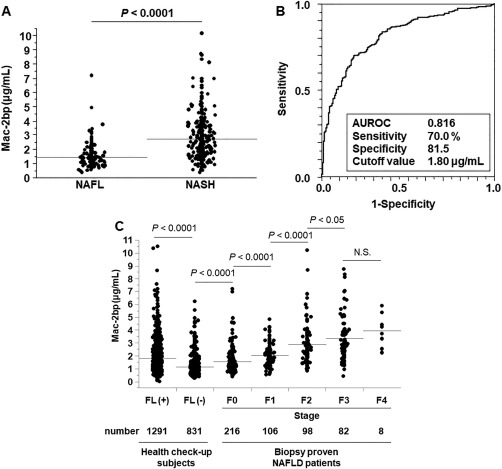
Mac‐2bp is a useful biomarker for nonalcoholic steatohepatitis diagnosis and fibrosis prediction. (A) Serum Mac‐2bp levels in patients with NAFL and NASH. The horizontal gray lines indicate the mean values for Mac‐2bp in each group. (B) ROC curve for Mac‐2bp for the discrimination of NASH in biopsy‐proven NAFL disease patients. (C) Serum Mac‐2bp levels in subjects with health check‐ups and in biopsy‐proven NAFL disease patients. Abbreviation: N.S., not significant.
Mac‐2bp IS A USEFUL BIOMARKER FOR NASH DIAGNOSIS AND LIVER FIBROSIS PREDICTION
Next, we used ROC analysis to evaluate the utility of Mac‐2bp as a NASH diagnosis biomarker (510 patients with NAFLD; Fig. 1B). When the cutoff value was 1.80 μg/mL, the values for the AUROC and for sensitivity and specificity for Mac‐2bp for NASH diagnosis were 0.816, 70.0%, and 81.5%, respectively. In contrast, the values for the AUROC and for sensitivity and specificity were 0.733, 58.6%, and 91.4% (FIB4 index) and 0.654, 50.9%, and 74.9% (NFS), respectively. Mac‐2bp was superior to these scoring systems for NASH diagnosis, although the FIB4 index showed high specificity.
Our previous study revealed that Mac‐2bp is a useful biomarker for the prediction of liver fibrosis severity in patients with NAFLD.26 In the present study, we measured serum Mac‐2bp levels in the NAFLD and check‐up cohorts. In patients with biopsy‐proven NAFLD, serum Mac‐2bp levels increased stepwise as the liver fibrosis stage increased (F0/F1/F2/F3/F4, 1.55 ± 0.91, 2.05 ± 0.88, 2.89 ± 1.59, 3.34 ± 1.72, 3.96 ± 1.28 μg/mL, respectively) (Fig. 1C). In the check‐up cohort, the mean serum Mac‐2bp value was higher in the FL(+) group than in the FL(–) group (1.81 ± 1.09, 1.11 ± 0.69 μg/mL, respectively). The serum Mac‐2bp levels of the FL(+) group subjects were between the levels of F0 and F1 in the patients with biopsy‐proven NAFLD. Using ROC analysis, we set Mac‐2bp cutoff values to predict each fibrosis stage in patients with biopsy‐proven NAFLD (Supporting Table S1). The cutoff values were 1.80 μg/mL (≤stage 1), 2.21 μg/mL (≤stage 2), and 2.24 μg/mL (≤stage 3). In 1,291 FL(+) subjects, the serum Mac‐2bp levels were >1.80 μg/mL in 498 subjects (38.6%) and >2.24 μg/mL in 318 subjects (24.6%).
SERUM Mac‐2bp LEVELS AND WFA+‐M2BP LEVELS WERE WELL CORRELATED IN THE HEALTH CHECK‐UP GROUP
We measured serum levels of Mac‐2bp and WFA+‐M2BP in 1,830 subjects receiving health check‐ups (1,337 male subjects, 493 female subjects). The results indicated that serum Mac‐2bp and WFA+‐M2BP levels were well correlated (R = 0.44, P < 0.0001) (Fig. 2).
Figure 2.
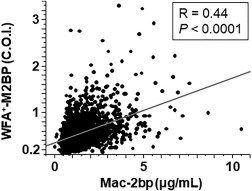
Serum Mac‐2bp and WFA+‐M2BP levels were well correlated in subjects with health check‐ups.
SERUM Mac‐2bp LEVELS AND WFA+‐M2BP LEVELS WERE EQUALLY USEFUL BIOMARKERS FOR NASH DIAGNOSIS
We measured both Mac‐2bp and WFA+‐M2BP in 116 (48 NAFL, 68 NASH) of 510 patients in the NAFLD cohort. The results for the clinical and biochemical characteristics of the 116 patients with NAFLD are presented in Table 2. Age, AST, ALT, ALP, IRI, and ferritin levels were significantly higher and FBS, Alb, and platelet count were significantly lower in the patients with NASH. The ratio of female to male patients was significantly higher in the NASH patient group. Serum Mac‐2bp and WFA+‐M2BP levels were significantly higher in the patients with NASH compared with the patients with NAFL.
Table 2.
CLINICAL AND SEROLOGICAL CHARACTERISTICS OF THE PATIENTS WITH BIOPSY‐PROVEN NAFLD WHO WERE MEASURED FOR BOTH Mac‐2bp AND WFA+‐M2BP
| NAFL | NASH | P Value | |
|---|---|---|---|
| Number | 48 | 68 | |
| Age (y) | 51.8 ± 9.8 | 55.6 ± 12.4 | <0.05 |
| Sex (M/F) | 35/13 | 28/40 | <0.01 |
| BMI (kg/m2) | 26.7 ± 3.9 | 26.9 ± 4.8 | N.S. |
| AST (U/L) | 37.9 ± 26.8 | 64.3 ± 37.0 | <0.0001 |
| ALT (U/L) | 56.2 ± 39.6 | 93.9 ± 60.3 | <0.0005 |
| GGT (U/L) | 104.9 ± 133.4 | 100.7 ± 121.4 | N.S. |
| ALP (U/L) | 244.4 ± 101.8 | 275.3 ± 100.2 | <0.05 |
| CHE (U/L) | 349.7 ± 54.8 | 352.3 ± 71.5 | N.S. |
| T‐Chol (mg/dL) | 208.8 ± 33.2 | 206.4 ± 39.9 | N.S. |
| TG (mg/dL) | 139.0 ± 59.3 | 161.4 ± 92.8 | N.S. |
| HDL‐C (mg/dL) | 51.6 ± 11.5 | 48.9 ± 15.6 | N.S. |
| FBS (mg/dL) | 111.2 ± 22.1 | 103.9 ± 28.2 | <0.05 |
| IRI (mU/mL) | 8.16 ± 3.83 | 14.7 ± 11.2 | <0.05 |
| Albumin (g/dL) | 4.36 ± 0.29 | 4.12 ± 0.35 | <0.0001 |
| Ferritin (ng/mL) | 185.8 ± 148.2 | 375.2 ± 419.8 | 0.07 |
| Platelet count (×104/μL) | 22.6 ± 5.7 | 19.2 ± 5.4 | <0.01 |
| Hyaluronic acid (ng/mL) | 39.6 ± 60.6 | 79.1 ± 109.5 | <0.05 |
| Mac‐2bp (μg/mL) | 1.23 ± 0.56 | 2.05 ± 1.12 | <0.0001 |
| WFA+‐M2BP (COI) | 0.67 ± 0.67 | 1.26 ± 1.10 | <0.0001 |
Abbreviations: CHE, choline esterase; GGT, gamma glutamyltranspeptidase; HDL‐C, high‐density lipoprotein cholesterol; N.D., not determined; N.S., not significant; T‐Chol, total cholesterol; TG, triglyceride.
We compared the significance of NASH diagnostic ability between Mac‐2bp and WFA+‐M2BP in the NASH patient group. The serum levels of Mac‐2bp and WFA+‐M2BP were well correlated (Fig. 3A). The ROC analysis revealed that the utility of Mac‐2bp and WFA+‐M2BP as NASH biomarkers was similar for each marker (Fig. 3B).
Figure 3.
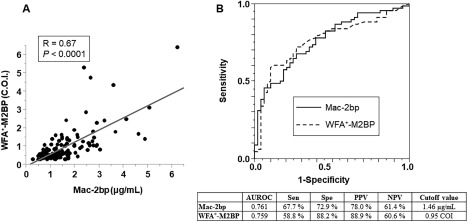
Serum Mac‐2bp levels and WFA+‐M2BP levels were equally useful biomarkers for NASH diagnosis. (A) Correlation between serum Mac‐2bp and WFA+‐M2BP in patients with biopsy‐proven NAFLD. (B) ROC curves of Mac‐2bp and WFA+‐M2BP for the discrimination of NASH in patients with biopsy‐proven NAFLD. The table presents the results for the efficacy of each biomarker for NASH diagnosis. Abbreviations: NPV, negative predictive value; PPV, positive predictive value; Sen, sensitivity; Spe, specificity.
COMPARISON OF Mac‐2bp AND WFA+‐M2BP AS LIVER FIBROSIS PREDICTION BIOMARKERS
The serum Mac‐2bp and WFA+‐M2BP levels increased as liver fibrosis severity increased (116 patients with NAFLD; Fig. 4A,B). Serum Mac‐2bp levels were significantly increased between F0 and F1 and between F1 and F2. We used ROC analysis to compare the utility of these two biomarkers for the assessment of advanced liver fibrosis (≥F2) in patients with NAFLD (Fig. 4C). The AUROC for Mac‐2bp was greater than that of WFA+‐M2BP (0.809 versus 0.758, respectively). Logistic regression analysis revealed that Mac‐2bp was an independent determinant for the prediction of advanced liver fibrosis, even after adjustment for WFA+‐M2BP results (Table 3). The AUROC values for Mac‐2bp and WFA+‐M2BP were 0.750 and 0.731, respectively, for the prediction of early liver fibrosis (≥F1); the values were 0.738 and 0.701, respectively, for the prediction of severe advanced fibrosis (≥F3) (Supporting Fig. S3). In contrast, the values for the AUROC for the assessment of advanced liver fibrosis (≥F2) in patients with NAFLD were 0.791 (FIB4 index) and 0.719 (NFS). Mac‐2bp was superior to these scoring systems for the prediction of advanced liver fibrosis in NAFLD.
Figure 4.
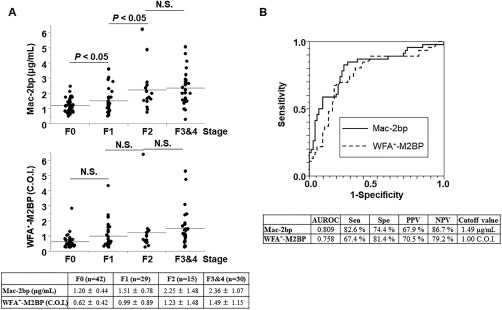
Comparison of Mac‐2bp and WFA+‐M2BP as liver fibrosis prediction biomarkers. (A) Correlation between serum Mac‐2bp levels and liver fibrosis stage (upper panel). Correlation between serum WFA+‐M2BP levels and liver fibrosis stage (middle panel). The table presents the results for the mean values of Mac‐2bp and WFA+‐M2BP at each liver fibrosis stage. (B) ROC curves of Mac‐2bp and WFA+‐M2BP for the prediction of advanced liver fibrosis (≥F2) in patients with biopsy‐proven NAFLD. The table presents the results for the efficacy of each biomarker. NPV, negative predictive value; N.S., not significant; PPV, positive predictive value; Sen, sensitivity; Spe, specificity.
Table 3.
LOGISTIC REGRESSION ANALYSIS FOR THE PREDICTION OF EARLY LIVER FIBROSIS (≥F1)
| Parameters | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Mac‐2bp (μg/mL) | 4.43 | 2.12‐10.40 | <0.0001 |
| WFA+‐M2BP (COI) | 1.31 | 0.68‐3.05 | N.S. |
Abbreviations: CI, confidence interval; N.S., not significant.
Mac‐2bp PROTEIN IS STRONGLY EXPRESSED IN NAFLD PATIENT HEPATOCYTES
We used IHC analysis of Mac‐2bp to determine the kinds of cells that were the source of Mac‐2bp protein production in livers of patients with NAFLD (Fig. 5). The Mac‐2bp protein was barely expressed in normal liver; strong cytoplasmic staining was apparent in the hepatocytes from patients with NAFLD. Hepatocytes of patients with NAFLD had whole cytoplasmic staining or peripheral nuclear lesion staining of Mac‐2bp. Surprisingly, there were significant negative correlations between serum Mac‐2bp levels and degree of hepatic Mac‐2bp staining (Supporting Fig. S2).
Figure 5.
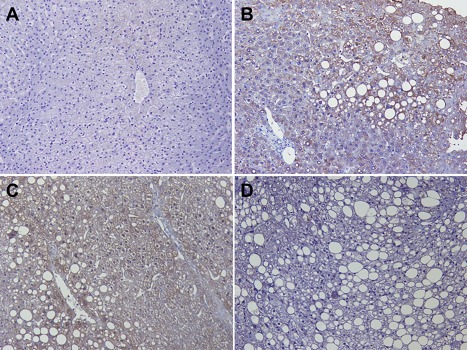
Mac‐2bp protein was strongly expressed in hepatocytes from patients with NAFLD. Photomicrographs of representative samples from human livers stained with anti‐human Mac‐2bp antibody (original magnification ×200). (A) Normal liver tissues obtained from livers of patients who underwent surgery for hepatic colorectal carcinoma metastasis. (B‐D) Livers from patients with NAFLD. Serum Mac‐2bp levels were (B) 0.932 μg/mL, (C) 1.26 μg/mL, and (D) 3.65 μg/mL.
Discussion
We investigated serum Mac‐2bp and WFA+‐M2BP in a large sample size of NAFLD subjects. Serum Mac‐2bp levels increased in a stepwise pattern in patients with biopsy‐proven NAFLD. The diagnostic ability for NASH was not different between Mac‐2bp and WFA+‐M2BP; however, the utility of Mac‐2bp for the prediction of advanced liver fibrosis was slightly superior to that of WFA+‐M2BP. IHC staining of Mac‐2bp revealed that hepatocytes strongly expressed Mac‐2bp in the livers of some patients with NAFLD; Mac‐2bp was barely expressed in the non‐NAFLD patients' livers. Our results indicated that hepatocytes were a main source of circulating Mac‐2bp in the patients with NAFLD; however, our study demonstrated that serum Mac‐2bp levels negatively correlated with the degree of Mac‐2bp staining in liver. Not only production but also secretion of Mac‐2bp should be involved in these phenomena.
Kuno et al.32 studied a group of 125 CHC patients and found that serum Mac‐2bp levels are greater and that WFA has the best correlation with liver fibrosis progression in these patients. They developed an automatic measurement system that can detect WFA+‐M2BP within 20 minutes. WFA+‐M2BP has a characteristic glycan structure that is recognized by WFA. WFA recognizes terminal N‐acetylgalactosaminides and specifically binds with the disaccharide LacdiNAc (β‐D‐GalNAc‐[1→4]‐D‐GlcNAc; GalNAc N‐acetylgalactosamine, GlcNAC N‐acetylglucosamine).40 WFA+‐M2BP is very useful as a liver fibrosis biomarker for CHC, but the liver fibrosis prediction abilities for the other chronic liver diseases are relatively lower than that for CHC.32, 33, 41, 42, 43 Our study revealed that compared with WFA+‐M2BP, Mac‐2bp had greater ability to predict NAFLD fibrosis stage. Mac‐2bp is a glycoprotein with seven N‐glycosylation sites29; the Mac‐2bp measured in this study included whole types of Mac‐2bp, including WFA+‐M2BP. Investigation of the NAFLD‐specific glycan modification of Mac‐2bp is necessary to find more useful NAFLD glycol‐biomarkers.
Bekki et al.44 found that hepatic stellate cells (HSCs) produce WFA+‐M2BP. WFA+‐M2BP produced from HSCs stimulate Mac‐2 (galectin 3) production from Kupffer cells and accelerate further HSC activation, which results in liver fibrosis progression. In our study, the hepatocytes in the patients with NAFLD mainly produced Mac‐2bp. Secreted glycoproteins have different types of glycosylation patterns due to the different origins of cell types and stimuli.45, 46 The differences in the Mac‐2bp‐producing cells or in the type of stimulation or both should account for the variant glycosylation patterns of Mac‐2bp. With comprehensive analysis of glycosylation patterns on Mac‐2bp, we would like to establish, in a future study, novel NAFLD biomarkers that can predict the severity of the NAFLD clinical course more precisely.
We found that Mac‐2bp was superior to WFA+‐M2BP for the prediction of NAFLD fibrosis stage, especially for early stage fibrosis. More than 90% of virus‐associated hepatitis patients with liver cirrhosis will develop HCC.7 In contrast, more than 50% of male patients with NAFLD with NAFLD‐associated HCC do not have liver cirrhosis.7, 8, 9 Therefore, it is important to diagnosis NAFLD at an early stage. These findings indicated that compared with WFA+‐M2BP, Mac‐2bp would be superior as a NAFLD biomarker for prediction of the extent of early liver fibrosis. In the subjects who received health check‐ups, the serum Mac‐2bp levels of the FL(+) subjects were between the levels of F0 and F1 found in the patients with biopsy‐proven NAFLD (Fig. 1C). We set the fibrosis‐predicting cutoff value as 1.80 (F1), 2.21 (F2), and 2.24 μg/mL (F3) for the group of 510 patients with biopsy‐proven NAFLD. Serum Mac‐2bp levels were >1.80 μg/mL in 38.6% and >2.24 μg/mL in 24.6% of the FL(+) subjects. These findings indicated that approximately 25% of the NAFLD subjects would have severe advanced fibrosis (≥F3).
Recent studies demonstrated that there is a significant association between the patatin‐like phospholipase domain containing 3 (PNPLA3) genotype and fibrosis progression in patients with NAFLD.47, 48 Although we did not analyze the PNPLA3 genotype in our study subjects, the information about the PNPLA3 genotype should be useful for the clinical follow‐up of patients with NAFLD.
Our previous study revealed that serum Mac‐2bp levels are higher in patients with NAFL compared with healthy volunteers.26 Serum Mac‐2bp was increased in a mouse simple fatty liver model and was further elevated in mouse NASH models by use of our enzyme‐linked immunosorbent assay system.49 However, in human patients with NAFLD, serum Mac‐2bp levels do not change with the degree of hepatic steatosis but do increase with hepatic inflammation and liver fibrosis progression.26, 27 In the present study, serum Mac‐2bp levels did not increase with steatosis degree in patients with NAFLD (Supporting Fig. S4). Taken together, these results indicate that serum Mac‐2bp increases in patients with fatty liver disease regardless of steatosis degree; Mac‐2bp is further elevated by inflammation and fibrosis. We found that there was significant negative correlation between serum Mac‐2bp levels and hepatic Mac‐2bp staining degree in patients with NAFLD (Supporting Figs. S1 and S2). We hypothesized that the mechanism of secretion of Mac‐2bp from the hepatocytes would be different in each NAFLD subject and that the balance between secretion and production of Mac‐2bp would contribute to the different accumulation patterns that occur in NAFLD hepatocytes. There are many etiologies for NAFLD (e.g., free fatty acids, inflammatory cytokines, and lipopolysaccharide).50, 51, 52, 53 These factors would have significant effects on the secretion of Mac‐2bp from hepatocytes in patients with NAFLD. The molecular mechanism underlying the induction of Mac‐2bp during fatty liver changes should be investigated.
In conclusion, both Mac‐2bp and WFA+‐M2BP were useful biomarkers for NASH diagnosis. Mac‐2bp would be superior to WFA+‐M2BP for the prediction of liver fibrosis stage in patients with NAFLD. Our study revealed that hepatocytes are the main producer of Mac‐2bp in patients with NAFLD. The balance between production and secretion of Mac‐2bp contributes to the determination of Mac‐2bp serum levels. The mechanisms that contribute to serum Mac‐2bp levels in patients with NAFLD should be investigated.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1080/full.
Supporting Information Figures.
Supporting Information Tables.
Acknowledgment
We thank Atsuko Sawanobori, Tomomi Maeda, and Haruka Maeda for their excellent technical assistance.
Potential conflict of interest: Tetsuo Takehara and Eiji Miyoshi received research funding from Sysmex Co. (Kobe, Hyogo, Japan). The other authors have no conflicts of interests to disclose.
Supported by a Japan Society for the Promotion of Science KAKENHI grant (grant number 15H04810, 16H05226, and 16K15428), the Program for Basic and Clinical Research on Hepatitis from the Japan Agency for Medical Research and Development, and research funding from Sysmex Co. (Kobe, Hyogo, Japan) (number J770701633).
REFERENCES
- 1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356‐359. [DOI] [PubMed] [Google Scholar]
- 2. Shirabe K, Takeishi K, Taketomi A, Uchiyama H, Kayashima H, Maehara Y. Improvement of long‐term outcomes in hepatitis C virus antibody‐positive patients with hepatocellular carcinoma after hepatectomy in the modern era. World J Surg 2011;35:1072‐1084. [DOI] [PubMed] [Google Scholar]
- 3. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 4. Liang TJ, Heller T. Pathogenesis of hepatitis C‐associated hepatocellular carcinoma. Gastroenterology 2004;127:S62‐S71. [DOI] [PubMed] [Google Scholar]
- 5. Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med 1998;129:94‐99. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med 1999;131:174‐181. [DOI] [PubMed] [Google Scholar]
- 7. Ertle J, Dechene A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non‐alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436‐2443. [DOI] [PubMed] [Google Scholar]
- 8. Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al.; Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan . Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428‐433. [DOI] [PubMed] [Google Scholar]
- 9. Tokushige K, Hyogo H, Nakajima T, Ono M, Kawaguchi T, Honda K, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol 2016;51:586‐596. [DOI] [PubMed] [Google Scholar]
- 10. Piscaglia F, Svegliati‐Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al.; HCC‐NAFLD Italian Study Group . Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827‐838. [DOI] [PubMed] [Google Scholar]
- 11. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495‐500. [DOI] [PubMed] [Google Scholar]
- 12. Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, et al. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology 2002;36(5 Suppl. 1):S161‐S172. [DOI] [PubMed] [Google Scholar]
- 13. Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 1986;2:165‐173. [DOI] [PubMed] [Google Scholar]
- 14. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al.; LIDO Study Group . Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898‐1906. [DOI] [PubMed] [Google Scholar]
- 15. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, et al. Transient elastography in patients with non‐alcoholic fatty liver disease (NAFLD). Gut 2007;56:1330‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, et al. Nonalcoholic fatty liver disease: US‐based acoustic radiation force impulse elastography. Radiology 2010;256:640‐647. [DOI] [PubMed] [Google Scholar]
- 18. Castera L, Forns X, Alberti A. Non‐invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835‐847. [DOI] [PubMed] [Google Scholar]
- 19. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander‐Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441‐1447. [DOI] [PubMed] [Google Scholar]
- 20. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 21. Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG‐NAFLD) . A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol 2011;46:257‐268. [DOI] [PubMed] [Google Scholar]
- 22. Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91‐100. [DOI] [PubMed] [Google Scholar]
- 23. Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology 2000;118:1117‐1123. [DOI] [PubMed] [Google Scholar]
- 24. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 25. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 26. Kamada Y, Fujii H, Fujii H, Sawai Y, Doi Y, Uozumi N, et al. Serum Mac‐2 binding protein levels as a novel diagnostic biomarker for prediction of disease severity and nonalcoholic steatohepatitis. Proteomics Clin Appl 2013;7:648‐656. [DOI] [PubMed] [Google Scholar]
- 27. Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Mori K, et al. A novel noninvasive diagnostic method for nonalcoholic steatohepatitis using two glycobiomarkers. Hepatology 2015;62:1433‐1443. [DOI] [PubMed] [Google Scholar]
- 28. Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, et al. 90K (Mac‐2 BP) and galectins in tumor progression and metastasis. Glycoconj J 2002;19:551‐556. [DOI] [PubMed] [Google Scholar]
- 29. Przybylo M, Martuszewska D, Pochec E, Hoja‐Lukowicz D, Litynska A. Identification of proteins bearing beta1‐6 branched N‐glycans in human melanoma cell lines from different progression stages by tandem mass spectrometry analysis. Biochim Biophys Acta 2007;1770:1427‐1435. [DOI] [PubMed] [Google Scholar]
- 30. Artini M, Natoli C, Tinari N, Costanzo A, Marinelli R, Balsano C, et al. Elevated serum levels of 90K/MAC‐2 BP predict unresponsiveness to alpha‐interferon therapy in chronic HCV hepatitis patients. J Hepatol 1996;25:212‐217. [DOI] [PubMed] [Google Scholar]
- 31. Cheung KJ, Tilleman K, Deforce D, Colle I, Van Vlierberghe H. The HCV serum proteome: a search for fibrosis protein markers. J Viral Hepat 2009;16:418‐429. 19226329 [Google Scholar]
- 32. Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet‐doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin‐positive Mac‐2 binding protein and the fibrosis stage of non‐alcoholic fatty liver disease. J Gastroenterol 2015;50:776‐784. [DOI] [PubMed] [Google Scholar]
- 34. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 35. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 36. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745‐750. [DOI] [PubMed] [Google Scholar]
- 37. Hamano M, Kamada Y, Kiso S, Furuta K, Kizu T, Chatani N, et al. Adiponectin negatively correlates with alcoholic and non‐alcoholic liver dysfunction: health check‐up study of Japanese men. Hepatol Res 2013;43:238‐248. [DOI] [PubMed] [Google Scholar]
- 38. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuno A, Sato T, Shimazaki H, Unno S, Saitou K, Kiyohara K, et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin‐assisted glycan profiling. Proteomics Clin Appl 2013;7:642‐647. [DOI] [PubMed] [Google Scholar]
- 40. Haji‐Ghassemi O, Gilbert M, Spence J, Schur MJ, Parker MJ, Jenkins ML, et al. Molecular basis for recognition of the cancer glycobiomarker, LacdiNAc (GalNAc[beta1‐‐>4]GlcNAc), by Wisteria floribunda agglutinin. J Biol Chem 2016;291:24085‐24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zou X, Zhu MY, Yu DM, Li W, Zhang DH, Lu FJ, et al. Serum WFA+ ‐M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int 2017;37:35‐44. [DOI] [PubMed] [Google Scholar]
- 42. Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. Impact of serum Wisteria floribunda agglutinin positive Mac‐2‐binding protein and serum interferon‐gamma‐inducible protein‐10 in primary biliary cirrhosis. Hepatol Res 2016;46:575‐583. [DOI] [PubMed] [Google Scholar]
- 43. Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac‐2‐binding protein level and high‐sensitivity C‐reactive protein concentration in autoimmune hepatitis. Hepatol Res 2016;46:613‐621. [DOI] [PubMed] [Google Scholar]
- 44. Bekki Y, Yoshizumi T, Shimoda S, Itoh S, Harimoto N, Ikegami T, et al. Hepatic stellate cells secrete WFA+ ‐M2BP: its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 2017;32:1387‐1393. [DOI] [PubMed] [Google Scholar]
- 45. Hart GW, Copeland RJ. Glycomics hits the big time. Cell 2010;143:672‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Connelly MA, Gruppen EG, Otvos JD, Dullaart RP. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clin Chim Acta 2016;459:177‐186. [DOI] [PubMed] [Google Scholar]
- 47. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Development of hepatocellular carcinoma in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res 2016, doi: 10.1111/hepr.12840. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48. Bugianesi E, Bizzarri C, Rosso C, Mosca A, Panera N, Veraldi S, et al. Low birthweight increases the likelihood of severe steatosis in pediatric non‐alcoholic fatty liver disease. Am J Gastroenterol 2017, doi: 10.1038/ajg.2017.140. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49. Iwata A, Kamada Y, Ebisutani Y, Yamamoto A, Ueda Y, Arai H, et al. Establishment of mouse Mac‐2 binding protein enzyme‐linked immunosorbent assay and its application for mouse chronic liver disease models. Hepatol Res 2016, doi: 10.1111/hepr.12819. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50. Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll‐like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013;57:577‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009;50:1827‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000;343:1467‐1476. [DOI] [PubMed] [Google Scholar]
- 53. Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, et al. Hyperresponsivity to low‐dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin‐mediated signaling. Cell Metab 2012;16:44‐54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1080/full.
Supporting Information Figures.
Supporting Information Tables.


