Abstract
Cholestatic drug‐induced liver injury (DILI) can be a diagnostic challenge due to a large differential diagnosis, variability in clinical presentation, and lack of serologic biomarkers associated with this condition. The clinical presentation of drug‐induced cholestasis includes bland cholestasis, cholestatic hepatitis, secondary sclerosing cholangitis, and vanishing bile duct syndrome. The associate mortality of cholestatic DILI can be as high as 10%, and thus prompt recognition and removal of the offending agent is of critical importance. Several risk factors have been identified for drug‐induced cholestasis, including older age, genetic determinants, and properties of certain medications. Antibiotics, particularly amoxicillin/clavulanate, remain the predominant cause of cholestatic DILI, although a variety of other medications associated with this condition have been identified. In this review, we summarize the presentation, clinical approach, risk factors, implicated medications, and management of drug‐induced cholestatic liver injury. (Hepatology Communications 2017;1:726–735)
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- BSEP
bile salt export pump
- DILI
drug‐induced liver injury
- HLA
human leukocyte antigen
- MDR
multidrug resistance
- MRP
multidrug resistance protein
- PBC
primary biliary cholangitis
- RUCAM
Roussel Uclaf assessment model
- ULN
upper limit of normal
Introduction
Drug‐induced liver injury (DILI) presents a significant burden to the health care system.1 Furthermore, recognition of DILI may be challenging as it is often a diagnosis of exclusion, clinical presentation is variable, there is a paucity of data available regarding risk factors, and standardized diagnostic testing for this condition does not exist.2 In the setting of DILI, there are several patterns of liver enzyme elevation; drug‐induced cholestasis can be defined as an increase in alkaline phosphatase (ALP) greater than 2 times the upper limit of normal (ULN) and/or an alanine aminotransferase (ALT)/ALP ratio of less than 2.3 One can also differentiate hepatocellular versus cholestatic DILI by calculating the R value, which uses ALP and ALT levels and is defined as: (ALT/ULN of normal ALT)/(ALP × ULN of normal ALT).4 When using this formula, the clinician should use the local laboratory's ULN for ALT and ALP.5 Hepatocellular injury presents with a predominant elevation of serum aminotransferases and an R factor greater than 5, often before the onset of jaundice, while cholestatic DILI has a value of less than 2. A mixed liver injury pattern has characteristics of both cholestatic and hepatocellular injury, with an ALT/ALP ratio greater than 2 but less than 5, although mixed injury is often considered to be a similar entity to cholestatic DILI.6 Among these three patterns, cholestatic injury occurs in 20%‐40% of cases.7
Drug‐induced cholestasis can have several histologic features. Cholestatic hepatitis is the most common form of DILI leading to cholestasis.8, 9 Bland cholestasis, typically associated with oral contraceptives and anabolic steroids, is characterized by canalicular dilatation and bile plugs but without significant inflammation. Idiosyncratic liver injury can also lead to ductal injury, including vanishing bile duct syndrome.8, 10, 11
In the majority of cases, liver test abnormalities reverse with the cessation of the offending drug.12 However, the time course for improvement with cholestatic injury is often slower than for hepatocellular injury.13, 14
Case
A 46‐year old man was seen for evaluation of abnormal liver enzymes, jaundice, and pruritus. He is an actor who was taking doxycycline for acne for a period of 3 months prior to presentation. At the time, he was also taking multiple vitamins and herbal supplements, including lysine, glycine, arginine, alpha lipoic acid, chlorophyll, and turmeric also for a 3‐month period. At the time of presentation, his ALT was 817, AST was 293, and total bilirubin was 10.5 mg/dL. Viral hepatitis and autoimmune hepatitis serologies were negative. A magnetic resonance cholangiogram was normal without ductal structuring or dilatation. Repeat enzymes performed 11 days after presentation revealed an ALT of 177 and AST of 93, but a significant rise in total bilirubin to 25.9 mg/dL. Therefore, a liver biopsy was performed that demonstrated cholestatic hepatitis (Fig. 1). The patient was managed with supportive care, including the use of plasmapharesis to manage his pruritus. Repeat enzymes at 15 days after presentation revealed an improvement in total bilirubin to 23.7 mg/dL. Follow‐up testing every 2 weeks demonstrated progressive improvement in the patient's transaminitis and hyperbilirubinemia, and at 3 months after presentation his aminotransferase and bilirubin levels were within normal limits.
Figure 1.
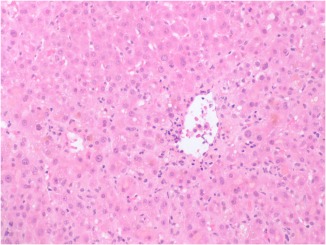
Central vein with lymphocytic infiltration and hepatocellular cholestasis, consistent with cholestatic hepatitis.
Pathophysiology
Hepatic drug transport has been shown to be involved in the pathophysiology of cholestatic effects from drugs.15, 16 In the liver, transport at the apical membrane into hepatocytes involves the organic anion transporting polypeptide, and inhibition of this efflux protein can lead to cholestasis from certain medications or their metabolites.15 The movement of drugs into bile involves canalicular transporters of the multidrug resistance (MDR) protein (MRP) family, which includes glycoproteins MDR1 (ABCB1), MDR3 (ABCB4), MRP2 (ABCC2), and bile salt export pump (BSEP).15 The BSEP has been shown to be a major transporter of bile salts and drug metabolites from hepatocytes into bile.15, 16 Drugs that inhibit export on the canalicular side through inhibition of BSEP can lead to cholestasis in susceptible individuals.15 For instance, patients with mutations in genes that encode BSEP or MDR3 have a 3‐fold increased risk of cholestatic DILI from oral contraceptives, psychotropic drugs, proton pump inhibitors, and certain antibiotics.17 Additional molecular mechanisms involved in drug‐induced cholestasis include destruction of the cytoskeleton, impaired trafficking and disruption of the tight junction network, and inhibition of adenosine triphosphate‐dependent transporters.18
Clinical Approach
The clinical presentation of cholestatic DILI is variable, ranging from asymptomatic elevation in ALP to symptoms of jaundice, pruritus, and fever. Unfortunately, there are no serologic markers that can reliably diagnose DILI, and therefore a thorough history is necessary regarding use of prescription and over‐the‐counter medications as well as vitamin and herbal supplements, along with the timing of when these products were used.
The most common method to assess causality between the liver injury and the suspected offending medication is the Roussel Uclaf assessment model (RUCAM).3, 19 The RUCAM, published in 1990 by the Council for International Organizations of Medical Sciences, is a causality assessment tool that assigns a score to six domains based on chronologic and clinical criteria.20, 21 The final score, ranging from –5 to 14, determines the likelihood of a causal relationship between the suspected offending drug and liver injury. Although the RUCAM offers an objective and standardized assessment of causality regarding DILI, its reliability has been questioned.22 However, RUCAM can be useful in clinical practice as it places focus on the major determinants that the diagnosis of DILI should be based on, such as exact exposure time, development of liver tests after dechallenge, exclusion of competing causes, and documented hepatotoxicity of the implicated agent. The diagnosis of DILI should ultimately be determined based on these parameters as well as clinical judgment.
The differential diagnoses for cholestatic DILI is large, and a discussion regarding the number of conditions with associated cholestatic liver injury is beyond the scope of this article. Signs or symptoms of infection should guide toward a more thorough exclusion of hepatitis that can sometimes show features of cholestasis. Thus, cholestasis can occur with certain viral infections, such as hepatitis A and E,23 Epstein‐Barr virus,24 typhoid fever,25 and acute Q fever.26 In general, drug‐induced cholestasis is a rare phenomenon, with one large case series of over 4,000 patients evaluated for acute or chronic liver disease demonstrating cholestatic DILI was the etiology of liver disease in <1% of patients evaluated.27 Abdominal pain can occur as part of drug‐induced cholestatic hepatitis, although if it is the predominant symptom, then an alternate cause, such as choledocolithiasis and/or ductal obstruction, should be evaluated with an ultrasound or cholangiography.6 In patients with normal hepatobiliary imaging and long‐standing cholestasis, primary biliary cholangitis (PBC) or autoimmune hepatitis with overlap syndrome of PBC should be assessed by appropriate serologic markers. Bland cholestasis can occur in association with sepsis and cardiac failure.28 Cases have also reported cholestasis from secondary sclerosing cholangitis due to large‐vessel vasculitis.29
There is no standard guideline regarding when to perform a liver biopsy, although this can be considered in the patient with progressively worsening liver enzymes and otherwise unremarkable evaluation to rule out diseases, such as anti‐mitochondrial antibody‐negative PBC, small‐duct primary sclerosing cholangitis or autoimmune hepatitis with overlapping features. A liver biopsy can also differentiate between acute and chronic cholestasis, which can ultimately have different prognoses.8
Risk Factors
CHEMICAL PROPERTIES OF DRUGS
The chemical properties of certain drugs may predispose to cholestatic DILI, although notably this association is not specific to cholestatic DILI and may also be associated with hepatocellular injury. The quinolones temafloxacin and trovafloxacin have a difluorinated side chain, making them highly lipophilic and subsequently associated with cholestatic liver disease.21 In a study using data from two pharmaceutical databases in the United States, daily doses of oral medications greater than 50 mg were shown to be significantly associated with severe DILI, of which one third of cases had a cholestatic injury pattern.30 In another analysis of approximately 600 patients from the Spanish Hepatotoxicity Registry, 77% of patients with DILI received medications with daily doses greater than 50 mg, with 50% having a cholestatic or mixed pattern of liver injury.14 These findings suggest that administration of medications in dosages greater than 50 mg a day may increase the risk of DILI, including cholestatic DILI. This risk may be further enhanced with medications that are excreted by the biliary system compared to drugs with minimal biliary excretion (74% versus 40%).31
AGE
Cholestatic pattern of DILI is more common among the elderly, whereas hepatocellular
DILI seems to be more common in younger individuals,14, 32 with one study demonstrating that 61% of DILI cases in patients older than 60 years were of a cholestatic pattern compared to 39% of patients younger than 60.14 A mixed pattern was also significantly more common in older patients than younger patients.14 The reason for this age‐related susceptibility to cholestatic liver injury is unclear but may be related to reduced expression of hepatocellular transporters.33
UNDERLYING LIVER DISEASE
In general, it is uncertain whether preexisting liver disease is a risk factor for the development of DILI. It is notable, though, that ALT and ALP levels trended toward being lower in the setting of DILI among those with underlying hepatitis C virus infection or nonalcoholic fatty liver disease.34 However, certain conditions may increase susceptibility to drug‐induced cholestasis. For instance, in patients with a history of intrahepatic cholestasis of pregnancy, there appears to be greater predisposition toward a cholestatic liver injury from oral contraceptives or postmenopausal hormone replacement.35, 36 Rifampicin seems to be associated with an increased risk for hepatotoxicity in patients with primary biliary cirrhosis.37, 38 There are few data to support the notion that a previous episode of cholestasis predisposes to an increased risk of DILI in the future. Data from the Spanish Hepatotoxicity Registry suggest that multiple episodes of DILI associated with different drugs in the same patient are rare, occurring only in 9 of 742 patients studied (1.2%).21
GENETIC DETERMINANTS
One of the most common medications leading to cholestatic DILI is
amoxicillin/clavulanate (Augmentin).6 The associated human leukocyte antigen (HLA) haplotypes HLA B1*1501‐DRB5*0101‐DQB1*0602,149 have been seen in 57% of patients with amoxicillin/clavulanate‐induced DILI compared to only 12% of patients who do not experience DILI when taking this medication.39 Data from the Spanish Registry, however, did not confirm this association but revealed a significantly higher prevalence of the HLA‐DQR1*06 allele compared to controls.40 Those with cholestatic/mixed DILI from amoxicillin/clavulanate were also found to have a significantly higher frequency of HLA‐DRB1*15 and HLADQB1*06 alleles and a lower frequency of DRB1*07 and DQB1*02 alleles.40
Flucloxacillin is a commonly used antibiotic in the United Kingdom, Australia, Sweden, and some other countries. In a genome‐wide association study of flucloxacillin‐induced DILI, Daly et al.41 reported an association in the major histocompatibility complex region, with the strongest association observed for rs2395029, a marker in complete linkage disequilibrium with HLA‐B*5701. Direct genotyping for HLA‐B*5701 in 51 cases and 63 drug‐exposed controls revealed a strong relationship between this allele and flucloxacillin‐induced liver injury (odds ratio 80.6).41 Increased susceptibility of cholestatic injury due to oral contraceptives has a reported association with the T to C polymorphism in BSEP 1331.42
Clinical Patterns
VANISHING BILE DUCT SYNDROME
Vanishing bile duct syndrome is diagnosed when less than 50% of bile ducts are seen on biopsy. It is a rare syndrome considered to occur in only 0.5% of cases of small duct biliary disease, although it can potentially cause cirrhosis by leading to near complete absence of ducts.43, 44, 45 Even though the pathogenesis of vanishing bile duct syndrome secondary to DILI has not been fully elucidated, it is thought that an immunologic response, predominantly from T cells, leads to recognition of antigen on biliary epithelial cells, resulting in immune cell infiltration into the intraepithelial layer of the bile ducts, apoptosis, and T‐cell cytotoxicity.46
Vanishing bile duct syndrome is seen mainly in patients with prolonged cholestasis for months or years. This was most commonly associated with the drug chlorpromazine,11 although more than 40 medications44, 47 have been implicated in vanishing bile duct syndrome, including amoxicillin,48 carbamazepine,49, 50 meropenem,51 and flucloxacillin.45, 48 In a recent prospective report from the DILI network, 26 of 363 (7%) patients were reported to experience vanishing bile duct syndrome secondary to drug injury, which presented most commonly as cholestatic hepatitis.10 The most commonly implicated agents were amoxicillin/clavulanate, temozolomide, herbal products, and azithromycin. Those who developed bile duct loss were more likely to have chronic liver injury compared to those who did not (94% versus 47%; P < 0.001).10 The clinical course associated with vanishing bile duct syndrome is variable, ranging from reversibility and full liver recovery to prolonged bile duct loss leading to death from cholestatic cirrhosis.50, 52 In a recent study from the DILI network cohort, approximately 19% of liver‐related mortality was observed among patients with bile duct loss, either as the primary or contributing cause of death.7 This mortality rate is numerically greater than the overall 6.2% overall mortality seen in a recently published report of 899 patients with DILI, of which half of the deaths were ultimately liver related, thus underscoring the seriousness of vanishing bile duct syndrome compared to other presentations of DILI.34
SECONDARY SCLEROSING CHOLANGITIS
Cholestatic DILI from development of secondary sclerosing cholangitis has been reported with chemotherapeutic agents,53, 54 and cases have been reported with ketamine and methimazole.55, 56, 57 Secondary sclerosing cholangitis associated with the use of docetaxel was recently reported.58 A recent study of cholangiographies among patients with DILI suggested that up to 10% of DILI cases may have sclerosing cholangitis‐like changes on magnetic resonance cholangio pancreatography.59 Recently presented findings from Ahmad et al.60 from the DILI network cohort identified four cases of drug‐induced secondary sclerosing cholangitis (7%) from a total of 56 patients with biliary imaging. Of these four cases, one was from moxifloxacin, one from atorvastatin, and two from herbal supplements. One of the four case subjects needed liver transplantation.
ACUTE CHOLESTASIS IN DILI
Acute cholestasis from DILI can be categorized into pure or bland cholestasis, and cholestatic hepatitis, which is accompanied by inflammatory cell infiltrate, degenerative changes, and possible hepatocellular necrosis.8 In pure cholestasis, bile plugs are detected in canaliculi and/or hepatocytes, predominantly zone 3 of the liver parenchyma. Inflammation, hepatocytic degeneration, and necrosis are absent, and no bile duct damage is seen.6, 23 This pattern of liver injury is commonly associated with oral contraceptives, anabolic steroids, warfarin, prochlorperazine, and thiabendazole.6 It should be noted that cholestasis from sepsis, cardiac failure, or after surgery can lead to bland cholestasis and should be considered in the differential.28
In cholestatic hepatitis, bile accumulation in the liver is accompanied by inflammation and hepatocellular injury. The presence of eosinophils in the inflammatory infiltrate suggests a better prognosis,61, 62 A number of medications have been implicated in causing cholestatic hepatitis. The differential diagnosis includes but is not limited to acute viral hepatitis, autoimmune hepatitis, and acute large duct obstruction.63 Histologically, cholestatic DILI, whether bland or with inflammation, is predominantly found in zone 3 of the liver.63, 64 A list of agents associated with acute cholestasis from DILI is presented in Table 1.
Table 1.
DRUGS IDENTIFIED AS POTENTIALLY LEADING TO ACUTE CHOLESTATIC LIVER INJURY
| Bland Cholestasis |
| Oral contraceptives |
| Anabolic steroids |
| Warfarin |
| Thiabendazole |
| Procholperazine |
| Cholestatic hepatitis |
| Cholestatic Hepatitis |
| Penicillins |
| Sulfonamides |
| Fluoroquinolones |
| Tetracyclines |
| Antifungals (terbinafine, griseofulvin, ketoconazole, itraconazole) |
| Antiretroviral therapy (stavudine, didanosine, nevirapine) |
| Anti‐inflammatory (diclofenac, sulindac, piroxicam, ibuprofen, phenylbutazone, gold, pencilamine, allopurinol, azathioprine) |
| Psychotropes (chlorpromazine, prochlorperazine, fluphenazine, thiroridazine, tricyclic antidepressants, risperidone, duloxetine, benzodiazepines, diazepam) |
CHRONIC CHOLESTASIS IN DILI
Chronic cholestasis is diagnosed when cholestatic liver injury persists for more than 3 months.63 Medications that have been typically associated with chronic cholestasis are primarily antibiotics and antifungals, chlorpromazine, ibuprofen, and amiodarone; rarely oral contraceptives have been associated with chronic cholestatic DILI45, 65, 66, 67 (Table 2). Unfortunately, persistent cholestasis even without inflammation can lead to long‐term liver damage due to ductal sclerosis, periportal fibrosis, and bile duct loss.6
Table 2.
DRUGS IDENTIFIED AS POTENTIALLY LEADING TO CHRONIC CHOLESTATIC LIVER INJURY
| Vanishing Bile Duct Syndrome |
| Psychotropes (chlorpromazine, imipramine, carbamazepine, amitriptyline, haloperidol, cyproheptadine, phenytoin) |
| Antibiotics (amoxicillin/clavulanate, flucloxacillin, quinolones, clindamycin, macrolides, tetracyclines) |
| Nonsteroidal anti‐inflammatory drugs (diclofenac, ibuprofen) |
| Others (amiodarone, cimetidine, thiabendazole, zonisamide, ajmaline) |
| Secondary Sclerosing Cholangitis |
| Docetaxel |
| Ketamine |
| Methimazole |
| Chemotherapeutic agents |
| Atorvastatin |
| Moxifloxacillin |
| Various herbal supplements |
Drugs Commonly Associated With Cholestatic Liver Injury
Although a comprehensive list of drugs reported to have induced hepatotoxicity has been published68 and can also be found on the website http://www.livertox.nih.gov, we will describe the most common categories of medications leading to cholestatic DILI.
ANTIBIOTICS AND ANTIFUNGALS
In several published reports, antibiotics have been found to be the most common category of drugs that lead to cholestasis.69, 70, 71 Specific examples of antibiotics/antifungals that can lead to cholestatic DILI are reviewed below.
AMOXICILLIN/CLAVULANATE
This particular antibiotic is among the most common antibiotics leading to DILI.32, 69 In a study from the United Kingdom, one third of drug‐induced jaundice was associated with amoxicillin/clavulanate.71 In the Spanish Hepatotoxicity Registry, amoxicillin/clavulanate was the most common cause of DILI, with 59/461 (13%) of all DILIs related to amoxicillin/clavulanate.32 In the United States, amoxicillin/clavulanate was the most common antibiotic associated with DILI.70 Liver injury usually develops early in the course of treatment but also late in the course of prolonged treatment and even after discontinuation of therapy.72 Liver injury is mainly related to the clavulanic acid component as the incidence of DILI with amoxicillin/clavulanate is markedly higher than that of amoxicillin alone.73 Risk factors for hepatotoxicity are age greater than 65 years, female sex, and repeated courses of the antibiotic.71, 72, 73 Most cases are mild, but protracted courses have been seen, and cases can rarely lead to acute liver failure or require transplantation.72, 74, 75
PENICILLINS
Flucloxacillin‐induced cholestasis is well documented.44, 48, 76, 77 Flucloxacillin is commonly prescribed in the United Kingdom, Sweden, and Australia, and is the most common reason for idiosyncratic liver injury in Sweden, accounting for 16% of cases, with 5% of those cases leading to mortality.12, 71 Female sex, and older age have been shown to have greater risk of liver injury caused by flucloxacillin.45, 76 Although often resolving after discontinuation of the drug, more severe presentations include ductopenia48 and cholestatic liver failure.45, 77 Other semisynthetic penicillinase‐resistant penicillins, such as cloxacillin, dicloxacillins, and oxacillins, have been shown to induce cholestatic hepatitis.70, 77
MACROLIDES
Erythromycin may cause a cholestatic liver injury.78 Erythromycin was the second most commonly reported antibiotic to cause DILI in Sweden.12 In a collection of case reports, cholestatic injury was observed in 69% of cases.61 The prognosis is generally favorable, and reports of acute liver failure and death are rare.61, 69 Clarithromycin and azithromycin have also been associated with cholestatic liver injury.79, 80, 81
TRIMETHOPRIM/SULFAMETHOXAZOLE
Trimethoprim/sulfamethoxazole was the fourth most common antibiotic inducing DILI in North America according to a study from the DILI network, after amoxicillin/clavulanate, isoniazid, and nitrofurantoin.69, 70 The sulfonamide component is considered to be responsible for the cholestatic pattern of liver injury.82 Almost 60% of reactions are of a cholestatic nature.61 Approximately 10% of case subjects with cholestatic jaundice caused by trimethoprim/sulfamethoxazole either died or underwent liver transplantation.12
TETRACYCLINES
With low‐dose tetracyclines, such as doxycycline, the incidence seems to be lower compared to other antibiotics leading to DILI.83, 84 Liver injury induced by tetracyclines can be cholestatic, hepatocellular, or mixed pattern, with similar frequencies.83
OTHER ANTI‐INFECTIOUS MEDICATIONS
The antifungal terbinafine can cause a cholestatic injury that can be potentially life‐threatening.85, 86 Terbinafine‐induced hepatotoxicity has been reported in several patients from large series from Sweden and the United States.12, 70 Quinolones that can also lead to cholestatic hepatitis87 include ciprofloxacin86, 88 and levofloxacin, which have in fact been linked to vanishing bile duct syndrome.89
DILI caused by cephalosporins has previously been considered to be extremely rare. However, a recent study from the DILI network in the United States described 33 cases of 1,212 patients enrolled between 2004 and 2012.90 Most patients had a cholestatic liver injury pattern, and in several cases the toxicity occurred after therapy had been stopped, with two fatalities.90
PSYCHOTROPIC DRUGS
Cholestatic‐type injury caused by chlorpromazine is well documented and leads to cholestatic hepatitis, ductopenia, and cholestatic cirrhosis.11 Cholestatic hepatitis has also been reported after tricyclic antidepressants, such as imipramine and amitryptiline,4 and the serotonin re‐uptake inhibitor duloxetine.91
ANTI‐INFLAMMATORY DRUGS
Azathioprine, a commonly used immunomodulator, has been reported to cause potentially fatal cases of cholestatic hepatitis.92, 93, 94 Most patients develop liver injury during the first 3 months of therapy.95 The frequency of azathioprine‐induced DILI ranges from 3% according to data from a retrospective study96 to as high as 10% based on a prospective study.95 Diclofenac, a commonly used nonsteroidal anti‐inflammatory drug, is mostly associated with a hepatocellular pattern of liver injury,95 although cholestatic reactions associated with diclofenac have also been reported.97 Ibuprofen‐induced vanishing bile duct syndrome leading to cirrhosis has been reported in a previously healthy child.98 Nimesulide is almost exclusively hepatocellular.
ORAL CONTRACEPTIVES
The long‐term use of oral contraceptives has been associated with an increased risk of several different presentations of liver injury, including intrahepatic canalicular cholestasis.36, 99 However, cholestatic and hepatocellular liver enzyme elevations are equally frequent from low‐dose estrogen oral contraceptives. The range of time between treatment and the onset of liver injury can range from 3 to 360 days, with a median time of 60 days.100
TREATMENT
There is no medical therapy for the treatment of cholestatic DILI. Instead, treatment involves withdrawal of the suspected drug, avoiding drug rechallenge, and treatment of symptoms.6 Cessation of the offending drug is imperative if the patient develops severe injury as evidenced by jaundice or elevation of serum aminotransferases to greater than 3 times the ULN, known as Hy's law.101
Ursodeoxycholic acid may be prescribed, but the data in cholestatic DILI are largely lacking. The major benefit from ursodeoxycholic acid in cholestatic liver disease in general is protection against cytotoxicity caused by toxic bile salts, stimulation of hepatobiliary secretion, antioxidant activity, enhancement in glutathione levels, and the inhibition of liver cell apoptosis.102 Management of pruritus secondary to severe cholestasis includes the use of cholestyramine, antihistamines, rifampin, phenobarbital, and opioid analogues, which can be used to ameliorate pruritus. Ultraviolet B phototherapy and plasmapharesis are alternative treatments in those who have failed medical therapy.28
Conclusion
Drug‐induced cholestasis can result in acute or chronic liver injury. Most cases of cholestatic DILI appear to resolve shortly after drug withdrawal, but a subset of cases can progress to chronic cholestasis, cirrhosis, and subsequent hepatic decompensation. Early recognition and prompt withdrawal of the offending drug is the mainstay of initial management. This review summarizes the implicated drugs, diagnostic tools, and management of drug‐induced cholestasis. Proper reporting of adverse drug reactions and outcomes are important as new drugs are being developed and approved.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clin Liv Dis 2012;16:199‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghabril M, Chalasani N, Bjornsson E. Drug‐induced liver injury: a clinical update. Curr Opin Gastroenterol 2010;26:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs–II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993;46:1331‐1336. [DOI] [PubMed] [Google Scholar]
- 4. DeLeve LD, Kaplowitz N. Mechanisms of drug‐induced liver disease. Gastroenterol Clin North Am 1995;24:787‐810. [PubMed] [Google Scholar]
- 5. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ; Practice Parameters Committee of the American College of Gastroenterology . ACG clinical guideline: the diagnosis and management of idiosyncratic drug‐induced liver injury. Am J Gastroenterol 2014;109:950‐966. [DOI] [PubMed] [Google Scholar]
- 6. Bjornsson ES, Jonasson JG. Drug‐induced cholestasis. Clin Liver Dis 2013;17:191‐209. [DOI] [PubMed] [Google Scholar]
- 7. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology 2002;36:451‐455. [DOI] [PubMed] [Google Scholar]
- 8. Kleiner DE. The pathology of drug‐induced liver injury. Semin Liver Dis 2009;29:364‐372. [DOI] [PubMed] [Google Scholar]
- 9. Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al.; Drug‐Induced Liver Injury Network (DILIN) . Hepatic histological findings in suspected drug‐induced liver injury: systematic evaluation and clinical associations. Hepatology 2014;59:661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al.; U.S. Drug Induced Liver Injury Network Investigators . Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017;65:1267‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moradpour D, Altorfer J, Flury R, Greminger P, Meyenberger C, Jost R, et al. Chlorpromazine‐induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology 1994;20:1437‐1441. [DOI] [PubMed] [Google Scholar]
- 12. Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug‐induced liver disease. Hepatology 2005;42:481‐489. [DOI] [PubMed] [Google Scholar]
- 13. Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al.; DILIN Investigators . Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol 2015;110:1450‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucena MI, Andrade RJ, Kaplowitz N, Garcia‐Cortes M, Fernandez MC, Romero‐Gomez M, et al.; Spanish Group for the Study of Drug‐Induced Liver Disease . Phenotypic characterization of idiosyncratic drug‐induced liver injury: the influence of age and sex. Hepatology 2009;49:2001‐2009. [DOI] [PubMed] [Google Scholar]
- 15. Pauli‐Magnus C, Meier PJ. Hepatobiliary transporters and drug‐induced cholestasis. Hepatology 2006;44:778‐787. [DOI] [PubMed] [Google Scholar]
- 16. Pauli‐Magnus C, Stieger B, Meier Y, Kullak‐Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol 2005;43:342‐357. [DOI] [PubMed] [Google Scholar]
- 17. Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug‐induced liver injury. Pharmacogenet Genomics 2007;17:47‐60. [DOI] [PubMed] [Google Scholar]
- 18. Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med 1998;339:1217‐1227. [DOI] [PubMed] [Google Scholar]
- 19. Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol 1993;46:1323‐1330. [DOI] [PubMed] [Google Scholar]
- 20. Hayashi PH. Causality assessment in drug‐induced liver injury. Semin Liver Dis 2009;29:348‐356. [DOI] [PubMed] [Google Scholar]
- 21. Lucena MI, Kaplowitz N, Hallal H, Castiella A, Garcia‐Bengoechea M, Otazua P, et al. Recurrent drug‐induced liver injury (DILI) with different drugs in the Spanish Registry: the dilemma of the relationship to autoimmune hepatitis. J Hepatol 2011;55:820‐827. [DOI] [PubMed] [Google Scholar]
- 22. Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, et al.; Drug‐Induced Liver Injury Network (DILIN) . Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug‐induced liver injury. Hepatology 2008;48:1175‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhamidimarri KR, Schiff E. Drug‐induced cholestasis. Clin Liver Dis 2013;17:519‐531. [DOI] [PubMed] [Google Scholar]
- 24. Maggio MC, Liotta A, Cardella F, Corsello G. Stevens‐Johnson syndrome and cholestatic hepatitis induced by acute Epstein‐Barr virus infection. Eur J Gastroenterol Hepatol 2011;23:289. [DOI] [PubMed] [Google Scholar]
- 25. Ratnayake EC, Shivanthan C, Wijesiriwardena BC. Cholestatic hepatitis in a patient with typhoid fever ‐ a case report. Ann Clin Microbiol Antimicrob 2011;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi HC, Lee SH, Kim J, Kim SH, Hwang JH, Kim JW, et al. A case of acute q Fever with severe acute cholestatic hepatitis. Gut Liver 2009;3:141‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug‐induced hepatitis in a United States tertiary referral center [corrected]. J Clin Gastroenterol 2005;39:64‐67. Erratum in: J Clin Gastroenterol 2005;39:176. [PubMed] [Google Scholar]
- 28. Nguyen KD, Sundaram V, Ayoub WS. Atypical causes of cholestasis. World J Gastroenterol 2014;20:9418‐9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Bjornsson ES, Sundaram V. Severe cholestatic hepatitis due to large vessel vasculitis: report of two cases. Gastroenterol Rep (Oxf) 2015;pii:gov061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug‐induced liver injury: search for signals. Hepatology 2008;47:2003‐2009. [DOI] [PubMed] [Google Scholar]
- 31. Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology 2010;51:615‐620. [DOI] [PubMed] [Google Scholar]
- 32. Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia‐Ruiz E, et al.; Spanish Group for the Study of Drug‐Induced Liver Disease . Drug‐induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10‐year period. Gastroenterology 2005;129:512‐521. [DOI] [PubMed] [Google Scholar]
- 33. Meier Y, Pauli‐Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, et al. Interindividual variability of canalicular ATP‐binding‐cassette (ABC)‐transporter expression in human liver. Hepatology 2006;44:62‐74. [DOI] [PubMed] [Google Scholar]
- 34. Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al.; United States Drug Induced Liver Injury Network . Features and outcomes of 899 patients with drug‐induced liver injury: the DILIN Prospective Study. Gastroenterology 2015;148:1340‐1352.e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leevy CB, Koneru B, Klein KM. Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease. Gastroenterology 1997;113:966‐972. [DOI] [PubMed] [Google Scholar]
- 36. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med 1992;7:199‐209. [DOI] [PubMed] [Google Scholar]
- 37. Bachs L, Pares A, Elena M, Piera C, Rodes J. Effects of long‐term rifampicin administration in primary biliary cirrhosis. Gastroenterology 1992;102:2077‐2080. [DOI] [PubMed] [Google Scholar]
- 38. Prince MI, Burt AD, Jones DE. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut 2002;50:436‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, et al. HLA association of amoxicillin‐clavulanate–induced hepatitis. Gastroenterology 1999;117:1181‐1186. [DOI] [PubMed] [Google Scholar]
- 40. Andrade RJ, Lucena MI, Alonso A, Garcia‐Cortes M, Garcia‐Ruiz E, Benitez R, et al. HLA class II genotype influences the type of liver injury in drug‐induced idiosyncratic liver disease. Hepatology 2004;39:1603‐1612. [DOI] [PubMed] [Google Scholar]
- 41. Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al.; DILIGEN Study; International SAE Consortium . HLA‐B*5701 genotype is a major determinant of drug‐induced liver injury due to flucloxacillin. Nat Genet 2009;41:816‐819. [DOI] [PubMed] [Google Scholar]
- 42. Meier Y, Zodan T, Lang C, Zimmermann R, Kullak‐Ublick GA, Meier PJ, et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive‐induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol 2008;14:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Degott C, Feldmann G, Larrey D, Durand‐Schneider AM, Grange D, Machayekhi JP, et al. Drug‐induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology 1992;15:244‐251. [DOI] [PubMed] [Google Scholar]
- 44. Desmet VJ. Vanishing bile duct syndrome in drug‐induced liver disease. J Hepatol 1997;26(Suppl. 1):31‐35. [DOI] [PubMed] [Google Scholar]
- 45. Olsson R, Wiholm BE, Sand C, Zettergren L, Hultcrantz R, Myrhed M. Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J Hepatol 1992;15:154‐161. [DOI] [PubMed] [Google Scholar]
- 46. Geubel AP, Sempoux CL. Drug and toxin‐induced bile duct disorders. J Gastroenterol Hepatol 2000;15:1232‐1238. [PubMed] [Google Scholar]
- 47. United States National Library of Medicine; National Insitute of Diabetes and Digestive and Kidney Diseases . LiverTox. http://livertox.nlm.nih.gov. Accessed March 2017.
- 48. Davies MH, Harrison RF, Elias E, Hubscher SG. Antibiotic‐associated acute vanishing bile duct syndrome: a pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatol 1994;20:112‐116. [DOI] [PubMed] [Google Scholar]
- 49. Forbes GM, Jeffrey GP, Shilkin KB, Reed WD. Carbamazepine hepatotoxicity: another cause of the vanishing bile duct syndrome. Gastroenterology 1992;102:1385‐1388. [PubMed] [Google Scholar]
- 50. Ramos AM, Gayotto LC, Clemente CM, Mello ES, Luz KG, Freitas ML. Reversible vanishing bile duct syndrome induced by carbamazepine. Eur J Gastroenterol Hepatol 2002;14:1019‐1022. [DOI] [PubMed] [Google Scholar]
- 51. Schumaker AL, Okulicz JF. Meropenem‐induced vanishing bile duct syndrome. Pharmacotherapy 2010;30:953. [DOI] [PubMed] [Google Scholar]
- 52. Vuppalanchi R, Chalasani N, Saxena R. Restoration of bile ducts in drug‐induced vanishing bile duct syndrome due to zonisamide. Am J Surg Pathol 2006;30:1619‐1623. [DOI] [PubMed] [Google Scholar]
- 53. Phongkitkarun S, Kobayashi S, Varavithya V, Huang X, Curley SA, Charnsangavej C. Bile duct complications of hepatic arterial infusion chemotherapy evaluated by helical CT. Clin Radiol 2005;60:700‐709. [DOI] [PubMed] [Google Scholar]
- 54. Sandrasegaran K, Alazmi WM, Tann M, Fogel EL, McHenry L, Lehman GA. Chemotherapy‐induced sclerosing cholangitis. Clin Radiol 2006;61:670‐678. [DOI] [PubMed] [Google Scholar]
- 55. Schwab GP, Wetscher GJ, Vogl W, Redmond E. Methimazole‐induced cholestatic liver injury, mimicking sclerosing cholangitis. Langenbecks Arch Chir 1996;381:225‐227. [DOI] [PubMed] [Google Scholar]
- 56. Seto WK, Ng M, Chan P, Ng IO, Cheung SC, Hung IF, et al. Ketamine‐induced cholangiopathy: a case report. Am J Gastroenterol 2011;106:1004‐1005. [DOI] [PubMed] [Google Scholar]
- 57. Turkish A, Luo JJ, Lefkowitch JH. Ketamine abuse, biliary tract disease, and secondary sclerosing cholangitis. Hepatology 2013;58:825‐827. [DOI] [PubMed] [Google Scholar]
- 58. Horsley‐Silva JL, Dow EN, Menias CO, Smith ML, Carballido EM, Lindor KD, et al. Docetaxel induced sclerosing cholangitis. Dig Dis Sci 2015;60:3814‐3816. [DOI] [PubMed] [Google Scholar]
- 59. Gudnason HO, Bjornsson HK, Gardarsdottir M, Thorisson HM, Olafsson S, Bergmann OM, Bjornsson ES. Secondary sclerosing cholangitis in patients with drug‐induced liver injury. Dig Liv Dis 2015;47:502‐507. [DOI] [PubMed] [Google Scholar]
- 60. Ahmad J, Rossi S, Rodgers SK, Fontana RJ, Chalasani NP, Stolz A, et al. Drug induced liver injury associated with sclerosing cholangitis like changes on magnetic resonance cholangiography imaging. [Abstract] Hepatology 2016;64:65A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bjornsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug‐induced liver injury. Aliment Pharmacol Ther 2007;25:1411‐1421. [DOI] [PubMed] [Google Scholar]
- 62. Devarbhavi H, Karanth D, Prasanna KS, Adarsh CK, Patil M. Drug‐Induced liver injury with hypersensitivity features has a better outcome: a single‐center experience of 39 children and adolescents. Hepatology 2011;54:1344‐1350. [DOI] [PubMed] [Google Scholar]
- 63. Ramachandran R, Kakar S. Histological patterns in drug‐induced liver disease. J Clin Pathol 2009;62:481‐492. [DOI] [PubMed] [Google Scholar]
- 64. Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 2003;83:633‐671. [DOI] [PubMed] [Google Scholar]
- 65. Chang CC, Petrelli M, Tomashefski JF Jr, McCullough AJ. Severe intrahepatic cholestasis caused by amiodarone toxicity after withdrawal of the drug: a case report and review of the literature. Arch Pathol Lab Med 1999;123:251‐256. [DOI] [PubMed] [Google Scholar]
- 66. Friis H, Andreasen PB. Drug‐induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med 1992;232:133‐138. [DOI] [PubMed] [Google Scholar]
- 67. Larrey D, Vial T, Micaleff A, Babany G, Morichau‐Beauchant M, Michel H, et al. Hepatitis associated with amoxycillin‐clavulanic acid combination report of 15 cases. Gut 1992;33:368‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bjornsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology 2016;63:590‐603. [DOI] [PubMed] [Google Scholar]
- 69. Bjornsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug‐induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005;40:1095‐1101. [DOI] [PubMed] [Google Scholar]
- 70. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al.; Drug Induced Liver Injury Network (DILIN) . Causes, clinical features, and outcomes from a prospective study of drug‐induced liver injury in the United States. Gastroenterology 2008;135:1924‐1934, 1934 e1921‐e1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hussaini SH, O'Brien CS, Despott EJ, Dalton HR. Antibiotic therapy: a major cause of drug‐induced jaundice in southwest England. Eur J Gastroenterol Hepatol 2007;19:15‐20. [DOI] [PubMed] [Google Scholar]
- 72. Lucena MI, Andrade RJ, Fernandez MC, Pachkoria K, Pelaez G, Duran JA, et al.; Spanish Group for the Study of Drug‐Induced Liver Disease (Grupo de Estudio para las Hepatopatías Asociadas a Medicamentos (GEHAM)) . Determinants of the clinical expression of amoxicillin‐clavulanate hepatotoxicity: a prospective series from Spain. Hepatology 2006;44:850‐856. [DOI] [PubMed] [Google Scholar]
- 73. Garcia Rodriguez LA, Stricker BH, Zimmerman HJ. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327‐1332. [DOI] [PubMed] [Google Scholar]
- 74. deLemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, et al.; Drug‐Induced Liver Injury Network (DILIN) . Amoxicillin‐clavulanate‐induced liver injury. Dig Dis Sci 2016;61:2406‐2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005;50:1785‐1790. [DOI] [PubMed] [Google Scholar]
- 76. Fairley CK, McNeil JJ, Desmond P, Smallwood R, Young H, Forbes A, et al. Risk factors for development of flucloxacillin associated jaundice. BMJ 1993;306:233‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Turner IB, Eckstein RP, Riley JW, Lunzer MR. Prolonged hepatic cholestasis after flucloxacillin therapy. Med J Aust 1989;151:701‐705. [DOI] [PubMed] [Google Scholar]
- 78. Polson JE. Hepatotoxicity due to antibiotics. Clin Liver Dis 2007;11:549‐561 [DOI] [PubMed] [Google Scholar]
- 79. Brown BA, Wallace RJ Jr, Griffith DE, Girard W. Clarithromycin‐induced hepatotoxicity. Clin Infect Dis 1995;20:1073‐1074. [DOI] [PubMed] [Google Scholar]
- 80. Lockwood AM, Cole S, Rabinovich M. Azithromycin‐induced liver injury. Am J Health Syst Pharm. 2010;67:810‐814. [DOI] [PubMed] [Google Scholar]
- 81. Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, et al. Clinical and histologic features of azithromycin‐induced liver injury. Clin Gastroenterol Hepatol 2015;13:369‐376. e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mainra RR, Card SE. Trimethoprim‐sulfamethoxazole‐associated hepatotoxicity ‐ part of a hypersensitivity syndrome. Can J Clin Pharmacol 2003;10:175‐178. [PubMed] [Google Scholar]
- 83. Bjornsson E, Lindberg J, Olsson R. Liver reactions to oral low‐dose tetracyclines. Scand J Gastroenterol 1997;32:390‐395. [DOI] [PubMed] [Google Scholar]
- 84. Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case‐control study. J Clin Pharm Ther 2007;32:483‐487. [DOI] [PubMed] [Google Scholar]
- 85. Agarwal K, Manas DM, Hudson M. Terbinafine and fulminant hepatic failure. N Engl J Med 1999;340:1292‐1293. [DOI] [PubMed] [Google Scholar]
- 86. Hautekeete ML, Kockx MM, Naegels S, Holvoet JK, Hubens H, Kloppel G. Cholestatic hepatitis related to quinolones: a report of two cases. J Hepatol 1995;23:759‐760. [DOI] [PubMed] [Google Scholar]
- 87. Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, et al.; DILIN Research Group . Clinical and histopathologic features of fluoroquinolone‐induced liver injury. Clin Gastroenterol Hepatol 2011;9:517‐523.e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bataille L, Rahier J, Geubel A. Delayed and prolonged cholestatic hepatitis with ductopenia after long‐term ciprofloxacin therapy for Crohn's disease. J Hepatol 2002;37:696‐699. [DOI] [PubMed] [Google Scholar]
- 89. Levine C, Trivedi A, Thung SN, Perumalswami PV. Severe ductopenia and cholestasis from levofloxacin drug‐induced liver injury: a case report and review. Semin Liver Dis 2014;34:246‐251. [DOI] [PubMed] [Google Scholar]
- 90. Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC; Drug‐Induced Liver Injury Network (DILIN) Study Investigators . Identification and characterization of cefazolin‐induced liver injury. Clin Gastroenterol Hepatol 2015;13:1328‐1336.e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vuppalanchi R, Hayashi PH, Chalasani N, Fontana RJ, Bonkovsky H, Saxena R, et al.; Drug‐Induced Liver Injury Network (DILIN) . Duloxetine hepatotoxicity: a case‐series from the drug‐induced liver injury network. Aliment Pharmacol Ther 2010;32:1174‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bjornsson ES, Gu J, Kleiner DE, Chalasani N, Hayashi PH, Hoofnagle JH; DILIN Investigators . Azathioprine and 6‐mercaptopurine‐induced liver injury: clinical features and outcomes. J Clin Gastroenterol 2017;51:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roda G, Caponi A, Belluzzi A, Roda E. Severe cholestatic acute hepatitis following azathioprine therapy in a patient with ulcerative pancolitis. Dig Liver Dis 2009;41:914‐915. [DOI] [PubMed] [Google Scholar]
- 94. Romagnuolo J, Sadowski DC, Lalor E, Jewell L, Thomson AB. Cholestatic hepatocellular injury with azathioprine: a case report and review of the mechanisms of hepatotoxicity. Can J Gastroenterol 1998;12:479‐483. [DOI] [PubMed] [Google Scholar]
- 95. Bastida G, Nos P, Aguas M, Beltran B, Rubin A, Dasi F, et al. Incidence, risk factors and clinical course of thiopurine‐induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2005;22:775‐782. [DOI] [PubMed] [Google Scholar]
- 96. Gisbert JP, Gonzalez‐Lama Y, Mate J. Thiopurine‐induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol 2007;102:1518‐1527. [DOI] [PubMed] [Google Scholar]
- 97. Watanabe N, Takashimizu S, Kojima S, Kagawa T, Nishizaki Y, Mine T, et al. Clinical and pathological features of a prolonged type of acute intrahepatic cholestasis. Hepatol Res 2007;37:598‐607. [DOI] [PubMed] [Google Scholar]
- 98. Srivastava M, Perez‐Atayde A, Jonas MM. Drug‐associated acute‐onset vanishing bile duct and Stevens‐Johnson syndromes in a child. Gastroenterology 1998;115:743‐746. [DOI] [PubMed] [Google Scholar]
- 99. Dourakis SP, Tolis G. Sex hormonal preparations and the liver. Eur J Contracept Reprod Health Care 1998;3:7‐16. [DOI] [PubMed] [Google Scholar]
- 100. Lindgren A, Olsson R. Liver damage from low‐dose oral contraceptives. J Intern Med 1993;234:287‐292. [DOI] [PubMed] [Google Scholar]
- 101. Zimmerman H. Hepatotoxicity. The Adverse Effects of Drugs and Other Chemicals on the Liver. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 102. Perez MJ, Briz O. Bile‐acid‐induced cell injury and protection. World J Gastroenterol 2009;15:1677‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]


