Abstract
Objective
Catatonia, a condition characterized by motor, behavioral and emotional changes, can occur during critical illness and appear as clinically similar to delirium, yet its management differs from delirium. Traditional criteria for medical catatonia preclude it’s diagnosis in delirium. Our objective in this investigation was to understand the overlap and relationship between delirium and catatonia in ICU patients and determine diagnostic thresholds for catatonia.
Setting
Convenience cohort, nested within 2 ongoing randomized trials at a single institution.
Patients
We enrolled 136 critically ill patients on mechanical ventilation and/or vasopressors, randomized to two usual care sedation regimens.
Measurements and Main Results
Patients were assessed for delirium and catatonia by independent and masked personnel using Confusion Assessment Method for the ICU (CAM-ICU) and the Bush Francis Catatonia Rating Scale (BFCRS) mapped to DSM-5 criterion A for catatonia. Of 136 patients, 58 (43%) patients had only delirium, 4 (3%) had only catatonia, 42 (31%) had both and 32 (24%) had neither. In a logistic regression model, more catatonia signs were associated with greater odds of having delirium. For example, patient assessments with ≥3 DSM-5 symptoms (75th percentile) had, on average, 27.8 times the odds (IQR 12.7, 60.6) of having delirium compared with patient assessments with 0 DSM-5 criteria (25th percentile) present (p< 0.001). A cut-off of ≥4 Bush Francis Catatonia Screening Instrument (BFCSI) items was both sensitive (91%) 95% CI (82.9 – 95.3) and specific (91%) 95% CI (87.6 – 92.9) for DSM-5 catatonia.
Conclusions
Given that about 1 in 3 patients had both catatonia and delirium, these data prompt reconsideration of DSM-5 criteria for “Catatonic Disorder Due to Another Medical Condition” that preclude diagnosing catatonia in the presence of delirium.
Keywords: catatonia, delirium, acute brain dysfunction, mechanical ventilation, DSM-5, CAM-ICU
Introduction
This clinical vignette represents an unusual twist on an ICU patient in whom the vast majority of the time, it would be the management choice to avoid benzodiazepines. However, in this patient who met the criteria for delirium, there was a co-occurrence of an overwhelmingly positive set of criteria for catatonia. This patient’s story represents an excellent example of what sparked us to undertake the current “Delirium and Catatonia in Critically Ill Patients” (DeCat) investigation.
Catatonia, a potentially lethal phenomenon characterized by its prominent motor (e.g., hypo- or hyper-activity), behavioral and affective abnormalities was previously thought to represent a subtype of schizophrenia. Catatonia has been described as occurring in mood disorders and medical illnesses, including critical illness (1–4). Delirium, a form of acute brain organ dysfunction manifested by inattention and changes in cognition, is a known predictor of excess mortality, length of stay, cost of care, and long-term cognitive impairment (5–12). While delirium is recommended as a part of the standard organ dysfunction monitoring for all intensive care unit (ICU) patients (13), catatonia is not routinely screened for in the ICU, although it has been recommended in some circumstances in the pediatric population (14).
Our understanding of catatonia in the ICU is being hampered by a diagnostic dilemma. The last 3 editions of the Diagnostic Statistical Manual (DSM), including the current DSM-5, hold that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium (15–17) but others doubt this (18, 19). The DSM-5 does allow the co-occurrence of delirium and catatonia with the use of the diagnosis “Catatonia Associated with Another Mental Disorder (Catatonia Specifier)” and with the “Unspecified Catatonia” diagnosis category, however specifically precludes this co-occurrence in the context of medical illness. Criterion D for “Catatonic Disorder Due to Another Medical Condition” (medical catatonia) disallows this diagnosis exclusively in the presence of delirium. Because of this, there is a virtual absence of data concerning the prevalence of catatonic signs in delirious patients and many believe that catatonia is under-recognized in the medically ill (1, 2, 20, 21). Some have even suggested that despite the DSM exclusionary criteria, delirium can co-exist with catatonic features in medical illness (22).
Management options for ICU patients are drastically different for patients with catatonia versus delirium. The treatment of catatonia generally includes avoidance of antipsychotics (due to the potential to worsen catatonia or precipitate a lethal form of catatonia similar to neuroleptic malignant syndrome) and treatment with benzodiazepines (typically lorazepam) and/or electroconvulsive therapy (ECT). Delirium, alternatively, is approached via treatment of the patient’s underlying diseases and environmental factors, along with avoidance of benzodiazepines and other psychoactive medications and then often with use of antipsychotics (23–25).
The objective of this investigation was to describe the relationship between delirium and catatonia in ICU patients and to determine diagnostic thresholds for catatonia in this population.
Materials and Methods
The DeCat (Delirium and Catatonia in Critically Ill Patients) investigation is a single center convenience sample, prospective observational cohort study nested within two ongoing blinded randomized NIH sponsored clinical trials. The parent trials are comprised of critically ill patients on mechanical ventilation and/or vasopressors, randomized to two usual care sedation regimens. The outcomes assessed in this cohort investigation were completely original and distinct from the parent study outcomes. For this DeCat investigation, data were collected between January 2014 and December 2015. The institutional review board (IRB) at Vanderbilt University Medical Center, Nashville, Tennessee approved this study. Informed consent was obtained from the patient or surrogate decision-maker.
Catatonia Assessment with Bush Francis Catatonia Rating Scale
Two psychiatrists (JW and RC) measured catatonia signs daily in the ICU and general medical ward from the date of enrollment until the point of censoring and remained blinded to the patient’s CAM-ICU status. Catatonia assessors were instructed to perform a targeted exam of catatonia and to not ascertain delirium status through use of the CAM-ICU or DSM-5, etc. Catatonia assessments were performed using the Bush Francis Catatonia Rating Scale (BFCRS), a 23 item rating scale obtained through observation, physical evaluation and interview (26–28). BFCRS ratings were not provided to the clinical treatment teams as no clear guidelines exist on how to manage comorbid delirium and catatonia. Additional information regarding the BFCRS and the Bush Francis Catatonia Screening Instrument (BFCSI), the first 14 item screen can be found in the supplemental materials.
DSM-5 Criteria for Catatonia
We sought to define catatonia by DSM-5 criterion A of the “Catatonic Disorder Due to Another Medical Condition” diagnosis. To meet a case definition of catatonia, according to the DSM-5 criterion A, ≥3 items need to be present (16). By applying a previously used algorithm to the BFCRS items we prospectively obtained we were able to approximate a DSM-5 score (29). The BFCRS score was not used to assign a case definition of catatonia, it was only used to screen for specific catatonia signs. More information about the BFCRS to DSM-5 algorithm can be found in the supplemental materials online. We ignored DSM-5 criterion D (cannot diagnose catatonia exclusively in the presence of delirium) as this was one of the primary aims of this investigation.
Delirium and Coma Assessments
We measured delirium twice daily in the ICU and daily thereafter until hospital discharge (or for up to 16 days) using the Confusion Assessment Method for the ICU. All delirium assessments were performed by trained study personnel who were masked to the catatonia assessment. Prior to assessment with the CAM-ICU, the Richmond Agitation and Sedation Scale (RASS) was performed to assess the patient’s level of arousal (30, 31). If a patient scored a -4 (deep sedation) or -5 (unarousable), the patient was considered to be comatose and the CAM-ICU was not performed and marked as ‘unable to assess’ (UTA). Measurement of delirium status was undertaken regardless of sedatives or analgesics, as the main measure for ascertaining whether delirium could be assessed, was the patient’s level of arousal via the RASS.
Study Outcomes
We were interested in assessing the proportion of assessments where patients met screening criteria for both delirium and catatonia (according to DSM-5 criterion A) in matched delirium and catatonia assessments. We were also interested in describing if severity of catatonia (as measured by the number of DSM-5 criterion A items present) was associated with greater odds of being delirious. Additionally, we aimed to describe the sensitivity and specificity of various cut-off points of the BFCSI in comparison to the DSM-5 criterion A for catatonia, in the setting of critical illness.
Statistical Analysis
Descriptive statistics were performed as median with interquartile range for continuous variables and frequency for categorical variables. We determined the percentage of critically ill patient assessments that concomitantly met screening criteria for both delirium and catatonia based on the CAM-ICU and the DSM-5 criterion A for catatonia. For these analyses, the unit of analysis was the patient’s matched delirium and catatonia assessments. We calculated the hours in between the delirium and catatonia assessments, and the distribution of CAM-ICU scores (unable to assess, negative or positive). We additionally assessed the distribution of patients and patient assessments in 4 groups of interest: delirious only, delirious and catatonic, catatonic only, neither.
To determine if critically ill patient assessments in the highest quartile of catatonia rating scale scores had greater odds of delirium than those assessments in the lowest quartile of catatonia rating scale scores, we described the frequency of individual DSM-5 signs, stratified by delirium status (CAM-ICU+/−). Additionally, we modeled this using a logistic regression model with delirium as the outcome and the number of DSM-5 catatonia signs present (0–8) as a continuous exposure. The highest number of DSM-5 catatonia items present was 8. Because each patient had multiple assessments, we used a cluster sandwich covariance estimator with the patient ID as a cluster in order to adjust the variance in our model to account for these repeated measures. We allowed the number of catatonia symptoms present to have a nonlinear relationship with delirium status using restricted cubic splines. To estimate the sensitivity and specificity of the BFCSI at various cut off points we fit logistic regression models comparing different BFCSI cut off values to DSM-5 criteria. The 95% confidence intervals were calculated using Wilson’s score method (32). All analyses were performed using statistical software R version 3.3.0.
Results
Patient Population
We enrolled 136 critically ill patients between January 2014 and December 2015. Median age was 59 years (IQR 52, 68). Males made up 62% of our cohort, 88% were White/Caucasian and 43% were admitted with sepsis (Table 1). Patients were not significantly cognitively impaired at baseline, with a median IQCODE of 3 (IQR 3, 3.27). Median Charlson comorbidity score at enrollment was 2 (IQR 1, 4) and median SOFA (severity of illness) score was 4.5 (3.3, 6.2). Mortality at 30 and 90 days was 29% and 35%, respectively. Median ICU length of stay was 6.5 days (IQR 4, 11.2) and median hospital length of stay was 12 days (6.2, 17.8).
Table 1.
Demographic and Baseline Characteristics*.
| Variable | Patients N = 136 |
|---|---|
|
| |
| Age at enrollment | 59 (52, 68) |
|
| |
| Gender | |
| Male | 62% |
| Female | 38% |
|
| |
| Race | |
| Multiracial heritage | 3% |
| White/Caucasian | 88% |
| Black/African American or Negro | 8% |
| Other | 1% |
|
| |
| Education | |
| Less than high school | 14% |
| High school or GED | 38% |
| Some college | 27% |
| Associates degree | 6% |
| Bachelors degree | 8% |
| Masters degree | 5% |
| Terminal degree | 3% |
|
| |
| Insurance | |
| None | 6% |
| Government/VHA | 5% |
| Medicaid only | 5% |
| Medicare + Medicaid | 12% |
| Medicare + private | 28% |
| Medicare only | 10% |
| Private only | 33% |
|
| |
| IQCODE at enrollment ǂ | 3 (3, 3.27) |
|
| |
| Mean SOFA score at enrollment† | 3.3 (4.5, 6.2) |
|
| |
| Mean Charlson score at enrollment‡ | 2 (1, 4) |
|
| |
| Primary reason for ICU admission¶ | |
| Sepsis/septic shock | 43% |
| ARDS, with or without infection | 13% |
| Airway protection/upper airway obstruction | 9% |
| COPD/asthma | 4% |
| Other pulmonary (inc. pneumonia, PE/DVT) | 11% |
| Acute MI/cardiogenic shock | 3% |
| Arrhythmia | 1% |
| GI bleed | 1% |
| Renal failure | 1% |
| Metabolic/endocrine/electrolyte | 1% |
| Malignancy | 1% |
| Seizures | 1% |
| Surgery, any type | 11% |
Median values (IQR, Interquartile Range)
The Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) ranges from 1 to 5, with a score of 3 indicating no change in cognition over the past 10 years, a score lower than 3 indicating improvement, and a score higher than 3 indicating decline in cognition, as compared with 10 years before. A score of 3.6 or higher indicates preexisting cognitive impairment.
No patients had congestive heart failure or cardiomyopathy, drug overdose or withdrawal, hemorrhagic shock, other infectious disease, cirrhosis/hepatic failure, pancreatitis, or neurologic disease as the primary reason for ICU admission.
Scores on the Sequential Organ Failure Assessment (SOFA) range from 0 to 24 (from 0 to 4 for each of six organ systems), with higher scores indicating more severe organ dysfunction. We used a modified SOFA score, which excluded the Glasgow Coma Scale components, since coma was included separately in our models.
Scores on the Charlson comorbidity index range from 0 to 33, with higher scores indicating a greater burden of illness; a score of 1 or 2 is associated with mortality of approximately 25% at 10 years.
Epidemiology of Catatonia and Delirium
Of the 136 patients included for study, 100 (74%) were delirious at one point during the investigation. Throughout their hospital stay, 58 (43%) of patients had delirium at any time without catatonia, 42 (31%) had both delirium and catatonia at some point during their study enrollment, 4 (3%) had catatonia alone (using DSM-5 criterion A), and 32 (24%) had neither delirium nor catatonia (Figure 1).
Figure 1. Distribution of Delirium and/or Catatonia.
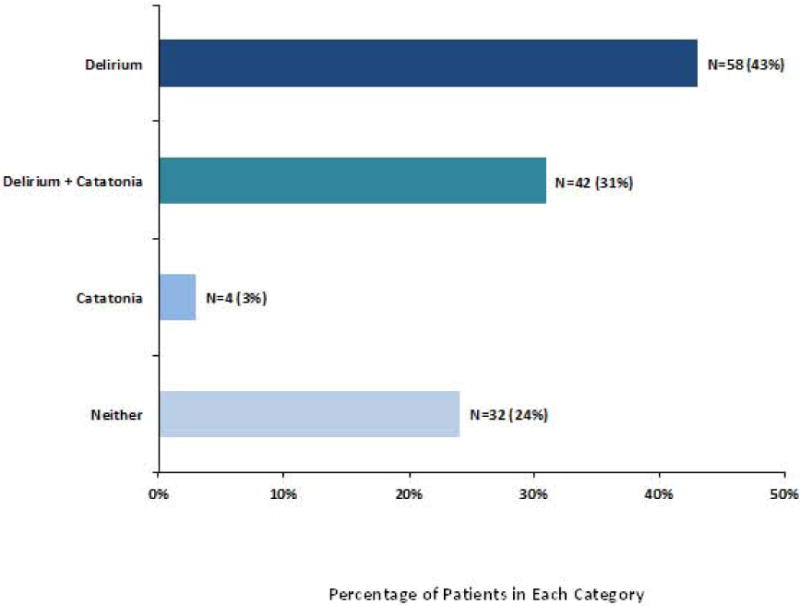
Bar Graph showing distribution of delirium and/or catatonia over the entire ICU stay by individual patients (N=136 patients). This figure demonstrates that 31% of patients met criteria for both delirium and catatonia during their ICU or hospital stay, which goes against current DSM catatonia nosology precluding diagnosis of catatonia due to another medical condition when delirium is present. †Delirium diagnosed using CAM-ICU: Confusion Assessment Method for the ICU. Catatonia diagnosed using ≥ 3 DSM-5 Criterion A items.
† Delirium was diagnosed with the CAM-ICU: Confusion Assessment Method for the ICU.
* Catatonia was defined according to DSM 5 Criterion A.
Predicting CAM-ICU status by catatonia severity
Due to persistent coma (RASS -4 or -5), 8 patients (6%) were unable to be assessed. The median number of matched delirium and catatonia assessments per patient was 3 (IQR 2, 5), with a median of 2.2 hours (IQR 1.2, 3.0) between delirium and catatonia assessments. Among delirious patient assessments (CAM-ICU+), 29% met diagnostic criteria for catatonia according to DSM-5 criterion A (please refer to supplemental material). In our cohort, 49% of patient assessments meeting DSM-5 criterion A for catatonia (≥3 of 12 signs), concomitantly met criteria for delirium. Median number catatonia signs according to the BFCRS (when score >0) were 5 (4, 7) in patient assessments with delirium and 2 (1, 4) in patient assessments without delirium, suggesting that more severe catatonia may be associated with delirium. The most frequently occurring catatonic signs in our delirious patients were autonomic abnormalities (96%), immobility/stupor (87%), staring (77%), mutism (60%) and posturing (60%) (Figure 2).
Figure 2. Frequency and Distribution of Catatonic Signs According to Delirium Status.
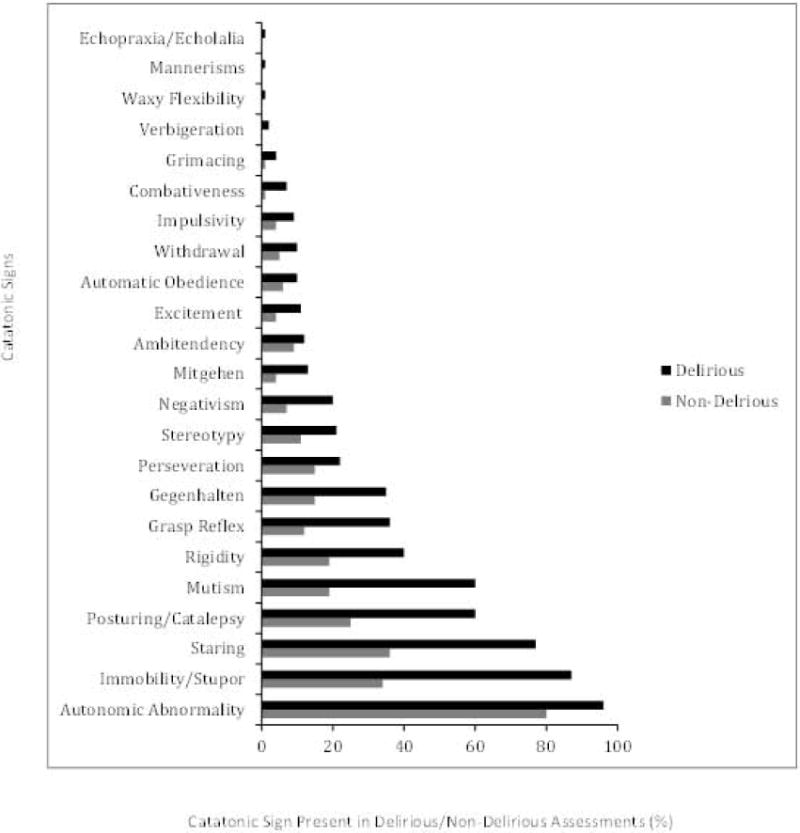
Percent of catatonic signs that were present in delirious (CAM-ICU +) versus non-delirious (CAM-ICU −) assessments (N=452, all paired assessments rather than by patient). Note that for each catatonic sign shown on the Y axis, the frequency is higher in delirious than in non-delirious patients, demonstrating overlap of individual catatonic signs and delirium in critically ill patients. †Delirium diagnosed using CAM-ICU: Confusion Assessment Method for the ICU.
‡ Catatonic signs were measured using the Bush Francis Catatonia Rating Scale.
† Delirium was diagnosed with the CAM-ICU: Confusion Assessment Method for the ICU.
In a logistic regression model, a higher number of catatonia signs was strongly associated with a greater odds of having delirium. For example, patient assessments with ≥3 DSM-5 signs had, on average, 27.75 times the odds (IQR 12.7, 60.6) of having delirium compared with patient assessments with 0 DSM-5 signs present (p< 0.001). (Figure 3)
Figure 3. Probability of Delirium at Different Catatonia Thresholds.
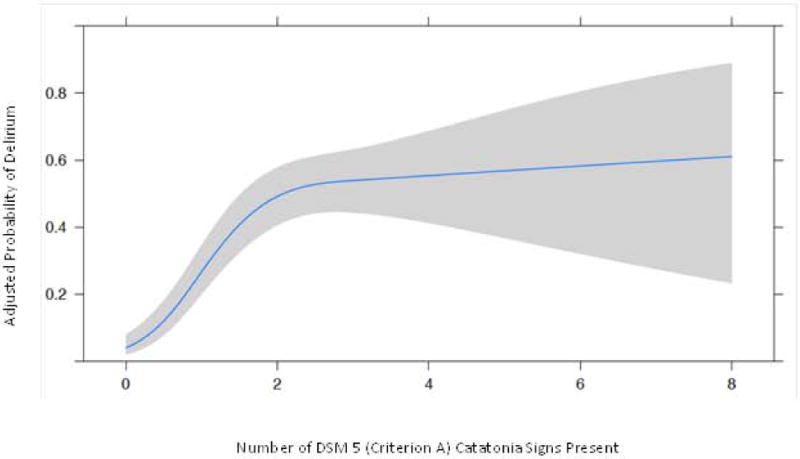
Probability of being delirious (CAM-ICU+) at various DSM-5 Criterion A cut-off points. * Clinical Interpretation: Again using the interquartile ranges of the cohort distributions to paint a clinically interpretable picture of the meaning of these data, a patient with 3 DSM-5 catatonia signs present had 27.8 times the odds (95% CI: 12.7, 60.6) of being delirious in comparison to a patient with 0 DSM-5 catatonia signs present; p= <0.001. NOTE: This figure uses the reference standard DSM-5 criteria for catatonia. This threshold of ≥3 DSM-5 items for catatonia is distinct from the Bush Francis Catatonia Screening Instrument (BFCSI) thresholds shown in Table 2, which are presenting validation data of a bedside instrument that could be used by non-psychiatrists in routine ICU care.
See Figure Legend for clinical examples that explain the application of these data.
‡ Delirium was diagnosed using CAM-ICU: Confusion Assessment Method for the ICU.
† The gray shaded area represents the 95% Confidence Interval (CI).
Comparison between BFCSI cut offs and DSM-5 criteria
Due to concern that the standard BFCSI criteria for catatonia (≥2 items) would be sensitive but not specific enough for the diagnosis and management of catatonia in critical illness, we calculated the sensitivity and specificity of various BFCSI cut offs in comparison to DSM-5 (Table 2). Catatonia as defined by BFCSI ≥3 signs was associated with a 100% sensitivity but only a 65% specificity. Using a BFCSI cut off of ≥4 signs yielded a 91% sensitivity and 91% specificity. Using a cut off of BFCSI ≥5 yielded a sensitivity of 59% and a specificity of 99%.
Table 2.
| BFCSI cut-off point | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| BFCSI ≥2 signs | 1 (0.958 – 1) | 0.468 (0.423 – 0.514) |
| BFCSI ≥3 signs | 1 (0.958 – 1) | 0.65 (0.605 – 0.692) |
| BFCSI ≥4 signs | 0.908 (0.829 – 0.953) | 0.906 (0.876 – 0.929) |
| BFCSI ≥5 signs | 0.586 (0.481 – 0.684) | 0.989 (0.975 – 0.995) |
BFCSI: Bush Francis Catatonia Screening Instrument
The reference standard for this comparison was ≥3 DSM 5 Criterion A items for diagnosing catatonia, as compared with the BFCSI. These data offer validation thresholds for the BFCSI as a bedside instrument that could be used by non-psychiatrists in routine ICU care depending on the desired sensitivity and specificity.
Discussion
One potential rationale for the DSM not allowing concomitant diagnoses of delirium and catatonia in medical illness, could be the real challenge in distinguishing these two entities from one another. However, nearly one-third of our patient assessments met criteria for both delirium and catatonia concomitantly, suggesting that catatonia can be readily recognized in critical illness. Additionally, it could be that the authors of the DSM correctly speculated that medical catatonia is frequently co-morbid with delirium. This exclusion by the DSM would be useful if the goal were to identify a group of medically ill catatonic patients who were not delirious, however, we have shown that 91% of patients (n=42) who were catatonic were also delirious during our study. The prevalence of catatonia in our cohort is higher than Rizos et. al. reported, however their study specifically excluded delirious patients, a group which we and others have proposed might be particularly vulnerable to the development of catatonia (3, 18, 19).
Despite increasing recognition and concern for delirium in critical illness, a significant gap in our understanding of the relationship between delirium and catatonia exists. In our study, a majority of patients were delirious at one point (n=100, 74%), which is consistent with the previous literature on delirium (7, 33–35). Patients who had delirium also frequently screened positive for catatonia (31% of patients over course of the study and 29% of all paired assessments). This finding is consistent with Grover et. al.’s, report of 12.7–30.2% prevalence of catatonia in critically ill patients with delirium (2).
Catatonic signs were more prevalent in the delirious group, in comparison to the non-delirious group. This could be due to signs and symptoms that define the critical illness phenotype, such as autonomic abnormalities, immobility/stupor, etc., or the fact that our tools for diagnosing each could use refinement. Of note, in our cohort, 96% of critically ill patients with delirium and 80% of those without delirium had autonomic abnormalities. In catatonic patients who had autonomic abnormalities, the authors do not believe that the autonomic abnormalities can be explained solely by catatonia, except for the rare circumstance of neuroleptic malignant syndrome or malignant catatonia (without another(35) medical explanation for the presence of the autonomic abnormality).
One of the salient points of this study was to provide practical recommendations, regarding accurate cut-off values on the BFCSI to providers who care for patients with features of both delirium and catatonia, to make clinical diagnoses clear and precise. In this investigation, we showed that a higher than traditional cut-off value on the BFCSI was required to diagnose catatonia with high specificity in critical illness. We recommend a cut-off value of 4 or more items when a widely sensitive and specific screen is desired (e.g., when a patient suspected of having catatonia is evaluated by a psychiatrist). A more stringent cut-off of 5 or more items, would be most useful when considering treatment (e.g., benzodiazepines or ECT).
The primary purpose of this investigation was to suggest that catatonia, a condition characterized by it’s prominent motor and behavioral abnormalities (akin to delirium), can and does exist in critical illness, including in the context of delirium. Our goal was not to suggest an abolition of terms such as “hypoactive” or “hyperactive” delirium nor an abolition of these commonly used descriptors for delirium or mechanisms by which we diagnose delirium. In our experience, patients can and do have hypoactive delirium or hypoactive catatonia in isolation and at times together. How to handle these two overlapping conditions alone and/or together is not answered by this research. Based on our above findings, the authors recommend that in the case of a patient with persistent delirium, catatonia should be considered on the differential in addition to delirium and a BFCRS checked. If such a patient should have ≥4 BFCRS signs present, Psychiatry should be consulted early for assistance in the further evaluation and management of the patient with presumed medical catatonia.
The study has some limitations. First, our cohort was nested within two ongoing trials, thus we were blinded to potential interventions that study participants might have received. Both trials are testing commonly used medications (antipsychotics vs. placebo and dexmedetomidine vs. propofol) that would be part of many ICU patients’ medications. Importantly, it is a basic tenet of the use of these tools—CAM-ICU, DSM-5 and BFCRS— that clinicians assess for delirium and catatonia without regard to the patients’ medications, and then once the assessment is complete, consider medications as a potential etiology of the diagnosis. Thus, this study does not rest on what medication the patient was on, but rather on whether or not the patient was meeting diagnostic criteria for delirium and/or catatonia. Future DeCat publications and those of other investigators will be uniquely poised to answer questions regarding response to various pharmacologic agents in delirious and catatonic patients, particularly as this relates to relevant clinical outcomes.
It was our explicit intent to determine whether critically ill patients (with and without delirium) could meet DSM-5 criterion A for catatonia. In order to achieve this objective we had to ignore the exclusionary for “Catatonic Disorder Due to Another Medical Condition” (catatonia can not be diagnosed exclusively within the context of delirium). This step was necessary to allow this investigation to determine whether delirium and catatonia could be diagnosed concomitantly. A natural evolution of thought stemming from this investigation, would be: “Does the phenomenology of delirium and catatonia overlap to such an extent that they therefore exist on a continuum?” While our study was not set up to definitively answer this question, our study suggests that they do and services as a strong hypothesis generating investigation. DeCat establishes the path by which future research can study shared risk factors and management-related outcomes differences.
The DSM-5 criteria for catatonia is an expert consensus, therefore it’s criteria have never been validated in an ICU setting. Due to this limitation, we recognize that there may be some critically ill patients who meet DSM-5 criterion A for catatonia but would not respond to traditional treatments for catatonia. Due to this limitation the authors urge caution in the application of these data to treatment approaches. Further studies are required to replicate our findings and to guide our treatment approach to the critically ill patient with catatonia. These data also call into question, but do not answer, whether delirious patients with catatonia should be managed differently than patients with delirium or catatonia alone. These questions must be definitively answered with future randomized trials.
This investigation represents the largest and most rigorously conducted study to evaluate the novel relationship between delirium and catatonia in critical illness. In approximately one out of three of patients, delirium and catatonia co-occurred, which suggests a reconsideration of the nosology of catatonia, demonstrating that catatonia due to another medical condition does occur and can be reliably diagnosed in the setting of delirium. The DSM-5 is intended to be a “living document” and therefore new and compelling evidence should spur thoughtful considerations of changes in the diagnostic criteria of catatonia (36). Furthermore, for the bedside clinician, these data represent a change in thinking since delirium is a commonly considered form of brain dysfunction in critically ill patients, while catatonia is rarely ever considered. Moving forward, while ongoing studies are being completed, the ICU practitioner should consider co-occurrence of catatonia in patients with refractory “delirium” and the divergent management options that this diagnosis begets.
Supplementary Material
Clinical Vignette.
A 69-year-old female with severe COPD was admitted to the hospital following a hip fracture. After surgical repair, she required mechanical ventilation for 24 hours in the ICU. Following extubation, she was noticed to have confusion and screened positive for delirium with the Confusion Assessment Method for the ICU (CAM-ICU). A workup including complete blood count, complete metabolic panel, urinalysis, head CT and EEG was unrevealing. Her mental status declined and she refused oral intake and medications, refused to speak or follow commands, and was intermittently combative without provocation. On exam, she had a fixed gaze and intermittently closed her eyes. When asked questions she would echo the examiner’s speech. She had mildly increased tone diffusely. Given her fixed gaze, mutism, echolalia (echoing speech), verbigeration (repeating speech), and negativism (not following commands), catatonia was suspected. Psychiatry was consulted and performed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a score of 18. A trial of 1mg of IV lorazepam was given, and within 2 minutes, the patient was able to communicate and follow commands. A diagnosis of catatonia was confirmed following the positive lorazepam trial. While she remained CAM positive for two more days, her mental status, cooperativeness, and agitation all improved with benzodiazepine treatment.
Acknowledgments
Source of Funding: Dr. Wilson would like to acknowledge that a portion of the preparation of the statistics for this manuscript was supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH. Dr. Wilson and Dr. Carlo would like to acknowledge that this work is partially supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program and with resources and the use of facilities at VA Tennessee Valley Healthcare System, Nashville TN. Ms. Thompson and Drs. Ely, Pandharipande, Girard, and Chandrasekhar all receive funding for their time working on this investigation from AG035117 and HL111111. Dr. Pandharipande would additionally like to recognize research grant funding from Hospira Inc. in collaboration with the NIH. Drs. Girard and Ely would additionally like to acknowledge salary support from the Tennessee Valley Healthcare System Geriatric Research Education and Clinical Center (GRECC). Dr. Ely will also disclose additional funding for his time from AG027472 and having received honoraria from Orion and Hospira for CME activity; he does not hold stock or consultant relationships with those companies. Dr. Heckers would like to acknowledge NIMH grant funding from MH70560-07A1.
Footnotes
Author contribution statement:
All authors listed above have contributed substantially to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work and have participated in drafting the work or revising it critically for important intellectual content. Additionally, each author has given their approval to the final version of the manuscript and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: The remaining authors have disclosed that they do not have any potential conflicts of interest.
Copyright form disclosure: Drs. Wilson, Pandharipande, Girard, and Ely received support for article research from the National Institutes of Health (NIH). Dr. Girard’s institution received funding from NIH (AG034257) and NIH (AG027472). Dr. Chandrasekhar received funding from internal funds. Dr. Dittus disclosed government work. Dr. Ely’s institution received funding from the NIH and Veteran Affairs funding; he received funding from Abbott and Pfizer; and he disclosed government work.
References
- 1.Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: an important and underdiagnosed cause of altered mental status. a case series and review of the literature*. Crit Care Med. 2014;42(3):e234–241. doi: 10.1097/CCM.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 2.Grover S, Ghosh A, Ghormode D. Do patients of delirium have catatonic features? An exploratory study. Psychiatry Clin Neurosci. 2014;68(8):644–651. doi: 10.1111/pcn.12168. [DOI] [PubMed] [Google Scholar]
- 3.Rizos DV, Peritogiannis V, Gkogkos C. Catatonia in the intensive care unit. Gen Hosp Psychiatry. 2011;33(1):e1–2. doi: 10.1016/j.genhosppsych.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Gelenberg AJ. The catatonic syndrome. Lancet. 1976;1(7973):1339–1341. doi: 10.1016/s0140-6736(76)92669-6. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 7.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 8.Lat I, McMillian W, Taylor S, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 11.Pandharipande PP, Girard TD, Jackson JC, et al. Delirium as an independent predictor of long-term cognitive impairment: Results from the BRAIN ICU (Bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors) study. Am J Respir Crit Care Med. 2013;187:A5237. [Google Scholar]
- 12.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 14.Esseveld MM, Leroy PL, Leue C, et al. Catatonia and refractory agitation in an updated flow chart for the evaluation of emotional-behavioral disturbances in severely ill children. Intensive Care Med. 2013;39(3):528–529. doi: 10.1007/s00134-012-2763-1. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric A. Diagnostic and statistical manual of mental disorders. Fourth. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 16.Association. AP. Diagnostic and statistical manual of mental disorders: DSM-5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 17.American Psychiatric A. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 18.Schieveld JN, Wolters AM, Blankespoor RJ, et al. The forthcoming DSM-5, critical care medicine, and pediatric neuropsychiatry: which new concepts do we need? J Neuropsychiatry Clin Neurosci. 2013;25(2):111–114. doi: 10.1176/appi.neuropsych.12020028. [DOI] [PubMed] [Google Scholar]
- 19.European Delirium A, American Delirium S. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. Bmc Med. 2014;12:141. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn DK. “Burn catatonia”: a case report and literature review”. J Burn Care Res. 2014;35(2):e135–142. doi: 10.1097/BCR.0b013e31828c73c7. [DOI] [PubMed] [Google Scholar]
- 21.Oldham MA, Lee HB. Catatonia vis-a-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015 doi: 10.1016/j.genhosppsych.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Francis A, Lopez-Canino A. Delirium With Catatonic Features: A New Subtype? Psychiatric Times. 2009;29:32–36. [Google Scholar]
- 23.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: A survey of 912 healthcare professionals. Crit Care Med. 2004;32(1):106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 24.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermus IP, Willems SJ, Bogman AC, et al. “Delirium” Is No Delirium: On Type Specifying and Drug Response”. Crit Care Med. 2015;43(12):e589. doi: 10.1097/CCM.0000000000001251. [DOI] [PubMed] [Google Scholar]
- 26.Bush G, Petrides G, Francis A. Catatonia and other motor syndromes in a chronically hospitalized psychiatric population. Schizophr Res. 1997;27(1):83–92. doi: 10.1016/S0920-9964(97)00084-4. [DOI] [PubMed] [Google Scholar]
- 27.Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 28.Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137–143. doi: 10.1111/j.1600-0447.1996.tb09815.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1–3):256–262. doi: 10.1016/j.schres.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Ely EW, Gautam S, May L, et al. A comparison of different sedation scales in the ICU and validation of the Richmond Agitation Sedation Scale (RASS) Am J Respir Crit Care Med. 2001;163:A954. [Google Scholar]
- 31.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 32.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. Journal of the American Statistical Association. 1927;22(158):209–212. [Google Scholar]
- 33.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 34.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 35.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.[cited 05/22/2017]Available from: https://www.surveygizmo.com/s3/2699329/PROPOSAL-IN-SUPPORT-OF-CHANGE-TO-DSM-5
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


