Abstract
Ion channels facilitate diffusion of ions across cell membranes for such diverse purposes as neuronal signalling, muscular contraction, and fluid homeostasis. Solute transporters often utilize ionic gradients to move aqueous solutes up their concentration gradient, also fulfilling a wide variety of tasks. Recently, an increasing number of ion channel-transporter (“chansporter”) complexes has been discovered. Chansporter complex formation may overcome what could otherwise be considerable spatial barriers to rapid signal integration and feedback between channels and transporters, the ions and other substrates they transport, and environmental factors to which they must respond. Here, current knowledge in this field is summarized, covering both heterologous expression structure/function findings and potential mechanisms by which chansporter complexes fulfil contrasting roles in cell signalling in vivo.
Keywords: myo-inositol, solute transporter, ion channel
1. Introduction
Cells in biological systems require constant and highly regulated movement of ions across cell membranes, to perform or regulate virtually all biological processes. Rapidly gating (opening and closing) voltage-gated sodium channels and potassium channels generate and propagate the action potentials required for nervous signalling, the heartbeat and other processes in excitable cells [1–3]. An array of calcium channels, including voltage-gated and store-operated channels on the plasma membrane, and channels in the sarcoplasmic/endoplasmic reticulum membrane that are opened by a variety of stimuli including Ca2+ and coupling to plasma membrane Ca2+ channels, increase cytosolic Ca2+ levels to trigger processes as diverse as wound healing, fertilization, gene transcription, immune responses, and long-term potentiation [4–9]. Other classes of ion channels with various grades of ion selectivity are required for ion and fluid homeostasis and secretion, regulation of resting membrane potential, and hormone production and secretion, often in non-excitable cells such as polarized epithelial cells [10–14].
Ion channels do not actively transport ions, but instead they facilitate ion diffusion down an electrochemical gradient, by creating an aqueous pathway through otherwise inhospitable cell membranes. These pathways are typically selective either for specific ions or classes of ions, and diffusion is highly regulated. This regulation often takes the form of gates intrinsic to the channel protein, which open and close to permit or stem ion flow; other forms of regulation can involve extrinsic moieties, e.g., inward rectification of some ion channels via block by Mg2+ and other molecules at depolarized voltages. Most ion channels, in addition to opening (activation) and closing (deactivation) also have an inactivation or desensitization gate that can close to stem the flow of ions even when the activating stimulus (e.g., membrane depolarization, ligand binding) is still present [15–18]. Another type of protein termed a uniporter, distinct from channels, also mediates movement of solutes across the cell membrane down their concentration gradient, but instead works by facilitative diffusion. Thus, solutes bind to e.g., the extracellular side of the protein, which then changes conformation to expose the bound solute to the intracellular side, permitting diffusion into the cell. Examples include the facilitated glucose transporters GLUT1-4 [19, 20].
In contrast, many other classes of transporters move solutes across membranes up their concentration gradient. This is achieved via one of various mechanisms. Secondary active transporters utilize a downhill ionic gradient, to move a solute up its concentration gradient concomitant with downhill transport of the ion [21–23]. For example, SGLT1 utilizes a downhill Na+ gradient to move glucose into cells, up its concentration gradient. Sodium-coupled transporters represent a major class of secondary active transporters for a range of ions and solutes, and several will be covered in this review. They are termed “secondary” active transporters because energy from ATP hydrolysis is required to establish the Na+ gradient they require for function, but the hydrolysis is not performed by the sodium-coupled transporter itself [24, 25]. Instead, the Na+ gradient is established by another protein, such as the Na+/K+-ATPase, which is often described as pump. The Na+/K+-ATPase is a primary active transporter because it hydrolyzes ATP to provide energy to pump K+ into the cell and Na+ out of the cell, both against their electrochemical gradients [26].
For many cellular processes, ion channels must work in concert with ion and solute transporters. The Na+/K+-ATPase is a prime example. Aside from establishing the Na+ gradient for Na+-coupled solute transport through other transporter proteins, the Na+ and K+ gradients established by the Na+/K+-ATPase are essential for maintaining resting membrane potential and setting the stage for the action potential [27–30], a process orchestrated by rapidly-gating ion channels [2, 31–35]. The importance of the Na+/K+-ATPase for nervous signalling is amply illustrated by the calculation that this one type of pump protein accounts for as much as two-thirds of neuronal energy expenditure [36].
By the same token, solute transporters, including pumps, may be reliant upon ion channels. As an example, gastric acidification in mammals requires the gastric H+/K+-ATPase to secrete protons into the stomach. The H+/K+-ATPase is positioned intracellularly in vesicles, until stimulated to traffic to gastric (oxyntic) pits on the apical surface of parietal cells, and then pump protons into the stomach. As protons move out, K+ ions move through the H+/K+-ATPase into the cell, where they would accumulate in the absence of a suitable conduit to release them back into the stomach lumen (where they are required for further cycles of the H+/K+-ATPase) [37, 38]. Several apically located ion channels are thought to serve the function of the K+ recycling conduit in parietal cells. KCNQ1-KCNE2 is a constitutively active potassium channel apically localized in parietal cells, which is potentiated by low extracellular pH, making it an ideal candidate for this process. Accordingly, genetic deletion from mice of either Kcnq1 or Kcne2 results in achlorhydria (loss of gastric acid secretion), indicating an essential role for KCNQ1-KCNE2 in gastric acid secretion, by supporting H+/K+-ATPase function [38–45]. Inward rectifying K+ channels including Kir1.1, Kir2.1, Kir4.1 and Kir4.2 are also thought to be important for gastric acid secretion [46–50].
Beyond functional cooperation between ion channels and transporters, some channels have been found to form physical complexes with solute transporters, a phenomenon that has not yet been described for the H+/K+-ATPase or Na+/K+-ATPase. Novel channel-transporter (“chansporter”) complexes are being discovered more frequently in the last 5 years than ever before, suggesting they may represent a previously overlooked, yet important mode of cellular signalling. In this review, the author describes examples of channel-transporter complexes reported in the literature, together with their known or suspected physiological roles and the types of signal integration they facilitate. The complexes covered in this review are summarized in Table 1.
Table 1.
Chansporter complexes covered in this review.
| Transporter class | Transporter (other names) | Interacting channels (other names) | Channel class | Native expression | References |
|---|---|---|---|---|---|
| Sodium-coupled sugar/polyol transporters | SLC5A3 (SMIT1) | KCNQ1, 2, 2/3 (Kv7.1, 2, 2/3) | KCNQ family voltage-gated potassium channels | Nervous system | [110, 130] |
| SLC5A11 (SMIT2) | KCNQ1, 2, 2/3 | Nervous system | [110, 130] | ||
| dSLC5A11 (cupcake) | dKCNQ | Nervous system | [137] | ||
| Chloride and bicarbonate exchangers | SLC26A5 (prestin) | KCNQ2, 3, 4 (Kv7.2, 3, 4) | Putative complex formation in outer hair cells | [150] | |
| SLC26A2, 3, 4 (pendrin), 5, 6, 9 | CFTR | Chloride channel/ABC transporter-like | Epithelia, e.g., pancreas, salivary glands | [151] | |
| ABC-family non-transporters | Sulfonylurea receptors (Sur1, Sur2) | Kir6.1, 6.2 | Inward rectifier K+ channels | Pancreas, heart | [166] |
| TRPM4 | Calcium-activated, monovalent cation-nonselective channel | Post-injury nervous system | [168] | ||
| Calcium ATPase | secretory pathway Ca2+-ATPase isoform 2 (SPCA2; ATP2C2) | Orai1 | calcium channel | Mammary epithelium | [170] |
| Amino acid transporters | SLC6A11 (GAT3) | MaxiK (BK; Slo1) | calcium-activated potassium channel | Nervous system | [176] |
| SLC61A2 (EAAT2; GLT-1) | AQP4 | water channel | Astrocytes | [178] | |
| Sodium chloride co-transporter | SLC12A3 (NCC) | ENaC | epithelial sodium channel | Distal convoluted tubules | [185] |
d, Drosophila melanogaster. All other complexes listed involve mammalian proteins.
2. SLC5A sodium-coupled solute transporter interactions with KCNQ potassium channels
Voltage-gated potassium (Kv) channels are formed by pore-forming α subunits that co-assemble into tetramers around an aqueous ion-conducting pore. They are in general highly selective (>10:1) for K+ over Na+, a property important for their role in repolarizing excitable cells to end each action potential. Kv channel α subunits are encoded by 40 different genes in the human genome, each of which fall into one of ~12 (depending on which nomenclature is used) subfamilies. Most Kv α subunits are functional as homomers, but many form heteromers with other α subunits (typically but not always from the same subfamily) that display distinct functional characteristics and may be prerequisite for some biological processes [2].
Most if not all Kv α subunits also co-assemble with some form of ancillary or β subunit to facilitate their surface trafficking or targeting, and/or modify their function for specific biological activities [51–54]. Still others, termed “silent” α subunits, either silence other α subunits upon heterotetramerization, or require heteromerization with other α subunit types for ion conduction [55, 56]. Kv α subunits, including silent α subunits, each possess six transmembrane domains split into a voltage sensing domain (VSD; transmembrane helices S1–S4) and a pore module (S5 and S6) [57–59] (Figure 1A). Kv channel ancillary subunits can be single transmembrane-spanning (1TM) such as the KCNEs (Figure 1A) or entirely cytosolic and their importance to normal channel function in vivo is emphasized by the pathogenic effects of their disruption [60–65].
Figure 1. KCNQ1-KCNE channel structure and function.
A. Representative electrophysiological recordings [65] from Xenopus oocytes expressing KCNQ1 alone (centre), or with KCNE1 (left) or KCNE3 (right). Voltage protocol inset. Subunit topologies shown beneath corresponding traces. VSD, voltage sensing domain.
B. High-resolution structures obtained by cryo-EM of Xenopus KCNQ1 [186] (red) bound to calmodulin (blue); left, one subunit; right, tetramer. View from inside membrane perspective.
C. A tetramer of KCNQ1 [186] viewed from inside the membrane (left) or outside the cell (right) perspectives. Individual subunits are coloured orange, green, light green and gold (KCNQ1) versus red and blue (calmodulin). Putative position of KCNEs [187] (black circles) shown in right-hand image. For all figures, structures were obtained from the Rutgers/UC San Diego/SDSC (RCSB) protein data bank and viewed using Jsmol (Javascript) (this panel) or NGL Viewer [188, 189] (all other structure images).
Kv α subunits in the KCNQ (Kv7) subfamily are represented by 5 isoforms in the human genome, KCNQ1-5. KCNQ channels are notable for their slow gating kinetics, small and flickery unitary conductance, and relatively little inactivation. KCNQ1 is a ubiquitous channel which is both important for human cardiomyocyte repolarization, and prominent in the essential functions of many different types of non-excitable, polarized epithelial cells. These contrasting roles are made possible by interaction of KCNQ1 with a variety of β subunits, most notably the KCNE 1TM subunits [66].
Each of the five KCNE isoforms co-assembles with and regulates KCNQ1 with divergent functional outcomes. For example, KCNE1 slows KCNQ1 activation 5–10-fold, positive-shifts KCNQ1 voltage-dependence of activation such that more comprehensive membrane depolarization is required for channel activation (Figure 1A), removes inactivation [67–70], and increases sensitivity 100-fold to current potentiation by phosphatidylinositol 4,5-biphosphate (PIP2) [71]. PIP2 is a minor membrane phospholipid constituent that plays a major role in regulating membrane excitability by binding to and regulating the gating of many different ion channels [72–74], and features heavily in the discussions below. KCNE1 slows KCNQ1 activation by a mechanism involving probably both the transmembrane domain and C-terminal domain of KCNE1. One school of thought is that KCNE1 slows down movement of the charged, voltage-sensing S4 segment of KCNQ1 to slow its activation, possibly also involving regulation of how S4 interacts with S5, and formation of a stable pre-open state [75–78]; another school of thought is that in addition to S4 slowing, KCNQ1-KCNE1 channels require all 4 voltage sensors to activate before channel opening, while KCNQ1 activation without KCNE1 requires only 1 voltage sensor to move [79, 80]. KCNQ1-KCNE1 channels generate the slowly activating potassium current (IKs) that is important for cardiomyocyte repolarization, and also from a channel required for endolymph secretion in the inner ear. Hence, individuals with severe loss-of-function mutations, typically in both alleles, suffer from the cardioauditory Jervell and Lange-Nielsen syndrome, which presents as Long QT Syndrome (delayed ventricular repolarization) and congenital profound bilateral sensorineural hearing loss [39, 81–83]. KCNQ1 requires co-assembly with calmodulin for surface expression and normal function (Figure 1B). KCNE1 is thought to fit into a pocket between the VSD of one KCNQ1 subunit and the pore module of another, within IKs channel complexes (Figure 1C). It is likely that other KCNEs are similarly localized. Two KCNE1 subunits are thought to exist in each functional IKs complex with four KCNQ1 α subunits (Figure 1C) although some suggest this can be variable and rise to 4:4 stoichiometry [84–88].
In contrast, as mentioned above, KCNQ1-KCNE2 (and KCNQ1-KCNE3; Figure 1A) channels are constitutively active, and also non-inactivating, meaning that they can provide a constant repolarizing force when cells are positive to the potassium equilibrium potential (EK), which is typically around −80 mV under physiological conditions. Thus, in polarized epithelial cells, which often remain at potentials between −40 mV and −20 mV, KCNQ1-KCNE2 can facilitate steady K+ efflux unless closed by other forms or regulation [40, 42, 43, 89]. KCNQ1-KCNE2 channels are required for gastric acid secretion (as explained above) and also for optimal thyroid hormone secretion; genetic deletion of either protein causes hypothyroidism [90–92]. KCNQ1 and KCNE2 are also expressed in pancreatic β cells, where they may also form a heteromeric channel complex. Germline deletion of Kcnq1 increases glucose tolerance in mice [93], whereas deletion of Kcne2 decreases glucose tolerance, decreases insulin sensitivity, and impairs insulin secretion in response to glucose [94, 95]. This hints at the complex effects of KCNE2 on KCNQ1: the channel is converted to a constitutively active one, but channel outward current is decreased (possibly by reduced surface expression and/or other mechanisms). Thus, KCNE2 co-assembly has opposite effects on KCNQ1 current magnitude depending on the membrane potential at which is it measured [89]. KCNE2 appears to be most highly expressed in the choroid plexus epithelium, where it regulates KCNQ1 and also the Kv1.3 (KCNA3) potassium channel on the apical side [96].
KCNQ1-KCNE3 channels are also constitutively activated and have been mechanistically studied more than KCNQ1-KCNE2, largely because they generate more robust currents, making it easier to study effects of mutagenesis, etc. We and others have shown that KCNE3 locks open the KCNQ1 VSD, via residues including V72 in the transmembrane segment and D54 and D55 in the KCNE3 N-terminal segment [97–104]. KCNQ1-KCNE3 generates a K+ current important for regulating cAMP-stimulated chloride secretion in the intestine [105]. KCNE4 and KCNE5, in contrast, each inhibit KCNQ1 activity; in the case of KCNE4 this effect involves calmodulin but probably not impaired trafficking [106–108], while the KCNE5 effect involves a positive-shift in the voltage dependence of activation [109]. For detailed reviews of KCNE family regulation of KCNQ1 and other α subunits, see [63–66].
KCNQ1 interaction with sodium-coupled myo-inositol transporters
The choroid plexus epithelium is the primary site for cerebrospinal fluid (CSF) production and secretion; deletion of KCNE2 depolarizes choroid epithelial cells, alters their cellular Kv currents, increases seizure susceptibility, and decreases immobile time in the tail suspension test – both of the latter suggesting increased neuronal excitability. Metabolomics analysis revealed that KCNE2 deletion in mice decreases CSF levels of the major osmolyte, myo-inositol, by ~30%. Mega-dosing with myo-inositol by intraperitoneal injections restored normal seizure susceptibility and tail suspension behaviour in Kcne2−/− mice without affecting wild-type mice, suggesting a causative role for altered myo-inositol handling in the neural defects in Kcne2−/− mice [96, 110]. Accordingly, KCNQ1-KCNE2 channels were found to form physical complexes with the sodium-coupled myo-inositol transporter, SMIT1 (encoded by SLC5A3), in mouse choroid plexus and when heterologously co-expressed in Chinese Hamster ovary (CHO) cells [110]. SMIT1 is predicted to be a ~14-transmembrane-segment protein (Figure 2A), closely related to the sodium-coupled glucose transporter, SGLT1 [25, 111–113], but favouring myo-inositol versus glucose as a substrate.
Figure 2. KCNQ-transporter interactions.
A. Transmembrane topologies of vSGLT (a representative of the SLC5A transporters) [190] and SLC26Dg (a representative of the SLC26 transporters) [191]. Substrates (magenta) shown in binding sites.
B. In this and subsequent panels, high-resolution structures obtained by X-ray crystallography or cryo-EM are shown for proteins known to participate in chansporter complexes, or structures of their closest relatives for which high resolution structural information is available. Unless otherwise stated, colouring progresses from (red to blue) N-terminal to C-terminal highlighting individual secondary structural elements such as individual transmembrane helices. This panel: Xenopus KCNQ1 with calmodulin bound [186] represents KCNQ1, 2, 3 or 4; vSGLT [190] is in place of SMIT1 or 2; Deinococcus geothermalis SLC26 [191] is in place of prestin. Red arrows indicate known or putative (“?”) direct physical interaction. Green lines approximate membrane boundaries. Also shown is the myo-inositol (MI) pathway, indicating how SMIT-transported myo-inositol can generate PIP2 which then regulates KCNQ gating. Abbreviations: PI4K, phosphatidylinositol 4-kinase; PI5K, phosphatidylinositol 5-kinase; PLC, phospholipase C; DAG, diacylglycerol; IP3, inositol(1,4,5)-triposphate, IP2, inositol(1,4)-bisphosphate; IP, inositol monophosphate; IPP, inositol-1,4 bisphosphate 1-phosphatase; IMPase, inositol-1(or 4)-monophosphatase; G6P, glucose 6-phosphate; Ino-1, inositol synthase.
C. Hypothetical configuration of individual SLC26 and SLC5A transporters with a KCNQ1 tetramer viewed from outside the cell.
D. Hypothetical 4:4 stoichiometry (KCNQ1:SLC5A) viewed from outside the cell.
Myo-inositol performs a variety of crucial cellular functions aside from its osmoregulatory properties. Myo-inositol is converted to phosphatidylinositol (PI) and phosphatidylinositol 4,5-biphosphate (PIP2) by phosphatidylinositol 4-kinase (PI4K) and phosphatidylinositol 5-kinase (PI5K). PIP2 is a prerequisite for normal functioning of many ion channels (e.g., KCNQs, inward rectifier K+ channels, transient receptor potential (TRP) channels and ryanodine receptors), transporters (e.g., sodium/hydrogen exchangers), and pumps (e.g., plasmalemmal calcium pumps) [72–74]. In addition, Gq-coupled receptor stimulation activates phospholipase C (PLC), cleaving PIP2 into inositol(1,4,5)-triposphate (IP3), and diacylglycerol (DAG) (Figure 2B), each of which contributes to protein kinase C (PKC) activation and subsequent downstream signalling cascades. IP3 also activates IP3 receptors, releasing Ca2+ from intracellular stores such as the endoplasmic reticulum.
While KCNQ1 co-expression enhanced SMIT1 myo-inositol uptake, KCNQ1-KCNE2 inhibited it, as did co-expression with R231A-KCNQ1, a constitutively active mutant with the VSD locked in the active state [110]. This suggests a more complicated channel-transporter relationship than merely Kv channel cellular hyperpolarization increasing the electrical gradient to enhance Na+ coupled myo-inositol influx, as R231A-KCNQ1 would be predicted to serve this role very efficiently. R231A-KCNQ1 also inhibited activity of SMIT2, a SMIT1-related myo-inositol transporter encoded by SLC5A11, while SMIT2 inhibited KCNQ1 activity, when the two were co-expressed in Xenopus oocytes [110].
Later work showed that SMIT2 regulates KCNQ channels in mammalian and Drosophila neurons (see below). Further, the closely related KCNQ4 channel did not augment or otherwise alter SMIT1 activity when co-expressed in oocytes, also suggesting against solely the hyperpolarizing effects of KCNQ1 as being important for SMIT1 regulation. However, it appears that KCNQ1 pore activity is important for this role, as inhibiting with KCNQ channel blocker XE991 also inhibited co-expressed SMIT1 activity [110]. The author’s lab is currently investigating how SMIT1 co-expression alters KCNQ1 channel functional properties and pharmacology. Initial findings suggest intimate association of SMIT1 with the KCNQ1 channel pore module. Thus, SMIT1 co-expression, in the absence of extracellular myo-inositol or other SMIT1 substrates, changes ion selectivity, voltage-dependence and pharmacology of KCNQ1 in a KCNE1-dependent manner, consistent with a model in which SMIT1 physically interacts with the KCNQ1 pore or close enough to it to alter its fundamental attributes. This association may include altering the way in which KCNE1 modulates KCNQ1, or even competition between SMIT1 and KCNE1 for interacting with similar sites in the KCNQ1 pore or gating apparatus [114].
One could speculate that transporters such as SMIT1 might interact one per KCNQ1 tetramer, potentially leaving room for other types of transporter to co-assemble as well (Figure 2C), or 4:4 giving an octameric complexes with the tetramer of KCNQ1 α subunits (Figure 2D).
KCNQ1-KCNE2 channels also functionally interact with the sodium/iodide symporter (NIS; encoded by SLC5A5), a close relative of SMIT1. Germline deletion of either Kcnq1 or Kcne2 in mice causes hypothyroidism, and KCNQ1-KCNE2 channels are required for optimal thyroid cell iodide uptake by NIS, for the purpose of thyroid hormone production [90, 92]. The mechanisms by which KCNQ1-KCNE2 regulates NIS have yet to be fully established.
KCNQ2/3 interaction with myo-inositol transporters
While KCNQ1 is synonymous with human ventricular repolarization and roles in various secretory epithelia, the remainder of the KCNQ family members (2–5) are better known for generating the muscarinic-inhibited (M) current in mammalian neurons [115–119]. KCNQ2 and KCNQ3 in particular are expressed widely in the brain, where they from heteromeric Kv channels in axon initial segments (AIS) of neurons. In addition, KCNQ2 and KCNQ3 form channels alone or with one another in nodes of Ranvier in nerves including the sciatic nerve [120–123]. KCNQ2/3 channels act as gatekeepers, controlling neuronal excitability by raising the threshold for action potential firing. Upon activation of the muscarinic acetylcholine receptor, KCNQ2/3 channels are inhibited, depolarizing the cell sufficiently to initiate an action potential [121].
As was also found for KCNQ1 channels, KCNQ2/3 channels are regulated by PIP2, which negative-shifts their voltage dependence such that they activate at more hyperpolarized membrane potentials, further quelling membrane excitability [73, 124–128]. Aside from being an important osmolyte, myo-inositol is a substrate for PIP2, and therefore SMIT1 can increase cellular PIP2 content by transporting myo-inositol into the cell. Dai and colleagues showed that this is one mechanism by which neuronal electrical activity can be linked to extracellular osmotic changes: increased extracellular myo-inositol is transported into the cell by SMIT1, raising PIP2 levels, negative-shifting the voltage dependence of KCNQ2/3 channel activation, hyperpolarizing the cell and reducing cellular excitability [129].
We found that not only is SMIT1-transported myo-inositol able to generate sufficient PIP2 to modulate KCNQ2/3 activity, as reported by Dai et al., but also that SMIT1 forms physical complexes with KCNQ2, and that KCNQ2 and KCNQ3 each co-localize with SMIT1 and/or SMIT2 in axon initial segments and sciatic nerve nodes of Ranvier [130]. Co-localization/co-assembly of KCNQ2/3 channels with SMIT1/2 may facilitate the channel being able exploit locally high PIP2 concentrations generated from SMIT1/2-transported myo-inositol, an hypothesis supported by previous studies showing that PIP2 diffusion is quite slow in the presence of intact cytoskeleton [131], and our finding that disruption of the cytoskeleton impairs the ability of SMIT1 to potentiate KCNQ2/3 current after 3 h incubation in 1 mM myo-inositol [130]. Reciprocally, KCNQ2/3 channels protect SMIT1 activity from the otherwise inhibitory effect of cellular depolarization via high extracellular K+ [130]. In tight confines such as axons, where diffusion might be slower for some molecules than in the soma, close physical proximity between KCNQ2/3 and SMITs could be particular advantageous, but what other advantages might KCNQ2/3-SMIT1/2 co-assembly confer? Ongoing work in the author’s laboratory suggests that SMIT1 binds directly to the KCNQ2 pore module, and in the absence of myo-inositol, SMIT1 binding itself alters KCNQ2/3 ion selectivity, gating and pharmacology, indicative of direct influence on pore architecture and fundamental channel properties [114]. Thus, SMIT-KCNQ co-regulation probably involves both direct physical contact and regulation by myo-inositol-derived PIP2 (Figure 2B).
The physiological significance in vivo of the regulatory modes described above remains to be determined. However, there is a striking similarity between the phenotypes of mice with either Kcnq2 or Slc5a3 (SMIT1) germline-deleted: disruption of either gene causes death within hours after birth from hypoventilation [132, 133]. Likewise, some human KCNQ2 sequence variants also cause respiratory dysfunction [134]. In Slc5a3−/− mice, this phenotype can be avoided by supplementing the drinking-water of the dam during gestation and lactation with 1 mM myo-inositol. Thus, the myo-inositol transported into phrenic nerves by SMIT1 is required for normal development and function of peripheral nerves, and for innervation of the diaphragm and ventilation [135]. An interesting experiment would be to similarly try to rescue Kcnq2−/− mice using myo-inositol, but this may not work, as just having myo-inositol, but not the channel that the PIP2 it generates presumably regulates in phrenic nerves (KCNQ2), may not suffice. Nevertheless, the phenotypic similarity of the two mouse lines, and the KCNQ2/3-SMIT1/2 co-localization we observed in sciatic nerve, are highly suggestive of requisite cooperation and chansporter complex formation in vivo. Unlike other family members, KCNQ4 and KCNQ5 have not yet been found to interact with SLC5A family transporters, but KCNQ4 is suggested to interact with SLC26A5 (Prestin) (see below).
dKCNQ interaction with cupcake
Vinegar flies (Drosophila melanogaster) express an orthologue of mammalian SMIT2 (gene name SLC5A11 in either case), termed cupcake [136]. Deletion of SLC5A11 renders Drosophila insensitive to the nutritional value of sugars. Cupcake is required for the increased excitability of ellipsoid body R4 neurons observed during fly starvation, which normally drives feeding and other hunger-driven behaviours. Cupcake expression in the brain, accordingly, is increased during starvation and decreased shortly after feeding of starved flies. It appears, however, that cupcake is not a sugar transporter, but rather it may act as a sugar sensor. Thus, unlike related mammalian transporters such as SGLT1, cupcake lacks a consensus sugar binding site. Neither does cupcake generate glucose-dependent currents when it is studied in Xenopus oocytes, although it does pass a large constitutive current that requires sodium and a conserved sodium-binding site [136, 137]. However, as described for mammalian SMITs above, cupcake co-assembles with dKCNQ, a Drosophila Kv channel orthologous to mammalian KCNQs. Cupcake co-assembly inhibits dKCNQ (as we previously found for the mammalian cupcake orthologue, SMIT2, with KCNQ1 [110]), and dKCNQ is required for normal feeding behaviour and food selection by controlling the excitability of neurons expressing cupcake [137].
3. SLC26A family anion transporter interactions with ion channels
Prestin interaction with KCNQ channels
KCNQ4 is a Kv channel α subunit structurally and functionally closely related to KCNQ2, 3 and 5. KCNQ4 is distinguished from these other KCNQ family members primarily by its expression in mammalian cochlea and auditory neurons, and its association when disrupted with human and mouse hearing loss. KCNQ4 is required for normal cochlear ion homeostasis and inherited sequence variants in KCNQ4 cause DFNA2, which presents as progressive sensorineural hearing loss at all frequencies [138]. Many of the KCNQ4 DFNA-linked mutations impair KCNQ4 trafficking to the plasma membrane and/or impair channel conductance when at the membrane [139, 140].
Prestin (encoded by SLC26A5) is part of the SLC26 family of anion transporters that also includes pendrin (encoded by SLC26A4) (Figure 2A). While pendrin is a more conventional, sodium-independent, electroneutral chloride/iodide transporter (anion exchanger), prestin is a motor protein in addition to being an anion transporter. Mechanical elongation and contraction of prestin underlies electromotility of outer hair cells of the inner ear, important for the amplification of sound waves that is required for high sensitivity hearing [141, 142]. Prestin is thought to be intrinsically voltage-sensitive by virtue of the ability to change shape in response to binding of intracellular chloride ions. Independent of this voltage sensing, prestin is also thought to have the capacity to transport anions, although was unable to do so when heterologously expressed in Xenopus oocytes, suggesting the possibility that oocytes lack an essential component required for the transport function of prestin [143–147].
Like KCNQ4, inherited sequence variants in SLC26A5, the gene encoding prestin, cause non-syndromic hearing loss [148, 149]. It is therefore very interesting that prestin expressed in CHO cells left-shifted the voltage dependence of activation of co-expressed KCNQ4, by about −15 mV; pendrin co-expression, in contrast, did not affect KCNQ4 activation. A similar −15 mV shift in the voltage dependence of activation was found when KCNQ2/3 channels were co-expressed with prestin, but a lesser effect (−5 mV) was observed when prestin was co-expressed with Kv1.1 (KCNA1) [150]. It is not yet known whether the functional effects of prestin on KCNQ channels arise from direct physical interaction (Figure 2B, C), nor whether the interaction occurs in native outer hair cells.
SLC26A chloride and bicarbonate exchanger interaction with CFTR
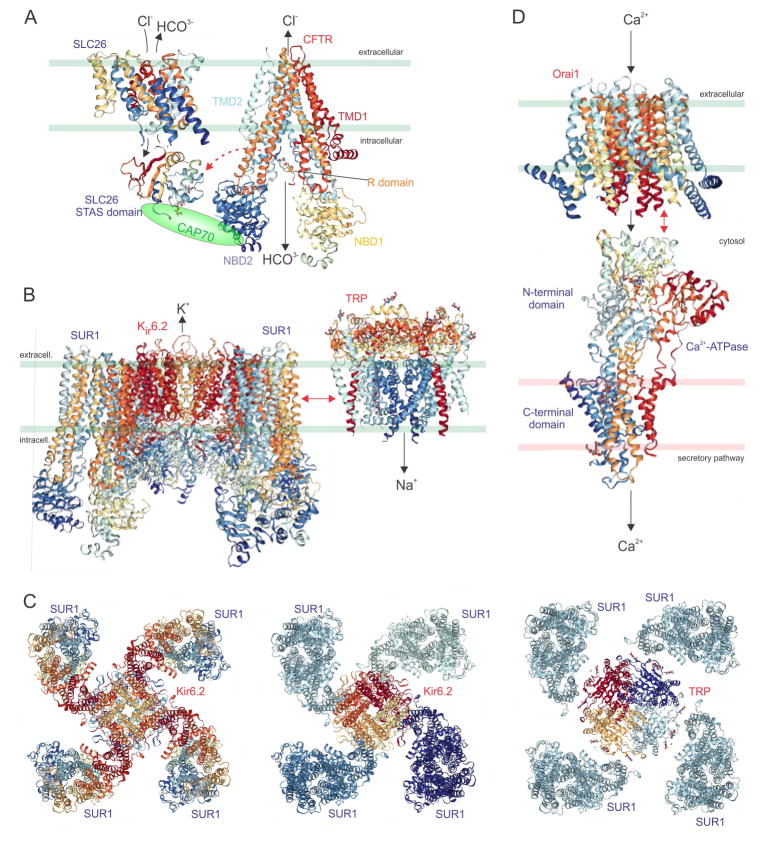
Prestin, pendrin, and also related SLC26 chloride and bicarbonate exchanger family members A2 and A3, form complexes with another ion channel - the cystic fibrosis transmembrane conductance regulator (CFTR) [151]. CFTR is a highly unusual protein that displays some functional properties of a chloride channel but also those of an ATP-binding cassette ABC transporter; while most ABC transporters operate by active transport, CFTR is a Cl− channel through which Cl− passes by diffusion [152, 153]. SLC26Ax-CFTR complexes are thought to form in, e.g., the pancreas and salivary glands, where the native epithelial chloride absorption and bicarbonate secretion characteristics are more similar to those of the complexes than of CFTR alone; dysfunction of the complexes may contribute to diseases including cystic fibrosis and congenital chloride diarrhoea [154, 155]. Thus, CFTR mutations cause cystic fibrosis [156–158]; SLC26A2, A3 and A4 mutations cause diastrophic dysplasia [159], congenital chloride-losing diarrhoea [160], and Pendred syndrome (deafness, goitre and organification defects) [161], respectively.
The molecular basis of SLC26A-CFTR interaction has been well studied. The STAS domain of some SLC26A transporters has been found to mediate interaction with CFTR via the phosphorylation site-rich R domain of the latter, assisted by PDZ ligand-mediated binding of both proteins to scaffolding proteins such as CAP70 (Figure 3A). When non-phosphorylated, the CFTR R domain binds to CFTR NBD1 to stop it interacting with NBD2, which would result in CFTR Cl- channel activity. The SLC26A STAS domain stabilizes this auto-inhibition. When phosphorylated by PKA, R domain-NBD1 interaction is altered and R domain-STAS domain binding is enhanced – this activates both CFTR and SLC26A anion transport leading to fluid and electrolyte secretion [152, 154, 162, 163]. The isolated STAS domain from SLC26A3 potentiates CFTR open probability (Po) >threefold, and the number of active channels (n) × channel open probability (nPo) sixfold, while leaving total surface CFTR protein levels unaffected. Similarly, CFTR co-assembly potentiates SLC26A6 activity [154, 155]. There, is, however, further complexity to STAS-CFTR interactions – for SLC26A3 or SLC26A6 this interaction results in reciprocal potentiation, but in the case of SLC26A9, CFTR is activated but SLC26A9 is inhibited by the interaction [164, 165].
Figure 3. ABC and ATPase chansporter complex interactions.
A. High-resolution structures obtained by X-ray crystallography or cryo-EM are shown for proteins known to participate in chansporter complexes, or structures of their closest relatives for which high resolution structural information is available. Red arrows indicate direct physical interaction. Green or pink lines approximate membrane boundaries.
A. Deinococcus geothermalis SLC26 [191] in place of mammalian SLC26A transporters (e.g., prestin, pendrin), the rat prestin STAS domain [192, 193] known to interact with CFTR, and human CFTR [153]. CAP70 (a PDZ-domain containing scaffolding protein) is shown to mediate the interaction. TMD, transmembrane domain; NBD, nucleotide binding domain.
B. Mouse/Syrian hamster Kir6.2-SUR1, for which the complex structure has been solved by cryo-EM [194]; and polycystin-2 (TRPP2) [195] in place of TRPM4, which interacts with SUR1 (probably not while it is in complexes with Kir6.2).
C. Known 4:4 stoichiometry (Kir6.2:SUR1) viewed from outside the cell, coloured (left) from N-terminal (red) to C-terminal (blue) or (center) by individual subunits; right, hypothetical 4:4 stoichiometry (TRP:SUR1) viewed from outside the cell, coloured by individual subunits.
D. Drosophila melanogaster Orai1 [196] and rabbit SERCA (Sarco/Endoplasmic Reticulum Ca2+-ATPase) [197] in place of SPCA2 (Secretory Pathway Ca2+-ATPase 2).
ABC and ATPase interactions with ion channels
Sulfonylurea receptor interactions with ion channels
Sulfonylurea receptors (SURs) are integral membrane proteins that hydrolyse ATP. SURs are members of the ABC superfamily of active transporters, but unlike the majority of family members, SURs are not active transporters, nor do they even pass ions themselves (unlike CFTR). Instead, SURs are best known for acting as regulatory subunits for KATP channels, which comprise an octamer of four SURs (Sur1 or 2) and four Kir6.1 or Kir6.2 α subunits (Figure 3B, C). KATP channels act as metabolic sensors in tissues including the pancreas and heart; ATP binding to the Kir6.x subunits closes the channel, MgATP binding to SURs counteracts this by inducing a conformational change in the channel [166]. It is interesting, therefore, to view KATP channels as another example of channel-transporter complexes, albeit the “transporter” belongs to a transporter family without actually transporting anything. The theme of the “transporter” in a chansporter complex not actually transporting anything when in the complex appears to also apply to the Drosophila cupcake (encoded by SLC5A11) protein related to mammalian SMIT2 (also encoded by SLC5A11) (see above).
SUR1 also participates in another type of channel complex, with TRPM4, a calcium-activated monovalent cation-nonselective channel (Figure 3B). SUR1/TRPM4 channels are not expressed under normal physiological conditions, but are upregulated in neurons, astrocytes and capillary endothelial cells following various types of brain or spinal cord injury, and are thus important mediators of cerebral oedema formation. Co-assembly with SUR1 renders TRPM4 channel activity sensitive to drugs that bind to SUR1, including inhibitors glibenclamide and tolbutamaide, and activator diazoxide [167]. TRPM4/SUR1 channels only activate after ATP depletion [168]. As is observed for KATP channels, one could speculate a 4:4 octameric stoichiometry for SUR1/TRPM4 channels (Figure 3C).
Ca2+-ATPase interaction with Orai1
The secretory pathway Ca2+-ATPase isoform 2 (SPCA2, encoded by ATP2C2) is a Golgi-localized P-type calcium ion transporter that has the capacity, as with other ATPases, to catalyse the hydrolysis of ATP into ADP and a free phosphate ion. In the case of SPCA2, this energy is used to power uphill transport of Ca2+ to prime the secretory pathway (Golgi and post-Golgi vesicles). SPCA2 was found to form physical complexes with Orai1, a Ca2+ channel better known for its role in the plasma membrane mediating store-operated Ca2+ influx by communicating with the ER-localized STIM1 protein, which senses ER Ca2+ depletion [169]. In contrast, Orai1-SPCA2 complexes mediate Ca2+ signalling without STIM1 binding, and are thought to operate store-independently.
SPCA2-Orai1 complexes influence Ca2+ regulation and tumorigenicity in human mammary epithelium [170]. SPCA2 acts in this context as an ion channel chaperone and store-independent activator of Orai1, via a C-terminal domain on the transporter [170]. This control of Orai1 activity by the SPCA2 C-terminus is regulated by the SPCA2 N-terminus. In addition, the SPCA2 N-terminal is also important for SPCA2 Ca2+ transport activity [170–173]. Interestingly, a prior study had shown that a 20 kDa C-terminal fragment of SPCA2 is expressed by a short transcript, under the control of the MIST1 transcription factor, in pancreatic acinar cells and various glandular tissues. Germline deletion of MIST1 in mice eliminated the short transcript and also disrupted Ca2+ signalling [174], shown subsequently to be because of the ability of the C-terminal SPCA2 fragment to regulate Orai1. There is debate about exactly how SPCA2 and Orai1 work together to mediate store-independent Ca2+ entry, but one hypothesis is that SPCA2 positioned in membranes of the secretory pathway physically and functionally interacts with plasma membrane Orai1 to promote Ca2+ influx through both proteins, from the extracellular side into the secretory vesicles and/or Golgi [173] (Figure 3D).
4. Amino acid transporter (SLC6A11 and SLC1A2) interactions with ion and water channels
MaxiK-GAT3
Although no functional effects have yet been reported for this interaction, a recent proteomic study uncovered complex formation between MaxiK (a.k.a. BK or Slo1, a Ca2+-activated K+ channel), and GAT3, an electrogenic sodium-dependent gamma-aminobutyric acid (GABA) transporter encoded by SLC6A11 in mouse brain. GAT3 probably transports 2 Na+, one GABA and one Cl− per transport cycle, although the Cl- transport is under debate [175] (Figure 4A). Subsequent co-immunoprecipitation and co-localization studies using heterologous co-expression in human embryonic kidney (HEK) 293 cells supported the proteomic findings [176]. It will be fascinating to evaluate whether there are functional consequences of this interaction, which potentially has implications for regulation of neuronal excitability.
Figure 4. Amino acid transporter interactions with ion and water channels.
High-resolution structures obtained by X-ray crystallography or cryo-EM are shown for proteins known to participate in chansporter complexes, or structures of their closest relatives for which high resolution structural information is available. Red arrows indicate known or putative (“?”) direct physical interaction. Green lines approximate membrane boundaries.
A. Aplysia Slo1 channel [198, 199] and the SERT human serotonin receptor (SLC6A4) [200] in place of GAT3 (SLC6A11).
B. Human AQP4 [201], human EAAT1 [202] in place of EAAT2 (EAAT1 does not bind to AQP4), and mouse μ-opioid receptor [203].
AQP4-EAAT2
Deficiency in the water channel aquaporin 4 (AQP4) has long been known to downregulate glutamate uptake and expression of the sodium-dependent glutamate transporter, EAAT2 (Excitatory Amino Acid Transporter 2; also known as GLT-1, encoded by SLC1A2). This relationship is relatively specific as EAAT1 (GLAST, the sodium-dependent glutamate aspartate transporter, encoded by SLC1A3) was reportedly not affected by AQP4 deficiency [177]. Very recently, AQP4 was found to form a tripartite physical complex with EAAT2 and the μ-opioid receptor in astrocytes (Figure 4B). The co-assembly between AQP4 and EAAT2 is mediated by a region within amino acids 252–323 of the AQP4 C-terminus. Interestingly, morphine was found to regulate expression of the complex via activation of protein kinase C, and it is suggested that the complex might play a role in morphine dependence [178]. In another recent study, the astrocytic Fc receptor was found to be required for autoantibody-induced internalization of AQP4 and EAAT2, a finding which may aid development of therapies to treat neuromyelitis optica, an autoimmune disease in which AQP4 autoantibodies cause CNS immunopathology and secondary CNS demyelination [179]. The findings of these two studies together with others described above underline the potential importance of chansporter complexes and their disruption to human disease processes.
5. Sodium-chloride symporter (SLC12A3) interaction with ENaC
The sodium-chloride symporter, NCC (encoded by SLC12A3) is another secondary active transporter, which uses the sodium ion gradient across the apical membrane of distal convoluted tubule cells to electroneutrally transport sodium and chloride ions into the cells from tubular fluid [180]. NCC forms physical complexes in distal convoluted tubule cells with ENaC, an epithelial sodium channel that also brings Na+ into distal convoluted tubule cells, by diffusion down an electrochemical gradient [181]. NCC and ENaC reciprocally regulate one another, and blocking NCC using thiazide also impairs co-expressed ENaC function, by reducing its open probability [182].
6. Conclusions and Perspectives
New ion channel-transporter (chansporter) complexes are being discovered at an increasing rate. Chansporter complexes appear to represent a novel paradigm in cell signalling, facilitating crosstalk between channels and transporters and their responses to voltage, membrane lipids, osmolarity, and various ions and other solutes. In some cases, the “transporter” lacks the capacity to transport, (e.g., SUR1); in other cases small fragments of the transporter can achieve regulatory effects upon the channel in the absence of the rest of the transporter (e.g., SPCA2). Outstanding questions include the stoichiometry and specific interaction interfaces of chansporter complexes, which are mostly unknown, and how widely these complexes are in utilized in mammalian cell signalling. For instance, do the majority of channels or transporters participate in these types of complexes? Also, what were the primary evolutionary driving forces that favoured selection of the ability to form specific chansporter complexes? The answers will differ depending on the specific complex but there may be patterns or family-specific generalities regarding which specific evolutionary advantages might be conferred by formation of each of the various chansporter complexes. Several hypotheses are given below.
Nanodomains. Chansporter complexes may act in nanodomains that exist to reduce spatiotemporal barriers between different signalling mechanisms. Diffusion of substrates imported by the transporters may be important in some cases for regulating co-assembled channel function. In these instances, it may be advantageous to position specific channel-transporter pairs close to one another. In the case of myo-inositol transporters such as SMIT1, for example, the myo-inositol they bring in is converted to PIP2, which happens to be a potent regulator of channels that form complexes with SMIT1. Because PIP2 diffusion within the membrane is quite slow, KCNQ-SMIT proximity could help ensure locally high PIP2 concentrations for activation of KCNQ channels. This may be especially important in constrained spaces such as axons [130].
-
Biosensor sharing. Channels and transporters might co-assemble because it is advantageous to exploit one another’s biosensory capabilities. This is particularly obvious in the cases where one of the partners has lost its transport capabilities. In the case of KATP channels, although SURs do not possess transport machinery, they sense the metabolic state of the cell and report this to the Kir6 channels to regulate their function [166]. Likewise, cupcake may not possess sugar transport capabilities, but it is suggested to sense sugar levels [136, 137] and it is possible that via co-assembly, it can communicate these to dKCNQ to regulate feeding behaviour.
KCNQ1 is adept at sensing its immediate environment, and it is possible that by responding to this in the form of altered channel activity and/or conformation, it may be able to regulate co-assembled SMIT activity and thus act as a biosensor for its transporter partner. KCNQ1 requires bound calmodulin for channel trafficking and function. One possibility is that Ca2+ binding to KCNQ1-bound calmodulin exerts conformational effects that can be transmitted to co-assembled SMITs to Ca2+-dependently regulate myo-inositol intake. KCNQs, and all other voltage-gated ion channels, sense membrane potential via their VSDs. Thus, KCNQs can communicate information about the membrane potential of the cell to SMIT1 via either S4 position or channel activity, or perhaps both. Thus, we found that KCNQ2/3 co-expression enabled SMIT1 to maintain its transport activity during cellular depolarization, whereas without KCNQ2/3, cellular depolarization lowered SMIT1 transport activity [130]. In contrast, locking the KCNQ1 S4 open (to mimic aspects of cell depolarization without actually depolarizing the cell) inhibited activity of co-expressed SMIT1 [110]. In addition, the sensitivity of KCNQ1 to extracellular pH (pHo) is modulated by co-expressed KCNEs. For example, homomeric KCNQ1 is inhibited by low pHo whereas KCNQ1-KCNE2 activity is potentiated by low pHo [43]. It is possible, then, that depending on the co-assembled KCNE subunit, KCNQ1 could transmit information about extracellular pH to SMITs or other co-assembled transporters to regulate their activity.
Feedback. As described above, KCNQ1 activity is sensitive to PIP2 concentration, and may therefore communicate information about myo-inositol-derived PIP2 levels to bound SMITs to regulate their myo-insoitol uptake activity. This could be important in certain circumstances, e.g., if a strong downhill Na+ gradient was favouring excessive myo-inositol uptake but local PIP2 levels were already inappropriately high. Co-assembly with different KCNEs can change the PIP2 sensitivity of KCNQ1, the established example being that KCNE1 increases PIP2 sensitivity 100-fold compared to KCNQ1 alone [71]. Thus, cells may be able to tune how KCNQ1 controls SMIT activity by differentially expressing the various KCNEs.
Tuning of channel properties. We recently found that SMIT1 can alter the ion selectivity, gating kinetics and pharmacology of co-expressed KCNQs, via direct interaction with the pore module [114]. This is good evidence of close physical interaction, but some of these modifications may also serve a physiological role. We found that SMIT1 increased KCNQ Na+ permeability, which could alter action potential morphology or tune the resting membrane potential in the cells where these complexes occur. Interestingly, SMIT1 co-assembly increases KCNQ2/3 Cs+ permeability, potentially resolving a long-standing discrepancy between the relative permeability for Cs+ of cloned KCNQ2/3 versus native M current generated by this channel [114]. In addition, modification of KCNQ gating kinetics by SMIT1, which in the case of KCNQ1 was KCNE1-dependent, could also modify action potential morphology. We do not yet know whether addition of substrate changes the effects of SMIT1 on KCNQ channel properties, but this could add another dynamic mode of regulation or feedback.
Location and surface stability. Finally, membrane proteins each contain within their primary sequences information that determines how, when and where they traffic to the cell surface, how long they remain there, and what is their fate once they are recycled from the plasma membrane. When one or more different membrane proteins co-assemble, they can thus influence one another’s fate. In KATP channels, for example, Kir6 and SUR subunits contain ER retention motifs that dictate they can only reach the surface if co-assembled [183, 184]. For other chansporter complexes, the effects may be more subtle, but if complexes form early in biogenesis, could ensure that a specific channel only traffics to a particular axonal region or side of a polarized cell, when a specific transporter partner co-assembles with it. KCNEs can also dictate Kv α subunit subcellular localization, subunit composition, and endocytosis rate, and therefore could also dictate these same properties for specific chansporter complexes if the channel were the dominant partner in this respect. In this model, expression by the cell of specific channel subunit combinations could ensure that a partnered transporter reached the appropriate part of a cell to fulfil a specific task, in a specific manner dictated by the channel, and could also regulate how long the transporter remained in that location. Of course, the reciprocal is equally likely to be the case.
High-resolution structures are now available for either the proteins, or their close surrogates, within many of the known chansporter complexes (Figures 1–4). Thus, future in silico modelling guided by site-directed mutagenesis and functional assays, and vice-versa, may help elucidate how chansporter complexes fit together, how the partners regulate one another, and the reasons why complex formation offers an advantage over more conventional, long-range signalling.
Acknowledgments
GWA is grateful for financial support from the US National Institutes of Health (GM115189, DK41544, DC015736, DC015982).
Nonstandard abbreviations
- ABC
ATP-binding cassette
- CFTR
cystic fibrosis transmembrane conductance regulator
- CSF
cerebrospinal fluid
- ENaC
epithelial sodium channel
- NCC
sodium chloride cotransporter
- NIS
sodium iodide symporter
- PI
phosphatidyl inositol
- PIP2
phosphatidylinositol 4,5-bisphosphate
- SGLT1
sodium-dependent glucose transporter 1
- SMIT1
sodium-dependent myo-inositol transporter 1
- SMIT2
sodium-dependent myo-inositol transporter 2
- SPCA2
secretory pathway Ca2+-ATPase isoform 2
- STIM1
stromal interaction molecule 1
- SUR
sulfonylurea receptor
References
- 1.Deal KK, England SK, Tamkun MM. Molecular physiology of cardiac potassium channels. Physiol Rev. 1996;76(1):49–67. doi: 10.1152/physrev.1996.76.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Jan LY, Jan YN. Voltage-gated potassium channels and the diversity of electrical signalling. J Physiol. 2012;590(Pt 11):2591–9. doi: 10.1113/jphysiol.2011.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKinnon R. Potassium channels. FEBS Lett. 2003;555(1):62–5. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 4.Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991;64(5):871–4. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Molecular properties of sodium and calcium channels. J Bioenerg Biomembr. 1996;28(3):219–30. doi: 10.1007/BF02110697. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 7.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95(4):1383–436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SL, et al. Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc Natl Acad Sci U S A. 2011;108(43):17838–43. doi: 10.1073/pnas.1114821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296(2):L145–55. doi: 10.1152/ajplung.90525.2008. [DOI] [PubMed] [Google Scholar]
- 11.Bleich M, Warth R. The very small-conductance K+ channel KvLQT1 and epithelial function. Pflugers Arch. 2000;440(2):202–6. doi: 10.1007/s004240000257. [DOI] [PubMed] [Google Scholar]
- 12.Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25(4):239–49. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- 13.Hubner CA, Jentsch TJ. Channelopathies of transepithelial transport and vesicular function. Adv Genet. 2008;63:113–52. doi: 10.1016/S0065-2660(08)01005-5. [DOI] [PubMed] [Google Scholar]
- 14.Jentsch TJ, Maritzen T, Zdebik AA. Chloride channel diseases resulting from impaired transepithelial transport or vesicular function. J Clin Invest. 2005;115(8):2039–46. doi: 10.1172/JCI25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catterall WA. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found Symp. 2002;241:206–18. discussion 218–32. [PubMed] [Google Scholar]
- 16.Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol. 2010;588(Pt 17):3149–56. doi: 10.1113/jphysiol.2010.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perozo E. New structural perspectives on K(+) channel gating. Structure. 2002;10(8):1027–9. doi: 10.1016/s0969-2126(02)00812-2. [DOI] [PubMed] [Google Scholar]
- 18.Jentsch TJ, et al. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82(2):503–68. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 19.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219(3):713–25. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8(2):113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronson PS. Identifying secondary active solute transport in epithelia. Am J Physiol. 1981;240(1):F1–11. doi: 10.1152/ajprenal.1981.240.1.F1. [DOI] [PubMed] [Google Scholar]
- 22.Feng L, et al. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010;330(6004):635–41. doi: 10.1126/science.1195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe A, et al. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature. 2010;468(7326):988–91. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright EM, Hager KM, Turk E. Sodium cotransport proteins. Curr Opin Cell Biol. 1992;4(4):696–702. doi: 10.1016/0955-0674(92)90091-p. [DOI] [PubMed] [Google Scholar]
- 25.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 26.Scheiner-Bobis G. The sodium pump. Its molecular properties and mechanics of ion transport. Eur J Biochem. 2002;269(10):2424–33. doi: 10.1046/j.1432-1033.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- 27.Gorman AL, Marmor MF. Long-term effect of ouabain and sodium pump inhibition on a neuronal membrane. J Physiol. 1974;242(1):49–60. doi: 10.1113/jphysiol.1974.sp010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorman AL, Marmor MF. Steady-state contribution of the sodium pump to the resting potential of a molluscan neurone. J Physiol. 1974;242(1):35–48. doi: 10.1113/jphysiol.1974.sp010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picton LD, et al. Sodium Pumps Mediate Activity-Dependent Changes in Mammalian Motor Networks. J Neurosci. 2017;37(4):906–921. doi: 10.1523/JNEUROSCI.2005-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picton LD, Zhang H, Sillar KT. Sodium pump regulation of locomotor control circuits. J Neurophysiol. 2017:jn 00066. doi: 10.1152/jn.00066.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catterall WA. Localization of sodium channels in cultured neural cells. J Neurosci. 1981;1(7):777–83. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catterall WA. Structure and function of voltage-gated sodium and calcium channels. Curr Opin Neurobiol. 1991;1(1):5–13. doi: 10.1016/0959-4388(91)90004-q. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952;116(4):473–96. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116(4):449–72. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952;116(4):424–48. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32(7):1222–32. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spenney JG. Biochemical mechanisms of acid secretion by gastric parietal cells. J Clin Gastroenterol. 1983;5(Suppl 1):7–15. doi: 10.1097/00004836-198312001-00002. [DOI] [PubMed] [Google Scholar]
- 38.Shin JM, et al. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457(3):609–22. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MP, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106(12):1447–55. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442(6):896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 41.Grahammer F, et al. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120(6):1363–71. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- 42.Heitzmann D, et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J Physiol. 2004;561(Pt 2):547–57. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heitzmann D, et al. KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cell Physiol Biochem. 2007;19(1–4):21–32. doi: 10.1159/000099189. [DOI] [PubMed] [Google Scholar]
- 44.Roepke TK, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281(33):23740–7. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 45.Roepke TK, et al. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5(7):e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forte JG. K+ channels in the secretory membrane of the parietal cell. focus on “Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels”. Am J Physiol Cell Physiol. 2004;286(3):C478–9. doi: 10.1152/ajpcell.00531.2003. [DOI] [PubMed] [Google Scholar]
- 47.Fujita A, et al. Specific localization of an inwardly rectifying K(+) channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K(+) recycling for the H(+)-K(+)-pump. J Physiol. 2002;540(Pt 1):85–92. doi: 10.1113/jphysiol.2001.013439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song P, et al. Kir4.1 channel expression is essential for parietal cell control of acid secretion. J Biol Chem. 2011;286(16):14120–8. doi: 10.1074/jbc.M110.151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vucic E, et al. Kir1.1 (ROMK) and Kv7.1 (KCNQ1/KvLQT1) are essential for normal gastric acid secretion: importance of functional Kir1.1. Pflugers Arch. 2015;467(7):1457–68. doi: 10.1007/s00424-014-1593-0. [DOI] [PubMed] [Google Scholar]
- 50.Yuan J, et al. Potassium channel KCNJ15 is required for histamine-stimulated gastric acid secretion. Am J Physiol Cell Physiol. 2015;309(4):C264–70. doi: 10.1152/ajpcell.00012.2015. [DOI] [PubMed] [Google Scholar]
- 51.Abbott GW, Goldstein SA, Sesti F. Do all voltage-gated potassium channels use MiRPs? Circ Res. 2001;88(10):981–3. doi: 10.1161/hh1001.091869. [DOI] [PubMed] [Google Scholar]
- 52.Abbott GW, Xu X, Roepke TK. Impact of ancillary subunits on ventricular repolarization. J Electrocardiol. 2007;40(6 Suppl):S42–6. doi: 10.1016/j.jelectrocard.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harkcom WT, Abbott GW. Emerging concepts in the pharmacogenomics of arrhythmias: ion channel trafficking. Expert Rev Cardiovasc Ther. 2010;8(8):1161–73. doi: 10.1586/erc.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanda VA, Abbott GW. KCNE Regulation of K(+) Channel Trafficking - a Sisyphean Task? Front Physiol. 2012;3:231. doi: 10.3389/fphys.2012.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Post MA, Kirsch GE, Brown AM. Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current. FEBS Lett. 1996;399(1–2):177–82. doi: 10.1016/s0014-5793(96)01316-6. [DOI] [PubMed] [Google Scholar]
- 56.Stocker M, Hellwig M, Kerschensteiner D. Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily. J Neurochem. 1999;72(4):1725–34. doi: 10.1046/j.1471-4159.1999.721725.x. [DOI] [PubMed] [Google Scholar]
- 57.Papazian DM, et al. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237(4816):749–53. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 58.Tempel BL, Jan YN, Jan LY. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988;332(6167):837–9. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- 59.Yellen G, et al. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991;251(4996):939–42. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- 60.Anantharam A, Markowitz SM, Abbott GW. Pharmacogenetic considerations in diseases of cardiac ion channels. J Pharmacol Exp Ther. 2003;307(3):831–8. doi: 10.1124/jpet.103.054569. [DOI] [PubMed] [Google Scholar]
- 61.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47(6):787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Panaghie G, Abbott GW. The impact of ancillary subunits on small-molecule interactions with voltage-gated potassium channels. Curr Pharm Des. 2006;12(18):2285–302. doi: 10.2174/138161206777585175. [DOI] [PubMed] [Google Scholar]
- 63.Abbott GW. The KCNE2 K(+) channel regulatory subunit: Ubiquitous influence, complex pathobiology. Gene. 2015;569(2):162–72. doi: 10.1016/j.gene.2015.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbott GW. KCNE4 and KCNE5: K+ channel regulation and cardiac arrhythmogenesis. Gene. 2016 doi: 10.1016/j.gene.2016.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbott GW. KCNE1 and KCNE3: The yin and yang of voltage-gated K(+) channel regulation. Gene. 2016;576(1 Pt 1):1–13. doi: 10.1016/j.gene.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbott GW. Biology of the KCNQ1 potassium channel. New Journal of Science. 2014;2014 [Google Scholar]
- 67.Barhanin J, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 68.Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 69.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112(6):651–63. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tristani-Firouzi M, Sanguinetti MC. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (minK) subunits. J Physiol. 1998;510(Pt 1):37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, et al. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc Natl Acad Sci U S A. 2011;108(22):9095–100. doi: 10.1073/pnas.1100872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001(111):re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 73.Loussouarn G, et al. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22(20):5412–21. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakajo K, Kubo Y. KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel. J Gen Physiol. 2007;130(3):269–81. doi: 10.1085/jgp.200709805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakajo K, Kubo Y. Steric hindrance between S4 and S5 of the KCNQ1/KCNE1 channel hampers pore opening. Nat Commun. 2014;5:4100. doi: 10.1038/ncomms5100. [DOI] [PubMed] [Google Scholar]
- 77.Ruscic KJ, et al. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc Natl Acad Sci U S A. 2013;110(7):E559–66. doi: 10.1073/pnas.1222616110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strutz-Seebohm N, et al. Structural basis of slow activation gating in the cardiac I Ks channel complex. Cell Physiol Biochem. 2011;27(5):443–52. doi: 10.1159/000329965. [DOI] [PubMed] [Google Scholar]
- 79.Osteen JD, et al. Allosteric gating mechanism underlies the flexible gating of KCNQ1 potassium channels. Proc Natl Acad Sci U S A. 2012;109(18):7103–8. doi: 10.1073/pnas.1201582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osteen JD, et al. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc Natl Acad Sci U S A. 2010;107(52):22710–5. doi: 10.1073/pnas.1016300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neyroud N, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15(2):186–9. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 82.Rivas A, Francis HW. Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: a model of Jervell and Lange-Nielsen syndrome. Otol Neurotol. 2005;26(3):415–24. doi: 10.1097/01.mao.0000169764.00798.84. [DOI] [PubMed] [Google Scholar]
- 83.Tyson J, et al. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet. 1997;6(12):2179–85. doi: 10.1093/hmg/6.12.2179. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, et al. Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40(1):15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 85.Plant LD, et al. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1323548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang KW, Goldstein SA. Subunit composition of minK potassium channels. Neuron. 1995;14(6):1303–9. doi: 10.1016/0896-6273(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Xia J, Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem. 1998;273(51):34069–74. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
- 88.Yu H, et al. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proc Natl Acad Sci U S A. 2013;110(21):8732–7. doi: 10.1073/pnas.1300684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tinel N, et al. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 2000;19(23):6326–30. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roepke TK, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med. 2009;15(10):1186–94. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frohlich H, et al. Hypothyroidism of gene-targeted mice lacking Kcnq1. Pflugers Arch. 2011;461(1):45–52. doi: 10.1007/s00424-010-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Purtell K, et al. The KCNQ1-KCNE2 K(+) channel is required for adequate thyroid I(−) uptake. FASEB J. 2012;26(8):3252–9. doi: 10.1096/fj.12-206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boini KM, et al. Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. Am J Physiol Regul Integr Comp Physiol. 2009;296(6):R1695–701. doi: 10.1152/ajpregu.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, et al. Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ Cardiovasc Genet. 2014;7(1):33–42. doi: 10.1161/CIRCGENETICS.113.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SM, et al. Kcne2 deletion impairs insulin secretion and causes type 2 diabetes mellitus. FASEB J. 2017 doi: 10.1096/fj.201601347. [DOI] [PubMed] [Google Scholar]
- 96.Roepke TK, et al. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. FASEB J. 2011;25(12):4264–73. doi: 10.1096/fj.11-187609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi E, Abbott GW. A shared mechanism for lipid- and beta-subunit-coordinated stabilization of the activated K+ channel voltage sensor. FASEB J. 2010;24(5):1518–24. doi: 10.1096/fj.09-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gage SD, Kobertz WR. KCNE3 truncation mutants reveal a bipartite modulation of KCNQ1 K+ channels. J Gen Physiol. 2004;124(6):759–71. doi: 10.1085/jgp.200409114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melman YF, Krumerman A, McDonald TV. A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J Biol Chem. 2002;277(28):25187–94. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- 100.Melman YF, Krummerman A, McDonald TV. KCNE regulation of KvLQT1 channels: structure-function correlates. Trends Cardiovasc Med. 2002;12(4):182–7. doi: 10.1016/s1050-1738(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 101.Melman YF, et al. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42(6):927–37. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 102.Panaghie G, Tai KK, Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol. 2006;570(Pt 3):455–67. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129(2):121–33. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Panaghie G, et al. Voltage-dependent C-type inactivation in a constitutively open K+ channel. Biophys J. 2008;95(6):2759–78. doi: 10.1529/biophysj.108.133678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schroeder BC, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403(6766):196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 106.Ciampa EJ, et al. KCNE4 juxtamembrane region is required for interaction with calmodulin and for functional suppression of KCNQ1. J Biol Chem. 2011;286(6):4141–9. doi: 10.1074/jbc.M110.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grunnet M, et al. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542(Pt 1):119–30. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grunnet M, et al. hKCNE4 inhibits the hKCNQ1 potassium current without affecting the activation kinetics. Biochem Biophys Res Commun. 2005;328(4):1146–53. doi: 10.1016/j.bbrc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 109.Angelo K, et al. KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophys J. 2002;83(4):1997–2006. doi: 10.1016/S0006-3495(02)73961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abbott GW, et al. KCNQ1, KCNE2, and Na+-Coupled Solute Transporters Form Reciprocally Regulating Complexes That Affect Neuronal Excitability. Sci Signal. 2014;7(315):ra22. doi: 10.1126/scisignal.2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwon HM, et al. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992;267(9):6297–301. [PubMed] [Google Scholar]
- 112.Berry GT, et al. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995;25(2):507–13. doi: 10.1016/0888-7543(95)80052-n. [DOI] [PubMed] [Google Scholar]
- 113.Hediger MA, et al. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330(6146):379–81. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 114.Manville RW, Neverisky DL, Abbott GW. SMIT1 modifies KCNQ potassium channel ion selectivity, gating and pharmacology by physical interaction with the pore. Biophysical Journal. 2017 doi: 10.1016/j.bpj.2017.06.055. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang HS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282(5395):1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 116.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 117.Main MJ, et al. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58(2):253–62. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- 118.Schroeder BC, et al. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275(31):24089–95. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- 119.Selyanko AA, et al. Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J Physiol. 2000;522(Pt 3):349–55. doi: 10.1111/j.1469-7793.2000.t01-2-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156(8):1185–95. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Devaux JJ, et al. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24(5):1236–44. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klinger F, et al. Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwarz JR, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573(Pt 1):17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kosenko A, et al. Coordinated signal integration at the M-type potassium channel upon muscarinic stimulation. EMBO J. 2012;31(14):3147–56. doi: 10.1038/emboj.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suh BC, et al. Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by Gq. J Gen Physiol. 2004;123(6):663–83. doi: 10.1085/jgp.200409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winks JS, et al. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25(13):3400–13. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q, et al. Dynamic PIP2 interactions with voltage sensor elements contribute to KCNQ2 channel gating. Proc Natl Acad Sci U S A. 2013;110(50):20093–8. doi: 10.1073/pnas.1312483110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou P, et al. Phosphatidylinositol 4,5-bisphosphate alters pharmacological selectivity for epilepsy-causing KCNQ potassium channels. Proc Natl Acad Sci U S A. 2013;110(21):8726–31. doi: 10.1073/pnas.1302167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dai G, et al. Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P2 levels. Proc Natl Acad Sci U S A. 2016;113(23):E3290–9. doi: 10.1073/pnas.1606348113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neverisky DL, Abbott GW. KCNQ-SMIT complex formation facilitates ion channel-solute transporter cross talk. FASEB J. 2017 doi: 10.1096/fj.201601334R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cho H, et al. Low mobility of phosphatidylinositol 4,5-bisphosphate underlies receptor specificity of Gq-mediated ion channel regulation in atrial myocytes. Proc Natl Acad Sci U S A. 2005;102(42):15241–6. doi: 10.1073/pnas.0408851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berry GT, et al. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J Biol Chem. 2003;278(20):18297–302. doi: 10.1074/jbc.M213176200. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe H, et al. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J Neurochem. 2000;75(1):28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- 134.Mulkey SB, et al. Neonatal nonepileptic myoclonus is a prominent clinical feature of KCNQ2 gain-of-function variants R201C and R201H. Epilepsia. 2017;58(3):436–445. doi: 10.1111/epi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chau JF, et al. Sodium/myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB J. 2005;19(13):1887–9. doi: 10.1096/fj.05-4192fje. [DOI] [PubMed] [Google Scholar]
- 136.Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nat Neurosci. 2013;16(5):526–8. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park JY, et al. Drosophila SLC5A11 Mediates Hunger by Regulating K(+) Channel Activity. Curr Biol. 2016;26(15):1965–74. doi: 10.1016/j.cub.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kubisch C, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96(3):437–46. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 139.Gao Y, et al. Impaired surface expression and conductance of the KCNQ4 channel lead to sensorineural hearing loss. J Cell Mol Med. 2013;17(7):889–900. doi: 10.1111/jcmm.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao Y, et al. Distinct roles of molecular chaperones HSP90alpha and HSP90beta in the biogenesis of KCNQ4 channels. PLoS One. 2013;8(2):e57282. doi: 10.1371/journal.pone.0057282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dallos P, Fakler B. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol. 2002;3(2):104–11. doi: 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- 142.Liberman MC, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419(6904):300–4. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 143.Bai JP, et al. Current carried by the Slc26 family member prestin does not flow through the transporter pathway. Sci Rep. 2017;7:46619. doi: 10.1038/srep46619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bai JP, et al. Prestin’s anion transport and voltage-sensing capabilities are independent. Biophys J. 2009;96(8):3179–86. doi: 10.1016/j.bpj.2008.12.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rybalchenko V, Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys J. 2008;95(9):4439–47. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Song L, Santos-Sacchi J. Conformational state-dependent anion binding in prestin: evidence for allosteric modulation. Biophys J. 2010;98(3):371–6. doi: 10.1016/j.bpj.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Song L, Seeger A, Santos-Sacchi J. On membrane motor activity and chloride flux in the outer hair cell: lessons learned from the environmental toxin tributyltin. Biophys J. 2005;88(3):2350–62. doi: 10.1529/biophysj.104.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheatham MA, et al. Cochlear function in Prestin knockout mice. J Physiol. 2004;560(Pt 3):821–30. doi: 10.1113/jphysiol.2004.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu XZ, et al. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet. 2003;12(10):1155–62. doi: 10.1093/hmg/ddg127. [DOI] [PubMed] [Google Scholar]
- 150.Chambard JM, Ashmore JF. Regulation of the voltage-gated potassium channel KCNQ4 in the auditory pathway. Pflugers Arch. 2005;450(1):34–44. doi: 10.1007/s00424-004-1366-2. [DOI] [PubMed] [Google Scholar]
- 151.Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4(2):107–116. doi: 10.1007/s12551-012-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440(7083):477–83. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu F, et al. Molecular Structure of the Human CFTR Ion Channel. Cell. 2017;169(1):85–95 e8. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 154.Ko SB, et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6(4):343–50. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shcheynikov N, et al. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006;273:177–86. discussion 186–92, 261–4. [PubMed] [Google Scholar]
- 156.Kerem B, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 157.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 158.Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059–65. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 159.Hastbacka J, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78(6):1073–87. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 160.Hoglund P, et al. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14(3):316–9. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 161.Everett LA, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17(4):411–22. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 162.Baker JM, et al. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14(8):738–45. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y, et al. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: relevance to cystic fibrosis. EMBO J. 2006;25(21):5049–57. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bertrand CA, et al. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133(4):421–38. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang MH, et al. Slc26a9 is inhibited by the R-region of the cystic fibrosis transmembrane conductance regulator via the STAS domain. J Biol Chem. 2009;284(41):28306–18. doi: 10.1074/jbc.M109.001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res. 2013;112(7):1059–72. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci. 2003;23(24):8568–77. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg. 2010;113(3):622–9. doi: 10.3171/2009.11.JNS081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feng M, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143(1):84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cross BM, et al. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS One. 2013;8(6):e67348. doi: 10.1371/journal.pone.0067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feng MY, Rao R. New insights into store-independent Ca(2+) entry: secretory pathway calcium ATPase 2 in normal physiology and cancer. Int J Oral Sci. 2013;5(2):71–4. doi: 10.1038/ijos.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smaardijk S, et al. SPCA2 couples Ca2+ influx via Orai1 to Ca2+ uptake into the Golgi/secretory pathway. Tissue Cell. 2017;49(2 Pt A):141–149. doi: 10.1016/j.tice.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 174.Garside VC, et al. MIST1 regulates the pancreatic acinar cell expression of Atp2c2, the gene encoding secretory pathway calcium ATPase 2. Exp Cell Res. 2010;316(17):2859–70. doi: 10.1016/j.yexcr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scimemi A. Structure, function, and plasticity of GABA transporters. Front Cell Neurosci. 2014;8:161. doi: 10.3389/fncel.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singh H, et al. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 2016;317:76–107. doi: 10.1016/j.neuroscience.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeng XN, et al. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci. 2007;34(1):34–9. doi: 10.1016/j.mcn.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 178.Wang H, et al. Aquaporin 4 Forms a Macromolecular Complex with Glutamate Transporter 1 and Mu Opioid Receptor in Astrocytes and Participates in Morphine Dependence. J Mol Neurosci. 2017;62(1):17–27. doi: 10.1007/s12031-017-0905-1. [DOI] [PubMed] [Google Scholar]
- 179.Hinson SR, et al. Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. Proc Natl Acad Sci U S A. 2017;114(21):5491–5496. doi: 10.1073/pnas.1701960114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moes AD, et al. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch. 2014;466(1):107–18. doi: 10.1007/s00424-013-1407-9. [DOI] [PubMed] [Google Scholar]
- 181.Barbry P, Hofman P. Molecular biology of Na+ absorption. Am J Physiol. 1997;273(3 Pt 1):G571–85. doi: 10.1152/ajpgi.1997.273.3.G571. [DOI] [PubMed] [Google Scholar]
- 182.Mistry AC, et al. The Sodium Chloride Cotransporter (NCC) and Epithelial Sodium Channel (ENaC) Associate. Biochem J. 2016 doi: 10.1042/BCJ20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwappach B, et al. Molecular basis for K(ATP) assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26(1):155–67. doi: 10.1016/s0896-6273(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 184.Zerangue N, et al. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22(3):537–48. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 185.Mistry AC, et al. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem J. 2016;473(19):3237–52. doi: 10.1042/BCJ20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun J, MacKinnon R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell. 2017;169(6):1042–1050 e9. doi: 10.1016/j.cell.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kroncke BM, et al. Structural basis for KCNE3 modulation of potassium recycling in epithelia. Sci Adv. 2016;2(9):e1501228. doi: 10.1126/sciadv.1501228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rose AS, Hildebrand PW. NGL Viewer: a web application for molecular visualization. Nucleic Acids Res. 2015;43(W1):W576–9. doi: 10.1093/nar/gkv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rose PW, et al. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017;45(D1):D271–D281. doi: 10.1093/nar/gkw1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321(5890):810–4. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Geertsma ER, et al. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nat Struct Mol Biol. 2015;22(10):803–8. doi: 10.1038/nsmb.3091. [DOI] [PubMed] [Google Scholar]
- 192.Lolli G, et al. The STAS domain of mammalian SLC26A5 prestin harbours an anion-binding site. Biochem J. 2016;473(4):365–70. doi: 10.1042/BJ20151089. [DOI] [PubMed] [Google Scholar]
- 193.Pasqualetto E, et al. Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. J Mol Biol. 2010;400(3):448–62. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 194.Li N, et al. Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell. 2017;168(1–2):101–110 e10. doi: 10.1016/j.cell.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 195.Shen PS, et al. The Structure of the Polycystic Kidney Disease Channel PKD2 in Lipid Nanodiscs. Cell. 2016;167(3):763–773 e11. doi: 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hou X, et al. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–13. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Clausen JD, et al. Crystal Structure of the Vanadate-Inhibited Ca(2+)-ATPase. Structure. 2016;24(4):617–23. doi: 10.1016/j.str.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 198.Hite RK, Tao X, MacKinnon R. Structural basis for gating the high-conductance Ca2+-activated K+ channel. Nature. 2017;541(7635):52–57. doi: 10.1038/nature20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tao X, Hite RK, MacKinnon R. Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel. Nature. 2017;541(7635):46–51. doi: 10.1038/nature20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532(7599):334–9. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ho JD, et al. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci U S A. 2009;106(18):7437–42. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Canul-Tec JC, et al. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature. 2017;544(7651):446–451. doi: 10.1038/nature22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Manglik A, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–6. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]