Abstract
Introduction
Despite improvements in treatment, survival rates of head and neck squamous cell carcinoma (HNSCC) are stagnant. The existing chemotherapeutic agents are non-selective and associated with toxicities. Combinations of the only the US FDA-approved epidermal growth factor receptor (EGFR)-targeted agent, cetuximab, with chemotherapy or radiation improves overall survival. However, the response rates to cetuximab are modest. Thus, there is an urgent need for new agents that can be safely integrated into current treatment regimens to improve outcome.
Areas covered
Current EGFR-targeted drugs under clinical development include mAbs and tyrosine kinase inhibitors. The modest efficacy of these drugs implicates intrinsic or acquired resistance. Novel molecular agents inhibiting alternative targets to overcome anti-EGFR resistance in HNSCC are under investigation. Gene therapy and immunotherapy are also promising strategies to improve efficacy and reduce toxicity.
Expert opinion
To date, only six drugs have been FDA-approved for the treatment of head and neck cancer. Cetuximab is the only approved molecular targeting agent for HNSCC and despite ubiquitous expression of EGFR in HNSCC tumors, clinical responses are limited. Genetic and epigenetic characterization of HNSCC tumors, coupled with improved preclinical models, should facilitate the development of more effective drugs.
Keywords: cetuximab, chemotherapy, clinical trial, drugs resistance, epidermal growth factor receptor, gene therapy, head and neck cancer, immunotherapy, squamous cell carcinoma, The Cancer Genome Atlas
1. Background
Head and neck cancers comprise a spectrum of malignancies arising in the oral cavity, pharynx and larynx with squamous cell carcinoma representing the most common histology (~ 85%) [1]. Worldwide, head and neck squamous cell carcinoma (HNSCC) is the/sixth most common cancer with an incidence of over 600,000 and over 350,000 deaths per year [2]. Of newly diagnosed patients, about two-thirds present with advanced-stage disease, usually with regional lymph node involvement [3], and 10% have distant metastases [4]. The most common predisposing factors include tobacco exposure and alcohol consumption. In addition, an increasing number of oropharyngeal cancers are linked with human papilloma virus (HPV) infection [5]. HNSCC treatment generally involves several modalities including surgery, radiotherapy (RT) and chemotherapy (CT) depending on the site of the primary tumor, TNM staging, expected oncological and functional outcomes and the treatment toxicities. Generally, single-modality treatment may be chosen for early stage disease while advanced stages require a multidisciplinary approach [1]. In the last few years, the integration of surgery, RT and CT has become the standard of care [1].
The US FDA to date has approved six agents for the treatment of HNSCC including five conventional CT drugs (cisplatin, methotrexate, 5-flurouracil [5-FU], bleomycin and docetaxel) and one targeted agent (cetuximab). Platinums, including cisplatin and carboplatin are the most commonly used CT agents for HNSCC treatment with responses in 13 – 40% of cases. They belong to the alkylating class of drugs that exert their effects by forming covalent bonds with DNA. Taxanes act by inhibiting microtubule disassembly, cell cycle arrest and apoptosis in the G2/M phase of the cell cycle with a response rate (RR) of 20 – 40%. Docetaxel is a second-generation taxane that displays potent and broad antineoplastic properties, however, with a similar RR of 20 – 42% as single agent in recurrent/metastasis (R/M) HNSCC [6]. Other agents, including methotrexate, 5-FU and bleomycin are associated with overall responses ranging from 20 to 40% [7]. The combination of cisplatin and 5-FU (PF regimen) is widely used [8]. However, a series of clinical trials and meta-analyses have suggested that docetaxel plus the standard PF regimen (the TPF regimen) given as induction CT may improve outcomes with acceptable toxicity rates in locoregionally advanced (LA) HNSCC compared with PF regimen [9–11]. Considering that these drugs can induce DNA damage, one theoretical complication from treatment of the primary tumor is a therapy-related secondary cancer that can result from the genotoxic activity of the drugs on healthy cells, irrespective of early phase toxicity [12,13]. In general, cytotoxic CT drugs are only cancer selective based on relative cell division rates in tumors compared with normal tissue. Targeted therapies have been developed to exploit unique tumor factors with the hope of improving efficacy and reducing toxicity.
Cetuximab, an epidermal growth factor receptor (EGFR) mAb, is currently the only FDA-approved EGFR targeting strategy for HNSCC. It is approved in three specific applications: in combination with radiation for LA disease, as a single agent for R/M disease after failure of platinum-based CT and in combination with platinum-based CT plus 5-FU for the first-line R/M HNSCC [14–16]. The National Comprehensive Cancer Network guidelines also suggest that cetuximab plus platinum (cisplatin or carboplatin) and 5-FU is a category one treatment option for patients with unresectable or R/M non-nasopharyngeal HNSCC [17]. Cetuximab is a chimeric human/murine IgG1 mAb, which binds with high affinity to EGFR. Its antitumor mechanisms include the inhibition of natural ligand-binding receptor activation, initiation of receptor endocytosis and the activation of antibody-dependent cell-mediated cytotoxicity (ADCC). It also inhibits DNA double-strand-break repair that contributes to RT and DNA damage-inducing CT resistance by preventing nuclear import of EGFR [18–21].
Drug resistance (intrinsic or acquired) is one of the major challenges in the current therapy of HNSCC. Despite wide-spread EGFR expression, many HNSCC tumors do not respond to EGFR-targeting therapies. Identification of predictive biomarkers to better select those patients who are likely to benefit from these costly treatments is likely to improve outcomes [22]. One major obstacle is the genetic heterogeneity of cancer, including HNSCC. Analysis of publically available databases that describe the genetic and epigenetic lesions in HNSCC, like The Cancer Genome Atlas (TCGA), is likely to provide valuable insight into the biology of this cancer [23–26].
This review focuses on the major drugs under clinical investigation in HNSCC treatment, including EGFR-targeted drugs and molecular agents inhibiting alternative targets.
2. Medical need
HNSCC is the sixth most common cancer in the world with an incidence of over 600,000 per year [2]. About two-thirds present with advanced-stage disease. For these patients, 5-year survival rates are < 50% [27]. Although conventional treatments have improved, survival rates for HNSCC have remained relatively stagnant over the past three decades [28], ignoring the intrinsic favorable prognosis in HPV-positive patients. The aggressive chemoradiation necessitated by advanced HNSCC disease often results in severe side effects (xerostomia, dysphagia, etc.) that compromise post-treatment quality of life [29]. Moreover, the existing cytotoxic chemotherapeutic agents are non-selective and considerable toxicity. Thus, there is an urgent need for new agents that can be safely integrated into current treatment regimens to improve both the tolerability and the efficacy. The only FDA-approved EGFR-targeted agent, cetuximab, shows improvement in overall survival (OS) for HNSCC patients when in combination of CT or RT. However, the RR as a single agent of cetuximab is consistently lower than 15% [30]. Therefore, novel molecular treatments in HNSCC are also needed in order to overcome drug resistance.
3. Existing treatment
3.1 Conventional treatment
Conventionally, HNSCC treatments include surgery, RT and CT. Surgery is a standard treatment for HNSCC and is frequently dependent on the anatomical site and extent of tumor and the desire to achieve organ preservation. Advances in reconstruction techniques make it possible for patients who need extensive surgical resections in LA-HNSCC and are substantially improved functional outcomes, even for the setting who need salvage surgery after failure of organ-preserving treatment. Neck dissection is usually carried out in primary surgical management or after chemoradiotherapy when residual disease is suspected. Nowadays, attention has been gained for the emerging sentinel lymph node biopsy and its ability to reliably detect nodal metastasis in HNSCC. Moreover, advances in minimally invasive endoscopic laser or robotic techniques have reduced postoperative complication and is related to a better organ and functional preservation [1,4,31]. Nevertheless, even continued evolution in surgical techniques, surgery alone is accompanied by a high risk of relapse in LA-HNSCC, and combination with other treatments is usually indicated. RT alone for early stage HNSCC can give rise to high tumor control and cure rates, but for LA-HNSCC, it is an integral part of primary or adjuvant treatment. Intensity-modulated radiation therapy (IMRT) represents an improvement of high-precision radiation in three dimensions that delivers radiation more precisely to the tumor while relatively sparing the surrounding normal tissues [4].
It has been well established that hypoxia is present in HNSCC and compromise therapeutic response to RT. This source of radiation resistance can be eliminated or modified by normobaric or hyperbaric oxygen or by the use of nitroimidazoles as hypoxic radiation sensitizers, which is called hypoxic modification. This treatment strategy has shown significant improvement of RT in a randomized trial [32] and using a genetic classifier [33] to identify patients with HPV/p16-negative HNSCC who might benefit from hypoxic modification is presently being investigated in a clinical trial (EORTC-1219-ROG-HNCG/DAHANCA29; http://www.eortc.org/).
CT is an integral part from palliative care to an important component of curative treatment for LA-HNSCC and is often administered concurrently with RT (known as concurrent chemoradiotherapy [CRT]) or before RT (known as induction CT) [7]. The platinum compounds cisplatin and carboplatin are regarded as standard agents in combination with RT or with other agents. They can serve as radiosensitizers due to their inhibition of sublethal damage repair, selective radiosensitization of hypoxic cells, reoxygenation and redistribution of cells towards more sensitive cell cycle phases. Although the radiosensitizing properties of the two platinum agents could be comparable, carboplatin is less active but well tolerated [31]. Taxane-based combination (TPF regimen) is very active and has been tested in the induction CT of LA-HNSCC with improved outcomes and acceptable toxicity compared with PF regimen [11].
Unlike early stage HNSCC can benefit from surgery or RT alone, LA-HNSCC represents the majority of patients with head and neck cancer and requires a combination of CT, RT or surgery. CRT has been demonstrated with better results than RT alone or the sequential administration of CT and RT in several Phase III clinical trials [34–37]. Meta-analyses of HNSCC showed an absolute 5-year survival benefit of 8% [38] for CRT, 6.5% for concomitant CT and RT and only 2.4% for induction CT followed by RT [39] compared with RT alone. However, CRT has an insignificant effect on distant recurrence rate and results in increased acute complications including mucositis, dermatitis and myelosuppression.
For R/M HNSCC, the goals of treatment are both to palliate symptoms and extend survival. Methotrexate, bleomycin, cisplatin, 5-FU and docetaxel are active as single agents in this setting [40]. However, multi-agent CT, mostly PF or TPF regimen, is the standard of care with superior RR. Despite therapeutic improvement, even optimization of surgery, RT and chemotherapeutic approaches in terms of balancing efficacy and safety/toxicity, the 5-year survival rate remains relatively stagnant. Higher doses of CT in an attempt to increase RR have given rise to unacceptable toxicity and healthy tissue damage [41]. Targeted therapies have been developed to exploit unique tumor factors with the hope of improving efficacy and reducing toxicity.
3.2 Targeted treatment – cetuximab
Cetuximab, an EGFR mAb, is currently the only FDA-approved EGFR-targeting strategy for HNSCC. It is approved based on a milestone randomized Phase III trial comparing cetuximab in combination with high-dose RT (211 patients) versus high-dose RT alone (213 patients) in patients with LA-HNSCC [14]. Cetuximab was initiated at a loading dose of 400 mg/m2 a week before RT, followed by 250 mg/m2/week during RT. The combination of cetuximab and RT significantly improved median OS (49.0 vs 29.3 months) and median progression-free survival (PFS; 17.1 vs 12.4 months) versus RT alone. It is noteworthy that even in combination with cetuximab, the incidence of radiation-associated acute toxicities was not increased. Five-year survival rates were 45.6% for cetuximab/RT versus 36.4% for RT alone [42]. This beneficial effect was seen mainly in patients with oropharyngeal cancers, the primary site of HPV-associated HNSCC, and was less evident in patients with laryngeal or hypopharyngeal cancers. Improvement in OS was limited to patients with prominent acneiform rash (hazard ratio: 0.49), suggesting that the development of a rash may predict cetuximab responses.
A number of ongoing Phase III trials are attempting to expand the use of cetuximab in LA-HNSCC, including induction TPF regimen CT followed by cisplatin/RT versus cetuximab/RT (NCT00716391, NCT00999700) and cetuximab/RT versus carboplatin/5-FU/RT (NCT01233843). Another four-arm Phase III trial included RT/concomitant CT or RT/concomitant cetuximab with induction TPF versus RT/concomitant CT or RT/concomitant cetuximab, without induction TPF (NCT01086826). Although the final results have not been published, the latest report from a Phase III trial conducted by the Radiation Therapy Oncology Group 0522 investigating the addition of cetuximab to the radiation-cisplatin platform for LA-HNSCC demonstrated no significant improvement in 30-day mortality (1.8 vs 2.0%, respectively; p = 0.81), 3-year PFS (61.2 vs 58.9%, respectively; p = 0.76), 3-year OS (72.9 vs 75.8%, respectively; p = 0.32), locoregional failure (19.9 vs 25.9%, respectively; p = 0.97) or distant metastasis (13.0 vs 9.7%, respectively; p = 0.08) and higher local toxicity was observed in the cetuximab arm [43]. They concluded that adding cetuximab to radiation-cisplatin did not improve outcome and hence should not be prescribed routinely. Outcomes did not differ by EGFR expression. As with all treatment regimens, higher PFS and OS were observed in patients with HPV-associated HNSCC.
Cetuximab has also been evaluated as monotherapy or in combination with CT in patients with R/M platinum-refractory HNSCC. Four hundred and forty-two patients with R/M HNSCC in a Phase III EXTREME trial [16] were randomized to receive platinum-based therapy or in combination with cetuximab as a first-line palliative regimen. A significant benefit of additional cetuximab with an improvement in median OS from 7.4 to 10.1 months (p = 0.04) was reported. No increase of CT-associated toxicities was observed in the cetuximab-containing arm. Combined analysis of p16 and HPV in this trial shows that adding cetuximab to CT improved survival, irrespective of tumor p16 or HPV status [44]. Three Phase II studies also demonstrated the efficacy of cetuximab in combination with platinum-based CT as second-line treatment in patients with R/M HNSCC who failed to respond to first-line platinum-based CT alone [15,45,46]. Reported RRs and median OS were ~ 10 – 13% and 5 – 6 months, respectively. An indirect comparison of these three Phase II trials suggests that cetuximab has the potential to increase median OS by ~ 2 months [30].
Despite the fact that combination of cetuximab with RT improves OS and PFS in LA-HNSCC compared with RT alone, addition of cetuximab with platinum-based CT improves median OS to 10.1 months in R/M HNSCC, latest Phase III trial demonstrated that adding cetuximab to CRT did not gain improvement of outcomes. Plus, the RR with cetuximab as a single agent is consistently lower than 15% [30] due to unclear resistance mechanisms. Hence, a better understanding of the molecular mechanisms of resistance to cetuximab may provide insights in new drugs development and identifying predictive biomarkers to optimize treatment strategies and lead to personalized therapy [47].
4. Market review
The expenditure of cancer therapy in the USA was $27 billion in 1990, and expanded to $90 billion in 2008, and is projected to jump to $157 billion by 2020 [48]. Much of this growth can be attributed to targeted therapies, as the globally cost grew to $13 billion in 2006 compared with $1.3 billion in 2001 [49]. Cetuximab sales for HNSCC treatment accounted for 30 – 40% of the worldwide market with a revenue of > $2 billion in 2009 [28]. As the only FDA-approved biological agent for HNSCC, cetuximab is expected to sustain continuing sales growth in the foreseeable future. Globally, the cost of cancer therapy is now growing at 21% per year [49]. As cancer therapy continues shifting towards biological agents and increasingly personalized-tailoring regimens, the cost of these drugs is expected to increase considerably due to the high cost of biological agents compared with conventional chemotherapeutics. Personalized medicine market in the USA was estimated at $232 billion in 2009 and is expected to grow 11% annually, nearly doubling to > $450 billion by 2015 [48]. Accordingly, the overall size of the head and neck cancer drugs market is projected to increase.
5. Current research goals
Conventional RT and CT are non-selective and therapies have been developed to exploit unique tumor factors with the hope of improving efficacy and reducing toxicity. The only FDA-approved molecular targeted agent, cetuximab, shows a RR lower than 15% [30] as a single agent in HNSCC treatment. Other EGFR-targeted agents such as tyrosine kinase inhibitors (TKIs) have not demonstrated improved survival in unselected populations. Thus, a better understanding of both predictive biomarkers and mechanisms of resistance to EGFR-targeted agents may lead to strategies to overcome resistance, and identify alternative targets for HNSCC treatment. The primary goal in the development of new drugs is to increase efficacy and reduce toxicity. As only a small subset of patients respond to EGFR-targeted therapy, identification of predictive biomarkers to select HNSCC patients most likely to benefit from cetuximab is warranted. Advances in basic research and analysis of genomic and epigenomic databases, like TCGA, are likely to facilitate deeper understanding of HNSCC biology and guide therapeutic developments.
6. Scientific rationale
6.1 EGFR biology and EGFR-targeted therapy
The EGFR is a transmembrane glycoprotein belonging to the EGFR tyrosine kinase family, which consists of EGFR/human EGFR 1 (HER1)/ErbB1, HER2/neu/ErbB2, HER3/ErbB3, HER4/ErbB4. All the four family members contain an extracellular ligand-binding domain, membrane-spanning domain and a cytoplasmic tyrosine kinase domain [47]. Aberrant expression or activity of EGFR has been identified in many human epithelial cancers, including HNSCC. Activation of EGFR by different ligands leads to homo- or heterodimerization of EGFR with another receptor of the HER family, and autophosphorylation of tyrosine residues leads to the activation of downstream signaling cascades including the well-known MAPK, phosphatidylinositol-3-kinase (PI3K)/Akt and STAT pathways that control gene transcription, cell proliferation and anti-apoptotic signals [50]. These ligands can be divided into three groups. The first group specifically binds to EGFR and includes epidermal growth factor (EGF), TGF-α and amphiregulin. The second group binds to both EGFR and HER4 and includes epiregulin, β-cellulin and heparin-binding EGF. The third group is composed of the neuregulins 1 – 4 and only binds to HER3 and HER4 [47]. HER2 has no known ligand. HER3 is the only family member that has no intrinsic kinase activity but downstream signaling is readily achieved through heterodimerization [47]. Despite the fact that clinical trials of EGFR-targeted therapies have rarely demonstrated a correlation between EGFR overexpression and the efficacy of EGFR-targeted therapies, the early studies suggest that increased EGFR expression and gene copy number correlate with poorer prognosis [51] and radio-resistance [52] in HNSCC, which indicate EGFR as a drug target.
Current EGFR-targeted drugs can be classified as either mAbs or TKIs (Figure 1). Monoclonal antibodies (mAbs) are intravenously administered and block ligand-binding induced receptor activation by specifically binding to the EGFR. Oral TKIs can reversibly or irreversibly inhibit the binding of ATP to the intracellular domain of EGFR and abrogate downstream signaling [22].
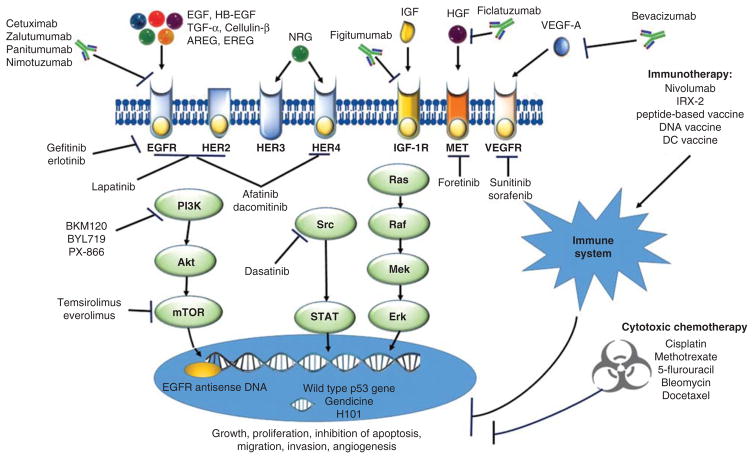
Figure 1. Currently applied and emerging investigated drugs in head and neck squamous cell carcinoma.
Current FDA-approved drugs for the treatment of HNSCC include five conventional chemotherapy drugs (cisplatin, methotrexate, 5-flurouracil, bleomycin, docetaxel) and one targeted agent (cetuximab). Emerging investigated drugs in clinical trials including other targeted agents, immunotherapy agents and gene therapy agents are represented here. Briefly, EGFR, VEGFR, c-MET and IGF-1R signals utilize a variety of downstream molecular pathways including the PI3K/Akt/mTOR, STAT and Ras/Raf/MEK/MAPK. Aberrant activation of these pathways are associated with tumor growth and angiogenesis. Agents targeting these pathways include monoclonal antibodies (cetuximab, zalutumumab, panitumumab, nimotuzumab, bevacizumab, ficlatuzumab and figitumumab), tyrosine kinase inhibitors (gefitinib, erlotinib, lapatinib, afatinib, dacomitinib), multikinase inhibitors (sorafenib, sunitinib), downstream inhibitors including Src family kinase inhibitors (dasatinib) and PI3K/Akt/mTOR inhibitors (BKM120, BYL719, everolimus, temsirolimus). Gene therapy includes Adp53/CRAdp53-based gene therapy (Gendicine, H-101, SCH-58500, ONYX-015) and EGFR antisense DNA. Immunotherapy includes non-specific immune stimulators/agents (nivolumab, IRX-2) and vaccine candidates (peptide-based vaccine, DNA vaccine and dendritic cell vaccine).
Akt: Protein kinase B; AREG: Amphiregulin; EREG: Epiregulin; ERK: Extracellular signal-regulated kinase; HB-EGF: Heparin-binding EGF-like growth factor; HER: Human EGFR; HGF: Hepatocyte growth factor; IGF-1R: IGF-1 receptor; MET: Also called c-MET tyrosine kinase or HGF receptor; MEK: MAPK/ERK kinase; mTOR: Mammalian target of rapamycin; NRG: Neuregulin; PI3K: Phosphatidylinositol-3-kinase; Raf: Rapidly accelerated fibrosarcoma kinase; Ras: Rat sarcoma protein; Src: Sarcoma-family kinase; STAT: Signal transducer and activator of transcription; VEGFR: VEGF receptor.
6.2 Molecular mechanism of EGFR-targeted therapy resistance
Cetuximab has a RR of only 13% [30] as a single agent in HNSCC treatment. Other EGFR-targeted agents such as TKIs have not demonstrated improved survival in unselected populations. The low efficacy suggests that other molecular mechanisms may exist that modulate intrinsic (primary) or acquired (secondary) resistance of EGFR inhibition. Identifying the mechanisms of resistance to EGFR-targeted agents may lead to strategies to overcome resistance, and identify alternative targets for HNSCC treatment (Figure 1). Possible strategies to overcome anti-EGFR resistance in HNSCC include (but are not restricted to) the inhibition of other receptor or non-receptor tyrosine kinases (e.g., MET, IGF-1R, sarcoma-family kinase [Src]), abrogation of VEGF/VEGFR, inhibition of downstream mediators in the EGFR signaling stream (e.g., PI3K inhibitors) and gene therapy (e.g., wild-type p53 restoration and EGFR antisense DNA) [47,53–56].
7. Competitive environment
The main characteristics of the major drugs and compounds currently applied or under development for HNSCC treatment are summarized in Table 1.
Table 1.
Competitive environment table of the major drugs and compounds currently applied or under development for HNSCC treatment.
| Compound | Company | Stage of development | Mechanism of action |
|---|---|---|---|
| Cisplatin | Bristol-Myers Squibb (BMS) | Marketed | DNA synthesis inhibitor |
| Methotrexate | Lederle (now Pfizer) | Marketed | Thymidylate synthase inhibitor |
| 5-Flurouracil | Simcere Pharmaceuticals | Marketed | DNA and RNA synthesis inhibitor |
| Bleomycin | PCI Biotech | Marketed | DNA inhibitor |
| Docetaxel | Sanofi | Marketed | Microtubule stimulant |
| Cetuximab | Eli Lilly, Bristol-Myers Squibb, Merck KGaA | Marketed | EGFR mAb |
| Zalutumumab | Genmab, MATOS Pharma | Phase III | EGFR mAb |
| Panitumumab | Amgen, Takeda | Phase III | EGFR mAb |
| Nimotuzumab | Center of Molecular Immunology, YM BioSciences | Phase III | EGFR mAb |
| Duligotuzumab | Roche | Phase II | Dual EGFR/HER3 mAb |
| Gefitinib | AstraZeneca | Phase III | Reversible EGFR TKI |
| Erlotinib | OSI Pharmaceuticals (now Astellas) | Phase III | Reversible EGFR TKI |
| Lapatinib | GlaxoSmithKline | Phase III | Reversible dual EGFR/HER2 TKI |
| Afatinib | Boehringer Ingelheim | Phase III | Irreversible pan-HER TKI |
| Dacomitinib | Pfizer | Phase II | Irreversible pan-HER TKI |
| Ficlatuzumab | AVEO | Phase II | HGF mAb |
| Foretinib | Exelixis, GSK | Phase II | SSKI (include c-MET) |
| Figitumumab | Pfizer | Phase II | IFG-1R mAb |
| Bevacizumab | Genentech (now Roche) | Phase III | Anti-VEGF mAb |
| Sunitinib | Pfizer | Phase II | Multiple TKI |
| Sorafenib | Bayer and Amgen | Phase II | Multiple TKI |
| Dasatinib | Bristol-Myers Squibb (BMS) | Phase II | Multiple TKI |
| BKM120 (Buparlisib) | Novartis | Phase II | PI3K inhibitor |
| BYL719 (Alpelisib) | Novartis | Phase II | PI3K inhibitor |
| PX-866 | Oncothyreon | Phase II | PI3K inhibitor |
| Temsirolimus | American Home Products (AHP) | Phase II | mTOR kinase inhibitor |
| Everolimus | Novartis | Phase II | mTOR kinase inhibitor |
| Gendicine | SiBiono GeneTech | Phase II | p53 stimulant |
| SCH-58500 | Canji (Schering-Plough; now Merck & Co) | Phase III | p53 stimulant |
| Advexin | Introgen Therapeutics | Phase III | p53 stimulant |
| ONYX-015 (Lontucirev) | Onyx Pharmaceuticals (now Amgen) | Phase III | p53 stimulant |
| H-101 | Shanghai Sunway Biotech | Phase III | p53 stimulant |
| EGFR antisense DNA | University of Pittsburgh | Phase II | EGFR antisense DNA |
| Nivolumab | Ono and Medarex (Bristol-Myers Squibb) | Phase III | Programmed cell death-1 mAb |
| IRX-2 | IRX Therapeutics | Phase II | Multi-cytokine immunostimulant |
| MAGE-A3 | GlaxoSmithKline | Phase I | Peptide epitope vaccine (immunostimulant) |
| MAGE-A3 Human papilloma virus-16 vaccine | University of Maryland | Phase I | Peptide epitope vaccine |
| INO-3112 DNA vaccine |
Inovio Pharmaceuticals | Phase I/II | DNA vaccine (immunostimulant) |
| DC vaccine | University of Pittsburgh | Phase I | DC vaccine (immunostimulant) |
DC: Dendritic cell; HER: Human EGFR; HGF: Hepatocyte growth factor; HNSCC: Head and neck squamous cell carcinoma; IGF-1R: IGF-1 receptor; MET: Also called c-MET tyrosine kinase or HGF receptor; mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositol-3-kinase; Src: Sarcoma-family kinase; SSKI: Spectrum selective kinase inhibitor; TKI: Tyrosine kinase inhibitor; VEGFR: VEGF receptor.
7.1 mAbs
Zalutumumab is a high-affinity human IgG1 anti-EGFR mAb, especially effective at inducing ADCC. Being completely human-derived, it is predicted to have a low immunogenicity profile compared with cetuximab, thus minimizing the risk of hypersensitivity reactions and compromising treatment efficacy in prolonged use [57]. In a Phase III trial, zalutumumab plus best supportive care (BSC) was associated with a prolonged PFS over BSC alone (median 9.9 vs 8.4 weeks; p = 0.0012) in patients with incurable R/M HNSCC. However, this study did not meet its end point of improving OS (median 6.7 vs 5.2 months; p = 0.0648) [58]. A Phase III trial to determine whether zalutumumab as a component of primary curative RT or CRT increases locoregional control in HNSCC patients is ongoing (NCT00496652).
Panitumumab is a human IgG2 anti-EGFR mAb, which may not elicit ADCC as strongly as cetuximab, reducing the incidence of life-threatening hypersensitivity reactions [59]. In a Phase I study, panitumumab plus paclitaxel, carboplatin and intensity-modulated RT was a tolerable regimen and was associated with at least a partial response (PR) in all 19 LA-HNSCC patients [60]. In a Phase III trial (SPECTRUM), panitumumab plus standard platinum-based CT versus CT alone in R/M HNSCC did not significantly improve the median OS (11.1 vs 9.0 months; p = 0.14) but did improve median PFS (5.8 vs 4.6 months; p = 0.004) [61]. When the results were analyzed by HPV status, both PFS and OS benefits were observed in HPV-negative patients [61]. A randomized Phase III trial comparing panitumumab/RT with cisplatin/RT (NCT00820248) in LA-HNSCC is ongoing. Several ongoing Phase II trials are evaluating combination of panitumumab with CT for R/M HNSCC (NCT00756444), as second-line monotherapy for R/M HNSCC (NCT00446446), or in combination with postoperative CRT for LA-HNSCC (NCT00798655). A Phase II biomarker-focused evaluation in LA-HNSCC patients receiving a single dose of panitumumab prior to definitive therapy (surgery or RT) (NCT01305772) was terminated for low accrual.
Nimotuzumab is another humanized anti-EGFR mAb with a lower incidence of skin toxicity compared with cetuximab or panitumumab. In contrast to other anti-EGFR antibodies, the intrinsic properties of nimotuzumab requires bivalent binding for stable attachment to cellular surface, thus selectively binding to cells with moderate-to-high EGFR expression. For normal cells with low EGFR expression, cetuximab and panitumumab still have high avidity target binding because their higher affinity constants lead to increased toxicities. Nimotuzumab with lesser affinity binds with less avidity and only causes transient target-binding interactions. Therefore, it spares healthy cells and avoids severe dose-limiting toxicities. All anti-EGFR antibodies can bind with a similar higher avidity to the cells with moderate-to-high EGFR expression and no clinical evidence from studies with panitumumab or cetuximab suggests greater efficacy than nimotuzumab [62]. Nimotuzumab is approved for HNSCC in several countries outside the USA [62]. A non-randomized Phase II trial of nimotuzumab plus RT in LA-HNSCC described its tolerability and increasing OS with increasing doses [63]. Subsequently, in a double-blind randomized trial in LA-HNSCC (n = 106), nimotuzumab plus RT had a significantly higher complete response rate (CRR) than placebo plus RT (59.5 vs 34.2%; p = 0.038), and no cases of skin rash were observed in nimotuzumab-treated patients. Median OS was improved (12.5 vs 9.5 months; p = 0.0491) [64]. In a Phase IIb study of CRT alone or plus nimotuzumab in HNSCC, median survival in the CRT-alone arm was 22 versus > 30 months in the CRT plus nimotuzumab arm (p < 0.003) [65]. There was a significant relationship between EGFR expression and OS in patients who received nimotuzumab plus CRT (p = 0.02), although few studies have found a correlation between EGFR expression and response to EGFR-targeted therapies. Ongoing Phase III trials in LA-HNSCC are testing the addition of nimotuzumab to RT (NCT01345084) and to adjuvant CRT (NCT00957086). Moreover, ongoing Phase II trials may provide additional insights into the use of nimotuzumab when added to CT for incurable HNSCC (NCT01425736) and to CRT for LA-HNSCC (NCT01516996; NCT00702481).
A dual-targeting anti-EGFR mAb simultaneously blocks two receptors, duligotuzumab (a human IgG1 anti-EGFR/HER3 mAb, NCT01577173) [66,67], is being studied in early phase trials to overcome resistance to cetuximab. While these newer anti-EGFR mAbs are being investigated in an attempt to improve outcomes or reduce toxicity in HNSCC, no preclinical or clinical evidence to date demonstrates superiority with respect to cetuximab.
7.2 Tyrosine kinase inhibitors
Unlike the mAbs, the TKIs have been developed to target the tyrosine kinase domain of EGFRs and to inhibit downstream signaling, eventually blocking the proliferation of tumor cells.
7.2.1 Reversible EGFR TKIs
Gefitinib is an oral small-molecule reversible EGFR TKI, and was the first TKI to reach Phase III investigation in HNSCC. However, due to recent negative study results, it is unlikely to be further developed [28]. In a Phase III trial, monotherapy of gefitinib 250 and 500 mg/day, or methotrexate 40 mg/m2/week was compared in 486 recurrent HNSCC patients after either CRT or surgery [68]. The RR was 2.7, 7.6 and 3.9%for gefitinib 250, 500 mg/day or methotrexate, with no significant differences between either dose of gefitinib or methotrexate. Neither dose of gefitinib was associated with improved survival compared with methotrexate (5.6 vs 6.7 vs 6.0 months; gefitinib 250, 500 mg/day and methotrexate, respectively). In another Phase III trial [69], 270 patients with R/M HNSCC were randomized to receive docetaxel plus gefitinib or plus placebo. No difference in OS was observed (7.3 months docetaxel/gefitinib vs 6.0 months docetaxel/placebo), although an unplanned subset analysis showed that gefitinib improved survival in patients younger than 65 years (median 7.6 vs 5.2 months; p = 0.04). Erlotinib is another oral, small-molecule, reversible EGFR TKI that has demonstrated efficacy in patients with HNSCC. Early Phase II trial showed tolerability and antitumor activity (overall objective RR was 4.3%) for erlotinib as monotherapy in R/MHNSCC [70]. However, another randomized Phase II trial [71] compared CRT plus erlotinib versus CRT alone and showed that complete response (CR) and PFS rates were not increased by the addition of erlotinib. Moreover, two Phase III trials of erlotinib, one as a component of first-line standard platinum containing CT for advanced HNSCC (NCT00448240) and the other as maintenance monotherapy after CRT or RT alone for resected HNSCC (NCT00412217), were terminated early for low accrual.
7.2.2 Dual and pan-HER TKIs
Heterodimerization of EGFR with other members of the HER family may be responsible for the limited activity of EGFR-targeted mAbs or TKIs. The ability to block more than just EGFR could therefore be of interest.
Lapatinib is an oral reversible dual EGFR and HER2 TKI. A randomized Phase II study assessed the activity and safety of CRT plus lapatinib followed by lapatinib maintenance treatment in 67 unresected LA-HNSCC and showed that lapatinib combined with CRT is well-tolerated with an increase in CRR (53 vs 36%) at 6 months post-CRT, and median PFS (55 vs 41%) at 18 months post-CRT compared with CRT plus placebo [72]. Another Phase II trial showed that a short-term course of lapatinib monotherapy (4 weeks) did not induce tumor apoptosis, but provided evidence of clinical activity (objective RR was 17 vs 0% placebo) in LA-HNSCC [73]. Conversely, a recently published Phase II trial of lapatinib as monotherapy in R/M HNSCC demonstrated no CR or PR, in either EGFR inhibitor-naive or refractory subjects [74]. A randomized Phase III trial is currently ongoing to study the combination of RT plus platinum-based CT with lapatinib or placebo in postoperative setting (NCT00424255). Other Phase II trials are testing the use of lapatinib with RT for LA-HNSCC who cannot tolerate CRT (NCT00490061), in combination with primary CRT in LA-HNSCC (NCT00387127), and in combination with CRT in HPV-negative patients (NCT01711658).
A new generation of TKIs, the irreversible small molecule pan-HER inhibitors, including afatinib and dacomitinib, have been developed. By covalently binding and irreversibly blocking multiple ErbB family kinases, sustained suppression of tumor growth may occur.
Afatinib is a TKI that irreversibly inhibits EGFR (including EGFR vIII), HER2 and HER4 kinases. In an open-label, randomized, Phase II trial conducted in 43 centers, 124 patients with R/M HNSCC failed with platinum-based therapy, were randomized to receive afatinib 50 mg/day or cetuximab 250 mg/m2/week until disease progression or intolerable adverse events (stage I), with optional crossover (stage II) [75]. Antitumor activity of afatinib was comparable with cetuximab with tumor shrinkage 10.4 versus 5.4%, ORR 16.1 versus 6.5%, disease control rates 50 versus 56.5%, respectively. Median OS (35.9 vs 47.1 weeks, p = 0.78) was also comparable. In stage II, the disease control rate was 38.9% with afatinib and 18.8% with cetuximab, suggesting sustained clinical benefit from sequential treatment with afatinib and cetuximab and a lack of cross-resistance [75]. Afatinib is now under development in several Phase III clinical trials investigating a comparison of afatinib with methotrexate as second-line treatment after failure of platinum-based CT in R/M HNSCC (NCT01345682), evaluating afatinib versus placebo as maintenance therapy after definitive CRT (NCT01345669) or after postoperative CRT (NCT01427478) for LA-HNSCC with a starting dose of 40 mg (instead of 50 mg) based on safety evaluations. A neoadjuvant afatinib window study conducted by the European Organisation for Research and Treatment of Cancer (NCT01538381) is ongoing to the pharmacodynamic activity of afatinib in the presurgical setting. Dacomitinib is another small irreversible pan-HER inhibitor (EGFR, HER2 and HER4 kinases), which was investigated as first-line treatment for R/M HNSCC in a Phase II trial [76] with a 12.7% PR rate and 12.1 weeks median PFS and 34.6% median OS. However, no evidence shows how it is compared with afatinib.
7.3 Other targeting agents investigated to overcome EGFR-targeted therapy resistance
7.3.1 c-MET
c-MET is a transmembrane tyrosine receptor primarily expressed on epithelial cells, which can be activated after binding to hepatocyte growth factor (HGF) secreted by mesenchymal cells. This epithelial–mesenchymal interaction mediates downstream signaling through MAPK, PI3K, STAT3 and NF-κB [77] and is associated with tumor proliferation, invasion and angiogenesis when aberrantly activated. To date, HGF is the sole known ligand binding to c-MET. Ficlatuzumab is a humanized anti-HGF mAb that inhibits HGF-induced c-MET activation. Preclinical studies and clinical trials to date have demonstrated antitumoral activity and acceptable toxicity of ficlatuzumab [78–80]. A Phase Ib trial of ficlatuzumab in R/M HNSCC is ongoing to find the recommended Phase II dose of the combination of ficlatuzumab and cetuximab (NCT02277197). Amplification of the MET oncogene [81] leads to increased expression and activation of c-MET [82,83], which is related to EGFR inhibitors resistance, radiation resistance [84] and cisplatin resistance [85]. c-MET is overexpressed in 58 – 84% HNSCC [84,86] and c-MET mutations have been identified in HNSCC tumor tissues and cell lines [84]. Foretinib is an oral multikinase inhibitor of c-MET and the VEGF receptor 2 (VEGFR2). In a Phase II trial, foretinib (240 mg/day) was orally administered to 14 R/M HNSCC. Fifty percent of patients (7/14) showed stable disease, 43% of patients (6/14) experienced tumor shrinkage and two patients had prolonged disease stabilization for > 13 months [87].
7.3.2 IGF-1 receptor targeted agent
The IGF-1 receptor (IGF-1R) is overexpressed in HNSCC [88,89] and may heterodimerize with EGFR in HNSCC cells when stimulated by either IGF or EGF [89], activating downstream signaling pathways, which are associated with cell growth, proliferation, cell differentiation, anti-apoptotic signaling and angiogenesis. It has been reported that elevated IGF-1R expression is associated with benefit of gefitinib in HNSCC patients with postoperative CRT [90]. Although cumulative evidence implicates IGF-1R as a target for HNSCC treatment, investigation of figitumumab (a fully human mAb IgG2 subtype targeting the IGF-1R) in incurable HNSCC with progressive disease on platinum-based therapy in Phase II trials found no evidence of clinical activity [91].
7.3.3 VEGF/VEGFR-targeted agents
Angiogenesis is an important process for primary tumor growth, cell proliferation, invasiveness, metastasis and radio-resistance. It is understood that under hypoxic conditions, multiple growth factors are released by cancer cells, including the VEGF [92–94]. VEGF-A is the most important member of the VEGF family, which mediates angiogenesis by binding to VEGFR1–3 [94]. In HNSCC, VEGF and VEGFR expression in tumor tissue is associated with worse prognosis [95,96]. Currently, the two dominant approaches to targeting angiogenesis are antibody-mediated inhibition of VEGF and small molecule inhibition of VEGFR tyrosine kinases [97]. Bevacizumab is a humanized anti-VEGF mAb that binds to all five isoforms of VEGF [98], reducing the total amount of circulating VEGF. A Phase II trial showed that the addition of bevacizumab to cisplatin plus IMRT did not increase toxicity in LA-HNSCC [99]. In another Phase II trial, combination of bevacizumab with cetuximab was well-tolerated and showed some activity in 46 R/M HNSCC patients [100]. The ORR, disease-control rate (DCR), median PFS and OS were 16, 73%, 2.8 and 7.5 months, respectively. However, in another Phase II trial investigating the addition of bevacizumab to pemetrexed in 40 R/M HNSCC patients, serious bleeding events occurred in 15% patients and were fatal in two patients [101]. A Phase III randomized trial comparing CT with or without bevacizumab in R/M HNSCC is ongoing (NCT00588770). Sunitinib is an oral TKI of VEGFRs, platelet-derived growth factor receptors (PDGF-Rs), Flt3 and c-kit tyrosine kinase. A Phase II trial was conducted in 38 HNSCC patients who had failed to respond to prior platinum therapy [102]. Only one PR was reported, which demonstrated modest activity in palliative HNSCC. In another Phase II trial [103], sunitinib (50 mg/day for 4 weeks in a 6-week cycle) was investigated in patients with incurable disease. Only one PR was observed in total 22 patients. No clinical activity was shown in another Phase II trial [104] with sunitinib monotherapy in R/M HNSCC and no further development of the drug is ongoing. Sorafenib is another multikinase inhibitor of VEGFR, PDGFR, Raf and c-kit kinase. Sorafenib monotherapy (400 mg three times a day continuously for a 28-day cycle) was investigated in a Phase II trial with 41 CT-naive R/M squamous cell carcinoma of the head and neck patients [105]. The estimated PR was 2%, the median PFS and OS were 4 and 9 months, respectively. Another Phase II trial comparing combination of sorafinib with cetuximab versus cetuximab alone is ongoing in refractory, R/M HNSCC patients (NCT00939627).
7.3.4 Src kinases inhibitor
Src kinases are intracellular tyrosine kinases that can affect cellular proliferation and survival by activation of STAT family of transcription factors, especially STAT3 [106]. Preclinical HNSCC models have demonstrated that inhibition of c-Src give rise to block invasion [107], induce apoptosis [108], blockage of DNA repair and EGFR nuclear translocation and sensitize HNSCC cell lines to radiation and enhance EGFR inhibition [109,110]. Dasatinib is a small molecule inhibitor of Src kinase. However, monotherapy of dasatinib failed to demonstrate activity in a Phase II trial with R/M HNSCC [111]. Of evaluated 12 patients, no OR was observed although 2 patients (16.7%) had stable disease at 8 weeks. As dasatinib has potential synergistic activity of anti-EGFR agents, addition of dasatinib to cetuximab in recurrent HNSCC who have received cetuximab-containing curative therapy in a Phase II trial (NCT01488318), to cetuximab and RT ± cisplatin in LA HNSCC in a Phase I/II trial (NCT00882583), and to erlotinib in a biomarker-focused evaluation for HNSCC with planned primary or salvage surgical resection (NCT00779389) are underway.
7.3.5 PI3K/Akt/mTOR pathway inhibitors
Activation of PI3K/Akt/mammalian target of rapamycin (mTOR) signaling plays a crucial role in the carcinogenesis of various human malignancies including HNSCC, independent from EGFR activation [112]. Consequently, alterations of this pathway might also play a role in resistance to anti-EGFR therapy, which makes this pathway attractive for molecular-oriented drug therapies. Three Phase II studies with PI3K inhibitors, BKM120 as monotherapy in patients with platinum refractory R/M disease (NCT01737450), BYL719 plus cetuximab versus cetuximab alone (NCT01602315) and PX-866 plus docetaxel versus docetaxel alone (NCT01204099) are ongoing in the setting of R/M HNSCC. One major downstream effector of Akt is the atypical serine/threonine kinase, mTOR, which regulates cell growth by coordinating growth factor and nutrient signaling [113]. Preclinical evidence in HNSCC models supports a synergistic interaction between the mTOR inhibitor temsirolimus when combined with EGFR inhibitors [114] or bevacizumab – cetuximab –irradiation [115]. However, a Phase II study evaluating the combination of erlotinib and temsirolimus in platinum-refractory R/M HNSCC patients was closed early after enrolling 12 patients due to toxicity. Other studies of temsirolimus (NCT01256385) and another mTOR inhibitor everolimus (NCT01283334, NCT00942734) as monotherapy or combination with other treatments are ongoing.
7.3.6 Gene therapy
Targeting the specific genetic alterations responsible for carcinogenesis and cancer progression is an attractive strategy for developing more effective anticancer therapeutics and reducing treatment-related toxicity. Gene therapy is generally delivered locally and HNSCC is ideally suited for gene therapy because lesions are readily accessible for injection or application of the agent. Several genetic alterations of tumor suppressor genes have been reported in the head and neck cancer including mutation of TP53, the retinoblastoma gene, p16 (CDKN2A) and PTEN [53,116]. Since the high incidence of TP53 mutation (69.8%) [117] and the protein p53 plays an important role in cell cycle and in apoptosis, gene therapy approaches delivering p53 have been tested in HNSCC by direct injection of an adenoviral vector expressing wild-type p53 gene (Adp53), mostly in the USA and the People’s Republic of China [118].
Gendicine (SBN-1) is the first Adp53-based gene therapy product in the world approved by the State FDA of the People’s Republic of China (SFDA) for the treatment of HNSCC in 2003, and was formally launched in 2004. It is a recombinant human serotype 5 adenovirus with the E1 region replaced by a human wild-type p53 expression cassette, and has been tested in clinical trials for the treatment of patients with various cancers [118]. The antitumor activities of the expressed wild-type p53 gene include triggering apoptotic pathways, activating immune response factors such as natural killer (NK) cells, inhibiting DNA repair and anti-apoptotic functions and blocking the transcription of survival signals [118]. In a Phase I clinical trial, Gendicine plus surgery was administered to 12 patients with advanced laryngeal cancer [119]. The 3-year relapse rate in Gendicine plus surgery arm was 0 versus 30% in surgery-alone arm. Three Phase II/III clinical trials demonstrated that Gendicine in combination with RT showed synergistic effects in HNSCC [120–122].
Other Adp53 vectors such as SCH-58500 (CANJI, Inc, San Diego, CA, USA) and Advexin (INGN-201; Introgen Therapeutics, Inc. Austin, TX, USA) have been developed and have been used in various clinical trials [123,124]. Although a few remarkable cases have been reported, Advexin did not show convincing results and therefore was not approved by the FDA [118]. Due to the low transduction rate of p53 gene introduction via Adp53 vector, several types of cancer-specific p53-expressing conditionally replicating adenovirus vectors (known as conditionally replicating Adp53 [CRAdp53] vectors), which can induce higher p53 expression and stronger antitumor effects have been developed [125].
ONYX-015 is currently the most extensively evaluated E1B gene deleted CRAdp53 vector, which can selectively proliferate in p53 mutant and induce adenovirus-mediated cytotoxicity, but not in p53 wild-type cells. Although this concept is controversial, ONYX-015 combined with CT is more effective than CT alone for patients with recurrent HNSCC [126]. Another Phase II trial reported modest antitumoral activity in HNSCC (10 – 14%) [127].
H-101 is a recombinant adenovirus with a total deletion of E1B gene similar to ONYX-015, and an additional partial E3 region deletion, which may enhance the safety profile of the administered adenovirus [128]. The SFDA approved H-101, especially for advanced nasopharyngeal carcinoma in combination with cisplatin and 5-FU in 2005. A Phase III randomized clinical trial compared effects of intratumoral H-101 injection plus PF regimen CT versus PF regimen CT alone in HNSCC and squamous cell carcinoma of esophagus, the ORR in H-101 plus PF was 78.8 versus 39.6% in PF-alone group (p = 0.000) [129]. Until now, only Gendicine and H-101 have been approved (SFDA), and both Adp53 and CRAdp53 vectors are not widely used.
In addition to delivering a tumor suppressor gene like p53, gene therapy can also be used to target oncogenes using antisense or siRNA strategies. EGFR antisense DNA therapy completed Phase I testing in HNSCC and a Phase II trial is ongoing (NCT00903461) [130,131]. The Phase I trial achieved a 29% clinical response and intratumoral EGFR antisense was safe and resulted in antitumor activity in patients with advanced HNSCC. Baseline levels of high EGFR and low STAT3 may be associated with antitumor effects. While effective to date, local delivery, even in HNSCC, remains a challenging strategy to employ in most clinical settings.
7.3.7 Immunotherapy
Immunotherapy is a promising area for HNSCC therapy as it mobilizes the immune system with limited effects on normal tissue, to target cancer cells that express tumor-specific antigens. HNSCC patients often present with a suppressed immune system, featuring dysregulation of immunecompetent cells and cytokines [132–134]. As innate and adaptive immunity play an important role in HNSCC pathogenesis, it is likely that the development of immunotherapeutic approaches will prove promising [135]. Compared with the traditional chemotherapies, immunotherapy is more specific, generally less toxic and has the potential for inducing memory responses that could provide long-term tumor immune surveillance. Immunotherapy may decrease the incidence of relapses and increase the long-term disease-free survival via continuous elimination of cancer cells by the primed immune system [136]. Immunotherapies can be classified into specific and non-specific immunotherapies. Specific immunity encompasses T cells and antibodies specifically recognizing and engaging a target while non-specific immunity includes antigen unspecified macrophages, dendritic cells (DC), NK cells and a multitude of factors and cytokines. Several immune-modulating agents have been tested in HNSCC patients [135]. These include below mentioned non-specific immune agents targeting specific tumor antigens and vaccine candidates based on different types of antigenic stimuli.
7.3.7.1 Anti-programmed cell death -1 antibody
Programmed death ligand-1 (PDL-1) is a ligand of the B7 superfamily expressed on tumor cells that inhibits T-lymphocyte function [137]. Programmed cell death-1 (PD-1), the receptor for PDL-1, is preferentially expressed on apoptotic cells. Binding of PDL-1 with PD-1 on T cells inhibits TCR-mediated IL-2 activation and T-cell proliferation signal [138]. Tumor infiltrating lymphocytes (TILs) that represent the host’s immune response to a malignant tumor are also part of this dynamic. PDL-1 expressed by HNSCC cells was found to correlate with decreased intratumoral TILs [139]. A Phase III trial investigating this emerging anti-PD-1 (nivolumab) versus cetuximab/methotrexate/docetaxel in R/M platinum-refractory HNSCC is ongoing (NCT02105636).
7.3.7.2 Other immunotherapeutic agents
Other immunotherapeutic agents are also in development for HNSCC. A Phase II trial showed that a multi-cytokine immunotherapy regimen (IRX-2) delivered in combination with cyclophosphamide, indomethacin and zinc followed by surgery in resectable HNSCC was well tolerated (NCT00210470) [140]. In addition, various ongoing Phase I – II trials are testing therapeutic vaccine strategies including peptide-based vaccines (NCT00257738, NCT00704041), a DNA vaccine (NCT02163057) and a DC vaccine (NCT00404339). The most effective immunotherapeutic regimen has yet to be defined in HNSCC.
8. Potential development issues
As is common in cancer therapy, a significant problem in the development of EGFR-targeted HNSCC therapies is the emergence of treatment resistance. Many HNSCC patients do not respond to EGFR inhibitor therapy (intrinsic resistance), and the majority of the patients who do achieve a clear tumor response to EGFR inhibitors will eventually manifest disease progression (acquired resistance) [47]. The elucidation of mechanisms of resistance to targeted therapies may provide valuable insights to improve outcomes by identifying new potential drugs as well as enabling rational combinations of molecular targeted therapies. The mechanism may include, but not restricted to, expression of other redundant receptor tyrosine kinase (i.e., HER2, MET) and activation of alternative or downstream pathways. For example, HER2 and HER3 have been linked to gefitinib resistance in HNSCC preclinical models [141]. c-MET is overexpressed in ~ 80% HNSCC and related to resistance to EGFR inhibitors and conventional RT and CT [84–86]. PI3K pathway can be activated by either receptor tyrosine kinase or via PIK3CA mutations or PTEN loss and play a role in resistance to anti-EGFR therapy [53]. MET inhibitors and PI3K inhibitors are under clinical investigation to overcome the resistance to anti-EGFR therapy. Such studies have the potential to improve patient outcome and may yield new insights into mechanisms of resistance, leading to opportunities for the design of rational combination therapies. Multi-target agents, such as irreversible pan-HER inhibitor afatinib currently in clinical trials, may also abrogate anti-EGFR resistance.
The second problem in the development of molecular targeted HNSCC therapies is the lack of predictive biomarkers for the response to the treatment. The high mutational heterogeneity of HNSCC suggests that no single targeted inhibitor is likely to benefit a large percentage of patients [117]. As such, the identification of responsive cases to existing agents may be necessary in order to identify novel predictive biomarkers. To date, the only predictive clinical marker for response to cetuximab is the severity of skin rash, which is correlated with outcome in HNSCC [42]. Patients who developed a moderate or severe rash had an increased median OS compared with those who had a mild or no rash (68.8 vs 25.6 months). Higher EGFR expression was thought to be a predictor of better response to anti-EGFR treatment. However, although EGFR overexpression has been linked to better response to conventional treatment [52], increased EGFR in tumor cells to date has not been demonstrated to predict better response to EGFR-targeted therapies in HNSCC. Conversely, activating EGFR mutations in lung cancer has been demonstrated to predict response to EGFR TKIs [142]. KRAS mutations which are frequent in colorectal cancer have also been shown to predict response to cetuximab [143], but these mutations are rare in HNSCC ranging from 0 to 9.1% [56]. HPV-positive HNSCC has been demonstrated to have better outcomes and improved survival compared with the HPV-negative patients [144]. This difference may be due to fewer genetic mutations occurring in HPV-positive cancers [145]. Thus, stratifying HNSCC patients with respect to HPV status into two groups seems warranted in clinical trials investigating molecular targeted therapy. However, more clinical benefit was observed in HPV-positive patients with cetuximab regimen treatment [43], while the HPV-negative patients were more likely to benefit from panitumumab regimen treatment [61]. Further studies investigating the predictive value of HPV status are warranted.
Recently, four whole-exome sequencing studies conducted on approximate 190 HNSCC specimens provided insights into the molecular progression of HNSCC [145–148]. More recently, several hundred additional tumors have now been analyzed by the TCGA project [149], affording the opportunity to utilize the genetic database of HNSCC in a large number of primary tumor. HNSCC appears to be generally driven by loss-of-function of tumor suppressors such as TP53, CDKN2A, CASP8 and NOTCH1, which may account for the unsatisfactory results of anti-EGFR therapy to date.
9. Conclusion
The existing CT agents approved for HNSCC therapy are non-selective and associated with considerable toxicity. Cetuximab is most likely to be effective in an as yet, incompletely defined subgroup of HNSCC patients. New EGFR mAbs are being investigated in an attempt to improve outcome and/or reduce toxicity. The clinical development of EGFR TKIs such as erlotinib and gefitinib has been limited by modest results in unselected HNSCC populations. It is expected that the second generation of TKIs with irreversible binding and dual/pan-HER-targeted functionality may prove more efficacious. Although some patients benefit from anti-EGFR therapy in HNSCC, the majority of tumors exhibit intrinsic or acquired resistance. Therefore, a better understanding of the molecular mechanisms of resistance is crucial. Strategies to overcome resistance to anti-EGFR therapeutics are being tested in clinical trials and may provide much-needed improvements in patient outcomes. These strategies include (but are not restricted to) the inhibition of other receptor or non-receptor tyrosine kinases, abrogation of VEGF/VEGFR, inhibition of downstream mediators in the EGFR signaling stream and gene therapy. Immunotherapy targeting cancer cells that express tumor-specific antigens, such as PD-1, with limited effects on normal tissue also represents a promising strategy.
10. Expert opinion
Key findings to date in HNSCC therapy suggest that the disease is heterogeneous and that increased understanding of the biologic underpinnings of key subgroups will help guide therapy. High EGFR expression is generally a poor prognostic factor and contributed to the clinical development of cetuximab. However, most studies have failed to identify EGFR expression or gene copy number as a predictive biomarker of clinical response to EGFR-targeted therapies. Cetuximab is FDA-approved for both newly diagnosed and R/M HNSCC, although the modest benefits of adding this agent to standard chemoradiation regimens has limited its use worldwide. Ongoing trials are investigating cetuximab in combination with other molecular targeted agents that have generally been selected based on preclinical evidence implicating the target in cetuximab resistance. HPV-associated HNSCC represents a defined subgroup, which is generally associated with improved prognosis. In the absence of HPV-selective therapies, ongoing studies are generally exploring de-intensification of standard regimens for HPV-positive HNSCC. Ultimately, judicious selection of preclinical models will help to define predictive biomarkers and guide emerging therapies.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Denaro N, Russi EG, Adamo V, Merlano MC. State-of-the-art and emerging treatment options in the management of head and neck cancer: news from 2013. Oncology. 2014;86:212–29. doi: 10.1159/000357712. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Albers AE, Strauss L, Liao T, et al. T cell-tumor interaction directs the development of immunotherapies in head and neck cancer. Clin Dev Immunol. 2010;2010:236378. doi: 10.1155/2010/236378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13:35–46. doi: 10.1007/s11864-011-0176-y. [DOI] [PubMed] [Google Scholar]
- 7.Purohit S, Bhise R, Lokanatha D, Govindbabu K. Systemic therapy in head and neck cancer: changing paradigm. Indian J Surg Oncol. 2013;4:19–26. doi: 10.1007/s13193-012-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browman GP, Cronin L. Standard chemotherapy in squamous cell head and neck cancer: what we have learned from randomized trials. Semin Oncol. 1994;21:311–19. [PubMed] [Google Scholar]
- 9.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15:1179–86. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 10.Chandana SR, Conley BA. Neoadjuvant chemotherapy for locally advanced squamous cancers of the head and neck: current status and future prospects. Curr Opin Oncol. 2009;21:218–23. doi: 10.1097/cco.0b013e328329abe5. [DOI] [PubMed] [Google Scholar]
- 11.Qin H, Luo J, Zhu YP, et al. Combination of taxanes, cisplatin and fluorouracil as induction chemotherapy for locally advanced head and neck cancer: a meta-analysis. PLoS One. 2012;7:e51526. doi: 10.1371/journal.pone.0051526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minicucci EM, da Silva GN, Salvadori DM. Relationship between head and neck cancer therapy and some genetic endpoints. World J Clin Oncol. 2014;5:93–102. doi: 10.5306/wjco.v5.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Relling MV. Can the genotoxicity of chemotherapy be predicted? Lancet. 2004;364:917–18. doi: 10.1016/S0140-6736(04)17038-4. [DOI] [PubMed] [Google Scholar]
- 14••.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. A milestone randomized Phase III trial compared cetuximab in combination with radiotherapy (RT) versus RT alone with survival benefit. [DOI] [PubMed] [Google Scholar]
- 15.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 16.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology™. Head and Neck Cancers. 2014;2 Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/head-andneck.pdf. [Google Scholar]
- 18.Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- 21.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–60. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz S, Ang KK, Vermorken J, et al. Targeted therapies for squamous cell carcinoma of the head and neck: current knowledge and future directions. Cancer Treat Rev. 2014;40:390–404. doi: 10.1016/j.ctrv.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–52. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- 24.Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nat Rev Drug Discov. 2012;11:201–14. doi: 10.1038/nrd3651. [DOI] [PubMed] [Google Scholar]
- 25.Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov. 2010;9:363–6. doi: 10.1038/nrd3181. [DOI] [PubMed] [Google Scholar]
- 26.Tabatabaeifar S, Kruse TA, Thomassen M, et al. Use of next generation sequencing in head and neck squamous cell carcinomas: a review. Oral Oncol. 2014;50(11):1035–40. doi: 10.1016/j.oraloncology.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Gregoire V, Lefebvre JL, Licitra L, Felip E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v184–6. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 28.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010;15:355–73. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirix P, Nuyts S. Evidence-based organsparing radiotherapy in head and neck cancer. Lancet Oncol. 2010;11:85–91. doi: 10.1016/S1470-2045(09)70231-1. [DOI] [PubMed] [Google Scholar]
- 30•.Vermorken JB, Herbst RS, Leon X, et al. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–19. doi: 10.1002/cncr.23442. Cetuximab as monotherapy with a low response rate. [DOI] [PubMed] [Google Scholar]
- 31.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46:135–46. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 33.Toustrup K, Sorensen BS, Lassen P, et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102:122–9. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 35.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–73. doi: 10.1200/JCO.2004.12.193. [DOI] [PubMed] [Google Scholar]
- 37.Budach V, Stuschke M, Budach W, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J Clin Oncol. 2005;23:1125–35. doi: 10.1200/JCO.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–55. [PubMed] [Google Scholar]
- 39.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–52. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 41.Agulnik M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN) Med Oncol. 2012;29:2481–91. doi: 10.1007/s12032-012-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 43••.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized Phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–50. doi: 10.1200/JCO.2013.53.5633. Adding cetuximab to concurrent chemoradiotherapy (CRT) did not improve outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermorken JB, Psyrri A, Mesia R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25:801–7. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baselga J, Trigo JM, Bourhis J, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568–77. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 46.Herbst RS, Arquette M, Shin DM, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–87. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keogh B. Era of personalized medicine may herald end of soaring cancer costs. J Natl Cancer Inst. 2012;104:12–13. 16–7. doi: 10.1093/jnci/djr536. [DOI] [PubMed] [Google Scholar]
- 49.McCabe C, Bergmann L, Bosanquet N, et al. Market and patient access to new oncology products in Europe: a current, multidisciplinary perspective. Ann Oncol. 2009;20:403–12. doi: 10.1093/annonc/mdn603. [DOI] [PubMed] [Google Scholar]
- 50.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–10. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler S, Siwak DR, Chai R, et al. Tumor epidermal growth factor receptor and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. 2012;18:2278–89. doi: 10.1158/1078-0432.CCR-11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentzen SM, Atasoy BM, Daley FM, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23:5560–7. doi: 10.1200/JCO.2005.06.411. [DOI] [PubMed] [Google Scholar]
- 53.Mountzios G, Rampias T, Psyrri A. The mutational spectrum of squamous-cell carcinoma of the head and neck: targetable genetic events and clinical impact. Ann Oncol. 2014;25:1889–900. doi: 10.1093/annonc/mdu143. [DOI] [PubMed] [Google Scholar]
- 54.Cohen RB. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC) Cancer Treat Rev. 2014;40:567–77. [Google Scholar]
- 55.Burtness B, Bauman JE, Galloway T. Novel targets in HPV-negative head and neck cancer: overcoming resistance to EGFR inhibition. Lancet Oncol. 2013;14:e302–e09. doi: 10.1016/S1470-2045(13)70085-8. [DOI] [PubMed] [Google Scholar]
- 56.Boeckx C, Baay M, Wouters A, et al. Anti-epidermal growth factor receptor therapy in head and neck squamous cell carcinoma: focus on potential molecular mechanisms of drug resistance. Oncologist. 2013;18:850–64. doi: 10.1634/theoncologist.2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera F, Salcedo M, Vega N, et al. Current situation of zalutumumab. Expert Opin Biol Ther. 2009;9:667–74. doi: 10.1517/14712590902932871. [DOI] [PubMed] [Google Scholar]
- 58.Machiels JP, Subramanian S, Ruzsa A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12:333–43. doi: 10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 59.Yang XD, Jia XC, Corvalan JR, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 60.Wirth LJ, Allen AM, Posner MR, et al. Phase I dose-finding study of paclitaxel with panitumumab, carboplatin and intensity-modulated radiotherapy in patients with locally advanced squamous cell cancer of the head and neck. Ann Oncol. 2010;21:342–7. doi: 10.1093/annonc/mdp477. [DOI] [PubMed] [Google Scholar]
- 61•.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. Combination of panitumumab to chemotherapy (CT) did not significantly improve the median overall survival (OS) versus CT alone in recurrent/metastasis (R/M) head and neck squamous cell carcinoma (HNSCC) [DOI] [PubMed] [Google Scholar]
- 62.Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1:41–8. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22:1646–54. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez MO, Rivero TC, del Castillo Bahi R, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. 2010;9:343–9. doi: 10.4161/cbt.9.5.10981. [DOI] [PubMed] [Google Scholar]
- 65.Basavaraj C, Sierra P, Shivu J, et al. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther. 2010;10:673–81. doi: 10.4161/cbt.10.7.12793. [DOI] [PubMed] [Google Scholar]
- 66.Kamath AV, Lu D, Gupta P, et al. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol. 2012;69:1063–9. doi: 10.1007/s00280-011-1806-6. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer G, Haber L, Crocker LM, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20:472–86. doi: 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 68••.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27:1864–71. doi: 10.1200/JCO.2008.17.0530. Randomized trial in R/M HNSCC with no observed benefit with gefitinib in comparison with methotrexate. [DOI] [PubMed] [Google Scholar]
- 69•.Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2013;31:1405–14. doi: 10.1200/JCO.2012.45.4272. Combination of gefitinib to docetaxel did not increase OS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 71•.Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31:1415–21. doi: 10.1200/JCO.2012.46.3299. Adding erlotinib to CRT did not gain benefit. [DOI] [PubMed] [Google Scholar]
- 72.Harrington K, Berrier A, Robinson M, et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer. 2013;49:1609–18. doi: 10.1016/j.ejca.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Del Campo JM, Hitt R, Sebastian P, et al. Effects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer. 2011;105:618–27. doi: 10.1038/bjc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.de Souza JA, Davis DW, Zhang Y, et al. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:2336–43. doi: 10.1158/1078-0432.CCR-11-2825. Very limited activity of lapatinib as monotherapy in R/M setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:1813–20. doi: 10.1093/annonc/mdu216. Comparison between cetuximab and afatinib and possible reversion of resistance with crossover therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdul Razak AR, Soulieres D, Laurie SA, et al. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck. Ann Oncol. 2013;24:761–9. doi: 10.1093/annonc/mds503. [DOI] [PubMed] [Google Scholar]
- 77.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 78.Patnaik A, Weiss GJ, Papadopoulos KP, et al. Phase I ficlatuzumab monotherapy or with erlotinib for refractory advanced solid tumours and multiple myeloma. Br J Cancer. 2014;111:272–80. doi: 10.1038/bjc.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tabernero J, Elez ME, Herranz M, et al. A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases. Clin Cancer Res. 2014;20:2793–804. doi: 10.1158/1078-0432.CCR-13-1837. [DOI] [PubMed] [Google Scholar]
- 80.Mittra ES, Fan-Minogue H, Lin FI, et al. Preclinical efficacy of the anti-hepatocyte growth factor antibody ficlatuzumab in a mouse brain orthotopic glioma model evaluated by bioluminescence, PET, and MRI. Clin Cancer Res. 2013;19:5711–21. doi: 10.1158/1078-0432.CCR-12-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agarwal S, Zerillo C, Kolmakova J, et al. Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR/Her2 inhibitor in non-small-cell lung cancer cells. Br J Cancer. 2009;100:941–9. doi: 10.1038/sj.bjc.6604937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol. 2010;177:415–23. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–31. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129:2337–48. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 86.Chau NG, Perez-Ordonez B, Zhang K, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:11. doi: 10.1186/1758-3284-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seiwert T, Sarantopoulos J, Kallender H, et al. Phase II trial of single-agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31:417–24. doi: 10.1007/s10637-012-9861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baserga R. The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin Ther Targets. 2005;9:753–68. doi: 10.1517/14728222.9.4.753. [DOI] [PubMed] [Google Scholar]
- 89.Barnes CJ, Ohshiro K, Rayala SK, et al. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13:4291–9. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 90.Thariat J, Bensadoun RJ, Etienne-Grimaldi MC, et al. Contrasted outcomes to gefitinib on tumoral IGF1R expression in head and neck cancer patients receiving postoperative chemoradiation (GORTEC trial 2004-02) Clin Cancer Res. 2012;18:5123–33. doi: 10.1158/1078-0432.CCR-12-1518. [DOI] [PubMed] [Google Scholar]
- 91.Schmitz S, Kaminsky-Forrett MC, Henry S, et al. Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02) Ann Oncol. 2012;23:2153–61. doi: 10.1093/annonc/mdr574. [DOI] [PubMed] [Google Scholar]
- 92.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerber PA, Hippe A, Buhren BA, et al. Chemokines in tumor-associated angiogenesis. Biol Chem. 2009;390:1213–23. doi: 10.1515/BC.2009.144. [DOI] [PubMed] [Google Scholar]
- 94.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta. 2014;1846:161–79. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–40. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 96.Neuchrist C, Erovic BM, Handisurya A, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003;25:464–74. doi: 10.1002/hed.10235. [DOI] [PubMed] [Google Scholar]
- 97.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 99.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118:5008–14. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 100.Argiris A, Kotsakis AP, Hoang T, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24:220–5. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Argiris A, Karamouzis MV, Gooding WE, et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2011;29:1140–5. doi: 10.1200/JCO.2010.33.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28:21–8. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 103.Choong NW, Kozloff M, Taber D, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs. 2010;28:677–83. doi: 10.1007/s10637-009-9296-7. [DOI] [PubMed] [Google Scholar]
- 104.Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, et al. A phase II study of sunitinib in patients with recurrent and/or metastatic nonnasopharyngeal head and neck cancer. Cancer Chemother Pharmacol. 2010;65:649–60. doi: 10.1007/s00280-009-1070-1. [DOI] [PubMed] [Google Scholar]
- 105.Williamson SK, Moon J, Huang CH, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: southwest Oncology Group Study S0420. J Clin Oncol. 2010;28:3330–5. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 107.Koppikar P, Choi SH, Egloff AM, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–91. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–32. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 109.Li C, Iida M, Dunn EF, Wheeler DL. Dasatinib blocks cetuximab- and radiation-induced nuclear translocation of the epidermal growth factor receptor in head and neck squamous cell carcinoma. Radiother Oncol. 2010;97:330–7. doi: 10.1016/j.radonc.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raju U, Riesterer O, Wang ZQ, et al. Dasatinib, a multi-kinase inhibitor increased radiation sensitivity by interfering with nuclear localization of epidermal growth factor receptor and by blocking DNA repair pathways. Radiother Oncol. 2012;105:241–9. doi: 10.1016/j.radonc.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Brooks HD, Glisson BS, Bekele BN, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011;117:2112–19. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molinolo AA, Hewitt SM, Amornphimoltham P, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 113.Freudlsperger C, Burnett JR, Friedman JA, et al. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets. 2011;15:63–74. doi: 10.1517/14728222.2011.541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cassell A, Freilino ML, Lee J, et al. Targeting TORC1/2 enhances sensitivity to EGFR inhibitors in head and neck cancer preclinical models. Neoplasia. 2012;14:1005–14. doi: 10.1593/neo.121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bozec A, Etienne-Grimaldi MC, Fischel JL, et al. The mTOR-targeting drug temsirolimus enhances the growth-inhibiting effects of the cetuximab-bevacizumab-irradiation combination on head and neck cancer xenografts. Oral Oncol. 2011;47:340–4. doi: 10.1016/j.oraloncology.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Bali A, Bali D, Sharma A. An overview of gene therapy in head and neck cancer. Indian J Hum Genet. 2013;19:282–90. doi: 10.4103/0971-6866.120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen GX, Zhang S, He XH, et al. Clinical utility of recombinant adenoviral human p53 gene therapy: current perspectives. Onco Targets Ther. 2014;7:1901–9. doi: 10.2147/OTT.S50483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han DM, Huang ZG, Zhang W, et al. Effectiveness of recombinant adenovirus p53 injection on laryngeal cancer: phase I clinical trial and follow up. Zhonghua Yi Xue Za Zhi. 2003;83:2029–32. [PubMed] [Google Scholar]
- 120.Chen CB, Pan JJ, Xu LY. Recombinant adenovirus p53 agent injection combined with radiotherapy in treatment of nasopharyngeal carcinoma: a phase II clinical trial. Zhonghua Yi Xue Za Zhi. 2003;83:2033–5. [PubMed] [Google Scholar]
- 121.Zhang SW, Xiao SW, Liu CQ, et al. Recombinant adenovirus-p53 gene therapy combined with radiotherapy for head and neck squamous-cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:426–8. [PubMed] [Google Scholar]
- 122.Zhang SW, Xiao SW, Liu CQ, et al. Treatment of head and neck squamous cell carcinoma by recombinant adenovirus-p53 combined with radiotherapy: a phase II clinical trial of 42 cases. Zhonghua Yi Xue Za Zhi. 2003;83:2023–8. [PubMed] [Google Scholar]
- 123.Wills KN, Maneval DC, Menzel P, et al. Development and characterization of recombinant adenoviruses encoding human p53 for gene therapy of cancer. Hum Gene Ther. 1994;5:1079–88. doi: 10.1089/hum.1994.5.9-1079. [DOI] [PubMed] [Google Scholar]
- 124.Gabrilovich DI. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther. 2006;6:823–32. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- 125.Tazawa H, Kagawa S, Fujiwara T. Advances in adenovirus-mediated p53 cancer gene therapy. Expert Opin Biol Ther. 2013;13:1569–83. doi: 10.1517/14712598.2013.845662. [DOI] [PubMed] [Google Scholar]
- 126.Khuri FR, Nemunaitis J, Ganly I, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 127.Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 128.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–8. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 129.Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–70. [PubMed] [Google Scholar]
- 130.Niwa H, Wentzel AL, Li M, et al. Antitumor effects of epidermal growth factor receptor antisense oligonucleotides in combination with docetaxel in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2003;9:5028–35. [PubMed] [Google Scholar]
- 131.Lai SY, Koppikar P, Thomas SM, et al. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanisms. J Clin Oncol. 2009;27:1235–42. doi: 10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duray A, Demoulin S, Hubert P, et al. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alhamarneh O, Amarnath SM, Stafford ND, Greenman J. Regulatory T cells: what role do they play in antitumor immunity in patients with head and neck cancer? Head Neck. 2008;30:251–61. doi: 10.1002/hed.20739. [DOI] [PubMed] [Google Scholar]
- 134.Whiteside TL. Anti-tumor vaccines in head and neck cancer: targeting immune responses to the tumor. Curr Cancer Drug Targets. 2007;7:633–42. doi: 10.2174/156800907782418310. [DOI] [PubMed] [Google Scholar]
- 135.Varilla V, Atienza J, Dasanu CA. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin Biol Ther. 2013;13:1241–56. doi: 10.1517/14712598.2013.810716. [DOI] [PubMed] [Google Scholar]
- 136.Schutt C, Bumm K, Mirandola L, et al. Immunological treatment options for locoregionally advanced head and neck squamous cell carcinoma. Int Rev Immunol. 2012;31:22–42. doi: 10.3109/08830185.2011.637253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Feng Y, Lu L, et al. Interferon-gamma-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–93. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 138.Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 139.Cho YA, Yoon HJ, Lee JI, et al. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47:1148–53. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 140.Wolf GT, Fee WE, Jr, Dolan RW, et al. Novel neoadjuvant immunotherapy regimen safety and survival in head and neck squamous cell cancer. Head Neck. 2011;33:1666–74. doi: 10.1002/hed.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Erjala K, Sundvall M, Junttila TT, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2006;12:4103–11. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 142.Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 143.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 144.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 145.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761–9. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pickering CR, Zhang J, Yoo SY, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cancer Genome Atlas Research N. Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–20. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]