Abstract
Mammalian Unc-51–like kinases 1 and 2 (ULK1 and ULK2) belong to the ULK/Atg1 family of serine/threonine kinases, which are conserved from yeast to mammals. Although ULK/Atg1 is best known for regulating flux through the autophagy pathway, it has evolutionarily conserved noncanonical functions in protein trafficking that are essential for maintaining cellular homeostasis. As a direct target of energy- and nutrient-sensing kinases, ULK/Atg1 is positioned to regulate the distribution and use of cellular resources in response to metabolic cues. In this review, we provide an overview of the molecular mechanisms through which ULK/Atg1 carries out its canonical and noncanonical functions and the signaling pathways that link its function to metabolism. We also highlight potential contributions of ULK/Atg1 in human diseases, including cancer and neurodegeneration.
Introduction
Cells redirect resources to adapt to changes in supply and demand. In response to nutrient or energy depletion, healthy cells switch from anabolic metabolic pathways that promote cellular growth to catabolic pathways that generate energy. Metabolic changes are often accompanied by the induction of autophagy, a catabolic process that degrades and recycles cellular components into intermediate metabolites to provide an efficient alternative to de novo synthesis [1].
The molecular mechanisms involved in autophagy have been best characterized in budding yeast [2]. Atg1 was among the first of more than 30 autophagy-related (ATG) genes identified [2]. Atg1 regulates multiple steps in the autophagy pathway, from initiating the response upon nutrient deprivation to facilitating membrane recycling during autophagosome biogenesis [3]. The spatiotemporal regulation of Atg1 repurposes machinery involved in protein trafficking to help meet the metabolic demands of the cell. Although the characterization of Atg1 homologues in higher eukaryotes has largely focused on their roles in autophagy, growing evidence indicates that this family of serine/threonine kinases has evolved to fulfill cell type–specific needs under physiologic conditions and may have a broader role in coordinating the use of resources in response to changing intracellular and environmental cues.
Here we review the canonical (i.e., autophagy-related) and noncanonical functions of metazoan Atg1 homologues (i.e., ULK/Atg1). We also discuss the potential role of ULK/Atg1 deregulation in the pathogenesis and prognosis of human diseases. A deeper understanding of ULK/Atg1 function is essential now that several ULK kinase inhibitors have been developed and are being considered for therapeutic purposes [4••,5].
ULK/Atg1 redirects resources to autophagy in response to cellular stress
Under vegetative conditions in yeast, inactive forms of vacuolar enzymes are transported from the cytoplasm to the vacuole via the cytoplasm-to-vacuole targeting (Cvt) pathway [6]. Upon nutrient deprivation, autophagy fulfills the transport function, and vacuolar hydrolases are included among cytosolic cargo, which are nonspecifically sequestered into autophagosomes. The Cvt pathway and autophagy are similar in that both sequester cellular material in vesicles that fuse with the vacuole, but they differ in specificity of the cargo and the size of the vesicles. Each pathway has unique genes, but many ATG genes, including Atg1, participate in both.
Atg1 interacts with at least eight other proteins, some of which participate in autophagy (i.e., Atg17–Atg29–Atg31), and others exclusively participate in the Cvt pathway (i.e., Atg11–Atg20–Atg24 and Vac8) [7]. Interaction with Atg13 mediates the self-association and activation of Atg1 via autophosphorylation of the evolutionarily conserved threonine 226 residue [8,9]. Meanwhile, the scaffolding complexes promote clustering and stimulation of Atg1’s catalytic activity [10••]: Atg11–cargo complexes recruit Atg1 to the vacuole to promote sequestration of cargo for selective autophagy, and the Atg17–Atg29–Atg31 complex recruits Atg1–Atg13 to the preautophagosomal structure (PAS) for bulk autophagy. Thus, spatiotemporal activation of Atg1 is regulated via association with Atg13 and the autophagy or Cvt-specific scaffolding complexes.
The activation of yeast Atg1 is linked to metabolic status via posttranslational modifications by energy sensors, including TORC1 and PKA. For example, phosphorylation of Atg13 by TORC1 reduces its binding affinity for Atg1 and Atg17 [11,12], thereby limiting autophagy induction under nutrient-replete conditions. Under these conditions, the Cvt pathway is the preferred method of delivering hydrolases to the vacuole. Mitochondrial respiratory deficiency suppresses autophagic flux, at least in part by activating PKA, which phosphorylates Atg13 and impairs recruitment of Atg1–Atg13 to the PAS [13,14].
Although there are examples of autophagy occurring in the absence of ULK1 and ULK2 in mammals [15,16], these kinases are required for efficient stress-induced autophagy under most circumstances [3,17]. The interaction between ULK1/2 and ATG13–FIP200/ATG17–ATG101 stabilizes ULK/ATG1 and is required for the induction of autophagy in response to cellular stress [18]. Mammalian ULK1 and ATG13 are phosphorylated by mTOR under normal growth conditions and are rapidly dephosphorylated in response to amino acid deprivation, thus activating ULK/ATG1 activity [19–21]. Glucose and amino acid starvation also activate AMPK, leading to the phosphorylation of ULK1 and ULK2 at multiple sites within their unstructured serine–proline-rich domain [22,23]. Although the mechanism by which AMPK-mediated phosphorylation affects ULK/ATG1’s autophagy-inducing function remains unclear, we know that it induces autophagy in response to glucose- or amino acid–starvation [18,22–24] and can influence the localization of the ULK/ATG1 kinase complex [25]. Thus, mTOR and AMPK cooperatively sense the metabolic cues and relay them to the ULK/ATG1 complex. ULK1 is also activated by the GSK3–TIP60-signaling axis upon growth factor deprivation [26].
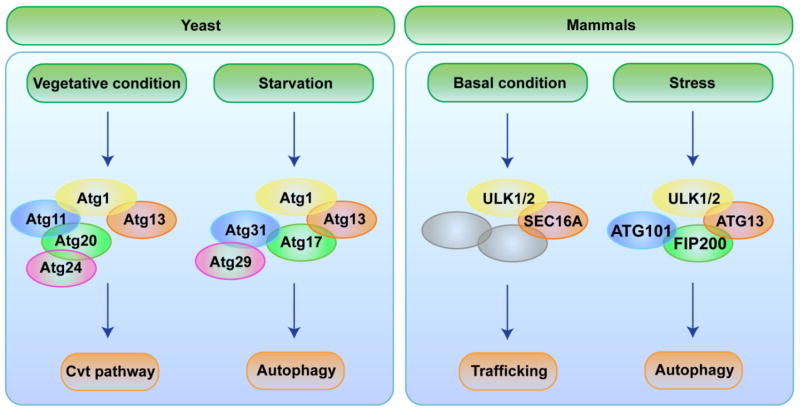
From these studies, it is evident that the spatiotemporal regulation of ULK/ATG1 activity is mediated by its interacting partners and that the signals for ULK/ATG1 activation may originate from the cargo or signaling pathways responsive to metabolic cues. Moreover, upon starvation in yeast, Atg1 helps repurpose the Cvt pathway’s machinery for autophagy (Figure 1). We speculate that ULK/ATG1 has a similar role in higher eukaryotes.
Figure 1.
ULK/Atg1 redirects machinery used under vegetative conditions for stress-induced autophagy. In yeast, the binding of Atg1 to Atg13 and the Atg11–Atg20–Atg24 complex facilitates the Cvt pathway under nutrient-rich conditions. Upon starvation, Atg1 forms an alternative complex that includes, Atg13 and Atg17–Atg29–Atg31 to induce autophagy. Depending on the stimulus, the cellular machineries involved in membrane nucleation and cargo sequestration are directed to the cargo or PAS by ULK/ATG1. Similarly, in mammals, ULK/ATG1 may exist in different complexes depending on the metabolic status of the cell. For example, the ULK/ATG1–SEC16A complex regulates ER-to-Golgi trafficking under normal growth conditions, while the ULK/ATG1–ATG13–FIP200–Atg101 complex induces autophagy in response to stress. It remains to be seen whether the redirection of COPII components that occurs in response to autophagy-inducing stimuli depends on ULK/ATG1.
ULK/Atg1 regulates multiple steps in the autophagy pathway
In response to metabolic stress, ULK/Atg1 phosphorylates several autophagy-related proteins, including components of the VPS34 Class III PI3 kinase complex, and ATG9. ULK1-mediated phosphorylation of ATG14 facilitates the recruitment of the ATG14-containing pro-autophagy VPS34 complex to phagophore initiation sites [27,28]. The subsequent phosphorylation of BECLIN1 and VPS34 by ULK1 promotes PI3P phosphorylation, recruitment of PI3P-binding proteins (e.g. ATG2, ATG18), and autophagosome nucleation [4••,27–29]. VPS34 complexes that do not contain ATG14 appear to be unaffected by ULK1 [29]. In yeast, the recruitment of Atg18 (and Atg8) to the PAS also requires Atg1-mediated phosphorylation of Atg9 [30]. ATG9 is a transmembrane protein that facilitates membrane recycling from various sources. In mammals, ATG9 traffics through the plasma membrane, the trans-Golgi network (TGN), early endosomes, late endosomes and recycling endosomes [31,32]. During starvation, ATG9 moves from the TGN and plasma membrane to the peripheral pool, colocalizing with endosome markers, and autophagosome markers, including ATG16L1 and LC3 [31]. Although certain aspects of ATG9 trafficking can be regulated in an ULK1-independent manner [24,30], the redistribution of ATG9 from the plasma membrane and juxta-nuclear region to the peripheral pool depends on ULK1 and its catalytic activity [33] . In yeast, Atg9 cycles between the preautophagosomal structure (PAS) and peripheral sites. This process requires the presence of Atg1, but not its catalytic activity [30,34].
ULK/Atg1 regulates ER-to-Golgi trafficking
In mammals, constitutive autophagy is essential for clearing long-lived proteins and organelles. Defective autophagy increases steady-state levels of the autophagy-adaptor protein p62 and intracellular accumulation of p62+;ubiquitin+ inclusions and abnormal mitochondria [1]. Constitutive autophagy involves genes from the ubiquitin-like conjugation pathway (e.g., Atg5 and Atg7) and Fip200/Atg17 [35–37]. However, in the CNS it proceeds in the absence of Ulk1 and Ulk2 [38••]. In the absence of exogenous stress, ULK/ATG1 regulates endoplasmic reticulum (ER)-to-Golgi trafficking of specific cargo in Caenorhabditis elegans and mammalian cells [38••]. ULK/ATG1-mediated phosphorylation of the COPII scaffold SEC16A regulates the assembly of SEC24C+ ER-exit sites (ERES) and the ER-to-Golgi trafficking of associated cargo [38••].
Certain components of the COPII pathway may be redirected to provide membrane sources for autophagy. Yeast strains with mutations in certain components of the secretory pathway (e.g., Sec12, Sec16, Sec23, and Sec24) fail to generate autophagosomes; this finding provided the first evidence that components of this pathway may also regulate autophagy [39]. Several additional proteins that regulate directional trafficking of COPII vesicles from the ER to the Golgi (e.g., Sar1, Ypt1/Rab1, and Hrr25) have also been implicated in autophagy [40–42]. Autophagosomes form in close proximity to ERES in yeast and mammals [43,44], and Sar1 inhibition also inhibits starvation-induced autophagy [40], suggesting that autophagy relies on ER export or certain components of the COPII machinery.
Recent studies have demonstrated that the induction of autophagy results in class-III PI3 kinase–dependent relocation of COPII components from ERES to an ER–Golgi intermediate complex (ERGIC) or ERGIC-like fraction [45•] and that membranes from this fraction promote lipidation of LC3/Atg8 in vitro [46]. Super-resolution microscopy has revealed that ATG13+ structures emerge from regions where ATG9+ vesicles align with the ERGIC. Although the formation of these PAS requires ER exit, being blocked by H89 and FLI06, two inhibitors of COPII transport, ATG13 did not localize to SEC16+ ERES [47•]. ATG13 served as a surrogate for ULK/ATG1 in the super-resolution studies [47•], but given that certain forms of autophagy proceed in the absence of ULK1 and ULK2 [15,16,38••], additional studies are required to determine whether the interaction between ULK/ATG1 and SEC16A is required for the formation of the ATG13+ PAS. Moreover, given that ULK/ATG1 activity is regulated by the metabolic status of the cell and that it interacts with and regulates the function of SEC16A, it is tempting to speculate that ULK1 and ULK2 redirect COPII components for autophagy, similar to the role of Atg1 in redirecting Cvt components.
ULK/ATG1 regulates vesicular trafficking
In addition to ER-to-Golgi trafficking, ULK/ATG1 has been implicated in several cell type–specific functions. In C. elegans, UNC-51 regulates axonal growth along the dorsoventral axis [48]. UNC-51 interacts with UNC-14 to regulate the trafficking of the Netrin receptor UNC-5 [49–51]. UNC-51 also interacts with VAB-8 to control SAX3/ROBO expression in the growth cones of touch neurons [49,52]. The severe axon guidance defects observed in unc-51-mutant C. elegans are not observed in epg-1 (Atg13) or epg-9 (Atg101) mutants, suggesting that the role of UNC-51 in neurodevelopment is not a result of defective autophagy [53,54].
Similar to unc-51 mutants, Drosophila melanogaster atg1 mutants exhibit abnormal axonal tracts in the ventral nerve cord [55] and abnormal defasciculation of the larval mushroom body axons [56]. The NCAM-like axon guidance molecule Fasciclin II is mislocalized in atg1-mutant D. melanogaster due to a defect in the kinesin-mediated vesicle-transport pathway [56]. ATG1-mediated phosphorylation of the kinesin adaptor UNC-76 increases its affinity for Synaptotagmin-1, a major transmembrane protein of synaptic vesicles, and promotes synaptic vesicle transport [55].
Mammalian ULK1 and ULK2 have also been implicated in regulating neurite outgrowth in various neuronal populations [57,58]. ULK1 modulates Ras- and Rab5-mediated endocytosis through its interactions with SynGAP and Syntenin, which recruits Rab5 and ULK1 to a subset of synaptic vesicles [59]. Rab5 marks a subset of signaling endosomes containing endocytosed nerve growth factor (NGF)–TrkA receptor complexes and promotes fusion of the vesicles with the endocytic/degradation pathway, thereby providing a means of titrating the NGF-signaling response [60,61]. The binding of NGF to TrkA induces TRAF6-mediated polyubiquitination of ULK1/2 at lysine 63 residue, thereby facilitating ULK1/2 interaction with p62 and its recruitment to the NGF–TrkA complex [58].
These studies highlight a multifaceted role of ULK/ATG1 in protein and membrane trafficking that may fulfill certain cell type–specific functions. Given that some ULK/Agt1-binding partners are also involved in autophagy, ULK/Atg1 may redirect these proteins in response to metabolic cues. For example, whereas p62 may recruit ULK1/2 to signaling endosomes under basal conditions [58], in response to proteasome inhibition or expression of polyQ-Htt, ULK1-mediated phosphorylation of p62 induces autophagic degradation of protein aggregates [62•]. Similarly, ULK1 interacts with GATE-16, an essential factor for intra-Golgi transport [63], and GABARAP [64], the γ2 subunit of GABA-A receptor–associated protein, which regulates receptor trafficking [65–67]. Both proteins are mammalian homologs of Atg8 and are involved in autophagy [68,69]. In yeast, the interaction between Atg8 and Atg1 targets Atg1 to autophagosomes, and also triggers vacuolar degradation of the Atg1-Atg13 complex [70].
ULK/Atg1 may influence disease
Data on the role of ULK/Atg1 in disease pathogenesis are limited. Nevertheless, alterations in ULK/Atg1 expression and autophagy-related functions have been implicated in the prognosis of some cancers [71]. In patients with esophageal squamous cell carcinoma [72] or hepatocellular carcinoma [73], Ulk1 overexpression is associated with poor prognosis. There are conflicting reports on the correlation between ULK1 expression and prognosis in breast cancer patients, with one report suggesting that ULK1 expression is associated with a poor prognosis [74], and another indicating that low ULK1 expression is a marker for disease progression [75]. Although the precise role of ULK/Atg1-dependent autophagy in tumor development and progression remains controversial, other functions of ULK/Atg1 may contribute to tumor growth.
First, ULK1/2 phosphorylates key glycolytic enzymes (i.e., hexokinase, phosphofructokinase 1, and enolase 1) and a gluconeogenic enzyme (i.e., fructose-1,6-bisphosphatase) to secure carbon flux into the pentose phosphate pathway and maintain cellular energy [76••]. Silencing other key autophagy genes (e.g., Atg5, Atg7, and Beclin-1) does not recapitulate the effect of ULK1/2 deficiency on glucose consumption under the same conditions [76••], dissociating this function from its role in autophagy. ULK1 and ULK2 have both been implicated in fatty acid metabolism in adipocytes [77]. Thus, future studies of metabolic alterations in ULK1-overexpressing tumors may reveal vulnerabilities that could be targeted using ULK/Atg1-specific inhibitors.
Second, ULK1 accelerates cell death in response to excessive accumulation of reactive-oxygen species (ROS). ULK1 and ULK2 are direct targets of p53, and treating osteosarcoma cells with a sublethal dose of camptothecin leads to p53-mediated upregulation of ULK1 and subsequent cell death [78–80]. Similarly, ULK1 sensitizes wild-type murine embryonic fibroblasts to cell death induced by ROS-inducing drugs [81•]. Oxidative stress induces translocation of ULK1 from the cytoplasm to the nucleus, where ULK1 promotes activation of poly (ADP-ribose) polymerase 1 (PARP1), thereby accelerating ATP depletion and necrotic cell death [81•], which can induce a protumor inflammatory response [82]. Thus, heterogeneity of ULK1 localization within a given tumor may benefit its growth. Although ULK1 overexpression may be associated with poor prognosis with current treatment regimens, the fact that it sensitizes cells to ROS-induced cell death could be exploited to select appropriate ROS-inducing drugs to advance treatment of patients with ULK1-overexpressing tumors.
Recent studies have implicated ULK1 and ULK2 in the interferon (IFN)-response pathway. ULK1 mediates a negative-feedback mechanism to limit the transcription of IFNs and related genes in multiple cell lines [83]. Upon IFN stimulation, AKT-dependent phosphorylation of ULK1 at serine residue 757 is required for phosphorylation of p38 MAPK and transcription of IFN-stimulated genes in multiple cell lines [84•]. ULK1 expression is elevated in patients with myeloproliferative disorders, compared to that in age-matched controls, and blocking ULK1 expression suppresses the antineoplastic effects of type-I IFNs against malignant erythroid precursors derived from patients [84•]. These results suggest that elevated ULK1 level could be favorable for type-I IFN treatment of myeloproliferative disorders that are IFN-responsive.
Conclusions
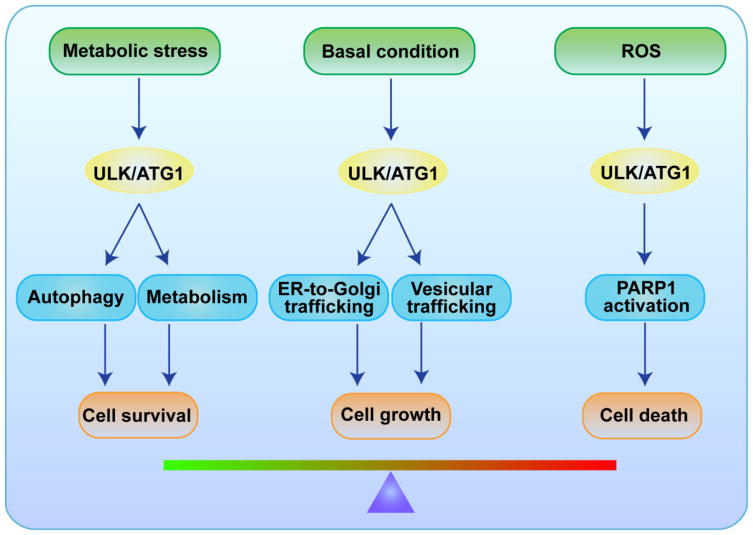
Recent studies have provided insight into the regulation and cellular function of the ULK/Atg1 family of proteins, but additional studies are required to better understand the factors directing the spatiotemporal activation of ULK/Atg1. Whether ULK/Atg1 redirects cellular resources for biosynthetic vs. degradative processes in response to metabolic cues as it does in yeast remains unknown. ULK/Atg1 appears to have related functions under different growth conditions (Figure 2) and is a direct target of metabolic sensors; therefore, it may be central to maintaining cellular homeostasis beyond its role in autophagy. These features may make ULK/Atg1 a more attractive target of therapy than autophagy-specific inhibitors and activators.
Figure 2.
ULK/ATG1 dictates cell fate under different physiologic states. Under normal physiological conditions, such as embryonic development, ULK/ATG1-mediated vesicular trafficking, which appears to be autophagy independent, promotes normal growth (i.e., axonal extension). Upon stress, the cell secures its energy supply by redirecting ULK/ATG1 to the autophagy and glycolysis pathways. When challenged with excessive ROS, the cell actively transports ULK/ATG1 into the nucleus, where the kinase initiates PARP1-mediated necrotic cell death.
Acknowledgments
We are grateful to Dr. Angela McArthur for editing the manuscript. The research was supported by the National Heart, Lung, and Blood Institute (R01 HL114697) and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noda NN, Fujioka Y. Atg1 family kinases in autophagy initiation. Cell Mol Life Sci. 2015;72:3083–3096. doi: 10.1007/s00018-015-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. This paper reported the optimal substrate motif of ULK1, which was identified by screening a peptide library. Additionally, the authors identified 15 novel phosphorylation sites on autophagy proteins, of which Ser249 of VPS34 was one. A selective inhibitor of ULK1, SBI-0206965, was developed and combined with mTOR inhibition to sensitize tumor cells to apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, Ganley IG. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem. 2015;290:11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding TM, Morano KA, Scott SV, Klionsky DJ. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Yeh YY, Shah KH, Herman PK. An Atg13 protein-mediated self-association of the Atg1 protein kinase is important for the induction of autophagy. J Biol Chem. 2011;286:28931–28939. doi: 10.1074/jbc.M111.250324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kijanska M, Dohnal I, Reiter W, Kaspar S, Stoffel I, Ammerer G, Kraft C, Peter M. Activation of Atg1 kinase in autophagy by regulated phosphorylation. Autophagy. 2010;6:1168–1178. doi: 10.4161/auto.6.8.13849. [DOI] [PubMed] [Google Scholar]
- 10••.Torggler R, Papinski D, Brach T, Bas L, Schuschnig M, Pfaffenwimmer T, Rohringer S, Matzhold T, Schweida D, Brezovich A, et al. Two Independent Pathways within Selective Autophagy Converge to Activate Atg1 Kinase at the Vacuole. Mol Cell. 2016;64:221–235. doi: 10.1016/j.molcel.2016.09.008. This study uncovered a new mechanism that spatiotemporally regulates Atg1 activity via two independent pathways that are mediated by Atg13 and Atg11 at the vacuole. [DOI] [PubMed] [Google Scholar]
- 11.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujioka Y, Suzuki SW, Yamamoto H, Kondo-Kakuta C, Kimura Y, Hirano H, Akada R, Inagaki F, Ohsumi Y, Noda NN. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat Struct Mol Biol. 2014;21:513–521. doi: 10.1038/nsmb.2822. [DOI] [PubMed] [Google Scholar]
- 13.Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci U S A. 2009;106:17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signalling pathways. EMBO J. 2011;30:2101–2114. doi: 10.1038/emboj.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alers S, Loffler AS, Paasch F, Dieterle AM, Keppeler H, Lauber K, Campbell DG, Fehrenbacher B, Schaller M, Wesselborg S, et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–1433. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack HI, Zheng B, Asara JM, Thomas SM. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy. 2012;8:1197–1214. doi: 10.4161/auto.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian W, Li W, Chen Y, Yan Z, Huang X, Zhuang H, Zhong W, Chen Y, Wu W, Lin C, et al. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett. 2015;589:1847–1854. doi: 10.1016/j.febslet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Lin SY, Li TY, Liu Q, Zhang C, Li X, Chen Y, Zhang SM, Lian G, Liu Q, Ruan K, et al. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science. 2012;336:477–481. doi: 10.1126/science.1217032. [DOI] [PubMed] [Google Scholar]
- 27.Wold MS, Lim J, Lachance V, Deng Z, Yue Z. ULK1-mediated phosphorylation of ATG14 promotes autophagy and is impaired in Huntington’s disease models. Mol Neurodegener. 2016;11:76. doi: 10.1186/s13024-016-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JM, Jung CH, Seo M, Otto NM, Grunwald D, Kim KH, Moriarity B, Kim YM, Starker C, Nho RS, et al. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy. 2016;12:547–564. doi: 10.1080/15548627.2016.1140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, Maiolica A, Hansmann I, Pfaffenwimmer T, Kijanska M, Stoffel I, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol Cell. 2014;53:471–483. doi: 10.1016/j.molcel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 32.Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154:1285–1299. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, Ma K, Gao R, Mu C, Chen L, Liu Q, Luo Q, Feng D, Zhu Y, Chen Q. Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 2017;27:184–201. doi: 10.1038/cr.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 35.Liang CC, Wang C, Peng X, Gan B, Guan JL. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. 2010;285:3499–3509. doi: 10.1074/jbc.M109.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 37.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 38••.Joo JH, Wang B, Frankel E, Ge L, Xu L, Iyengar R, Li-Harms X, Wright C, Shaw TI, Lindsten T, et al. The Noncanonical Role of ULK/ATG1 in ER-to-Golgi Trafficking Is Essential for Cellular Homeostasis. Mol Cell. 2016;62:491–506. doi: 10.1016/j.molcel.2016.04.020. This study provides the first evidence that constitutive autophagy can proceed in the absence of ULK1 and ULK2 in the adult mouse brain. Although not required for constitutive autophagy, ULK1 and ULK2 have an important homeostatic function in regulating the ER-to-Golgi trafficking of SEC24C-specific cargo, such as serotonin transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, Kihara A, Yoshimori T, Noda T, Ohsumi Y. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell. 2001;12:3690–3702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic. 2010;11:1246–1261. doi: 10.1111/j.1600-0854.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka C, Tan LJ, Mochida K, Kirisako H, Koizumi M, Asai E, Sakoh-Nakatogawa M, Ohsumi Y, Nakatogawa H. Hrr25 triggers selective autophagy-related pathways by phosphorylating receptor proteins. J Cell Biol. 2014;207:91–105. doi: 10.1083/jcb.201402128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc Natl Acad Sci U S A. 2012;109:6981–6986. doi: 10.1073/pnas.1121299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki K, Akioka M, Kondo-Kakuta C, Yamamoto H, Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126:2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 45•.Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. Elife. 2014;3:e04135. doi: 10.7554/eLife.04135. This paper demonstrates that PI3 kinase regulates recruitment of COPII machinary to the ERGIC, which promotes LC3 lipidation, a key step during autophagy induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge L, Melville D, Zhang M, Schekman R. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. Elife. 2013;2:e00947. doi: 10.7554/eLife.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Karanasios E, Walker SA, Okkenhaug H, Manifava M, Hummel E, Zimmermann H, Ahmed Q, Domart MC, Collinson L, Ktistakis NT. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat Commun. 2016;7:12420. doi: 10.1038/ncomms12420. Using an imaging-based approach, the authors found that the ULK1 structure originates from ER docked with ATG9 vesicles. The formation of this structure also depends on ER exit and COPII vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogura K, Wicky C, Magnenat L, Tobler H, Mori I, Muller F, Ohshima Y. Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 1994;8:2389–2400. doi: 10.1101/gad.8.20.2389. [DOI] [PubMed] [Google Scholar]
- 49.Lai T, Garriga G. The conserved kinase UNC-51 acts with VAB-8 and UNC-14 to regulate axon outgrowth in C. elegans. Development. 2004;131:5991–6000. doi: 10.1242/dev.01457. [DOI] [PubMed] [Google Scholar]
- 50.Ogura K, Shirakawa M, Barnes TM, Hekimi S, Ohshima Y. The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev. 1997;11:1801–1811. doi: 10.1101/gad.11.14.1801. [DOI] [PubMed] [Google Scholar]
- 51.Ogura K, Goshima Y. The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans. Development. 2006;133:3441–3450. doi: 10.1242/dev.02503. [DOI] [PubMed] [Google Scholar]
- 52.Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10:169–176. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- 53.Liang Q, Yang P, Tian E, Han J, Zhang H. The C. elegans ATG101 homolog EPG-9 directly interacts with EPG-1/Atg13 and is essential for autopha. Autophagy. 2012;8:1426–1433. doi: 10.4161/auto.21163. [DOI] [PubMed] [Google Scholar]
- 54.Tian E, Wang F, Han J, Zhang H. epg-1 functions in autophagy-regulated processes and may encode a highly divergent Atg13 homolog in C. elegans. Autophagy. 2009;5:608–615. doi: 10.4161/auto.5.5.8624. [DOI] [PubMed] [Google Scholar]
- 55.Toda H, Mochizuki H, Flores R, 3rd, Josowitz R, Krasieva TB, Lamorte VJ, Suzuki E, Gindhart JG, Furukubo-Tokunaga K, Tomoda T. UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev. 2008;22:3292–3307. doi: 10.1101/gad.1734608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mochizuki H, Toda H, Ando M, Kurusu M, Tomoda T, Furukubo-Tokunaga K. Unc-51/ATG1 controls axonal and dendritic development via kinesin-mediated vesicle transport in the Drosophila brain. PLoS One. 2011;6:e19632. doi: 10.1371/journal.pone.0019632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomoda T, Bhatt RS, Kuroyanagi H, Shirasawa T, Hatten ME. A mouse serine/threonine kinase homologous to C. elegans UNC51 functions in parallel fiber formation of cerebellar granule neurons. Neuron. 1999;24:833–846. doi: 10.1016/s0896-6273(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Babu JR, da Silva S, Shu Q, Graef IA, Oliver T, Tomoda T, Tani T, Wooten MW, Wang F. Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc Natl Acad Sci U S A. 2007;104:5842–5847. doi: 10.1073/pnas.0701402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomoda T, Kim JH, Zhan C, Hatten ME. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 2004;18:541–558. doi: 10.1101/gad.1151204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 61.Howe CL, Mobley WC. Signaling endosome hypothesis: A cellular mechanism for long distance communication. J Neurobiol. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- 62•.Lim J, Lachenmayer ML, Wu S, Liu W, Kundu M, Wang R, Komatsu M, Oh YJ, Zhao Y, Yue Z. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015;11:e1004987. doi: 10.1371/journal.pgen.1004987. This study provides strong biochemical evidence that ULK1 and ULK2 phosphorylate p62/SQSTM1 to regulate autophagy in response to proteasome inhibition or expression of polyglutamine-expanded huntingtin but not in response to nutrient starvation. Thus, this paper highlights the role of ULK1 and ULK2 in a specific type of autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagiv Y, Legesse-Miller A, Porat A, Elazar Z. GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28. EMBO J. 2000;19:1494–1504. doi: 10.1093/emboj/19.7.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okazaki N, Yan J, Yuasa S, Ueno T, Kominami E, Masuho Y, Koga H, Muramatsu M. Interaction of the Unc-51-like kinase and microtubule-associated protein light chain 3 related proteins in the brain: possible role of vesicular transport in axonal elongation. Brain Res Mol Brain Res. 2000;85:1–12. doi: 10.1016/s0169-328x(00)00218-7. [DOI] [PubMed] [Google Scholar]
- 65.Leil TA, Chen ZW, Chang CS, Olsen RW. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, Wang H, Vicini S, Olsen RW. The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc Natl Acad Sci U S A. 2000;97:11557–11562. doi: 10.1073/pnas.190133497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 68.Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29:1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joachim J, Jefferies HB, Razi M, Frith D, Snijders AP, Chakravarty P, Judith D, Tooze SA. Activation of ULK Kinase and Autophagy by GABARAP Trafficking from the Centrosome Is Regulated by WAC and GM130. Mol Cell. 2015;60:899–913. doi: 10.1016/j.molcel.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraft C, Kijanska M, Kalie E, Siergiejuk E, Lee SS, Semplicio G, Stoffel I, Brezovich A, Verma M, Hansmann I, et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012;31:3691–3703. doi: 10.1038/emboj.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang S, Li Y, Zhu YH, Wu XQ, Tang J, Li Z, Feng GK, Deng R, Li DD, Luo RZ, et al. Intensive expression of UNC-51-like kinase 1 is a novel biomarker of poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Sci. 2011;102:1568–1575. doi: 10.1111/j.1349-7006.2011.01964.x. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Yu H, Zhang X, Shen X, Zhang K, Sheng H, Dai S, Gao H. UNC51-like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6:711–717. [PMC free article] [PubMed] [Google Scholar]
- 74.Pike LR, Singleton DC, Buffa F, Abramczyk O, Phadwal K, Li JL, Simon AK, Murray JT, Harris AL. Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. Biochem J. 2013;449:389–400. doi: 10.1042/BJ20120972. [DOI] [PubMed] [Google Scholar]
- 75.Tang J, Deng R, Luo RZ, Shen GP, Cai MY, Du ZM, Jiang S, Yang MT, Fu JH, Zhu XF. Low expression of ULK1 is associated with operable breast cancer progression and is an adverse prognostic marker of survival for patients. Breast Cancer Res Treat. 2012;134:549–560. doi: 10.1007/s10549-012-2080-y. [DOI] [PubMed] [Google Scholar]
- 76••.Li TY, Sun Y, Liang Y, Liu Q, Shi Y, Zhang CS, Zhang C, Song L, Zhang P, Zhang X, et al. ULK1/2 Constitute a Bifurcate Node Controlling Glucose Metabolic Fluxes in Addition to Autophagy. Mol Cell. 2016;62:359–370. doi: 10.1016/j.molcel.2016.04.009. This paper reveals a role for ULK1 and ULK2 in glucose metabolism. The authors demonstrate that upon nutrient depletion, ULK1 and ULK2 not only initiate autophagy but also enhance glucose metabolism to maintain cellular energy and redox homeostasis via direct phosphorylation of key glycolytic enzymes. [DOI] [PubMed] [Google Scholar]
- 77.Ro SH, Jung CH, Hahn WS, Xu X, Kim YM, Yun YS, Park JM, Kim KH, Seo M, Ha TY, et al. Distinct functions of Ulk1 and Ulk2 in the regulation of lipid metabolism in adipocytes. Autophagy. 2013;9:2103–2114. doi: 10.4161/auto.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao W, Shen Z, Shang L, Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ. 2011;18:1598–1607. doi: 10.1038/cdd.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenzelmann Broz D, Spano Mello S, Bieging KT, Jiang D, Dusek RL, Brady CA, Sidow A, Attardi LD. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27:1016–1031. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenzelmann Broz D, Attardi LD. TRP53 activates a global autophagy program to promote tumor suppression. Autophagy. 2013;9:1440–1442. doi: 10.4161/auto.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.Joshi A, Iyengar R, Joo JH, Li-Harms XJ, Wright C, Marino R, Winborn BJ, Phillips A, Temirov J, Sciarretta S, et al. Nuclear ULK1 promotes cell death in response to oxidative stress through PARP1. Cell Death Differ. 2016;23:216–230. doi: 10.1038/cdd.2015.88. This paper uncovered a noncanonical function of ULK1 and ULK2 in sensitizing cells to necrotic cell death after hydrogen peroxide treatment. By interacting with and enhancing PARP1 activity, ULK1 and ULK2 promote ATP depletion and death of hydrogen peroxide–treated cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155:688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Saleiro D, Mehrotra S, Kroczynska B, Beauchamp EM, Lisowski P, Majchrzak-Kita B, Bhagat TD, Stein BL, McMahon B, Altman JK, et al. Central role of ULK1 in type I interferon signaling. Cell Rep. 2015;11:605–617. doi: 10.1016/j.celrep.2015.03.056. This manuscript reports that in response to IFN stimulation, Akt promotes the phosphorylation of ULK1 at S757, which is essential for optimal phosphorylation of p38 MAPK and proper transcription of IFN-stimulated genes. The finding highlights a noncanonical function of ULK1 and ULK2 in an antiviral response. [DOI] [PMC free article] [PubMed] [Google Scholar]