Abstract
Objective
Smokers who are not motivated to quit are an important group for intervention, particularly if they have children with asthma. Research indicates that unmotivated smokers are less responsive to intensive interventions, although motivation by treatment interactions have not been tested. This study examines whether motivation to quit moderates the effect of a cessation induction intervention.
Methods
Parents had an asthmatic child requiring urgent care, and did not have to want to quit smoking to be eligible for the study. Two home visits included asthma education, motivational interviewing (MI) for cessation and feedback on child’s secondhand smoke exposure (SHSe). Participants were then randomized (n=339, 79.6% female) to receive Enhanced-PAM (Precaution Adoption Model; 6 MI calls including SHSe feedback) or PAM (6 contact control calls). Motivation to quit was assessed at baseline and point-prevalence abstinence (ppa) and SHSe outcomes were objectively measured.
Results
At baseline, 38.9% were not motivated to quit. Those who were not motivated to quit were 3 to 4 times more likely to be abstinent at six months in Enhanced-PAM vs. PAM (7-day ppa: OR=3.71, 95%CI=1.06, 12.99; 30-day: OR=4.15, 95%CI=1.20, 14.35); those receiving Enhanced-PAM achieved quit rates comparable to motivated smokers. Those who were not motivated to quit were more than 4 times as likely to have very low/undetectable SHSe at follow-up in Enhanced-PAM vs PAM (OR=4.46, 95%CI=1.31, 15.15). Among motivated smokers, neither outcome significantly differed by treatment arm.
Conclusions
It cannot be assumed that smokers who are unmotivated to quit will not be responsive to intensive interventions.
Keywords: motivational interviewing, biomarker feedback, asthma, smoking cessation, teachable moment, unmotivated smokers
Although 70% of smokers want to stop smoking at some point in their lives (Malarcher, Dube, Shaw, Babb, & Kauffmann, 2011), only 20–30% of smokers are ready to quit within 30 days (Etter, Perneger, & Ronchi, 1997; Reid, Hammond, Rynard, & Burkhalter, 2014). Evidenced-based treatments are primarily designed for smokers who are ready to quit within 30 days. Thus, these treatments are less likely to attract and retain smokers who are not motivated to quit (Catley et al., 2012; Harris et al., 2016), and the effect on their smoking may even be counterproductive because the treatment approach is not matched to their level of motivation (Aveyard et al., 2006). Similarly, the vast majority of research studies recruit only those smokers who are ready to quit within 30 days, excluding smokers who are not ready to quit. Studies have shown that smokers who are not motivated to quit are older, Caucasian, unemployed, smoke more cigarettes per day and are more likely to have used non-guideline based approaches to quit smoking in their previous attempts versus smokers who are motivated to quit (Borrelli, Gaynor, et al., 2016; Borrelli et al., 2015; Bartlett et al., 2015). Therefore, treatments are needed that show effectiveness in helping smokers who are not motivated to quit smoking.
Parents who smoke and are not motivated to quit smoking are an important group to target for intervention, given that secondhand smoke exposure (SHSe) increases the risk of pediatric asthma and other respiratory illnesses, otitis media and poor cognitive development (Burke et al., 2012; Chen, Clifford, Lang, & Anstey, 2013). SHSe also increases the likelihood that children will become smokers themselves (Kandel, Griesler, & Hu, 2015; Vuolo & Staff, 2013). Despite reductions in overall smoking prevalence, parental smoking and pediatric SHSe remain high, particularly among minority and low income families, and parents with children with asthma (Fedele et al., 2016; Kauffmann et al., 2010).
Our previous randomized controlled trial enrolled parents who smoked and had a child with asthma regardless of their motivation to quit smoking (Borrelli, McQuaid, Tooley, et al., 2016). Parents who brought their child to urgent care due to an asthma exacerbation were recruited. They were told that they did not have to quit smoking in order to enroll in the study, but that they would have to agree to talk about their smoking as part of the asthma education that they would receive. Thus, asthma education was provided as a ‘foot in the door’ to discuss the risks of smoking on their child’s health and their own health. After receiving two home visits that included asthma education and motivational interviewing (MI) for cessation induction (which included biomarker feedback on the child’s SHS and smoker’s level of carbon monoxide), parents were randomized to receive either Enhanced-PAM (Precaution Adoption Model; continued MI for cessation induction and repeated SHSe feedback) or PAM (contact control calls that included a brief check on asthma status) during six phone calls over 4 months. Enhanced-PAM was significantly more likely to quit smoking than PAM at short and long term follow-ups.
The current paper is a secondary analysis of the data from this previous trial to test whether the effect of treatment condition is moderated by motivation to quit. To our knowledge, only one other study examined whether smoking cessation treatment was moderated by baseline motivation to quit, and this study was done with pregnant smokers, did not control for treatment intensity, and did not use MI (Aveyard et al., 2006). Motivational Interviewing is particularly well-suited for smokers who are not motivated to quit, given the emphasis on resolving ambivalence, building intrinsic motivation, and identifying how one’s personal values are both helped and hindered by smoking (Borrelli, 2013). Meta-analyses have shown that MI increases quit rates (Heckman, Egleston, & Hofmann, 2010; Lai, Cahill, Qin, & Tang, 2010), and biomarker feedback on the health risks of smoking has been found to potentiate the effects of MI (Borrelli, McQuaid, et al., 2016).
We hypothesized that motivation to quit within 30 days would moderate the effect of the intervention on smoking outcome and SHSe, such that smokers who were not motivated to quit within 30 days would be more likely to quit smoking and reduce SHSe with Enhanced-PAM vs. PAM. On the other hand, we hypothesized that smokers who were motivated to quit within 30 days would be equally likely to quit smoking and reduce SHSe in either treatment condition. A rejection of the null hypothesis would suggest that more intensive, longitudinal treatments that use MI and repeated feedback are needed for smokers who are not motivated to quit, and that less resources may be used for interventions with smokers who are motivated to quit given their equal propensity to quit regardless of intervention intensity.
Our definition of ‘motivated to quit within 30 days’ was used because 1) the literature has previously used imprecise definitions of unmotivated smokers (Carpenter et al., 2011; Davis et al., 2011), 2) this is the guideline that practitioners use to determine that the smoker is serious about quitting and recommend pharmacotherapy and behavioral counseling, 3) it corresponds to the voluminous literature on the ‘preparation’ stage of change so that comparisons can be made with prior studies, 4) it has been used in the majority of systematic reviews and empirical articles (Asfar, Ebbert, Klesges, & Relyea, 2011), and 5) it is consistent with the definition used in the U.S. Public Health Service (USPHS, 2008) clinical practice guidelines.
Method
Sample and Participant Selection
Parents of children with asthma were recruited from emergency departments and urgent care centers in Rhode Island, USA. Parents were eligible if they 1) were the primary caregiver ≥ 18 years of age (i.e., the person who spends the most time with the child and > 4 hours per week), 2) have a child aged 3–17 years (asthma is difficult to diagnose in very young children), 3) smoked ≥ 3 cigarettes/day for the past year and > 100 cigarettes in their life-time (to ensure that the sample is comprised of ‘regular’ smokers, rather than ‘occasional’ smokers), 4) were not pregnant or planning to become pregnant, 5) spoke English, 6) had a telephone, and 7) not currently enrolled in smoking cessation treatment. Parents of children who had other pulmonary disease (e.g., cystic fibrosis) were excluded. Prospective participants were told that they would be required to accept asthma education visits in their home and discuss their smoking, although they did not have to want to quit smoking to be enrolled in the trial. Informed consent was obtained in writing from all participants. The study was approved by our institution’s human subjects review board.
Design
This paper is a secondary analysis from a larger study on motivating parents to quit smoking, which recruited a sample of parents who smoked and had a child with asthma, and a sample of parents who smoked and had healthy children (Borrelli et al., 2016). The current study utilizes only the sample of parents who smoked and had a child with asthma, because this group was the only group who were randomized to receive either a more intensive (Enhanced-PAM) or less intensive (PAM) smoking cessation intervention, a feature which is critical to testing the hypotheses proffered in the current study.
All participants who smoked and had a child with asthma received two, 1-hour home visits focused on asthma education and smoking cessation induction counseling. The asthma education was based on NIH guidelines (NAEPP, 2007). The smoking cessation induction counseling used Motivational Interviewing (Miller, 2002), which included communication strategies (e.g., open-ended questions, affirmations, reflections, evocation, empathy, autonomy promotion) and motivational techniques (costs/benefits of quitting, elicit-provide-elicit, typical day, barriers/facilitators of motivation and confidence, resolving ambivalence, and values clarification (Borrelli, 2013). Verbal and graphical feedback was provided regarding their 1) carbon monoxide (CO) level, associated symptoms, and how quitting could attenuate disease risk and symptoms, and 2) child’s SHSe based on objective measurements obtained by passive dosimetry: “Your child breathed in as much smoke as if s(he) smoked ‘X’ number of cigarettes last week.” Feedback on the level of SHSe in their home versus non-smokers’ homes was also provided. The risks of smoking on their child’s asthma and how risks could be attenuated by both quitting smoking and/or eliminating SHSe were discussed. If the smoker was ready to quit, options for quitting were explored and skill building and problem solving regarding how to cope with triggers to smoke were discussed.
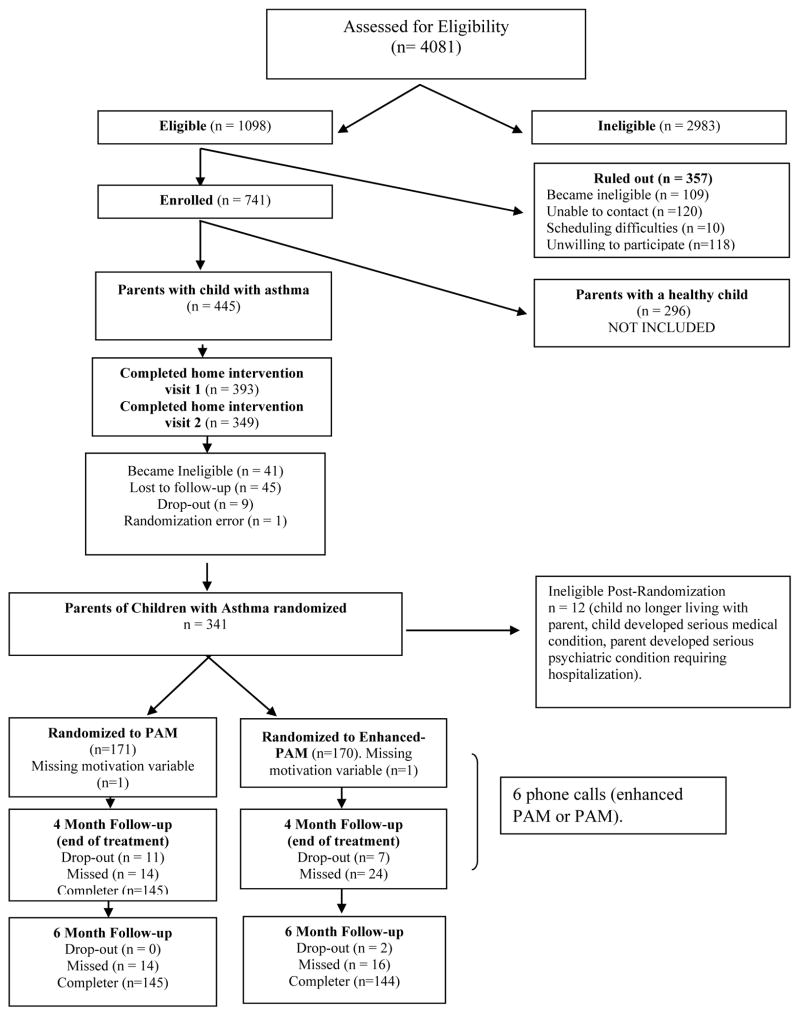
After the two home visits, parents of children with asthma were randomized to either PAM or Enhanced-PAM (Figure 1); both groups received six, 15–20 minute calls regarding asthma symptoms and management for four months after the home visits. Calls were given twice per month (every other week) for the first two months and once per month for the last two months. For PAM participants, changes in asthma symptoms (daytime and nighttime) and asthma medications were explored, as well as reasons for changes. The asthma management strategies previously discussed in visits 1 and 2 were reviewed, and counseling regarding asthma management continued in MI style. PAM did not receive any smoking cessation counseling during the phone calls. Enhanced-PAM received a ‘check-in’ on asthma symptoms and management, as well as strategies to help parents link their smoking to their child’s asthma. This included continued MI for smoking cessation, including how smoking fits in with (or hinders) the smokers’ values and goals, elicitation of change talk through strategic open-ended questions, and building readiness and confidence for change and, at the final phone call, a second round of SHSe feedback was given (to compare their child’s current levels SHSe with those obtained during the home visits). The number, duration, and timing of follow-up phone calls were identical for both Enhanced-PAM and PAM. Participants were offered 8 weeks of nicotine patch treatment at no cost if they were willing to set a quit date within 30 days.
Figure 1.
Participant recruitment flowchart
Treatment Fidelity
We followed best practice guidelines for treatment fidelity (Borrelli et al., 2005). Trainers were licensed Clinical Psychologists, one certified in Motivational Interviewing and the other in asthma education. Counselors had graduate-level degrees and were trained using didactics, role-plays, and video. Skill acquisition was determined by intervention delivery with pilot participants. Counselors were guided by a written treatment protocol, sessions were audiotaped, and a random 20% were reviewed weekly with counselors. Sessions were coded using the Motivational Interviewing Treatment Integrity coding system (version 3.1.1) by three coders (blind to condition and outcomes) who completed 40 hours of training (e.g., coding standardized transcripts).
Measures
Smoking variables
Nicotine dependence was measured at baseline with the Fagerstrom Test for Nicotine Dependence (FTND), a valid and reliable scale to assess the degree of participant’s nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Number of cigarettes smoked per day in the past week, total number of years smoked and the presence of household smoking ban (no smoking allowed anywhere in the home) were assessed by parent self-report.
Smoking status
Smoking status was assessed within one month of the last treatment call (4 months after baseline) and 2 months later (at the 6 month follow-up). Smoking status was assessed via self-report of no smoking, not even a puff, in the past 7 or 30 days (point prevalence abstinence (ppa)). Self-reported abstinence was verified using expired air carbon monoxide (Bedfont, CO Ecolyzer) at follow-up assessment. Abstinence was defined as CO ≤ 9 part per million (ppm) (Benowitz, 2002). Continuous abstinence was defined as no smoking since the previous assessment.
Motivation
Motivation to quit was assessed at baseline with the question “Are you seriously thinking about quitting smoking”? The answer choices were ‘yes, within the next 30 days’; ‘yes, within the next 6 months’ or ‘no, I am not thinking of quitting smoking.’ We considered those who endorsed ‘yes within 30 days’ as ‘motivated to quit.’ Endorsement to either ‘yes within the next 6 months’ or ‘no, I am not thinking of quitting smoking’ were classified as ‘not motivated to quit.’ We collapsed these two groups for several reasons. Conceptually, the two groups (‘I plan to quit within 6 months’ and ‘I am not thinking of quitting) are similar because they represent smokers who are not committed to quitting smoking, either having a distal and vague goal to quit, or no plan to quit. Furthermore, there were no significant differences in abstinence rates between these two groups, providing further evidence to combine them. This categorical method of assessing motivation to quit is preferable to continuous measures because it avoids the need to choose arbitrary cut off values (Abrams, 2003).
Secondhand smoke exposure (SHSe)
SHSe was assessed objectively using a passive nicotine monitor that uses nicotine as an index for SHSe (Hammond & Leaderer, 1987; Leaderer & Hammond, 1991). One passive nicotine monitor was placed in the room in which the child spent the most time (home environment) and one was worn by the child (child environment). The monitor detects nicotine in the environment by passively collecting ambient smoke at a sampling rate of 24 ml/min. The monitors were analyzed by a chemistry laboratory blind to treatment condition using gas chromatography (Hammond & Leaderer, 1987) with a limit of detection of 0.005 mcg/m3. Given the expected highly skewed distribution of the sample concentrations, baseline and follow-up values were discretized as ‘very low/undetectable’ (< 0.05 mcg/m3), ‘moderate’ (0.05 – 0.49 mcg/m3) and ‘high’ levels (≥ 0.50 mcg/m3) according to validated cut off values (Leaderer & Hammond, 1991). Home and the child monitors were each dichotomized into very low/undetectable vs. moderate/high levels. Home and child monitors were analyzed separately.
Analytic Plan
Analyses were performed with IBM SPSS Statistics version 20. Baseline differences in demographic variables between motivated and not motivated to quit groups were assessed using Analysis of Variance (ANOVA) for continuous variables and Chi squared tests for categorical variables. The association between motivation and the cessation outcomes (7 and 30 day ppa, and continuous abstinence) was examined with a series of longitudinal regression models implemented with generalized estimating equations (GEE’s) with robust standard errors (Hu & Lachin, 2001). Analysis was performed on the intention-to-treat sample, thus including all randomized participants (missing = smoking). Models allow for simultaneous testing of effects on outcomes at multiple time points. In this model, time was entered as a within-subjects factor whereas motivation to quit (yes vs. no) and treatment group (Enhanced-PAM vs PAM) were entered as between-subjects categorical factors. Covariates included parent’s age and sex, employment status and ethnicity that were selected due to significant differences between motivation groups on these variables (Table 1), as well as consistency with previously presented outcomes results (Borrelli et al., 2016). First, we specified a model that included a three-way interaction between motivation, treatment group, and time. Because the 3-way interaction did not reach statistical significance, we tested only the interaction between motivation to quit and treatment group and adjusted for the (fixed) main effect of time.
Table 1.
Baseline sociodemographic and smoking-related characteristics of parents by motivation to quit
| Variable | Motivated to Quit (n = 207) Mean (SD) or % |
Not Motivated to Quit (n = 132) Mean (SD) or % |
Total Sample (n = 339) Mean (SD) or % |
|---|---|---|---|
| Parent age* (yrs) | 35.3 (10.3) | 32.1 (8.8) | 34.1 (9.9) |
| Child age (yrs) | 5.2 (4.5) | 5.0 (4.5) | 5.2 (4.5) |
| Female* % | 75.8 | 85.6 | 79.6 |
| Employed* % | 48.5 | 35.1 | 43.3 |
| % < High school | 29.0 | 36.4 | 31.9 |
| % Hispanic† | 18.6 | 11.5 | 15.8 |
| % White | 50.7 | 49.2 | 50.1 |
| # of Cigarettes/day | 14.3 (9.8) | 14.8 (12.5) | 14.5 (10.9) |
| # Years smoked | 18.2 (10.3) | 16.2 (8.7) | 17.5 (9.8) |
| FTND | 4.2 (2.3) | 4.0 (2.4) | 4.2 (2.4) |
| % household ban | 64.3 | 62.1 | 63.4 |
Note: Percentages shown are column %; FTND = Fagerstrom Test for Nicotine Dependence.
p = ≤ 0.03 for differences between those who are motivated to quit and those who are not motivated to quit.
p=.08
Unadjusted and adjusted parameter estimates for the main effects and interactions (B, SE and 95% CI)are presented in Table 3. Statistically significant interactions are interpreted as between group comparisons (Enhanced-PAM vs. PAM) on the odds of being abstinent at end of treatment and at six-month follow-up for the motivated and not-motivated subgroups and are expressed as odd ratios (ORs) with 95% CI.
Table 3.
Unadjusted and adjusted analyses between study variables and cessation outcomes.
| 7-day ppa | 30-day ppa | Continuous Abstinence | |
|---|---|---|---|
|
| |||
| Model 1: Unadjusted | B (SE)a | B (SE) | B (SE) |
| 95% CI | 95% CI | 95%CI | |
| Time | −.14 (.17) | −.02 (.15) | .11 (.17) |
| (−.47, .19) | (−.33, .27) | (−.22, .45) | |
| Treatment | −1.32 (.63) | −1.42 (.62) | −1.47 (.68) |
| (−2.55, −.08)1 | (−2.64, −.20)3 | (−2.80, −.13)1 | |
| Motivation | −1.20 (.59) | −1.37 (.58) | −1.48 (.65) |
| (−2.36, −.04)2 | (−2.52, −.22)3 | (−2.76, −.20)3 | |
| Motivation *treatment | 1.17 (.71) | 1.36 (.70) | 1.66 (.76) |
| (−.22, 2.57) | (−.02, 2.70) † | (.17, 3.16) 1 | |
| Model 2: Adjusted | |||
| Time | −.09 (.18) | .03 (.16) | .06 (.17) |
| (−.45, .27) | (−.29, .35) | (−.28, .40) | |
| Parent sex | −.35 (.35) | −.38 (.36) | −.41 (.36) |
| (−1.04, .35) | (−1.09, .34) | (−1.13, .30) | |
| Parent age | .003 (.01) | .007 (.01) | .01 (.01) |
| (−.03, .03) | (−.03, .04) | (−.02, .04) | |
| Parent Hispanic | .01 (.42) | .02 (.38) | .20 (.39) |
| (−.81, .84) | (−.73, .78) | (−.57, .97) | |
| Employment | .32 (.32) | .33 (.31) | .30 (.34) |
| (−.30, .95) | (−.29, .96) | (−.37, .97) | |
| Treatment | −1.36 (.63) | −1.45 (.63) | −1.45 (.69) |
| (−2.61, −.12)1 | (−2.68, −.23)3 | (−2.81, −.09)2 | |
| Motivation | −1.16 (.60) | −1.33 (.60) | −1.43 (.65) |
| (−2.35, .01)† | (−2.51, −.16)1 | (−2.71, −.16)1 | |
| Motivation * Treatment | 1.53 (.73) | 1.68 (.72) | 1.58 (.77) |
| (.09, 2.97)2 | (.26, 3.00)3 | (.06, 3.10)2 | |
p=0.03
p=0.04
p=0.02
p=0.053
SE= Standard Error
Logistic regression models were used to examine the association between motivation to quit and objectively assessed SHSe in the home and child environment. At step 1, the same covariates selected above were entered plus child’s age and the proportion of households with a smoking ban. At step two, treatment group and motivation to quit were entered as predictors, and a treatment group by motivation to quit interaction was specified. Significant interactions were interpreted as between group comparisons (Enhanced-PAM vs. PAM) on the odds of being in the very low/undetectable SHSe category at follow-up for the motivated and not motivated group. Models additionally controlled for baseline value of the outcome (SHSe levels).
Results
Preliminary Analyses
Baseline demographics and smoking-related sample characteristics are summarized in Table 1. There were 207 participants who reported motivation to quit within 30 days (61.1%) and 132 (38.9%) who were not motivated to quit within 30 days. Smokers who were motivated to quit were more likely to be employed (χ2 = 4.74, df = 1, p = 0.03) and older (F1, 338 = 8.41, p = 0.004) compared to those who were not motivated to quit. Females were less likely to be motivated to quit (χ2 = 4.74, df = 1, p = 0.03). There was no significant difference in the proportion of motivated and not motivated participants by treatment group (χ2 = 0.032, df = 1, p = 0.86). There were also no significant differences between those who were motivated to quit and those who were not motivated to quit in drop-out rates (motivated: 20.8% vs not-motivated: 13.6%; χ2 = 2.78, df = 1, p = 0.09), home visit completion rates (home visit 1: χ2 =0.43, df=1, p=0.51; home visit 2: χ2 =0.64, df=1, p=0.42), or intervention dose as evidenced by phone calls received (Z=−0.44, p=0.67). As expected, a greater proportion of smokers who were motivated to quit were sent nicotine patches (86.5%) and used these patches (64.7%) compared to smokers not motivated to quit (56.1% sent patches and 42.2% used patches; χ2 = 12.61, df=1, p=0.0004). There were no significant differences in 7-day ppa by use of nicotine patches at either end of treatment (χ2 = 0.02, df=1, p=0.90) or at six-months (χ2 = 0.96, df=1, p=0.33). As reported in a previous paper, objectively coded data indicated satisfactory adherence to MI and there were no differences between treatment groups on treatment fidelity (Borrelli et al., 2016).
Motivation to quit and smoking cessation outcomes
Table 2 summarizes the unadjusted proportions of participants achieving abstinence at the four and six-month end-points by motivation to quit smoking in the two treatment groups. Regression models adjusted for covariates indicated that the group by motivation by time interaction was not significant (7-day ppa: Wald χ2 = 4.31, p = 0.36; 30-day ppa: Wald χ2 = 6.80, p = 0.15; continuous abstinence: Wald χ2 = 6.07, p = 0.19), therefore, time was dropped from the interaction term and the models were refit.
Table 2.
Unadjusted proportion of parents achieving abstinence at 4 month (end of treatment) and at 6 month follow-up by motivation to quit in the two treatment groups.
| Outcome | Motivated to quit (n = 207) | Not-motivated to quit (n = 132) | ||
|---|---|---|---|---|
|
| ||||
| Enhanced-PAM (n = 104) | PAM (n = 103) | Enhanced-PAM (n = 65) | PAM (n = 67) | |
| 7 day ppa | ||||
| 4-month | 16.30% | 14.60% | 15.40%1 | 3.00% |
| 6-month | 13.50% | 11.70% | 13.80% | 6.00% |
| 30 day ppa | ||||
| 4-month | 18.30% | 13.60% | 18.50%2 | 3.00% |
| 6-month | 14.40% | 17.50% | 13.80% | 6.00% |
| Continuous abstinence | ||||
| 4-month | 13.50% | 11.70% | 15.40%3 | 3.00% |
| 6-month | 11.50% | 17.50% | 13.80%† | 4.50% |
PPA = point-prevalence-abstinence.
Superscript numbers refer to significant unadjusted comparisons between Enhanced PAM and PAM for motivation and not motivated participants.
p=.013
p=.004
p=.013.
p=.06
After adjusting for pre-specified covariates, a significant motivation by treatment interaction was found for 7-day ppa, 30-day ppa, and continuous abstinence (Table 3). Among participants who were not motivated to quit, the odds of achieving 7-day ppa at follow-up were more than three times greater in Enhanced-PAM vs. PAM (OR = 3.71, 95% CI = 1.06, 12.99). The effects were stronger for 30-day ppa (OR = 4.15, 95% CI = 1.20, 14.35) and continuous abstinence (OR = 4.32, 95% CI = 1.11, 16.87). Importantly, among those not motivated to quit, Enhanced PAM had quit rates that were comparable to smokers who were motivated to quit. Among participants who were motivated to quit smoking, there were no significant differences between treatment groups in the odds of achieving 7-day ppa (OR = 1.13, 95% CI = 0.59, 2.18) or 30-day ppa (OR = 1.13, 95% CI = 0.59, 2.17) or continuous abstinence (OR = 0.89, 95% CI = 0.45, 1.76). In the unadjusted model (Table 3), a statistically significant interaction between motivation and treatment group was observed for continuous abstinence and, marginally (p=.053), for 30-day ppa, but not for 7 day ppa.
Motivation to quit and improvements in SHSe
Table 4 summarizes the unadjusted proportion of participants in each category of SHSe at baseline and follow-up for the home (A), and child (B) environment by motivation to quit in the two treatment conditions. There was a significant treatment group by motivation interaction (B = 1.63, SE = 0.75, p = 0.03) for predicting home SHSe at follow-up. Among participants who were not motivated to quit smoking, the odds of being in the very low/undetectable category at follow-up for participants randomized to Enhanced-PAM were more than 4 times that of PAM (OR = 4.46, 95% CI = 1.31, 15.15) after adjustment for baseline SHSe and the other study covariates.
Table 4-A.
Secondhand smoke exposure levels at baseline and 4-month follow-up in the home environment by motivation to quit in the two treatment groups.
| Time | Home SHSe category | Motivated to quit (n = 160) | Not-motivated to quit (n=111) | ||
|---|---|---|---|---|---|
|
| |||||
| Enhanced-PAM (n = 80) | PAM (n = 80) | Enhanced-PAM (n = 53) | PAM (n = 58) | ||
| Baseline | very low/undetectable | 16.30% | 23.30% | 15.40% | 16.40% |
| moderate/high | 83.70% | 76.70% | 84.60% | 83.60% | |
| Follow-up | very low/undetectable | 21.20% | 25.00% | 24.50% | 8.60% |
| moderate/high | 78.80% | 75.00% | 75.50% | 91.40% | |
Among motivated participants, there were no differences in the odds of having very low/undetectable levels of home SHSe at follow-up between treatment groups (OR = 0.96, 95% CI = 0.42, 2.23). These was not a significant treatment group by motivation interaction for predicting the odds of having very low/undetectable levels of SHSe at follow-up in the child sampler environment (B = 0.56, SE = 0.69, p = 0.41).
Discussion
Evidenced-based treatments are predominately designed for smokers who are ready to quit within 30 days. There is little guidance from research about what treatments are effective for smokers who are not ready to quit, particularly smokers who have a child with asthma. In the current study, parents of a child with asthma who were not motivated to quit smoking achieved better smoking cessation and SHSe outcomes with the more intensive intervention (Enhanced PAM) vs. the less intensive intervention (PAM), whereas those who were motivated to quit smoking at study entry did not significantly differ in their outcomes by treatment group. Further, those who were not motivated to quit and received the more intensive intervention achieved cessation rates comparable to those who were motivated to quit. This suggests that interventions should be provided to smokers who have children with asthma, regardless of their motivation to quit. Intensive, longitudinal treatments that focus on cessation induction using MI and SHSe feedback may be needed to achieve smoking cessation outcomes among smokers who are not motivated to quit smoking. Our results are consistent with meta-analyses that have shown that MI is particularly effective for smokers who are not motivated to quit (Hettema & Hendricks, 2010). To our knowledge, no previous studies have examined the interaction between motivation to quit and treatment condition.
Our results are opposite to those found by (Aveyard et al., 2006) who found that women who were ready to quit smoking at baseline benefited more from motivational and intensive materials than women who were in earlier stages of change. The authors suggested that smokers ‘in preparation’ for quitting may respond more favorably to interventions because they are primed and more interested to do so. However, that study was conducted with pregnant women, who may have different facilitators and barriers to quit smoking than the current sample. In addition, unlike the currently study, the Aveyard study did not control for contact time. Similarly, Danan and colleagues (Danan et al., 2016) found that, among predominately male smokers in the VA, those who were in later stages of change were more likely to quit smoking with proactive outreach (telephone counseling, pharmacotherapy) than usual care but these effects were not achieved by smokers in precontemplation.
Previous results support the use of MI with smokers who have children with asthma (Borrelli, McQuaid, Novak, Hammond, & Becker, 2010; Borrelli, McQuaid, et al., 2016). The current study suggests that MI is also effective among smokers who are not motivated to quit and have a child with asthma. Our study employed several features that have been shown to be associated with larger effects of MI on smoking cessation: longer interventions, rigorous treatment fidelity, medical comorbidities, and inclusion of samples of smokers who are not motivated to quit (Hettema & Hendricks, 2010; Lai et al., 2010; Lindson-Hawley, Thompson, & Begh, 2015). One meta-analysis (Hettema & Hendricks, 2010) found that studies that included samples with low levels of motivation had significant effects at both short and long term follow-ups (d=.39, d=.34 respectively) as did those that included participants who were not required to be motivated to quit smoking at study enrollment (d=.26). With regard to treatment fidelity, our study employed rigorous MI training, assessment of MI skill acquisition, ongoing skills maintenance and assessment of MI delivery using validated instruments. It is difficult to determine the effect of MI on smoking cessation on studies that do not employ treatment fidelity and indeed, lower effects have been noted (Cook et al., 2016; Hettema & Hendricks, 2010). One study tested the effect of MI on smokers who were not interested in quitting in the next 30 days and did not find an effect of MI, but the study did not measure treatment fidelity (Cook et al., 2016).
Several other approaches have been used to motivate smokers who are not motivated to quit. Practice quit attempts, sampling nicotine replacement (Carpenter et al., 2011), and switching to e-cigarettes (Bullen et al., 2013) have been shown to lead to greater attempts to quit smoking among those not motivated to quit, but have not been shown to be associated with long term biochemically confirmed abstinence. Future studies could also examine the effects of these interventions in smokers who are not motivated to quit and have children with asthma or other chronic respiratory illness.
A strength of our study is that we used proactive, rather than reactive recruitment. Reactive recruitment appeals to smokers who are highly motivated to quit, which constitutes a small percentage of the smoking population. Proactive recruitment of smokers reach a larger percent of the population but generally achieve lower abstinence rates. The current study proactively recruited smokers and achieved quit rates that are in line with clinic-based interventions, despite having a sample that varied in their motivation to quit. At study entry, 38.9% of our sample was not motivated to quit within 30 days. This is a fairly large percentage, given that the children of the smokers were undergoing asthma exacerbations that required urgent care. In the current study, these unmotivated smokers achieved quit rates that are 2–3 times greater than the spontaneous quit rates noted in the literature (Fiore, Jaen, Baker, & et al, 2008). In a previous study of Latino parents who smoke and have children with asthma, the percent of parents who were unmotivated to quit at study entry was slightly higher (45%; Borrelli et al.., 2010), possibly due to several methodological factors (ie., smokers were not solely recruited from urgent care as done in the current study, and the previous sample consisted only of Latino smokers; Borrelli, et al., 2010).
Over 60% of the participants in the current study said they wanted to quit smoking within 30 days. This is higher than previously found in studies with general smokers. National data show that 40% of current smokers are in the contemplation stage (thinking about quitting smoking within six-months), 40% are in the pre-contemplation stage (not thinking about quitting smoking) and only 20% are in the preparation stage (planning to quit within 30-days; Etter et al 1997). More recent data indicate that about 30% of current smokers are ‘considering quitting within the next month’ (Reid et al., 2014). Our sample may have a higher percentage of smokers who want to quit possibly due to the context of a ‘teachable moment’ (e.g., child in urgent care for an asthma exacerbation) which heightens the salience of risk of smoking and increases receptivity to messages about quitting smoking (Borrelli et al., 2016). ‘Readiness to quit’ is typically higher among populations experiencing a teachable moment, such as parents who smoke and have a child with asthma, or smokers with medical comorbidities (Borrelli, Novak et al., 2005).
With regard to second-hand smoke exposure, 63.4% of our sample reported that they had household smoking bans. While this may seem high, objective measurement has been shown to be a more accurate representation of smoke exposure, particularly among parents of children with asthma. One study found that parents of children with asthma were more likely to self-report having household smoking bans than parents of healthy kids, but objective measurement of smoke exposure revealed equivalent levels of household smoke between the two groups (Borrelli, McQuaid & Wagener, 2014). Misrepresentation of smoke exposure could be a function of guilt about admitting exposure to their child (particularly if the child has asthma), or lack of knowledge. With regard to the latter, a parent might self-report a smoking ban in their home, but consider ‘smoking on the porch’ as ‘not smoking in their home’ (however, the smoke could drift into the house) or ‘smoking in a different room, away from the child’ as ‘not smoking in their home.’ Parents also may have a general smoking ban in their home, but allow certain visitors or family to smoke in their homes (e.g., parent who is grateful that her mother will watch the children but doesn’t want to ask her to not smoke). For these reasons, we used objective measurement of secondhand smoke exposure as an outcome variable in the study.
Studies are often limited by their ability to generalize to the larger population. The current study minimizes this bias by proactive recruitment and by offering smokers health education program for which they are highly motivated (asthma education regarding their child) during a teachable moment (the child’s asthma exacerbation) so that smoking cessation induction could be woven into these sessions as an opportunity to link the child’s health to their parent’s behavior. Another possible limitation of our study is that the half-life of carbon monoxide is 4–6 hours, so biochemical verification is limited to that time frame. Also, although the pattern of unadjusted proportions supported our hypotheses (Table 2), the unadjusted regression models revealed that the motivation × treatment interaction was significant (p<.05) or marginally significant (p=.053) for two of the three smoking outcome variables, but the adjusted analyses revealed that the motivation × treatment interaction was significant for all three smoking outcome variables. Therefore, the results should be interpreted with caution. A final limitation is that smoking status was not objectively verified at study entry. However, we have high confidence that those who admitted to smoking are actual smokers because smokers are usually reticent to admit smoking due to stigmatization or guilt, particularly parents of children with asthma.
Despite these limitations, all smokers who have children with asthma, regardless of their motivation to quit, should be offered intensive smoking cessation treatments over the longer term. It cannot be assumed that low motivation to quit means that smokers will not participate and will not respond to treatment. We believe that ‘the hook’ for treatment engagement was offering asthma education during a teachable moment. The challenge for smoking cessation is to find ‘hooks’ for other types of smokers who are not motivated to quit.
Table 4-B.
Secondhand smoke exposure levels at baseline and 4-month follow-up in the child environment by motivation to quit in the two treatment groups.
| Time | Child SHSe category | Motivated to quit (n = 159) | Not-motivated to quit (n = 111 ) | ||
|---|---|---|---|---|---|
|
| |||||
| Enhanced-PAM (n = 79) | PAM (n = 80) | Enhanced-PAM (n = 53) | PAM (n = 58) | ||
| Baseline | very low/undetectable | 16.70% | 17.60% | 21.50% | 12.30% |
| moderate/high | 83.30% | 82.40% | 78.50% | 87.70% | |
| Follow-up | very low/undetectable | 21.50% | 21.20% | 24.50% | 12.10% |
| moderate/high | 78.50% | 78.80% | 75.50% | 87.90% | |
Public health significance statement.
In the current study, parents of a child with asthma who were not motivated to quit smoking achieved better smoking cessation and secondhand smoke outcomes with the more intensive intervention vs. the less intensive intervention, whereas those who were motivated to quit smoking at study entry did not significantly differ in their outcomes by treatment group. This suggests that interventions should be provided to smokers who have children with asthma, regardless of their motivation to quit. Intensive, longitudinal treatments that focus on cessation induction using Motivational Interviewing and secondhand smoke exposure feedback may be needed to achieve smoking cessation outcomes among smokers who are not motivated to quit smoking.
Acknowledgments
This study was funded by NIH 5 R01 HL062165-09 (B. Borrelli, PI). The study was conducted at The Miriam Hospital, when Dr. Borrelli was employed there.
References
- Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstain MG, Monti PM. The Tobacco Treatment Handbook: A Guide to Best Practices. New York: Guilford Press; 2003. [Google Scholar]
- Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addictive Behavior. 2011;36(7):764–768. doi: 10.1016/j.addbeh.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P, Lawrence T, Cheng KK, Griffin C, Croghan E, Johnson C. A randomized controlled trial of smoking cessation for pregnant women to test the effect of a transtheoretical model-based intervention on movement in stage and interaction with baseline stage. British Journal of Health Psychology. 2006;11:263–278. doi: 10.1348/135910705x52534. [DOI] [PubMed] [Google Scholar]
- Bartlett YK, Borrelli B, Armitage CJ, Tooley E, Wearden A. Approaches used to quit smoking: Differences between smokers who are motivated to quit and those who are not motivated to quit. Paper to be presented at British Psychological Society, Division of Health Psychology Annual Conference; 16–18 September 2015; London: UK. [Google Scholar]
- Benowitz N, Jacob P. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Borrelli B. Advances in Smoking Cessation. Future Medicine Ltd; 2013. Motivational interviewing for smoking cessation; pp. 128–141. [Google Scholar]
- Borrelli B, Gaynor S, Bartlett YK, Tooley E, Armitage CA, Wearden A. Are there different types of smokers who are not motivated to quit? Results from a Latent Class Analysis. Paper presented at the SRNT Society for Research on Nicotine and Tobacco; Chicago IL. 2016. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Bartlett K, Tooley EM, Armitage CJ, Wearden A. Prevalence and Frequency of mHealth and eHealth Use Among Smokers in the US and UK and Differences by Motivation to Quit. Journal of Medical Internet Research. 2015;17(7):e164. doi: 10.2196/jmir.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: Outcomes from Project CARES (Community-nurse Assisted Research and Education on Smoking) Preventive Medicine. 2005;41:815–821. doi: 10.1016/j.ypmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. Journal of Consulting and Clinical Psychology. 2010;78(1):34–43. doi: 10.1037/a0016932. [DOI] [PubMed] [Google Scholar]
- Borrelli B, McQuaid EM, Tooley EM, Busch AM, Hammond K, Becker B, Dunsiger S. Motivating Parents of Kids with Asthma to Quit Smoking: The Effect of the Teachable Moment and Increasing Intervention Intensity Using a Longitudinal Randomized Trial Design. Addiction. 2016;111:1646–1655. doi: 10.1111/add.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, Orwig D. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology. 2005;73(5):852–860. doi: 10.1037/0022-006x.73.5.852. [DOI] [PubMed] [Google Scholar]
- Borrelli B, McQuaid EL, Wagener TL. Asthma vs. healthy children: Differences in smoke exposure and caregiver perceived risk. Nicotine & Tobacco Research. 2014;16(5):554–561. doi: 10.1093/ntr/ntt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. doi: 10.1016/s0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archive of Internal Medicine. 2011;171(21):1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Goggin K, Richter K, Williams K, Patten C, … Liston R. Motivational Interviewing for encouraging quit attempts among unmotivated smokers: study protocol of a randomized, controlled, efficacy trial. BMC Public Health. 2012;12:456. doi: 10.1186/1471-2458-12-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Quitting smoking among adults-United States, 2001–2010. Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Chen R, Clifford A, Lang L, Anstey KJ. Is exposure to secondhand smoke associated with cognitive parameters of children and adolescents?--a systematic literature review. Annal of Epidemiology. 2013;23(10):652–661. doi: 10.1016/j.annepidem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, … Mermelstein R. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111(1):117–128. doi: 10.1111/add.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danan ER, Joseph AM, Sherman SE, Burgess DJ, Noorbaloochi S, Clothier B, … Fu SS. Does Motivation Matter? Analysis of a Randomized Trial of Proactive Outreach to VA Smokers. Journal of General Internal Medicine. 2016;31(8):878–887. doi: 10.1007/s11606-016-3687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MF, Shapiro D, Windsor R, Whalen P, Rhode R, Miller HS, Sechrest L. Motivational interviewing versus prescriptive advice for smokers who are not ready to quit. Patient Education & Counselling. 2011;83(1):129–133. doi: 10.1016/j.pec.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: International comparison and association with smoking prevalence. Preventive Medicine. 1997;26(4):580–5. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- Fedele DA, Tooley E, Busch A, McQuaid EL, Hammond SK, Borrelli B. Comparison of secondhand smoke exposure in minority and nonminority children with asthma. Health Psychology. 2016;35(2):115–122. doi: 10.1037/hea0000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 Update. Clinical practice guideline. Rockville, MD: Public Health Service; 2008. [Google Scholar]
- Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology. 1987;21(5):494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Bradley-Ewing A, Goggin K, Richter KP, Patten C, Williams K, … Catley D. Recruiting unmotivated smokers into a smoking induction trial. Health Education Research. 2016;31(3):363–374. doi: 10.1093/her/cyw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tobacco Control. 2010;19(5):410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog TA, Blagg CO. Are most precontemplators contemplating smoking cessation? Assessing the validity of the stages of change. Health Psychology. 2007;26(2):222–231. doi: 10.1037/0278-6133.26.2.222. [DOI] [PubMed] [Google Scholar]
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. Journal of Consulting & Clinical Psychology. 2010;78(6):868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- Hu M, Lachin JM. Application of robust estimating equations to the analysis of quantitative longitudinal data. Statistics in Medicine. 2001;20(22):3411–3428. doi: 10.1002/sim.962. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Hu MC. Intergenerational Patterns of Smoking and Nicotine Dependence Among US Adolescents. American Journal of Public Health. 2015;105(11):e63–72. doi: 10.2105/ajph.2015.302775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann RB, Babb SD, O’Halloran A, Asman K, Bishop E, Tynan M, … Pechacek TF. Vital signs: nonsmokers’ exposure to secondhand smoke --- United States, 1999–2008. MMWR Morbidity & Mortality Weekly Report. 2010;59(35):1141–1146. [PubMed] [Google Scholar]
- Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Systematic Review. 2010;(1):Cd006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environmental Science & Technology. 1991;25(4):770–777. doi: 10.1021/es00016a023. [DOI] [Google Scholar]
- Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Systematic Review. 2015;(3):Cd006936. doi: 10.1002/14651858.CD006936.pub3. [DOI] [PubMed] [Google Scholar]
- Malarcher A, Dube S, Shaw L, Babb S, Kauffmann R. Quitting smoking among adults--United States, 2001–2010. MMWR Morbidity & Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Miller R. Motivational interviewing: preparing people for change. 2. Vol. 428. New York: Guilford Press; 2002. [Google Scholar]
- NAEPP. National Asthma Education and Prevention Program (NAEPP) Guidelines for the Diagnosis and Management of Asthma (National Asthma Education and Prevention Program Expert Panel Report 3) Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- Reid JL, Hammond D, Rynard VL, Burkhalter R. Tobacco Use in Canada: Patterns and Trends. Waterloo, ON: Propel Centre for Population Health Impact, University of Waterloo; 2014. [Google Scholar]
- USPHS. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respiratory Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- Vuolo M, Staff J. Parent and child cigarette use: a longitudinal, multigenerational study. Pediatrics. 2013;132(3):e568–577. doi: 10.1542/peds.2013-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]