Abstract
RORγt regulates TH17 differentiation, thymic T cell development and lymph node genesis. Although elimination of RORγt prevents TH17-mediated experimental autoimmune encephalomyelitis (EAE), it also disrupts thymocyte development, which could lead to lethal thymic lymphoma. Here we identified two amino acid mutations in RORγt (RORγtM) that preferentially disrupted TH17 differentiation but not thymocyte development. Mice expressing RORγtM were resistant to EAE associated with defective TH17 differentiation, but maintained normal thymocyte development and lymph node genesis, except for Peyer’s patches. RORγtM showed reduced ubiquitination at K69 that is selectively required for TH17 differentiation but not T cell development. This study will inform the development of treatments that selectively target TH17-mediated autoimmunity, but do not affect thymocyte development and induce lymphoma.
The transcription factor RORγt, encoded by the gene rorc2, instructs the differentiation of T helper 17 (TH17) cells. Activation of naïve T cells in the presence of TGF-β and interleukin 6 (IL-6) is sufficient to up-regulate RORγt and induce differentiation into TH17 cells 1. TH17 cells produce the effector cytokines including IL-17 to mediate the pathological inflammation responsible for autoimmunity such as psoriasis and multiple sclerosis 2, 3, 4. In addition, during thymocyte development, RORγt is up-regulated at the CD4+CD8+ (DP) stage to enhance their survival by up-regulating anti-apoptotic Bcl-xL5. RORγt−/− mice develop lethal thymic lymphomas likely resulting from abnormal thymocyte development 6. RORγt−/− mice also lack all lymph nodes, including gut-associated mesenteric lymph nodes and Peyer’s patches 5, 7, due to a requirement of RORγt for the development of lymphoid tissue inducer (LTi) cells, the progenitors for lymph node genesis 5, 8, 9. The mechanisms that distinguish the functions of RORγt in TH17 differentiation in the peripheral immune system and thymocyte development in the central immune system remain unknown. Dissecting these functions is critical for understanding how one central transcription factor regulates distinct differentiation processes in the peripheral and central immune systems. Given the essential function of RORγt in TH17 cells, RORγt inhibitors have been developed for treatment of TH17-dependent autoimmunity 10, 11, 12, 13, 14. Such inhibitors are also expected to interfere with RORγt function in thymocytes and thus impair T cell development, which could eventually lead to the development of lymphoma 6. Development of novel RORγt-based treatments that specifically target TH17-mediated autoimmunity is as such of high order 6.
RORγt is a steroid nuclear receptor that consists of three domains 15, 16: a conserved DNA-binding domain with two zinc finger motifs responsible for DNA-binding; a conserved ligand-binding domain with a C-terminal AF2 motif responsible for recruiting steroid receptor co-activator (SRC) to stimulate gene expression 17, 18; and a hinge domain between the DNA- and ligand-binding domains. Upon ligand binding, RORγt recruits SRC and binds to target DNA to regulate target gene expression. To date, no function has been assigned to the RORγt hinge domain, which is generally believed to serve as a flexible linker region between the conserved and functionally important DNA-binding and ligand-binding domains. However, the observation that the hinge domain is not conserved between three members of the ROR family 19, suggests a potentially unique function.
By mutagenesis, we identified a mutant form of RORγt, which contains two amino acid mutations within the non-conserved hinge region of RORγt (called RORγtM in this manuscript) that specifically disrupted RORγt function in TH17 differentiation, but not thymocyte development. Mice expressing RORγtM in the endogenous RORγt locus (called RORγtM/M mice here) lacked TH17 differentiation, but had normal overall T cell development and developed most lymph nodes, including gut-associated mesenteric lymph nodes, but lacked Peyer’s patches. RORγtM interfered with ubiquitination of RORγt at lysine 69 (K69). Similar to RORγtM, mutation of K69 to arginine (K69R) to prevent ubiquitination selectively impaired RORγt-mediated TH17 differentiation but not thymocyte development. Our study thus distinguishes between RORγt function in TH17 differentiation and thymocyte development, and reveals distinct mechanisms for these two RORγt-regulated processes.
Results
RORγt supports in vitro T cell development and TH17 differentiation
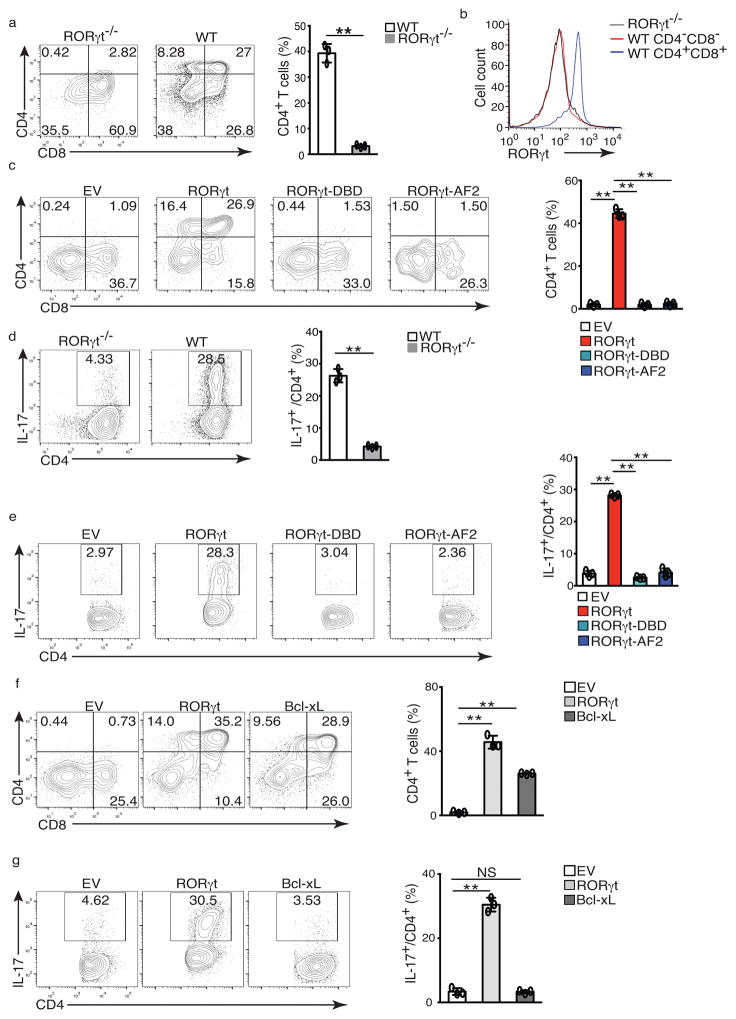
To evaluate the function of RORγt function in TH17 differentiation and thymocyte development, we investigated the structural requirements of the RORγt protein for these two functions. In an in vitro differentiation system, sorted CD4−CD8− (DN) thymocytes from wild-type and RORγt−/− mice were cultured on OP9-DL4 stromal cells to induce differentiation 20. Wild-type, but not RORγt−/− DN thymocytes differentiated into CD4+CD8+ DP and CD4+ (CD4SP) thymocytes (Fig. 1a). Both wild-type and RORγt−/− DN thymocytes were negative for RORγt expression, but DP thymocytes differentiated from wild-type DN thymocytes expressed RORγt (Fig. 1b). Consistent with previous observation, RORγt was up-regulated in DP thymocytes and was required for their survival 5, 7, 18. A population of TCRloCD24hiCD8+ thymocytes developed from both wild-type and RORγt−/− DN thymocytes (Supplementary Fig. 1a) prior to the DP stage 21. Thus, we used the percentage of CD4+ cells, including both CD4+CD8+ DPs and mature CD4+ SPs, to evaluate the efficiency of thymocyte development (Fig. 1a, right panel). To assess the role of RORγt in thymocyte development, we transduced RORγt−/− DN thymocytes with a retrovirus expressing RORγt-GFP or empty virus alone (EV-GFP) as control. Thymocyte development was rescued only in RORγt-GFP+ cells, but not in EV-GFP+ cells (Fig. 1c) or in GFP- cells that were not transduced by virus (Supplementary Fig. 1b). Furthermore, thymocyte development was not rescued by expression of RORγt mutants that cannot bind target DNA (RORγt-DBD) or the co-activator SRC (RORγt-AF2) (Fig. 1c), although both mutants were expressed at the equivalent levels as wild-type RORγt (Supplementary Fig. 1c), consistent with the notion that DNA-binding activity and AF2-mediated recruitment of co-activator SRC are required for RORγt to support thymocyte development 18.
Figure 1. RORγt supports in vitro T cell development and TH17 differentiation.
(a) Flow cytometer analysis of CD4 and CD8 in ex vivo development of CD4−CD8− thymocytes from wild type or RORγt−/− mice for three days. Right panel is the frequency of total CD4+ thymocytes. (b) Flow cytometer analysis for RORγt expression by cells shown in a. (c) Flow cytometer analysis of ex vivo development of CD4−CD8−RORγt−/− thymocytes transduced with retrovirus expressing GFP only (EV), or together with RORγt or mutants incapable of binding DNA (RORγt-DBD) or co-activator (RORγt-AF2). Right panel is the percentage of CD4+ among 7-AAD− Thy1.2+GFP+ cells. (d) Flow cytometer analysis for IL-17A+ cells in naïve WT or RORγt−/− CD4+ T cells polarized under Th17 priming conditions for three days. Right panel is the percentage of IL-17A+ cells among CD4+ cells. (e) Flow cytometer analysis of IL-17A+ cells in RORγt−/− CD4+ T cells transduced with indicated retrovirus. Right panel is the percentage of IL-17A+ cells among CD4+GFP+ cells. (f) Flow cytometer analysis of ex vivo development of CD4−CD8−RORγt−/− thymocytes transdced with indicated retrovirus. Right panel is the quantification of the results. (g) Flow cytometer analysis of IL-17A+ cells in RORγt−/− CD4+ T cells transduced with indicated retrovirus. Right panel is the quantification of results. Data in right panel of a,c,d,e,f,g are mean ± s.e.m. **P < 0.01, ***P < 0.001 (ANOVA test). Each symbol represents an individual experiment. Data are pooled from three experiments (right panel of a,c,d,e,f,g ), or are from one experiment representative of three experiments (b and left panel of a,c,d,e,f,g),
To establish an in vitro TH17 differentiation assay, splenic CD4+ T cells isolated from wild-type or RORγt−/− mice were cultured in TH17 priming conditions. RORγt−/− CD4+ T cells, but not wild-type CD4+ T cells, were defective in TH17 differentiation (Fig. 1d), and lacked RORγt expression (Supplementary Fig. 1d). TH17 differentiation of RORγt−/− T cells was rescued in retrovirally transduced RORγt-GFP+ cells, but not EV-GFP+ (Fig. 1e) or in GFP− non-transduced cells (Supplementary Fig. 1e). Similar to RORγt-mediated T cell development, retroviral expression of RORγt-DBD and RORγt-AF2 at the same level as RORγt-GFP (Supplementary Fig. 1f) failed to rescue Th17 differentiation (Fig. 1e). Therefore, both T cell development and TH17 differentiation depend on RORγt DNA-binding activity and AF2-mediated recruitment of co-activator SRC. Consistent with RORγt up-regulation of anti-apoptotic Bcl-xL to enhance thymocyte survival 5, 7, retroviral expression of anti-apoptotic Bcl-xL (Bcl-xL-GFP) partially rescued differentiation of DP thymocytes from RORγt−/− thymocytes (Fig. 1f). However, Bcl-xL-GFP could not rescue TH17 differentiation in RORγt/− T cells (Fig. 1g), indicating distinct mechanisms involved in RORγt-regulated TH17 differentiation and thymocyte development.
Two amino acids are critical for TH17 differentiation but not T cell development
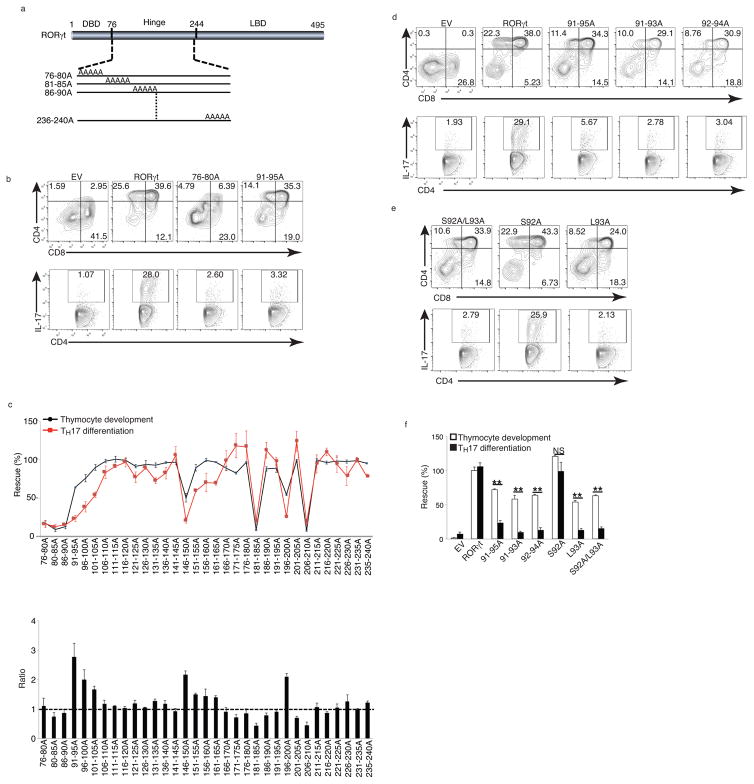
As mutations affecting the RORγt DNA-binding activity and the AF2 motif of ligand-binding domain interfered with both thymocyte development and TH17 differentiation, we extended the search for structural RORγt elements differentially required for these functions by introducing mutations in the RORγt hinge domain. By mutating consecutive stretches of five amino acids to alanines (alanine scanning), we created 33 mutants covering the hinge region (amino acids 76–240, Fig. 2a). RORγt−/− DN thymocytes or peripheral RORγt−/− CD4+ T cells transduced with retroviruses expressing RORγt-GFP or alanine-mutant RORγt were assessed for rescued thymocyte development and TH17 differentiation in vitro, as described above. 20 out of 33 mutants such as 176–180A and 191–195A (supplementary Fig. 1g) rescued both thymocyte development and TH17 differentiation. Seven mutants (e.g. 76–80A in Fig. 2b and 181–185A in supplementary Fig. 1g) markedly impaired both thymocyte development and TH17 differentiation. These mutants were scattered across the entire hinge domain (Fig. 2c, top panel), indicating that functionally important motifs of RORγt for both functions are not clustered together.
Figure 2. S92 and L93 are critical for TH17 differentiation but not T cell development.
(a) Schematic representation of RORγt structure and alanine-scanning mutants. Numbers indicate the positions of amino acids. DBD: DNA-binding domain. LBD: Ligand-binding domain. (b) Flow cytometer analysis of ex vivo thymocyte development (top panel) and TH17 differentiation of RORγt−/− cells retrovirally expressing indicated RORγt. (c) Top panel is the percentage of rescued thymocyte development (black/circles) and TH17 differentiation (red/squares) by indicated RORγt mutants relative to WT RORγt (defined as 100%). Bottom panel is the ratio of percentage of rescued thymocyte development to that of rescued Th17 differentiation. (d and e) Flow cytometer analysis of ex vivo thymocyte development (top panel) and TH17 differentiation of RORγt−/− cells retrovirally expressing indicated RORγt (f) Quantification of the results shown in d and e. Data in c,f are mean ± s.e.m. **P < 0.01, ***P < 0.001 (ANOVA test). Data are pooled from three experiments (c,f ), or are from one experiment representative of three experiments (b,d,e),
Six mutants, including 91–95A (Fig. 2b) and 151–155A (Supplementary Fig. 1g) preferentially impaired TH17 differentiation, with limited effect on thymocyte development, indicating a selective requirement of these two regions (amino acids 91–105 and 151–165) for TH17 differentiation (Fig. 2c, top panel). 151–165 mutants maintained more than 50% activity during TH17 differentiation, while mutant 91–95A maintained the majority of activity in thymocyte development, but could not support TH17 differentiation (Fig. 2c, top panel). When expressed as the ratio of the percentage of rescued thymocyte development versus percentage of rescued TH17 differentiation, mutant 91–95A showed the biggest difference between the ability to support thymocyte development and inability to support TH17 differentiation (Fig. 2c, bottom panel).
We further defined which amino acids were critical for distinguishing between the two RORγt-regulated functions in mutant 91–95A. The amino acid sequence from 91–95 is DSLHA and our alanine scan did not change the last A. To investigate the requirement for the first four amino acids, we created two three-alanine mutants covering these four amino acids: 91–93A and 92–94A. Similar to mutant 91–95A, 91–93A and 92–94A supported thymocyte development (Fig. 2d, top panel), but neither supported TH17 differentiation (Fig. 2d, bottom panel) when retrovirally expressed in RORγt−/− T cells. Additionally, a S92A-L93A mutant rescued thymocyte development (Fig. 2e, top panel) but not TH17 differentiation (Fig. 2e, bottom panel) in RORγt−/− T cells. The single mutant S92A preserved both functions, whereas mutant L93A significantly impaired both thymocyte development and TH17 differentiation (Fig. 2e). Immunoblotting confirmed that all mutants were expressed at equivalent levels with wild-type RORγt (Supplementary Fig. 1h). S92A-L93A was the minimum mutation sufficient to distinguishly regulate RORγt-dependent thymocyte development and TH17 differentiation (Fig. 2f). We have thus identified a RORγt mutation (hereafter called RORγtM) that specifically disrupts RORγt-mediated TH17 differentiation, but not thymocyte development.
RORγtM cannot induce genes critical for Th17 differentiation
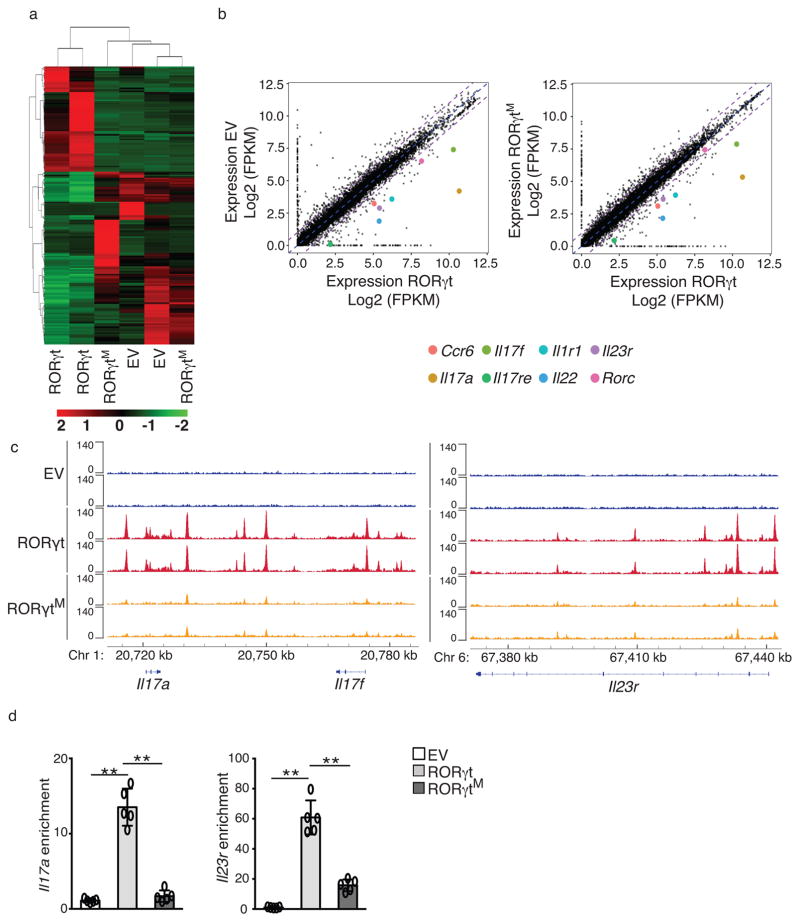
Th17 differentiation depends on RORγt-regulated gene expression 22. To assess the global effects of RORγtM on TH17 differentiation, we performed RNA sequencing (RNA-seq) and chromatin immunoprecipitation-DNA-seq (ChIP-seq) analyses of RORγt−/− T cells transduced with retrovirus expressing GFP only (EV-GFP), RORγt-GFP or RORγtM-GFP (Fig. 3). The flag-tag fused to RORγt to immunoprecipitate RORγt-binding DNA fragments for ChIP did not affect RORγt expression or function in Th17 differentiation (Supplementary Fig. 2a,b). We found similar expression patterns between RORγt−/− T cells reconstituted with EV-GFP and RORγtM-GFP, which was different for RORγt−/− T cells reconstituted with RORγt-GFP (Fig. 3a), an observation confirmed by hierarchical clustering analysis (Fig. 3a). Many TH17 signature genes, including Il17a, Il17f, Il23r, Il1r, Ccr6, IL-17re and Il22 were down-regulated in EV-GFP+RORγt−/− T cells compared to RORγt-GFP+RORγt−/− T cells (Fig. 3b, left panel), confirming the defective Th17 differentiation. These TH17 signature genes were also down-regulated in RORγtM-GFP+RORγt−/− T cells compared to RORγt-GFP+RORγt-−/− T cells (Fig. 3b, right panel). ChIP-seq identified 21,917 RORγt-DNA binding peaks within gene loci such as Il17 and Il23r (Fig. 3c) as well as Il1r, Il17re, Rorc and Ccr6 (Supplementary Fig. 2c) known to be critical for TH17 differentiation. Of 3,931 RORγtM-DNA binding peaks identified in RORγtM-GFP+ cells, almost all (3886 out of 3931) overlapped with RORγt-DNA binding peaks. However, RORγtM lost or showed significantly reduced DNA-binding peaks within important TH17 gene loci (Fig. 3c and supplementary Fig. 2c), which correlated with their diminished expression (Fig. 3b). Greatly deduced binding to Il17 and Il23r loci by RORγtM compared to RORγt was confirmed by individual ChIP assays (Fig. 3d). Therefore, RORγtM selectively loses DNA-binding sites and ability to activate genes required for TH17 differentiation.
Figure 3. RORγtM cannot induce genes critical for TH17 differentiation.
(a) Heatmap visualization of the up-regulated (red) and down-regulated (green) genes determined by RNA-seq assay in RORγt−/− CD4+ T cells retrovirally reconstituted with EV, WT RORγt, or RORγtM and polarized under TH17 conditions for three days. (b) Comparison of gene expression profiles between RORγt−/− CD4+ T cells reconstituted with EV and RORγt (left panel), and between RORγt−/− CD4+ T cells reconstituted with RORγtM and RORγt (right panel). Colored dots represent selected critical TH17 genes as indicated. Average fragments per kilobase of transcript per million mapped reads (FRKM) values of three replicates are shown. (c) ChIP assay identified RORγt DNA-binding peaks in Il17a, Il17f, and Il23r loci. (d) ChIP analysis of RORγt interaction with Il17a (left) and Il23r (right) loci in RORγt−/− CD4+ T cells retrovirally expressing EV, WT RORγt, or RORγtM. Data are pooled from three experiments (a,b), or are from one experiment representative of three experiments (c,d). Data in d are shown as mean ± s.e.m.; Each symbol represents an individual experiment. ** p<0.01 by ANOVA.
RORγtM supports thymocyte development in vivo
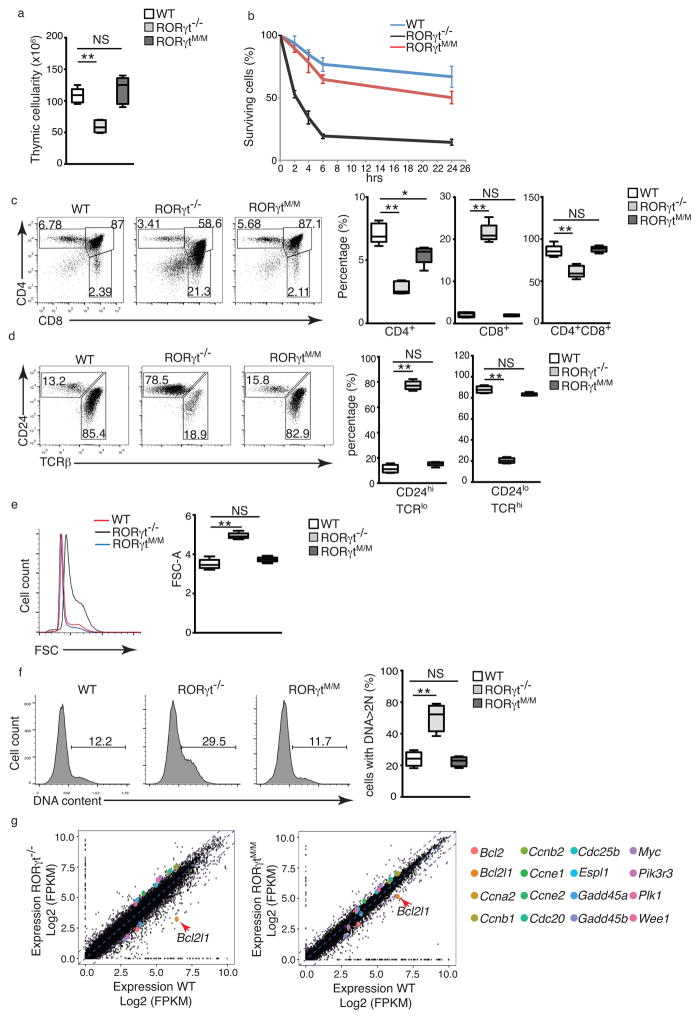
To test the in vivo function of RORγtM, we knocked-in exon 4, which contains the coding sequence for the two critical amino acids (S92-L93), in the RORγt locus (Supplementary Fig. 3a). A neomycin gene used for selection was also deleted by flp recombinase (Supplementary Fig. 3b) to avoid interfering with gene expression. RORγtM expression was indicated by the detection of S92A-L93A mutations by sequence analysis of RORγt mRNA (Supplementary Fig. 3c). Mice expressing RORγtM from the endogenous RORγt locus are called RORγtM/M hereafter. We first examined T cell development in RORγtM/M mice. Intracellular staining of RORγt showed equivalent expression of RORγtM and RORγt in CD4+CD8+ DPs of corresponding mice (Supplementary Fig. 4a), suggesting appropriate RORγtM expression during T cell development. RORγt−/− mice had dramatically reduced thymic cellularity (Fig. 4a) due to accelerated spontaneous apoptosis (Fig. 4b), while the thymic cellularity and survival of RORγtM/M thymocytes were almost equivalent to that of wild-type mice (Fig. 4a, b). Based on the surface CD44 and CD25 markers, DN thymocyte development in RORγt−/− or RORγtM/M mice was similar to wild-type (Supplementary Fig. 4b) 5. The percentage (Fig. 4c) and absolute number (Supplementary Fig. 4c) of CD4+ and CD4+CD8+ DP were reduced in RORγt−/− thymus compared to wild-type mice, while RORγtM/M mice had similar percentage and number of CD4+ and CD4+CD8+ DP cells to those detected in wild-type mice. Moreover, while CD8+ thymocytes in RORγt−/− mice were mostly immature TCRloCD24hi, CD8+ cells in RORγtM/M thymi were mostly TCRhiCD24lo mature cells, similar to wild-type mice (Fig. 4d). Compared to the enlarged cell size (FSC) (Fig. 4e) and markedly increased number of cells entering DNA synthesis (S) phase of the cell cycle, indicated by cells with >2N DNA (Fig. 4f) in RORγt−/− mice, RORγtM/M thymocytes had the same cell size and percentage of cells in S phase as the wild-type mice (Fig. 4e, f). In addition, RNA-seq indicated that the most prominent apoptotic molecule up-regulated in wild-type thymocytes compared to the RORγt−/− thymocytes was Bcl-xL (encoded by Bcl2I1) (Fig. 4g). Many cell cycle regulators, including cyclins and cell division cycle proteins, displayed changes greater than 2-fold in RORγt−/− vs wild-type thymocytes(Fig. 4g, top panel), consistent with the survival and cell cycle changes observed in RORγt−/− thymocytes. Changes in the expression of Bcl-xL and cell cycle molecules were significantly less pronounced between RORγtM/M and wild-type thymocytes (Fig. 4g, bottom panel) than between RORγt−/− and wild-type thymocytes (Fig. 4g, top panel), suggesting that RORγtM/M thymocytes were phenotypically equivalent to wild-type, but not RORγt−/− thymocytes. As such, RORγtM functions largely similarly to endogenous RORγt in supporting thymocyte development in vivo.
Figure 4. RORγtM supports thymocyte development in vivo.
(a) Thymic cellularity of indicated mice (n=5 per genotype). (b) Flow cytometer analysis of percentage of survival thymocytes stained with Annexin V and F-AAD after culturing for different times (n=3 per genotype). (c) Flow cytometer analysis of surface CD4 and CD8 in thymocytes. Right panel is the quantification of the percentage of indicated population of thymocytes (n=5 per genotype). (d) Flow cytometer analysis of CD24 and TCRβ expression for the maturity of CD8+ thymocytes. Right panel is the quantification of the percentage of indicated population of cells (n=5 per genotype). (e) Flow cytometer analysis of the forward scatter (FSC) in thymocytes of indicated mice. Right panel is the quantification of results (n=5 per genotype). (F) Flow cytometer analysis of DNA content with 7-AAD in indicated thymocytes. Numbers indicate the percentage of cells with >2N of DNA. Right panel is the quantification of results (n=3 per genotype). (g) Comparison of gene expression profiles determined by RNA-seq between RORγt−/− and WT thymocytes (top panel) or between RORγtM and WT thymocytes (bottom panel). Colored dots indicate selected genes. Average fragments per kilobase of transcript per million mapped reads (FRKM) values of two replicates are shown. Data in b are mean ± s.e.m. Central line of box-whisker plot (a and right panel of c, d, e, f) represents median and whiskers indicate extreme data points. *P < 0.05, **P < 0.01, NS non-significant (ANOVA test).Data are pooled from three experiments (h), are from one experiment representative of five experiments (left panel of c,d,e and f) or from one experiment representative of three experiments (g and left panel of f).
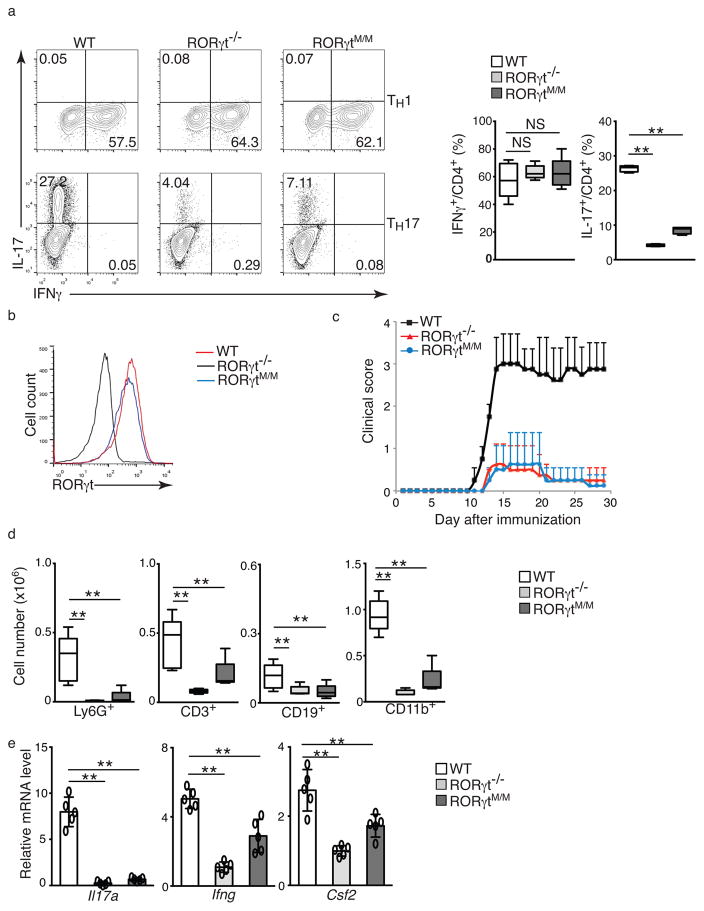
RORγtM/M mice have defective TH17 differentiation
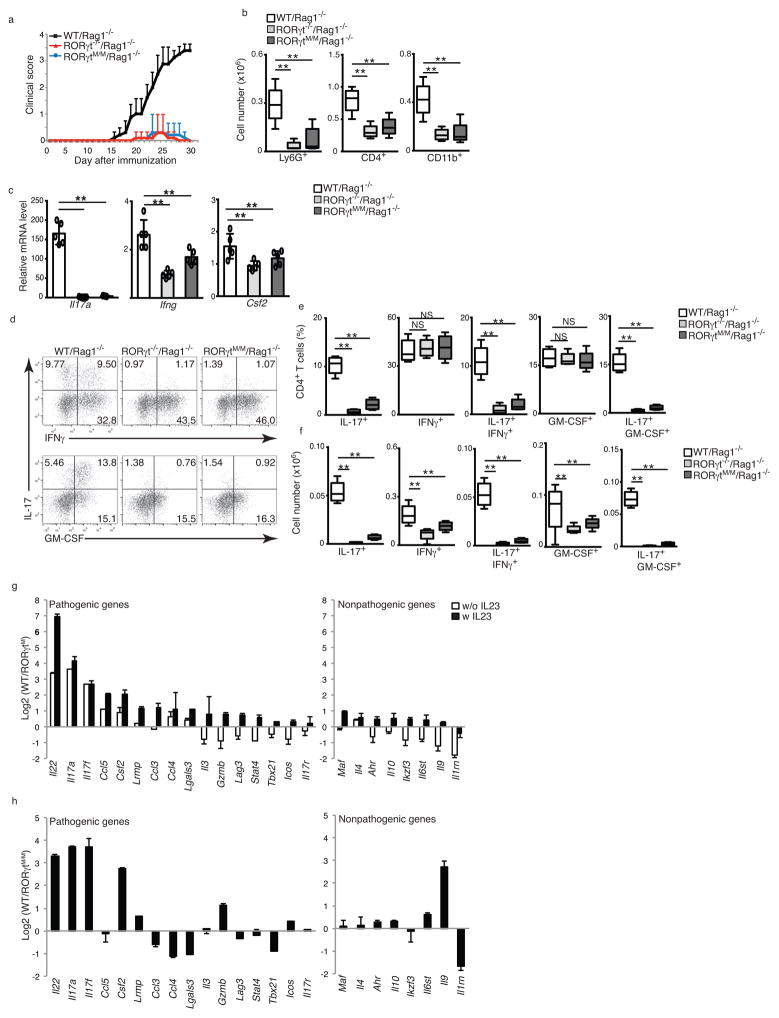
The distribution of naïve and memory T cells in RORγtM/M mice was similar to that observed in wild-type mice (Supplementary Fig. 5a,b). To determine the function of RORγtM in TH17 cells, we differentiated naïve spenic T cells from wild-type, RORγtM/M and RORγt−/− mice under Th1, TH17, Th2 or Treg conditions. In contrast to wild-type T cells, and similar to RORγt−/− T cells, RORγtM/M T cells failed to differentiate into IL-17 producing cells (Fig. 5a, bottom panel), while there were no obvious differences in their ability to differentiate into TH1 (Fig. 5a, top panel), TH2 (Supplementary Fig. 5c) or Treg cells (Supplementary Fig. 5d) compared to wild-type cells, suggesting a selective defect in TH17 differentiation. In in vitro-differentiated TH17 cells, RORγtM protein expression was equivalent to RORγt in wild-type cells (Fig. 5b), suggesting that defective TH17 differentiation was not due to lack of RORγtM expression. Development of EAE was attenuated in both RORγt−/− and RORγtM/M mice, compared to wild-type mice (Fig. 5c), with greatly reduced numbers of infiltrated CD45+ lymphocytes, including Ly6G+ neutrophils, CD3+ T cells, CD19+ B cells, and CD11b+ monocytes recovered from the central nervous system (CNS) of RORγt−/− and RORγtM/M mice compared to wild-type mice (Fig. 5d, Supplementary Fig. 6a), indicating reduced inflammation. Analysis of infiltrated lymphocytes indicated reduced expression of Il17a, Ifng and Csf2 mRNA in RORγtM/M and RORγt−/− mice compared to wild-type mice (Fig. 5e). Adoptive transfer of naïve CD4+ T cells into Rag1−/− mice followed by induction of EAE also induced greatly attenuated EAE in Rag1−/− mice reconstituted with RORγt−/− or RORγtM/M CD4+ T cells, in contrast to Rag1−/− mice reconstituted with wild-type CD4+ T cells (Fig. 6a), with reduced numbers of infiltrated lymphocytes, including Ly6G+ neutrophils, CD4+ T cells and CD11b+ monocytes (Fig.6b and supplementary Fig. 6b) and decreased Il17a, Ifng and Csf2 mRNA expression in infiltrating cells (Fig. 6c,d). Il17a mRNA was the most reduced, consistent with the greatest reduction in the percentage (Fig. 6e) and absolute number (Fig. 6f) of IL-17+IFNγ+ and IL-17+GM-CSF+ T cells, which are believed to be pathogenic for EAE 3, 4, 23. In contrast, the overall percentage of IFNγ+ and GM-CSF+ cells in RORγt−/− or RORγtM/M reconstituted Rag1−/− mice were not obviously changed compared to wild-type reconstituted Rag1−/− mice (Fig. 6e,f), demonstrating selective defects in the differentiation of TH17 cells. A similar specific reduction of IL-17+ cells, but not IFN-γ+ cells was also observed in CD4+ T cells from draining lymph nodes, and to a lesser extent in CD4+ T cells from spleens (Supplementary Fig. 6c). We did not observe differences in the expression of Foxp3 in T cells from EAE-induced wild-type and RORγtM/M mice (Supplementary Fig. 6d).
Figure 5. RORγtM/M mice have defective TH17 differentiation.
(a) Flow cytometer analysis of intracellular IFNγ or IL-17A in indicated naïve CD4+ T cells differentiated for three day under TH1 or TH17 priming conditions. Right panel is the quantification of the results (n=5 per genotype). (b) Flow cytometer analysis of RORγt expression in TH17 cells shown in a. (c) Mean EAE clinical score of indicated mice (female) different days after immunization with MOG35-55(n=10 for WT, n=8 for RORγt−/− and n=13 for RORγt M/M). (d) Flow cytometer analysis of surface markers for different types of CNS infiltrated lymphocytes from indicated EAE-induced mice at the peak of disease. (e) qPCR analysis of expression of indicated cytokines in CNS-infiltrated lymphocytes of EAE-induced mice. Data in e are mean ± s.e.m. Each symbol (e) represents an individual mouse. Central line of box-whisker plot (right panel of a, and d, e) represents median and whiskers indicate extreme data points. *P < 0.05, **P < 0.01, NS non-significant (ANOVA test).Data are pooled from three experiments (c), or from one experiment representative of three experiments (b and left panel of a).
Figure 6. RORγtM/M CD4+ T cells are defective in induction of EAE.
(a) Mean EAE clinical score of Rag1−/− recipient mice (female) reconstituted with CD4+ cells from indicated mice at different days after immunization with MOG35-55 (n=5 per genotype). (b) Flow cytometer analysis of indicated surface markers for different types of CNS infiltrated lymphocytes in EAE-induced mice at the peak of disease. (c) qPCR analysis of expression of indicated cytokines in CNS-infiltrated lymphocytes of EAE-induced mice. (d) Flow cytometer analysis of intracellular IFNγ and GM-CSF in CNS-infiltrated CD4+ T cells from EAE-induced mice. (e, f) Percentage (e) and absolute number (f) of indicated CNS-infiltrated lymphocytes in EAE-induced mice. (g) Ratio of the expression of pathogenic and nonpathogenic genes determined by qPCR between WT and RORγtM CD4+ T cells differentiated under regular Th17 priming conditions and pathogenic Th17 priming conditions (+IL-23) for three days (n=3 per genotype). (h) Ratio of the expression of pathogenic and nonpathogenic genes determined by qPCR between WT and RORγtMM CD4+ T cells obtained from EAE-induced mice (n=3 per genotype). Data c are mean ± s.e.m. **P < 0.01, NS non-significant (ANOVA test). Each symbol (c) represents an individual mouse. Central line of box-whisker plot (b, e and f) represents median and whiskers indicate extreme data points. Data are pooled from three experiments (a,b,c,e,f,g and h), or from one experiment representative of three experiments (d).
Next we assessed the ratio of representative pathogenic and nonpathogenic gene expression in wild-type and RORγtM/M CD4+ T cells differentiated with either TGF-β + IL-6 or pathogenic TGF-β + IL-6 + IL-23 TH17 priming conditions. The expression of nonpathogenic genes did not generally change more than 2 fold between wild-type and RORγtM/M T cells in either priming conditions (Fig. 6g). However, many pathogenic genes showed higher expression in wild-type compared to RORγtM/M T cells under non-pathogenic priming conditions (Fig. 6g). Some pathogenic genes had lower expression in wild-type compared to RORγtM/M T cells, but usually less than 2 fold change (Fig. 6g). All the pathogenic genes detected were expressed higher in wild-type than in RORγtM T cells in the presence of IL-23 (Fig. 6g). When analyzing pathogenic and nonpathogenic gene expression in T cells obtained from EAE-induced mice, many pathogenic genes showed higher expression in wild-type T cells, whereas some showed lower expression in wild-type, but usually less than 2 fold change compared to RORγtM T cells (Fig. 6h). Therefore, RORγtM/M T cells showed lower pathogenic potential, a loss of function in supporting TH17 differentiation and induction of EAE in vivo.
RORγtM/M mice develop lymph nodes, but not Peyer’s patches
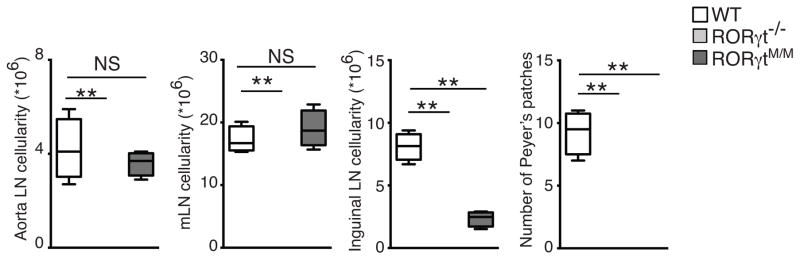
Because RORγt−/− mice typically lack all lymph nodes 5, we examined lymph nodes development in RORγtM/M mice. In contrast to RORγt−/− mice, RORγtM/M mice had almost all lymph nodes examined, including aorta lymph nodes and gut-associated mesenteric lymph nodes (Table 1 and Fig. 7a). While inguinal lymph nodes were developed but smaller than those found in wild-type mice, reflected by the reduced cellularity (Fig. 7a). Consistently, the number of ILC3, which are enriched for lymphoid tissue inducer (LTi) was comparable to wild-type in mesenteric lymph nodes of RORγtM/M mice (Supplementary Fig. 6e, f). However, RORγtM/M mice failed to develop Peyer’s patches similar to RORγt−/− mice (Table 1 and Fig. 7a), consistent with the evidence indicating that development of Peyer’s patches and other peripheral lymph nodes depend on different pathways 24, 25. RORγtM is thus able to support the development of most lymph nodes, except Peyer’s patches.
Table 1.
Visualization of presence and absence of aorta, mesenteric, and inguinal lymph nodes in indicated mice (n=10 per genotype). Data are pooled from 10 mice.
| Mouse strain | Aorta LN | Messenteric LN | Inguinal LN | Peyer’s patches |
|---|---|---|---|---|
| WT | + | + | + | + |
| RORγt−/− | − | − | − | − |
| RORγtM/M | + | + | + | − |
Figure 7. RORγtM/M mice develop lymph nodes, but not Peyer’s patches.
Cellularity of aorta, mesenteric, and inguinal lymph nodes and total number of Peyer’s patches in indicated mice (n=5 per genotype). Central line of box-whisker plot represents median and whiskers indicate extreme data points. **P < 0.01, NS non-significant (ANOVA test). Data are pooled from 5 mice.
RORγtM interferes with the ubiquitination of RORγt at lysine 69
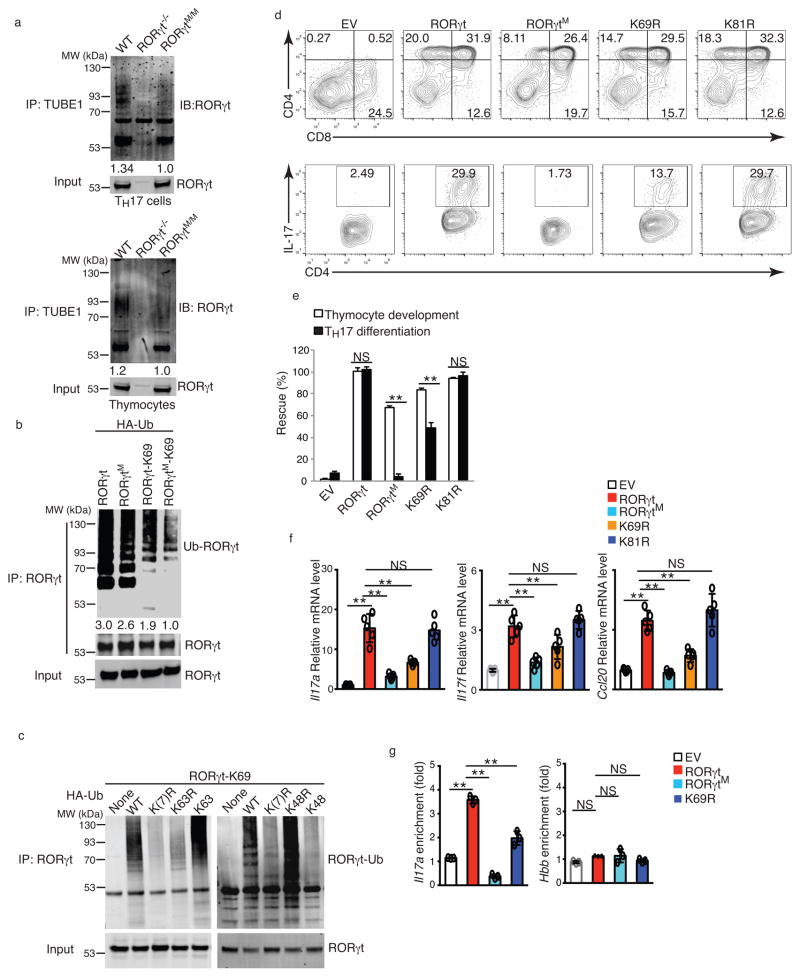
To understand how the two-alanine mutation in RORγtM affects RORγt function, we first monitored RORγtM activity using a RORγt-luciferase reporter. RORγt recruits the transcription co-activator SRC1 via an AF2 motif within the ligand-binding domain 18, 26. RORγtM interacted with SRC1 and stimulated RORγt reporter activity similarly to wild-type RORγt (Supplementary Fig. 7a,b). RORγt is ubiquitinated in TH17 cells 26. Comparison of the RORγt ubiquitination patterns in cells isolated from wild-type, RORγt−/− and RORγtM/M mice indicated that the ubiquitination of RORγtM was reduced in differentiated TH17 cells (Fig.8a, left panel) and thymocytes (Fig.8a, right panel). We then immunoprecipitated RORγt and RORγtM for mass spectrometric analysis of ubiquitinated lysines (K). Digesting ubiquitinated K leaves a GG isopeptide linked to K, which can be easily detected using mass spectrometry as an increased mass of 114.102 Da 27. We detected ubiquitination signals at lysine 69 (K69) in wild-type RORγt, but not RORγtM immunoprecipitated from differentiated TH17 cells (data not shown). We then monitored RORγt ubiquitination in HEK 293T cells transfected with wild-type RORγt or RORγtM and HA-tagged ubiquitin, followed by immunoprecipitation for RORγt. Ubiquitinated RORγtM was reduced compared to wild-type RORγt (Fig. 8b). To specifically evaluate K69 ubiquitination, we generated a mutant RORγt in which all 23 lysines, except K69, were mutated to arginines (RORγt-K69), so that only K69 can be ubiquitinated. RORγt-K69 was polyubiquitinated at K69 in HEK 293T cells (Fig. 8b), while introduction of the L92A-S93A mutation to RORγt-K69 (RORγtM-K69) markedly impaired K69 ubiquitination (Fig. 8b), confirming that two amino acid mutations in RORγtM interfere with K69 ubiquitination. Mutant 91–95A, but not three alanine scanning mutants 151–155A, 156–160A and 161–165A greatly impaired RORγt ubiquitination at K69 (Supplementary Fig.7c). We next determined whether the polyubiquitin chains on RORγt-K69 were K63- or K48-linked 28. Mutating all seven Ks within HA-ubiquitin to arginines [K(7)R] or mutating only K63 to arginine (K63R) prevented RORγt-K69 ubiquitination, whereas mutating all Ks within HA-ubiquitin except K63 to arginines (K63) did not affect RORγt-K69 ubiquitination (Fig. 8c). This suggests the formation of K63-linked polyubiquitin chains at RORγt K69 residue. In contrast, mutating K48 of HA-ubiquitin to arginine (K48R) did not affect RORγt-K69 ubiquitination, whereas mutating all Ks of HA-ubiquitin except K48 to arginines (K48) prevented RORγt-K69 ubiquitination (Fig. 8c), indicating that polyubiquitin chains at RORγt K69 were not K48-linked. An important function of ubiquitination is to regulate protein degradation 28, ubiquitination was reported to regulate RORγt stability 29, 30, 31, 32. However, RORγtM protein expression showed no difference compared to wild-type RORγt at various times after treatment of differentiated TH17 cells with the protein synthesis inhibitor cycloheximide (CHX) (Supplementary Fig. 7d,e), indicating that the degradation rate of RORγtM was same as wild-type RORγt and correlating with the observation that changes in RORγtM ubiquitination were not due to K48-linked polyubiquitin chains that target proteins for degradation 28.
Figure 8. RORγtM interferes with the ubiquitination of RORγt at lysine 69.
(a) Western blot analysis of RORγt in TUBE1-enriched ubiquitinated proteins from differentiated TH17 cells (left panel) or thymocytes (right panel). (b) Western blot analysis of ubiquitinated RORγt (Ub-RORγt) in HEK 293T cells expressing RORγt or RORγt-K69, together with HA-tagged ubiquitin for 48 hrs. (c) Western analysis of Ub-RORγt in HEK 293T cells expressing RORγt-K69, together with HA-tagged WT or mutated ubiquitin. (d) Flow cytometer analysis of CD4 and CD8 in ex vivo development of thymocytes (top panel) or IL-17A+ cells in TH17 differentiation for three days in RORγt−/− thymocytes or CD4+ cells retrovirally expressing indicated RORγt mutants. (e) Quantification of results shown in d. (f) qPCR analysis of mRNA levels of indicated genes in Th17 cells shown in e. (g) ChIP analysis of indicated RORγt binding to IL-17 promoter in RORγt−/− CD4+ T cells retrovirally expressing various RORγt and differentiated under TH17 priming conditions for three days. Hemoglobin gene (Hbb) locus is a control. Data in e,f,g are mean ± s.e.m. **P < 0.01, NS non-significant (ANOVA test). Each symbol (f, g) represents an individual experiment. Data are pooled from three experiments (e, g), from five experiments (f), or from one experiment representative of three experiments (a,b,c and d). Full length gel imagines are available in supplementary figure 8.
To determine the function of K69 ubiquitination in RORγt-regulated function, we mutated RORγt K69 to arginine (mutant K69R) to prevent ubiquitination. As a control, we mutated RORγt K81 to arginine (mutant K81R), as K81 was not ubiquitinated in mass spectrometric analysis. K69R interacted with SRC1 and stimulated RORγt reporter activity similar to RORγtM and RORγt in HEK 293T cells (Supplementary Fig. 7b,f). In thymocyte development and TH17 differentiation assays, retroviral expression of K69R rescued 90% of thymocyte development in RORγt−/− thymocytes, similar to wild-type RORγt and K81R, and slightly greater than thymocyte development supported by RORγtM (Fig. 8d,e), suggesting that K69 ubiquitination is not essential for RORγt-mediated thymocyte development. In contrast, K69R rescued only about 45% of TH17 differentiation in RORγt−/− T cells, which was markedly reduced compared to wild-type RORγt and K81R (Fig. 8d,e), and correlated with reduced expression of TH17 signature genes, including Il17a, IL17f, and Ccl20 (Fig. 8f), as well as binding to the IL-17 promoter (Fig. 8g) in K69R-transduced RORγt−/− T cells. Therefore, K69 ubiquitination, although dispensable for thymocyte development, is required to promote TH17 differentiation. However, because K69R was more potent than RORγtM in supporting TH17 differentiation, lack of K69 ubiquitination is only partially responsible for failed Th17 differentiation observed in the RORγtM/M T cells.
Discussion
In this study, we identified a two amino acid mutation in the hinge region of RORγt that allows normal thymocyte development, but not TH17 differentiation. While RORγt−/− mice exhibit multiple defects, including thymocyte and ILC3 development, lymph node genesis and TH17 differentiation, RORγtM/M mice exhibited a specific defect in Th17 differentiation, while maintaining thymocyte development. RORγtM/M mice also developed ILC3 cells, but these ILC3 were defective in the production of IL-17 cytokine (data not shown). Therefore, RORγtM preserves the function to instruct T and ILC3 development, but selectively disrupts the IL-17 production function of mature cells. We further demonstrated that the two amino acid mutation impaired ubiquitination of RORγt at K69 that is selectively required for TH17 differentiation but not thymocyte development.
RORγt−/− mice have a high incidence of thymic lymphoma, likely due to dysregulated thymocyte development, including cell cycle and apoptosis changes 6. Because thymocyte development in RORγtM mice is largely normal, lymphoma development seems unlikely. Indeed, we did not detected lymphoma among more than 160 RORγtM mice analyzed, including 25 mice 24 week old, 8 mice 37–39 week old and 7 mice older than 39 weeks (results not shown), while more than 50% of RORγt−/− mice die of lymphoma prior to 39 week of age 6. These result support the idea that lymphoma in RORγt−/− mice results from abnormal thymocyte development.
The RORγt S92A-L93A mutations were the minimum alterations in the hinge region that selectively disrupted RORγt function in TH17 differentiation without affecting thymocyte development. Although S92 is a potential phosphorylation site, mutant S92A, which cannot be phosphorylated, did not affect RORγt function. In contrast, mutant L93A affected both RORγt-mediated thymocyte development and TH17 differentiation. Whereas, two amino acid mutation, S92A-L93A (RORγtM), can maximally separate RORγt function in thymocytes and TH17 differentiation. RORγtM did not affect RORγt activity, recruitment of the co-activator SRC1 and stimulation of a RORγt reporter, suggesting that two amino acid mutation does not affect overall RORγt function. However, RORγtM impairs the ubiquitination of K69 that is required for TH17 differentiation, but not thymocyte development. Therefore, post-translational ubiquitination plays an important role in distinguishing RORγt function in TH17 differentiation versus thymocyte development. Although ubiquitination is known to regulate protein degradation 28, K69R and RORγtM do not affect RORγt protein levels, and thus does not support the function of K69 ubiquitination in the regulation of RORγt stability. This is further confirmed by K63- but not K48-linked ubiqutination at K69 of RORγt. The fact that K69 locates in the DNA-binding domain and K69R impairs RORγt-binding to the IL-17 locus supports that RORγtM affects DNA-binding activity selectively in TH17 cells via interfering with K69 ubiquitination. This possibility is strengthened by our global analysis indicated that RORγtM failed to bind and stimulate genes critical for TH17 differentiation, whereas it did not significantly affect the expression of cell survival and cell cycle genes critical for thymocyte development. It still remains to be determined how K69 ubiquitination is selectively required for TH17 differentiation but not thymocyte development. One of the possibilities is that K69 ubiquitination induces the recruitment of a co-factor to RORγt specifically in Th17 cells, which can be determined by comparing RORγt-associated proteins between Th17 cells and thymocytes. In addition, the enzymes responsible for modifying K69 of RORγt remain unknown. Identification of these enzymes will facilitate the development of drugs that specifically target RORγt-mediated pathological autoimmune TH17 responses, without interfering with thymocyte development or lymph node genesis.
RORγt mutations reported in humans result in susceptibility to fungal and mycobacteria infection 33. However, these RORγt mutations locate in either the DNA-binding domain or the ligand-binding domain. Humans bearing RORγt mutations also have defects in lymph node genesis and thymocyte apoptosis 33, consistent with the phenotypes observed in RORγt−/− mice, and suggesting function conservation between human and mouse RORγt. It will be important to investigate if modifications of the S92-L93 motif identified in mice also distinguishes the thymocyte development and Th17 differentiation function of RORγt in humans.
Materials & Methods
Mice
The RORγt−/− (Rorc2−/−) mouse strain was described previously 5. Both the targeting vector and the knock-in RORγtM/M mice were designed and generated by Biocytogen LLC. Rosa-Flp (008600) and Rag1−/− (002216) mice were purchased from the Jackson Laboratory. For all experiments, mice were 6–12 weeks of age; age-matched littermates were used in experimental groups, and all groups contained five mice unless stated otherwise. Mice were bred and housed under specific pathogen-free (SPF) conditions in the Animal Resource Center at the Beckman Research Institute of City of Hope under protocols approved by the Institutional Animal Care and Use Committee.
Plasmids and Retrovirus
The retroviral vector MIGR (murine stem cell virus [MSCV]-IRES-GFP) was a gift from Dr. Warren S. Pear (University of Pennsylvania, Philadelphia, PA) and used to clone RORγt or BCL-xL cDNA as described previously. Mutant RORγt vectors were prepared using a site-directed mutagenesis kit from Agilent Technologies. pRK5-HA-ubiquitin constructs (a gift from Ted Dawson, Addgene #17603 -17608) for expressing HA-tagged wild-type and mutant ubiquitin were obtained from Addgene. Retroviruses were packaged in a Platinum-Eco cell line from Cell Biolabs as described previously 26. Briefly, retroviral expression plasmids were transfected into Platinum-Eco packaging cells using Lipofectamine 2000 (thermofisher scientific). Viral supernatants were collected 72 h later, passed through 0.4-μm filters, and stored at −80°C until use.
Flow cytometry and cell sorting
Tissues were homogenized by crushing with the head of a 1-ml syringe in a petri dish, followed by straining through a 40-μm nylon filter. Red Blood Cell Lysing buffer (Sigma-Aldrich) was used for red cell lysis and remaining cells were counted. Antibodies used for flow cytometry staining are listed in Supplementary Table 1. For intracellular cytokine staining, cells obtained from in vitro cultures were incubated for 4–5 h with 50 ng/ml PMA, 750 ng/ml ionomycin (both from Sigma-Aldrich), and 10 μg/ml brefeldin A (BD Biosciences) in a tissue culture incubator at 37°C. Cells were resuspended in Fixation/Permeabilization solution and intracellular cytokine staining was performed (BD Cytofix/Cytoperm Kit; BD Biosciences). For sorting double-negative thymocytes, thymocytes from C57BL/6 or RORγt−/− mice were stained with 7-AAD and antibodies against Thy1.2, CD4 and CD8 (listed in Life Sciences Reporting System item 9). Specific Thy1.2+/CD4−/CD8− populations were sorted using a FACSAria (BD Biosciences).
T cell development assay
Electronically sorted double-negative thymocytes (Thy1.2+/CD4−/CD8−) were cultured at 5×105/ml overnight on an 80% confluent OP9-DL4 monolayer (generous gift from Dr. Ellen Rothenberg, Caltech) in flat-bottom 24-well culture plates with αMEM (MEM α medium; Invitrogen Life Technologies) supplemented with 20% FBS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine (Invitrogen Life Technologies), and 5 ng/ml recombinant murine IL-7 (PeproTech). The following day, co-cultures were transduced by spin infection (1200 g, 30°C for 2 h) using retroviral supernatants in the presence of 8 mg/ml polybrene (Sigma-Aldrich). After spin infection, viral supernatant was replaced with culture media containing 5 ng/ml recombinant murine IL-7. 72 h post-transduction, co-cultures were harvested for flow cytometry analysis.
CD4+ T cell isolation and in vitro TH17 differentiation assay
Naive CD4+ T cells were purified from C57BL/6, RORγt−/−, or RORγtM/M mice by negative selection using CD4+ T Cell Isolation Kit II (Miltenyi Biotec). Suspensions of 4×105 cells/ml Iscove’s modified DMEM (Cellgro) containing 2 mM L-glutamine, 50 mM 2-ME, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FBS were cultured in 24-well plates pre-coated with 0.2 mg/ml goat anti-hamster antibody (MP biomedicals) for three days. The medium was supplemented with 0.25 μg/ml hamster anti-CD3 (145-2C11; eBioscience), 1 μg/ml hamster anti-CD28 (37.51; eBioscience), and polarizing cytokines: 2 ng/ml TGF-β (Peprotech), 20 ng/ml IL-6 (Peprotech), 5 μg/ml anti-IL-4 (11B11), and 5 μg/ml anti-IFNγ (XMG 1.2) for TH17 differentiation; 20 ng/ml IL-23 (Miltenyi Biotec) was also added when inducing pathogenic TH17 cells. 20 ng/ml IL-12 (Peprotech) and 5 μg/ml anti-IL-4 for TH1 differentiation; 10 ng/ml IL-4 and 10 μg/ml anti-IFNγ for TH2 differentiation; or 5 ng/ml TGF-β for Treg differentiation. For retroviral transduction, T cells were first activated with 0.25 μg/ml hamster anti-CD3 (145-2C11; eBioscience), 1 μg/ml hamster anti-CD28 (37.51; eBioscience) in 24-well plates pre-coated with 0.2mg/ml goat anti-hamster antibody for 24 h, then spin-infected with viral supernatants (1200 g, 30°C for 2 h) in the presence of 8 mg/ml polybrene (Sigma-Aldrich). After spin infection, viral supernatant was replaced by culture media with polarizing cytokines for in vitro differentiation.
RNA sequencing (RNA-seq) and analysis
To measure gene expression in the thymus of C57BL/6, RORγt−/− or RORγtM/M mice, two separate samples were collected on different days, and thymocytes from four (two male and two female) were pooled each day. For demonstrating the gene expression profile regulated by wild-type RORγt or RORγtM/M in in vitro-differentiated Th17 cells, CD4+ T cells were enriched from RORγt−/− mice, transduced with retrovirus containing empty vector, wild-type RORγt or RORγtM/M, then polarized under Th17 conditions for three days. Cells were processed for RNA isolation (Qiagen). Quality verification, library preparation, and sequencing were performed at City of Hope Integrative Genomics Core facility. Samples were sequenced on an IlluminaHiSeq 2000 using 75-bp paired end reads. Reads were aligned to the mm9 mouse genome using TopHat version 2.1.0 and Bowtie version 1.1.1. Differential expression was computed with Cufflinks version 2.2.1. Differentially expressed genes were filtered by significance at FDR-adjusted P < 0.05, and the ratio of fragments per kilobase of transcript per million mapped reads (FPKM) were at least two-fold changes between two of the populations. Clustering and heatmapping were performed using hierarchical clustering in R with pheatmap package version 1.0.8. For heat maps, expression values were scaled per gene.
Chromatin immunoprecipitation and DNA sequencing (ChIP-seq)
2×107 cells were incubated with 1% formaldehyde, to cross-link proteins with chromatin, for 5 min at room temperature. 125 mM glycine was added to stop the cross-linking reaction. To shear genomic DNA into 200–500 bp fragments, cell lysates were sonicated using a water-bath sonicator (Covaris S200). Cell lysates were centrifuged (12000 g, 10 min) and incubated with FLAG antibody (M2, Sigma-aldrich, listed in Life Sciences Reporting System item 9) or IgG controls, and magnetic protein A/G beads (Millipore). After extensive washing, DNA was eluted followed by reversion of the protein–DNA cross-linking. DNA was recovered for DNA-seq or qRT-PCR to quantify specific DNA fragments that were precipitated. Primers used in qRT-PCR were listed in Supplementary Table 1. Samples were sequenced on an IlluminaHiSeq 2000 using 75-bp paired end reads. Reads were aligned to the mm9 mouse genome using Bowtie version 1.1.1. Differential peaks were computed with MACS version 1.4.2 and bedtools version 2.17.0. Neighboring genes in the mm9 mouse genome were searched using webtools Cistrome Analysis Pipeline (at website: Cistrome.org/ap).
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed using SsoFast EvaGreen Supermix (Bio-Rad) in a CFX96 Real-Time PCR Detection System (Bio-Rad), using primers as noted (Supplementary Table 1). The amplification efficiency of all primers has been tested, and the optimized conditions were used in all qRT-PCR reactions. Expression was calculated with the ΔΔxp method normalized to β-actin, and all measurements were performed in triplicate.
Apoptosis assays
Thymocytes were freshly isolated from wild-type, RORγt−/−, or RORγtM/M mice and cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine at 1×106 cells/ml. Thymocytes were incubated at 37°C with 5% CO2. Dead cells were detected by Annexin V-PE and 7-AAD staining (BD Bioscience), as described previously 34.
Induction and assessment of Experimental Autoimmune Encephalomyelitis (EAE)
Active EAE was induced and assessed as previously described 35. Briefly, mice were immunized subcutaneously in the abdominal flank with 200 mg MOG35-55 and 400 mg Mycobacterium tuberculosis H37RA (Difco) in a 400 μl emulsion of equal volumes of water and complete Freund’s adjuvant (Difco). Each mouse also received 0.4 mg purified Bordetella pertussis toxin in 200 μl PBS intraperitoneally on days 0 and 1. For reconstitution of T cells in Rag1−/− mice, 5×106 flow cytometry-sorted CD4+ T cells from C57BL/6, RORγt−/−, or RORγtM/M mice were intraperitoneally transferred into Rag1−/− mice, followed by active induction of EAE.
Western blot and immunoprecipitation
Cells were lysed in lysis buffer (0.5% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM EGTA, 20 mM aprotinin, 1 mM phenylmethylsulfonyl fluoride). Protein from cell extracts (15 mg) was resolved using SDS-PAGE (10% gel), transferred to PVDF Membrane (Millipore), and Western blot was performed using relevant antibodies. Immunoprecipitation experiments were performed as described previously. In brief, cell extracts were incubated overnight with relevant antibodies and protein A/G beads at 4°C. After incubation, beads were washed four times with lysis buffer, resolved using SDS-PAGE, and analyzed using Western blot. Western blot primary antibodies were listed in Life Sciences Reporting System item 9.
Enrichment of ubiquitinated proteins by Agarose-TUBE1
A total of 1.5×108 thymocytes from C57BL/6, RORγt−/−, or RORγtM/M mice, or 1.5×108 Th17 cells differentiated in vitro as described above, were lysed in 500 μl cell lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, 1 mM PMSF). Cell lysates were incubated with 20 μl equilibrated Agarose-coupled Tandem Ubiquitin Binding Entity 1 (Agarose-TUBE1) (LifeSensors) at 4°C for 4 h. Agarose-TUBE1 was washed with 1 ml TBS-T (20 mM Tris-HCl [pH 8], 150 mM NaCl, 0.1% Tween-20) and suspended in SDS reducing sample buffer. Eluted samples were analyzed by Western blotting.
Statistical analysis
Prism software (Graphpad) was used for all statistical analyses. Unpaired Student’s t tests and one-way analysis of variance (ANOVA) were used to compare experimental groups. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank J. C. Zuniga-Pflucker for providing DP9-DL4 stroma cell line, and Biocytogen for assisting with design and generation of the RORγtM mice. This work was supported by grants from NIH R01-AI053147, NIH R01-AI109644 and institutional pilot funding. In addition, research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572, which includes work performed in the Animal Resource Center, Integrative Genomics, Analytical Cytometry, and Mass Spectrometry and Proteomics Cores supported by this grant. Dr. Zhaofeng Huang is supported by Science and Technology Department of Guangdong Province (2016A050503023). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions. Z. Sun and He, Z. conceived the study. He, Z., Ma, J., Wang, R., Huang, Z., Sen, S., Zhang, J., and Wang, F. carried out the experiments. Z. Sun and He, Z. wrote the manuscript. E. R. instructed and provided critical reagents for in vitro thymocyte development experiments. All the authors reviewed and edited the manuscript.
Competing financial interests. The authors declare no competing financial interests.
RNA-seq and ChIP-seq data access code is SUB2581283
The data that support the findings of this study are available from the corresponding author upon request.
References
- 1.Ivanov, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature reviews. Immunology. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codarri L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 4.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 6.Ueda E, et al. High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORgamma. Cancer research. 2002;62:901–909. [PubMed] [Google Scholar]
- 7.Kurebayashi S, et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 10.Huh JR, Littman DR. Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–2237. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 12.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan C. Footrace to clinic heats up for T-cell nuclear receptor inhibitors. Nature biotechnology. 2013;31:370. doi: 10.1038/nbt0513-370. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Xie H, Wang R, Sun Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert opinion on therapeutic targets. 2007;11:737–743. doi: 10.1517/14728222.11.6.737. [DOI] [PubMed] [Google Scholar]
- 15.Sherlock JP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4−CD8− entheseal resident T cells. Nature medicine. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 16.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie H, Huang Z, Wang R, Sun Z. Regulation of thymocyte survival by transcriptional coactivators. Critical reviews in immunology. 2006;26:475–486. doi: 10.1615/critrevimmunol.v26.i6.10. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Sadim MS, Sun Z. RORgammat recruits steroid receptor coactivators to ensure thymocyte survival. J Immunol. 2005;175:3800–3809. doi: 10.4049/jimmunol.175.6.3800. [DOI] [PubMed] [Google Scholar]
- 19.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Progress in nucleic acid research and molecular biology. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 20.Holmes R, Zuniga-Pflucker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harbor protocols. 2009;2009 doi: 10.1101/pdb.prot5156. pdb prot5156. [DOI] [PubMed] [Google Scholar]
- 21.Yui MA, Rothenberg EV. Developmental gene networks: a triathlon on the course to T cell identity. Nature reviews Immunology. 2014;14:529–545. doi: 10.1038/nri3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staschke KA, et al. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J Immunol. 2009;183:568–577. doi: 10.4049/jimmunol.0802361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debard N, Sierro F, Kraehenbuhl JP. Development of Peyer’s patches, follicle-associated epithelium and M cell: lessons from immunodeficient and knockout mice. Seminars in immunology. 1999;11:183–191. doi: 10.1006/smim.1999.0174. [DOI] [PubMed] [Google Scholar]
- 25.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature reviews Immunology. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 26.He Z, et al. Ubiquitination of RORgammat at Lysine 446 Limits Th17 Differentiation by Controlling Coactivator Recruitment. J Immunol. 2016;197:1148–1158. doi: 10.4049/jimmunol.1600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MJ, Barrett-Wilt GA, Hua Z, Vierstra RD. Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16512–16517. doi: 10.1073/pnas.1004181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain R, et al. Interleukin-23-Induced Transcription Factor Blimp-1 Promotes Pathogenicity of T Helper 17 Cells. Immunity. 2016;44:131–142. doi: 10.1016/j.immuni.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarevic V, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L, et al. The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor gammat (RORgammat) in Th17 cells. J Biol Chem. 2014;289:25546–25555. doi: 10.1074/jbc.M114.565291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, et al. TRAF5-mediated Lys-63-linked Polyubiquitination Plays an Essential Role in Positive Regulation of RORgammat in Promoting IL-17A Expression. J Biol Chem. 2015;290:29086–29094. doi: 10.1074/jbc.M115.664573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, et al. Cutting edge: Ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORgammat. J Immunol. 2015;194:4094–4097. doi: 10.4049/jimmunol.1401451. [DOI] [PubMed] [Google Scholar]
- 33.Okada S, et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–613. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Wang R, Fang X, Ding Y, Sun Z. Critical role of TCF-1 in repression of the IL-17 gene. PLoS One. 2011;6:e24768. doi: 10.1371/journal.pone.0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.