Abstract
Objective
To assess whether an intensive lifestyle intervention (ILI) for weight reduction precipitates binge eating (BE) and whether BE attenuates 4-year weight loss among participants with type 2 diabetes and overweight/obesity.
Methods
Participants (n=4,901) were from Look AHEAD, an RCT that compared ILI to diabetes support and education (DSE). We used annual assessments of measured weight and self-reported BE. Using yearly time points a person endorsed BE, participants were classified as: no BE; remitted BE; incident BE; inconsistent BE (2–3 years including baseline); and consistent BE (≥4 years including baseline). We used Cox regression and mixed-effects models for analyses.
Results
ILI participants were marginally more likely to report incident BE at year 4 than those in DSE (p=0.06). At year 4, ILI participants with remitted BE lost more weight (4.7±0.8%) than those with consistent BE (1.9 ±1.0%; p=0.03). ILI participants with no BE lost more weight (4.6 ±0.2%) than those with incident BE (3.1 ±0.6%; p=0.02) and consistent BE (p=0.01). DSE participants with remitted BE lost more weight than those with incident and consistent BE.
Conclusions
Preexisting BE does not seem to be a contraindication for ILI, although persistent BE attenuates weight loss. Patients who report new or ongoing BE need additional treatment.
Keywords: Binge eating, weight, obesity, psychosocial behavior
Introduction
The health benefits of weight loss have been documented,1,2 yet the behavioral effects of participating in a lifestyle program have not been fully determined. In some3,4 but not all studies,5,6 dieting precedes unhealthy weight-control behaviors, leading to concerns that lifestyle interventions for weight reduction may induce or exacerbate disordered eating behaviors such as binge eating (BE). BE is characterized by consumption of a large amount of food, with a sense of loss of control over eating.7 A diagnosis of binge eating disorder (BED) is made if BE episodes occur at least once a week for 3 months or more, and are associated with marked distress without regular compensatory behaviors (e.g., purging).7 Among overweight adults with and without preexisting BE, moderate caloric restriction does not seem to precipitate this behavior in the short term (≤1 year).8–10 Further, in participants with BE at baseline, behavioral weight loss seems to decrease this behavior.11,12 Whether these improvements return to baseline with long- term follow-up (>1 year) is unknown.
Individuals with BE may be less successful with weight management, regardless of whether diagnostic thresholds are met. BE has been identified as a risk factor for poor response to weight loss treatment in several,9,13–15 but not all studies.16–18 Thus, some clinicians have suggested that BE should be treated before weight loss.19 Few studies have examined long-term effects (>1 year) of BE on weight loss, when behavioral adherence typically declines and weight regain is common.
In this study, we used data from the Action for Health in Diabetes (Look AHEAD) study to address these gaps. Look AHEAD is a 16-center randomized clinical trial that compared the effects of an intensive lifestyle intervention (ILI) to diabetes support and education (DSE) on incidence of major cardiovascular events.20,21 Gorin and colleagues (2008) previously reported that participants randomly assigned to the ILI were marginally less likely to binge eat at year 1 than those in the DSE group (OR=0.77, p=0.06). Across groups, individuals who stopped BE at 1 year or who reported no BE at either baseline or 1 year lost 5.3 kg and 4.8 kg, respectively, which was significantly more than the 3.1 kg and 3.0 kg, respectively, lost among those who continued to binge eat or who had begun to binge eat at year 1.9 The aims of this study were to: 1) assess the effects of the ILI on the precipitation (and possible remission) of BE over 4 years; and 2) examine whether 4-year weight loss outcomes were related to BE status over the 4-year period. We hypothesized that the ILI and DSE groups would not differ in incidence of BE and that the ILI group, as compared to the DSE group, would have a greater percent of participants who reported remission of BE. We also hypothesized that individuals who continued to report BE over 4 years would lose less weight than those who were continuously free of this behavior or reported remission of BE.
Methods
Participants
Details of the study’s procedures have been reported previously.22 In brief, 5,145 participants were recruited from 16 centers in the US. Participants were 45–76 years of age with overweight or obesity (body mass index (BMI) ≥25 kg/m2; BMI≥27 if on insulin) and type 2 diabetes. Participants were required to have a hemoglobin A1c<11%, systolic blood pressure<160 mm Hg, diastolic blood pressure<100 mm Hg and triglycerides<600 mg/dL. Local institutional reviews boards at each site approved the protocol and consent forms. The current analysis used the publically available data (n=4,901), which included consented and randomized participants and excluded people from Native American sites due to consent limitations. Participants with baseline compensatory behaviors (n=307) or with missing baseline BE data (n=12) were excluded from analyses.
Interventions
Participants were randomly assigned within each center to the ILI or DSE group.23 The ILI included individual and group sessions focused on improving diet and physical activity. The goal of the program was to achieve and maintain a mean loss of ≥7% of initial body weight. Participants were prescribed a 1200–1800 kcal/day calorie goal and 175 minutes/week of moderate-intensity physical activity. Participants in ILI were seen weekly for the first 6 months, 3 times per month for the next 6 months, and at least once a month from years 2–4, with a combination of group and individual contacts following a standardized protocol.23 BE was not explicitly addressed in sessions, though participants were encouraged to consume a regular pattern of meals. Intervention materials are available at https://www.lookaheadtrial.org/publicResources/interventionMaterial.cfm. Participants in DSE were invited to three group sessions each year that used a standardized protocol and focused on diet, physical activity, or social support.24
Assessments
Assessments were completed at baseline and annually thereafter by certified staff masked to intervention assignment. Participants were compensated $100 per visit.
Demographic and clinical characteristics
Height and weight were measured in duplicate using a stadiometer and digital scale (Tanita, model BWB-800, Willowbrook, IL) with participants wearing light clothing. Self-report forms were used to collect demographic data. Prescription medication use was assessed at baseline. Participants completed questions regarding diabetes duration and treatment regimen. Glycated hemoglobin level was measured with a dedicated ion-exchange high-performance liquid chromatography instrument [Bio-Rad Variant 11; Bio-Rad Laboratories, Hercules, California].
Binge eating
BE was assessed annually using a self-report questionnaire adapted from the Questionnaire on Eating and Weight Patterns (QEWP).25 At each time point, participants were categorized as having BE if they endorsed consumption of a large amount of food in a discrete period of time while feeling a loss of control over eating at least once in the past 6 months. Participants reported the average weekly frequency of BE as less than 1 day a week; 1 day a week; 2–3 days a week; 4–5 days a week; or almost every day. A midpoint average was used for frequency intervals. Use of compensatory behaviors (e.g., vomiting, excessing exercise) in the past 3 months was assessed using questions from the QEWP.25
Dietary intake
Reported dietary intake was measured in a subgroup of participants (n=2,612) using a self-administered 130-item food frequency questionnaire. The measure assessed usual consumption during the past 6-months and was used to calculate daily energy intake, and percent of energy intake from fat, carbohydrates, and protein.
Weight loss history
At baseline, participants were asked the number of times (0, 1–2, 3–4, 5–6, or ≥7 times) that they had intentional weight losses of 5–9 lbs, 10–19 lbs, 20–49 lbs, 50–79 lbs, 80–99 lbs, and 100+ lbs. Number of previous weight loss attempts was computed using the lower end of the frequency intervals and summing across categories. Total amount of past weight loss was calculated by multiplying the frequency of attempts by the amount of weight loss, using the lower bound of the frequency and amount intervals and totaling across categories.
Depressive symptoms and quality of life
The Beck Depression Inventory (BDI) 1A was used to assess depressive symptoms.26 The measure includes 21 symptoms with scores ranging from 0–63. A greater number of symptoms indicates higher depressive symptoms. Scores ≥10 suggest mild or greater depression. The 36-item Short-Form Health Survey was used to assess health-related quality of life.27 Two summary scores, physical composite and mental composite, were used in this analysis. Summary scores are norm-based T-scores with a mean of 50 and standard deviation of 10. Lower scores indicate worse health-related quality of life.
Tobacco and alcohol use
Tobacco and alcohol use were assessed by self-report. Participants indicated whether they were a current, former, or never smoker. Participants reported the ounces of alcoholic drinks they typically consumed per week. Binge drinking was evaluated using an item asking how many days during the past month they consumed ≥5 drinks on the same occasion.
Attendance
Staff recorded participants’ attendance to group and individual meetings. After the first year, attendance also incorporated scheduled bi-monthly phone or e-mail contacts and participants’ attendance in Refresher Groups and National Campaigns.28
Definition of BE Groups
Five mutually exclusive groups were constructed based on the presence or absence of BE at each year. Trajectories were created using observed values. The five trajectory groups were: “no BE” (BE absent at baseline, Y1, Y2, Y3, and Y4); “incident BE” (BE absent at baseline but present at Y1, Y2, Y3, or Y4); “fully remitted BE” (BE present at baseline but absent at Y1, Y2, Y3, and Y4); “inconsistent BE” (BE present for 2–3 years including at baseline); and “consistent BE” (BE present at ≥4 years including at baseline).
Statistical Analyses
Analyses were performed using SPSS version 24 (Armonk, NY) with an alpha of 0.05. Baseline characteristics were compared between participants with and without BE using t-tests, Wilcoxon’s rank-sum test, and chi-squared tests. We estimated the effect of the ILI (vs the DSE group) on BE incidence using multivariable Cox proportional hazards regression, with time to BE as the outcome. Cumulative incidence was estimated based on the number of first occurrences of BE divided by the number of people who were at risk (i.e., participants free of BE at baseline). Models were adjusted for factors that could affect weight loss and BE including baseline age, race/ethnicity, sex, education level, BMI, previous weight loss attempts, physical and mental health-related quality of life, depressive symptoms, number of prescription medications, and weekly alcohol consumption.9 Comparable analytics were used to examine BE remission among participants who began the trial with this behavior.
The co-primary outcome analysis compared 4-year percentage change in baseline weight based on the trajectory of BE across 4 years. Models were fit and analyzed stratified by treatment. A piecewise, linear mixed-effects model with a breakpoint at year 1 was selected based on model fit.29 Models adjusted for baseline differences between individuals with and without BE as used previously.9 Chi-squared tests were used to compare categorical weight losses based on BE trajectory.
Results
Baseline Differences Based on BE
At baseline, 546 (11.2%) of the cohort reported having one or more episodes of BE in the past 6 months. Of those with baseline BE, the average number of days per week individuals reported BE was 1.6 ±1.6, and 41.4% of participants reported episodes on less than 1 day/week; 22.5% reported 1 day/week; 26.0% reported 2–3 days/week; 4.0% reported 4–5 days/week; and 5.1% reported BE almost every day. At baseline, 54 participants (1.1%) met DSM 5 criteria for BED.
Baseline characteristics and weight losses at 1 year by BE status have been described in detail previously.9 Baseline differences between individuals with and without BE were recalculated based on the public access data set and yielded similar results (Table 1). Participants who reported BE, as compared with those who did not, were more likely to be younger, female, white, and to have >16 years of education (ps<0.05). Relative to participants without BE, those who reported BE had a higher BMI, more previous weight loss attempts, higher depressive symptoms, greater numbers of prescription medications, and lower physical and mental health-related quality of life (ps<0.05). Compared to participants without BE, those with BE reported consuming a higher number of calories per day, and a greater percentage of their intake came from fat (ps<0.05).
Table 1.
Participant characteristics of study sample by baseline binge eating (BE)
| No BE | BE | P-Value | |
|---|---|---|---|
| Participants, No (%) | 4026 (82.5) | 546 (11.2) | |
| Age, mean (SD), y | 59.22 (6.71) | 57.40 (6.57) | <0.001 |
| Sex | |||
| Male, No. (%) | 1705 (42.3) | 198 (36.3) | 0.007 |
| Female, No. (%) | 2321 (57.7) | 348 (63.7) | |
| Race, No. (%) | |||
| White | 2652 (65.9) | 418 (76.6) | <0.001 |
| Hispanic | 577 (14.3) | 54 (9.9) | |
| African American | 666 (16.5) | 55 (10.1) | |
| Other/Mixed | 131 (3.3) | 19 (3.5) | |
| Education, No. (%) | |||
| <13 yrs | 783 (19.4) | 76 (13.9) | 0.01 |
| 13–16 yrs | 1470 (36.5) | 198 (36.3) | |
| >16 yrs | 1688 (41.9) | 259 (47.4) | |
| Married, No. (%) | 2676 (66.5) | 356 (65.2) | 0.55 |
| BMI | 35.70 (5.77) | 37.26 (6.06) | <0.001 |
| Previous weight loss attempts, No. | 5.70 (5.42) | 8.32 (6.55) | <0.001 |
| Previous weight loss, lbs | 64.90 (84.64) | 102.28 (106.49) | <0.001 |
| Daily caloric intake, kcala | 1920.24 (836.83) | 2350.26 (980.71) | <0.001 |
| Daily fat intake, %a | 39.78 (7.04) | 40.86 (6.60) | 0.01 |
| Daily protein intake, %a | 17.22 (2.92) | 16.96 (2.69) | 0.14 |
| Daily intake carbohydrates, %a | 43.85 (7.73) | 43.12 (7.18) | 0.12 |
| BDI score, mean (SD) | 4.92 (4.54) | 7.60 (5.49) | <0.001 |
| Alcoholic drinks (oz/week; sum beer, wine, liquor/wk), mean (SD), No./wk | 9.00 (25.77) | 6.94 (19.82) | 0.03 |
| Smoking, No. (%) | |||
| Current | 173 (4.3) | 19 (3.5) | 0.32 |
| Former | 1828 (45.4) | 270 (49.5) | |
| Never | 2018 (50.1) | 256 (46.9) | |
| Binge drinking, No. (%) | 206 (5.1) | 16 (2.9) | 0.03 |
| Physical health, mean (SD) | 48.32 (7.74) | 46.20 (8.70) | <0.001 |
| Mental health, mean (SD) | 54.82 (7.47) | 50.69 (9.64) | <0.001 |
| Glycated hemoglobin level, mean (SD), % | 7.26 (1.15) | 7.26 (1.15) | 0.88 |
| Diabetes duration, mean (SD), y | 6.62 (6.35) | 7.18 (6.43) | 0.06 |
| Medications No., mean (SD) | 5.58 (2.93) | 5.87 (3.06) | 0.03 |
| Diabetes treatment, No. (%) | |||
| Diet only | 510 (13.1) | 70 (13.3) | 0.96 |
| Oral medications without insulin | 2667 (68.4) | 358 (67.8) | |
| Insulin | 720 (18.5) | 100 (18.9) |
Excludes participants reporting compensatory behaviors at baseline;
Scores for a subsample of participants who completed food frequency questionnaire (n=2108 for no BE and 314 for individuals with BE).
Binge Eating Behavior: Incidence and Remission by Treatment Group
At year 4, weight data were obtained on 90.1% of the sample, and both weight and questionnaire data were available for 88.6% of participants. Individuals with baseline BE were no less likely to complete the 4-year assessment than were those without BE (88.1% vs 89.0%, p=0.54).
Incidence
Of participants without BE at baseline, 201 (10.2%) of ILI participants and 171 (8.3%) of those in DSE developed BE over the 4 years. The Cox proportional hazard model revealed that participants in the ILI were marginally more likely to have incident BE at year 4 than those in the DSE (HR=1.22, 95% CI=0.99, 1.51, p=0.06; Figure 1). BE incidence was significantly associated with a greater number of previous weight loss attempts (HR=1.03, 95% CI=1.01, 1.05, p=0.004). Older age (HR=0.97, 95% CI=0.95, 0.98, p<0.001), better baseline physical health-related quality of life (HR=0.98, 95% CI=0.97, 0.99, p=0.02) and better mental health-related quality of life (HR=0.98, 95% CI=0.96, 0.99, p=0.005) were significantly associated with lower incidence of BE.
Figure 1.

Cumulative hazard ratio for intensive lifestyle intervention (ILI) versus diabetes support and education (DSE) for incidence of BE over 4 years in participants without baseline binge eating (BE).
Remission
Among the 546 participants who began the study with BE, at baseline, the ILI group reported a mean (±standard deviation) of 1.7 ±1.8 BE days per week and DSE participants reported 1.6 ±1.4 BE days per week. From year 1 to year 4, the average number of BE days per week significantly declined (p<0.001) in both groups. At year 4, the number of BE days per week in the ILI group was 0.5 ±1.3 and in the DSE was 0.4 ±0.9. Percentage reduction in the number of BE days per week did not differ between ILI (56.8%) and DSE groups (63.9%; p=0.50). Full BE remission was achieved by 46.0% of ILI and 46.7% of DSE participants. The ILI and DSE groups did not differ in the proportion of individuals who had full BE remission (adjusted HR=1.09, 95% CI=0.90, 1.32, p=0.37). None of the included covariates were significant predictors of full remission (ps>0.05).
BE trajectories
The ILI and DSE groups differed in BE trajectory (p=0.007) in unadjusted analyses. A greater percentage of DSE participants reported no BE at any time point (81.8%) compared to the ILI group (78.1%; adjusted residual = −3.2). A smaller proportion of DSE participants reported consistent BE (1.8%) compared to the ILI group (3.0%; adjusted residual=2.7). The number of participants in the DSE and ILI groups did not differ in the percentage of participants with fully remitted BE (5.0% vs. 6.0%), incident BE (7.4% vs 8.8%), or inconsistent BE (3.9% vs 4.1%).
4 Year Weight Loss Outcomes
ILI
At year 4, ILI participants who did not report BE at any time (over the 4 years) lost a mean (±standard error) of 4.6 ±0.2% of initial weight, compared to significantly smaller losses for participants with incident BE (3.1 ±0.6%; p=0.02) or those with consistent BE (1.9 ±1.0%; p=0.01). Percent weight loss did not differ among participants with no BE and those with inconsistent BE (3.5 ±0.9%). Individuals with fully remitted BE lost 4.7 ±0.8%, which was significantly greater than the loss for individuals with consistent BE (p=0.03), but did not differ from the other groups (Figure 2). At year 4, meeting a categorical weight loss of >7% differed by BE trajectory group (p=0.02; Figure 3a). Categorical weight losses also differed by BE trajectory for >0% (p=0.004) and ≥5% (p=0.02).
Figure 2.
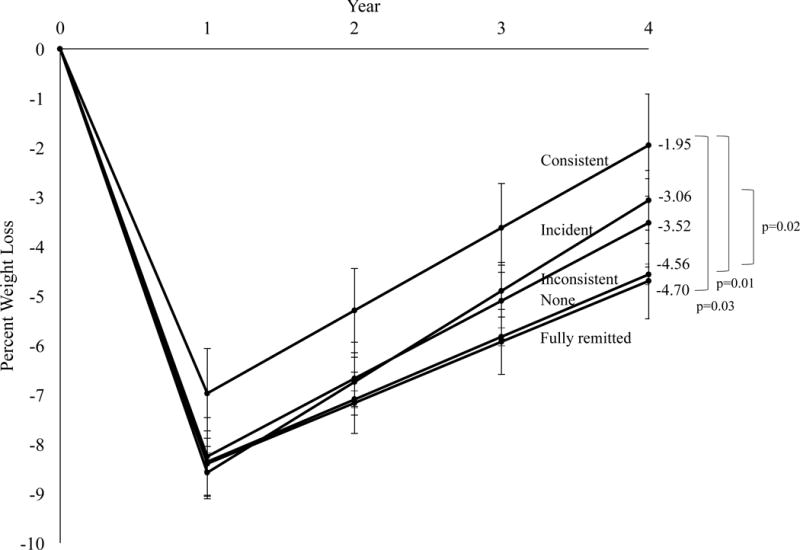
Percent reduction in initial weight in the intensive lifestyle intervention (ILI) group by binge eating (BE) trajectory. Values shown are mean (±standard error) and weight losses were estimated using linear mixed-effects models controlling for baseline age, race/ethnicity, sex, education level, body mass index, number of previous weight loss attempts, quality of life (physical and mental composite scores), depressive symptoms, number of prescription medications, and weekly alcohol consumption.
Figure 3.
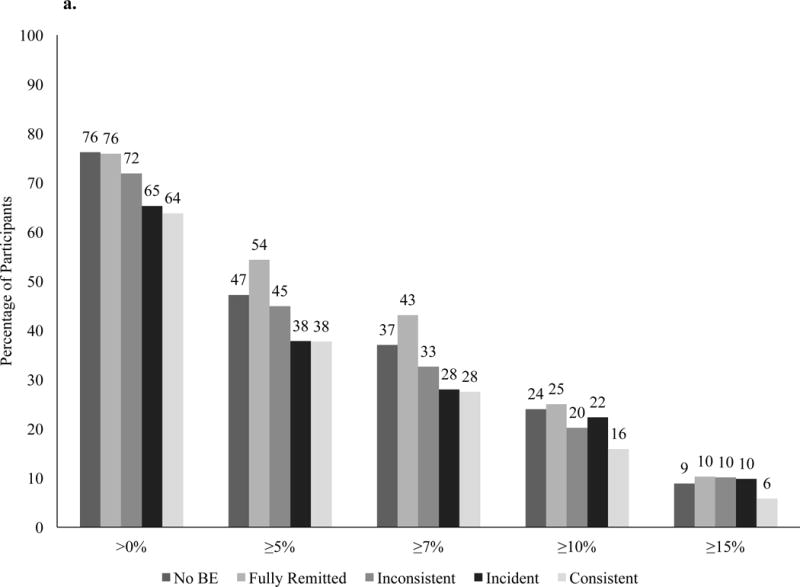
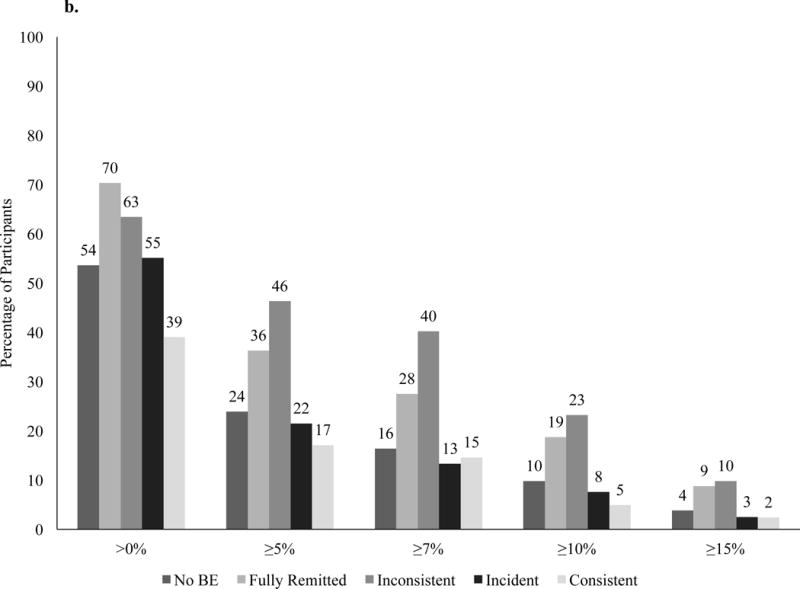
Percentage of participants in the intensive lifestyle intervention (a) and diabetes support and education (b) groups who at year 4 met different categorical weight losses.
DSE
At year 4, participants in the DSE group who did not report BE at any time lost 0.9 ±0.2% of initial weight, which was significantly smaller than the 3.8 ±0.9% lost by participants with fully remitted BE (p=0.001) and 3.3 ±1.0 lost by individuals with inconsistent BE (p=0.02; Figure 4). Participants who reported incident BE lost 0.8 ±0.7% and those with consistent BE gained 1.3 ±1.4%, which was significantly worse than participants with fully remitted BE (ps=0.007 and 0.002, respectively). Percent weight loss in participants with no BE was not significantly different from individuals with incident BE or those with consistent BE. Categorical percent weight losses differed by BE trajectory at >0% (p=0.003), ≥5% (p<0.001), ≥7%, ≥10% (p<0.001), and ≥15% of initial weight (p=0.01; Figure 3b).
Figure 4.
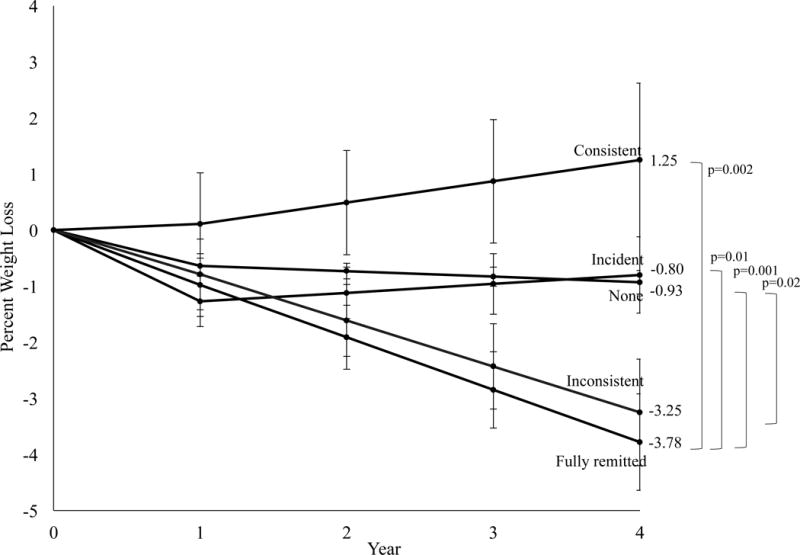
Percent reduction in initial weight in the diabetes support and education (DSE) group by binge eating (BE) trajectory. Values shown are mean (±standard error) and weight losses were estimated using linear mixed-effects models controlling for baseline age, race/ethnicity, sex, education level, body mass index, number of previous weight loss attempts, quality of life (physical and mental composite scores), depressive symptoms, number of prescription medications, and weekly alcohol consumption.
Binge Eating Behavior and Treatment Adherence
During the first year, ILI participants with baseline BE attended a mean (±standard deviation) of 34.4 ±8.3 of a possible 42 sessions, which was not different from the 34.8 ±8.2 sessions attended by participants without baseline BE. Mean treatment contacts per year during years 2–4 did not differ significantly by BE trajectory group (p=0.06). Individuals without BE had 20.2 ±10.9 contacts, those with fully remitted BE had 17.8 ±10.2 contacts, and individuals with incident BE, inconsistent BE, and consistent BE had 21.1 ±10.1, 19.4 ±8.8, and 18.8 ±9.2, respectively.
Discussion
The current study assessed the effects of BE on 4-year weight losses in participants in the ILI and DSE groups. Across treatment arms, retention was high and similar among participants with and without baseline BE (88.1% vs 89.0%). Treatment attendance did not differ by BE trajectory. As hypothesized, participants in the ILI who consistently reported BE lost approximately half as much weight at year 4 as individuals who reported full remission of their BE after baseline, or those who did not report such eating at any time. Those who developed BE during the ILI also had attenuated weight loss compared to people without BE. Modest weight loses of ≥5% produce clinically significant improvements in metabolic risk factors.30 While 47% of participants without BE achieved a ≥5% weight loss, only 38% of those with incident BE and 38% of individuals with consistent BE attained this goal. Participants who had full remission of BE lost similar amounts of weight as those who did not report BE at any time. More than half (54%) of individuals with fully remitted BE had a ≥5% weight loss at year 4.
The present findings support and extend previous results that participants who achieved remission from BE lost more weight that those who continued to binge eat.9,14–16 Consistent with previous studies of shorter durations9,14–16 and of patients who had bariatric surgery,31,32 participants who continued or developed BE had smaller long-term weight losses than those without BE. These results suggest that individuals with preexisting BE should not be excluded or discouraged from engaging in behavioral weight loss. However, we believe that BE should be assessed regularly during weight loss. Participants who report BE after baseline assessments may benefit from additional treatment, such as cognitive behavioral therapy, to promote BE remission.11 Cognitive behavioral therapy does not tend to produce weight loss, yet it does help to promote weight stabilization and prevent future weight gain.33 For individuals who continue to binge eat after a lifestyle intervention, a sequential or additive approach of behavioral weight loss followed by cognitive behavioral therapy may improve weight loss maintenance.
There was a trend at year 4 towards greater cumulative BE incidence in the ILI than in the DSE group, consistent with concerns that some investigators have raised.3 It is possible that in a subset of individuals, ILI increases rigid and extreme dietary restraint, which may result in feelings of deprivation and subsequent BE. Among these individuals, enhancing flexible dietary restraint might prevent BE and improve weight loss.34 Another explanation is that the ILI group, as a result of education regarding calories and portion sizes, may have become more aware of what constituted a large amount of food, as compared with DSE participants. This awareness may have led participants to use a more accurate standard in assessing when they had eaten a large amount of food.35 Further study is needed to examine these hypotheses.
Similar to previous results, we found that higher BE incidence was significantly associated with a greater number of previous weight loss attempts,36 younger age, and worse baseline physical and mental health-related quality of life.37 These factors may be useful in identifying who is at greatest risk for developing BE during ILI. However, effect sizes were modest, potentially reflecting the overall low incidence of BE in this sample. Additionally, lifetime history of BE was not assessed. It is possible that individuals with incident BE during ILI may have had BE before they were assessed at baseline.
Contrary to our hypothesis, we found minimal long-term effects of the ILI on BE remission relative to DSE. At 1 year, the majority of individuals who reported BE at baseline no reported longer this behavior (67.6% ILI vs 65.9% DSE).9 However, at year 4, the percentage of participants with full BE remission dropped to 46.0% of ILI participants and 46.7% of the DSE participants. This supports literature that the durability of BE remission after behavioral weight loss may not be sustained over time.38 Specialty treatment for BE may be necessary to attain long-term remission.
There are several limitations to this study. A self-report measure of BE was used to facilitate efficient and cost-effective data collection. However, inconsistencies between self-reported versus interview-assessed BE behavior have been noted and interview-assessments are generally thought to be more accurate.39 We created trajectories based on BE instead of full threshold BED due to the small percentage of participants who met full criteria. However, groupings based on BE behavior identified individuals at risk for suboptimal weight loss during ILI. Generalizability of these findings may be limited due to the study participant characteristics and procedures. Participants were 45–76 years of age, overweight with type 2 diabetes and recruited for a weight loss study. Since the peak age of onset of BE is late adolescence to early adulthood,37 the participants’ ages may have accounted for the low prevalence of BED and low incidence of BE. There may have been a “healthy volunteer effect”.40 Individuals interested in participating in an 11-year study may be more health conscious than people with diabetes in the general population. Since all participants had type 2 diabetes, results may not generalize to all individuals with overweight or obesity.
In conclusion, new or persistent BE was associated with attenuated 4-year weight loss outcomes in participants with overweight/obesity and type 2 diabetes. Individuals with new or persistent BE may need additional treatment during weight management to improve outcomes. Further research is needed to examine the efficacy of such interventions in improving BE abstinence rates and weight loss success.
What is known about this subject?
Studies have yielded mixed findings concerning whether intensive lifestyle interventions (ILI) for weight reduction precipitate binge eating (BE) and whether BE attenuates weight loss.
Most studies have been of short duration (i.e., ≤1 year).
What does this study add?
ILI participants were marginally more likely to report incident BE at year 4 than were those in diabetes support and education (DSE) control group.
At year 4, ILI participants with no BE lost significantly more weight (4.6 ±0.2%) than those with incident BE (3.1 ±0.6%; p=0.02) and those with consistent BE (1.9 ±1.0%; p=0.01).
These findings suggest that individuals with incident or persistent BE during ILI may have suboptimal long-term weight loss.
Acknowledgments
Funding: This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. AMC was supported by an NRSA postdoctoral fellowship from the NINR/NIH #T32NR007100. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); and the Frederic C. Bartter General Clinical Research Center (M01RR01346) The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. The analyses performed herein were not conducted at the Look AHEAD Data Coordinating Center. This does not represent the work of the Look AHEAD study group.
Dr. Chao reports grant support from Shire Pharmaceuticals, outside the submitted work. Dr. Wadden reports grants and personal fees from Novo Nordisk, personal fees from Weight Watchers, grants from Eisai Pharmaceutical, outside the submitted work. Dr. Shaw Tronieri reports personal fees from Novo Nordisk, outside the submitted work. Dr. Yanovski reports that her spouse receives research project support from Rhythm Pharmaceuticals. Dr. Berkowitz reports grant support from Novo Nordisk and Eisai Inc. and is a scientific consultant for Eisai Inc, outside the submitted work.
Footnotes
Clinical Trial Registry Name: Look AHEAD: Action for Health in Diabetes (LookAHEAD)
Registration ID Number: NCT00017953
URL for Registry: https://clinicaltrials.gov/ct2/show/NCT00017953
Disclosures: All other authors have no conflicts of interest to declare.
References
- 1.Wing RR, Espeland MA, Clark JM, et al. Association of weight loss maintenance and weight regain on 4-year changes in CVD risk factors: the Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care. 2016;39(8):1345–1355. doi: 10.2337/dc16-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. The Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polivy J, Herman CP. Dieting and binging: a causal analysis. Am Psychol. 1985;40(2):193. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Howard CE, Porzelius LK. The role of dieting in binge eating disorder: etiology and treatment implications. Clin Psychol Rev. 1999;19(1):25–44. doi: 10.1016/s0272-7358(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 5.Spurrell EB, Wilfley DE, Tanofsky MB, Brownell KD. Age of onset for binge eating: are there different pathways to binge eating? Int J Eat Disord. 1997;21(1):55–65. doi: 10.1002/(sici)1098-108x(199701)21:1<55::aid-eat7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Grilo C, Masheb R. Onset of dieting vs binge eating in outpatients with binge eating disorder. Int J Obes. 2000;24(4):404–9. doi: 10.1038/sj.ijo.0801171. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatry Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 8.Yanovski SZ, Billington CJ, Epstein LH, et al. Dieting and the development of eating disorders in overweight and obese adults. Arch Intern Med. 2000;160(17):2581–2589. doi: 10.1001/archinte.160.17.2581. [DOI] [PubMed] [Google Scholar]
- 9.Gorin AA, Niemeier HM, Hogan P, et al. Binge eating and weight loss outcomes in overweight and obese individuals with type 2 diabetes: results from the Look AHEAD trial. Arch Gen Psychiatry. 2008;65(12):1447–1455. doi: 10.1001/archpsyc.65.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, Foster GD, Sarwer DB, et al. Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. The American Journal of Clinical Nutrition. 2004;80(3):560–568. doi: 10.1093/ajcn/80.3.560. [DOI] [PubMed] [Google Scholar]
- 11.Berkman ND, Brownley KA, Peat CM, et al. Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2015. Management and outcomes of binge-eating disorder. [PubMed] [Google Scholar]
- 12.Munsch S, Biedert E, Meyer A, et al. A randomized comparison of cognitive behavioral therapy and behavioral weight loss treatment for overweight individuals with binge eating disorder. Int J Eat Disord. 2007;40(2):102–113. doi: 10.1002/eat.20350. [DOI] [PubMed] [Google Scholar]
- 13.Blaine B, Rodman J. Responses to weight loss treatment among obese individuals with and without BED: a matched-study meta-analysis. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. 2007;12(2):54–60. doi: 10.1007/BF03327579. [DOI] [PubMed] [Google Scholar]
- 14.Masheb RM, Lutes LD, Myra Kim H, et al. High-frequency binge eating predicts weight gain among veterans receiving behavioral weight loss treatments. Obesity. 2015;23(1):54–61. doi: 10.1002/oby.20931. [DOI] [PubMed] [Google Scholar]
- 15.Pacanowski CR, Senso MM, Oriogun K, Crain AL, Sherwood NE. Binge eating behavior and weight loss maintenance over a 2-year period. J Obes. 2014;2014 doi: 10.1155/2014/249315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladis MM, Wadden TA, Vogt R, Foster G, Kuehnel RH, Bartlett SJ. Behavioral treatment of obese binge eaters: do they need different care? J Psychosom Res. 1998;44(3):375–384. doi: 10.1016/s0022-3999(97)00262-6. [DOI] [PubMed] [Google Scholar]
- 17.Delinsky SS, Latner JD, Wilson GT. Binge eating and weight loss in a self-help behavior modification program. Obesity. 2006;14(7):1244–1249. doi: 10.1038/oby.2006.141. [DOI] [PubMed] [Google Scholar]
- 18.Sherwood N, Jeffery R, Wing R. Binge status as a predictor of weight loss treatment outcome. Int J Obes. 1999;23(5):485–493. doi: 10.1038/sj.ijo.0800846. [DOI] [PubMed] [Google Scholar]
- 19.Bruce B, Wilfley D. Binge eating among the overweight population: a serious and prevalent problem. J Am Diet Assoc. 1996;96(1):58–61. doi: 10.1016/S0002-8223(96)00016-8. [DOI] [PubMed] [Google Scholar]
- 20.Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;2013(369):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Look AHEAD Research Group. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diabetes and Vascular Disease Research. 2006;3(3):202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 23.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Look AHEAD Research Group. The development and description of the comparison group in the Look AHEAD trial. Clinical Trials. 2011;8(3):320–29. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer R, Yanovski S, Marcus M. The Questionnaire on Eating and Weight Patterns-Revised (QEWP-R) New York: New York State Psychiatric Institute; 1993. [Google Scholar]
- 26.Beck AT, Steer R, Brown G. Beck Depression Inventory Manual. Vol. 4. San Antonio, TX: The Psychological Corporation; 1987. pp. 561–571. Harcourt Brace Jovanovich Beck, AT, Ward, CH, Mendelson, M, Mock, J, & Erbaugh, J(1961) An inventory for measuring depression Archives of General Psychiatry. [Google Scholar]
- 27.Ware JE, Keller SD, Kosinski M. SF-36: Physical and mental health summary scales: A user’s manual. Health Assessment Lab; 1994. [Google Scholar]
- 28.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallop R, Tasca GA. Multilevel modeling of longitudinal data for psychotherapy researchers: II. The complexities. Psychotherapy Research. 2009;19(4–5):438–452. doi: 10.1080/10503300902849475. [DOI] [PubMed] [Google Scholar]
- 30.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao AM, Wadden TA, Faulconbridge LF, et al. Binge-eating disorder and the outcome of bariatric surgery in a prospective, observational study: two-year results. Obesity. 2016;24(11):2327–2333. doi: 10.1002/oby.21648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivezaj V, Kessler EE, Lydecker JA, Barnes RD, White MA, Grilo CM. Loss-of-control eating following sleeve gastrectomy surgery. Surg Obes Relat Dis. 2017;13(3):392–398. doi: 10.1016/j.soard.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masheb RM, White MA, Grilo CM. Substantial weight gains are common prior to treatment-seeking in obese patients with binge eating disorder. Compr Psychiatry. 2013;54(7):880–884. doi: 10.1016/j.comppsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Downe KA, Goldfein JA, Devlin MJ. Restraint, hunger, and disinhibition following treatment for binge-eating disorder. Int J Eat Disord. 2009;42(6):498–504. doi: 10.1002/eat.20639. [DOI] [PubMed] [Google Scholar]
- 35.Yanovski SZ, Gormally JF, Leser MS, Gwirtsman HE, Yanovski JA. Binge eating disorder affects outcome of comprehensive very-low-calorie diet treatment. Obesity. 1994;2(3):205–212. doi: 10.1002/j.1550-8528.1994.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 36.Marchesini G, Cuzzolaro M, Mannucci E, et al. Weight cycling in treatment-seeking obese persons: data from the QUOVADIS study. Int J Obes. 2004;28(11):1456–1462. doi: 10.1038/sj.ijo.0802741. [DOI] [PubMed] [Google Scholar]
- 37.Kessler RC, Berglund PA, Chiu WT, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73(9):904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67(1):94–101. doi: 10.1001/archgenpsychiatry.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg KC, Peterson CB, Frazier P, Crow SJ. Convergence of scores on the interview and questionnaire versions of the Eating Disorder Examination: a meta-analytic review. American Psychological Association; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allison KC, Crow SJ, Reeves RR, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity. 2007;15(5):1287–1293. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


