Abstract
The ever-increasing prevalence of metabolic diseases such as dyslipidemia and diabetes in the western world continues to be of great public health concern. Biologically active sphingolipids, such as sphingosine 1-phosphate (S1P) and ceramide, are important regulators of lipid metabolism. S1P not only directly functions as an active intracellular mediator, but also activates multiple signaling pathways via five transmembrane G-protein coupled receptors (GPCRs), S1PR1-5. S1P is exclusively formed by sphingosine kinases (SphKs). Two isoforms of SphKs, SphK1 and SphK2, have been identified. Recent identification of the conjugated bile acid-induced activation of S1PR2 as a key regulator of SphK2 opened new directions for both the sphingolipid and bile acid research fields. The role of SphKs/S1P-mediated signaling pathways in health and various human diseases has been extensively reviewed elsewhere. This review focuses on recent findings related to SphKs/S1P-medaited signaling pathways in regulating hepatic lipid metabolism.
Keywords: Sphingolipids, Sphingosine 1-phosphate, Sphingosine kinase, G protein-coupled receptor, Hepatic lipid metabolism, Metabolic diseases
2. Introduction
Sphingosine forms the backbone of most sphingolipids and was initially recognized as a component of the plasma membrane lipid bilayer. The advent of large scale data analyses including genomics and proteomics paved way for the identification of many regulatory receptors and enzymes involved in sphingolipid metabolism (1). Since then, sphingosine and its derivative sphingolipids have emerged as important signaling molecules in the regulation of different biological processes including migration, differentiation, cell survival and lipid metabolism. The phosphorylated derivative of sphingosine, sphingosine 1-phosphate (S1P), has attracted the attention of investigators for its potency as an activator of cell signaling and regulator of cell survival, growth, and immune cell trafficking which are important in inflammatory diseases and various cancers (2, 3). In addition, studies with genetically modified mouse models have provided physiological insight into the functions of sphingolipids and have yielded many important advances in the understanding of the role of sphingolipid-mediated signaling pathways in various human diseases, including inflammation, cancer, pulmonary arterial hypertension, diabetes, nonalcoholic fatty liver disease (NAFLD), and metabolic diseases (4–6)
S1P represents a key signaling sphingolipid molecule. It is exclusively formed by the phosphorylation of sphingosine via the action of sphingosine kinases (SphKs). Two major isoforms of SphK (SphK1 and SphK2) have been isolated and characterized. SphK1 and SphK2 have diverse and compensatory biological activities. S1P is not only an intracellular messenger, but also a natural ligand for five specific cell surface G-protein-coupled receptors (GPCRs). During the last two decades, numerous agonists and antagonists of S1P receptors (S1PRs) and chemical inhibitors of SphKs have been developed. One notable discovery is FTY720 (fingolimod), a modulator of the S1PRs except S1PR2, has been approved for the treatment of multiple sclerosis (7). However, the role of SphKs and S1P in lipid metabolism remains largely unknown. In this review, we will focus on the current understanding of the role of SphKs/S1P-mediated signaling pathways in regulating hepatic lipid metabolism and the development of potential therapeutics for the treatment of metabolic disorders by targeting SphKs and S1PRs.
3. Sphingosine Lipid Metabolism
Sphingolipids are lipids that contain the sphingoid backbone and can be N-acylated to form ceramide which occupies a central position in the biosynthetic pathway of sphingolipid metabolism (8). The first step in de novo synthesis of sphingolipids is the condensation of serine and palmitoyl CoA by the enzyme serine palmitoyltransferase to form 3-ketosphinganine. This reaction takes place in the endoplasmic reticulum (ER) and represents the rate-limiting step of this biosynthetic pathway. Subsequent reactions involve the N-acylation of 3-ketosphinganine and reduction to sphinganine. Sphinganine is further fatty acylated by ceramide synthesis to generate dihydroceramide followed by desaturation by dihydroceramide desaturase to form ceramide, which is the precursor of the majority of complex sphingolipids (9). Ceramide is membrane-bound molecule with very low aqueous solubility. It requires transport from ER to the Golgi complex by ceramide transfer protein or vehicular transport, where it serves as a substrate for the production of sphingolipids such as glycosphospholipids, sphingomyelin and sphingosine. In addition, ceramide can be converted to an important cell signaling molecule, ceramide 1-phosphate, by ceramide kinase. Furthermore, sphingosine can be converted to S1P by SphKs (10, 11).
4. Sphingosine Kinases
SphKs belong to the class of lipid kinases that contain five conserved domains and are evolutionarily conserved. Two mammalian isoforms have been identified, SphK1 and SphK2 (12). Human SphKs are encoded by two distinct genes, SPHK1 and SPHK2, which are located on chromosome 17 and 19, respectively (13). Human SphK1 and SphK2 share 80% similarity in amino acid sequence and 50% similarity in nucleotide sequence identity. SphK2 has 200 additional amino acids at the N-terminal region, containing a nuclear targeting sequence. The predicted molecular weights for SphK1 and SphK2 are 42 kDa and 68 kDa, respectively (14). SphK1 is highly expressed in cells in the lung and spleen, whereas SphK2 is more abundant in liver, kidney, brain and heart (15). Moreover, the intracellular localization of SphK1 and SphK2 is closely linked to their physiological functions. SphK1 predominately resides in the cytoplasm under normal physilogical conditions and upon various stimuli such as activation of mitogen-activated protein kinase (MAPK) by cytokines and growth factors, SphK1 is activated and translocated from the cytosol to the plasma membrane to carry out its catalytic conversion of sphingosine to S1P. Activation of SphK1 is associated with promotion of cell proliferation, survival, migration, differentiation, angiogenesis and inflammation (16–18). In contrast, SphK2 is mainly localized in the nucleus (19). Nuclear S1P produced by SphK2 has been shown to inhibit histone deacetylases (HDAC1/2) activity, leading to increased histone acetylation and increased gene transcriptional activity (20). The subcellular localization of SphK2 and its function are less well-studied. In addition, SphK2 is also found in mitochondria, where it binds with high affinity and specificity to prohibitin 2, a highly-conserved protein that regulates mitochondrial assembly and function (21). Activation of SphK2 has also been shown to increase mitochondrial membrane permeability and promote cytochrome c release (22, 23).
5. Sphingosine 1-Phosphate Signaling
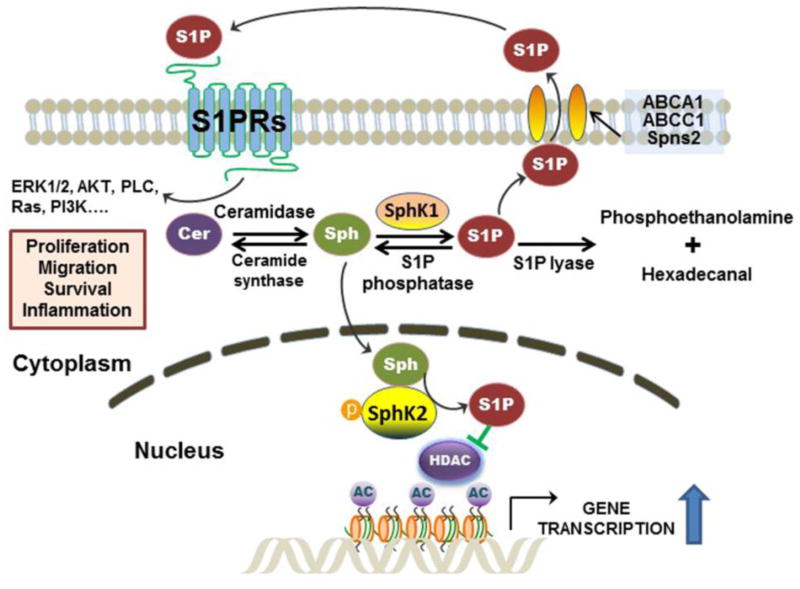
Sphingosine 1-phosphate (S1P) is a simple sphingolipid but potent activator of cellular signaling pathways. S1P plays a role in various cellular processes including cell proliferation, differentiation, angiogenesis, inflammation and cancer (24). Like most signaling molecules, intracellular S1P level is tightly regulated by its synthesis and degradation. S1P is exclusively synthesized via phosphorylation of the 1-hydroxyl group on sphingosine either by SphK1 or SphK2 in response to diverse stimuli, including inflammatory cytokines, growth factors, and activation of GPCRs. S1P can be converted back to sphingosine by S1P specific phosphatase in the cytosol or degraded by S1P lyase to ethanolamine phosphate and hexadecanal (25). Unlike sphingosine, which is sufficiently hydrophobic to diffuse cross membranes, S1P is a more hydrophilic molecule, which requires specific transporters to be exported to the extracellular space. Several membrane-associated transporters have been identified as active S1P transporters, including ATP-binding cassette (ABC) transporters, ABCA1 and ABCC1, and spinster homologue 2 (Spns2) (26–29). The exported S1P can activate the S1P-specific GPCRs on the cell membrane to induce various physiological responses (Figure 1) (30).
Figure 1. Biosynthetic and signaling pathways of sphingosine 1-phosphate (S1P).
S1P is synthesized by phosphorylation of sphingosine (Sph), which is exclusively mediated by sphingosine kinase 1 (SphK1) in the cytosol and Sphk2 in the nucleus. Cytosolic S1P can be exported by transporters (ABCA1, ABCC1, and Spns2) and activates GPCRs (S1PR1-5) on the cell surface. S1P also can be dephosphorylated by S1P phosphatase back to Sph for ceramide (Cer) synthesis or further degraded by S1P lyase into phosphoethanolamine and hexadecenal. Both SphK1 and SphK2 can be activated by ERK. Nuclear S1P generated by SphK2 inhibits HDAC1/2 activity, which results in increase of histone acetylation and up-regulation of gene transcription.
Identification of the S1P-specific GPCRs represents an important milestone in understanding S1P-mediated biological functions. Since the discovery of the first S1PR, S1PR1 (formerly named as EDG1) in 1998 (31), a total of five GPCRs have been identified as S1P-specific receptors (S1PR1-5) (20, 32–34). S1PRs are differentially expressed in different tissues and the expression levels vary under different physiological and pathological conditions (35). S1PR1 is ubiquitously expressed and deletion of S1PR1 is embryonically lethal (36). S1PR1 has been shown to play a key role in immune cell trafficking and angiogenesis (37, 38). S1PR2 is important for the development of the auditory and vestibular system (39). Unlike S1PR1, deletion of S1PR2 is not lethal, however mice deficient in S1PR2 have been shown to develop spontaneous seizures (40). S1PR3 is highly expressed in the brain, heart, lung, spleen, kidney, liver, intestine and skeletal muscle (41). In addition, it plays a role in vascular endothelial function and lung barrier integrity (42). S1PR4 is expressed in leukocytes and regulates T cell cytokine production (43). S1PR5 is highly expressed in oligodendrocytes, however the function of S1PR5 remains unknown (44). The various biological functions of S1P in different cells and tissues are largely due to the different expression patterns of S1PR subtypes and the various G proteins they couple with (45). The crystal structure of S1PR1 made a significant advancement in the understanding of S1P-mediated signaling (46). The S1P receptor subtype-specific agonists and antagonists have become novel therapeutic candidates for various diseases (47, 48).
6. SphKs and S1P in Lipid Metabolism
Dyslipidemia associated with metabolic diseases is complex and involves dysregulation of metabolic pathways of various lipid species (9, 49). In addition to phospholipid metabolites, sphingolipid metabolites are also involved in the regulation of metabolic lipid homeostasis (9). The function of SphKs in regulating cell proliferation, differentiation and migration as well as the inflammatory response has been studied extensively, but only until recently have studies demonstrated an eminent role for SphKs in regulating lipid metabolism (50–53). S1P is present in the circulation and bile and mainly associated with high density lipoproteins (HDL) and albumin (24). HDL-mediated release of S1P has been shown to have protective effects against atherosclerosis (54, 55). S1P-mediated activation of the inflammatory response represents a key event in atherosclerotic disease progression. Both pharmacologic inhibition and genetic silencing of S1PR2 attenuated atherosclerotic lesion formation in apolipoprotein E knockout mice (ApoE−/−) (56). Interestingly, several studies have shown that FTY720, a synthetic S1P analogue, reduces atherosclerosis in rodent models (57–61). A recent study has shown that the SphK1/S1P axis plays a critical role in hypoxia-mediated pulmonary hypertension (HPH). Pharmacological inhibition of SphK1 and S1PR2 or genetic deletion of SphK1 prevented the development of HPH in rodent HPH models (62). FTY720 is also found to reduce cholesterol and sphingolipid accumulation in Niemann-Pick type C (NPC) mutant fibroblasts by upregulating the expression of NPC1 and NPC2 and reducing cholesterol accumulation (63).
Obesity is closely associated with diabetes, nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH), and cardiovascular diseases (64). Numerous studies have reported that dysregulation of sphingolipid metabolism is linked to diabetes (4). However, most studies in this area are focused on ceremide (65–67) and only a few studies have examined the involvement of SphKs/S1P signaling in obesity-related diseases. It has been reported that the expression of SphK1, but not SphK2, is elevated in mouse 3T3-L1 adipocytes during adipogenesis and in ob/ob mouse adipose tissue (68). Recently, several studies reported that SphK/S1P-signaling plays an important role in hepatic lipid metabolism and insulin resistance (4, 50,52, 53, 69). However, there is a discrepancy regarding the role of SphK1 in the regulation of hepatic lipid metabolism in recent studies. NAFLD is characterized by aberrant accumulation of lipids in hepatocytes. Hepatic lipid homeostasis is controlled by the balance of hepatic fatty acid synthesis, dietary fat intakes, adipocyte lipolysis, fatty acid oxidation, and secretion of hepatic lipids (70). Kowalski, et al. reported that high-fat-high-glucose diet feeding resulted in accumulation of hepatic lipids and the reduction of total hepatic SphK activity, which was correlated to the down-regulation of SphK1, but not SphK2 (53). However, hepatic overexpression of SphK1 only reduced hepatic triglycerides in low-fat-diet-fed mice, but not in high-fat-diet-fed mice. SphK1/S1P-mediated signaling has been suggested to promote hepatic steatosis and inflammation. Expression of hepatic SphK1 is elevated in both high-fat-high-glucose-fed mice and human NASH patients (71). SphK1−/− mice were protected from high-fat-high-glucose diet-induced hepatic inflammation and lipid accumulation (71). Interestingly, another recent study done by Chen, et al reported that deletion of SphK1 ameliorated hepatic steatosis in high-fat-diet fed mice by the down-regulation of peroxisome proliferator-activated receptor gamma (PPARγ) expression in the liver (50). Furthermore, SphK1/S1P-mediated pro-steatotic effect is dependent on S1PR2/S1PR3, not S1PR1 (50). To solve these discrepancies, more in-depth mechanistic approaches will be needed, including liver/hepatocyte-specific deletion and overexpression of SphK1. In contrast to SphK1, the physiological and pathological function of SphK2 is less well-characterized. The role of SphK2 in regulating immune cell function and the inflammatory response is controversial in different disease settings (72, 73). Specific chemical inhibitors of SphK2 have been developed as potential tumor suppressors (74–78). Our recent study demonstrated that SphK2 is a key regulator of hepatic lipid metabolism (51). Both S1PR2−/− and SphK2−/− mice fed on a high-fat-diet rapidly develop overt fatty livers compared to wild type mice. Interestingly, key lipid metabolism genes such as SREBP-1c, FAS, LDLR, FXR and PPARγ are significantly downregulated in both S1PR2−/− and SphK2−/− mice (51). Our recent RNA-seq data further indicated that overexpression of S1PR2 significantly upregulated the expression of SphK2 and key genes in hepatic lipid metabolism (unpublished data). Similarly, another study reported by Lee, et al indicated that activation of SphK2 by endoplasmic reticulum (ER) stress ameliorates hepatic steatosis and insulin resistance (52). High fat diet-induced ER stress results in the upregulation of SphK2 through the activation of ATF4. Consistent with our findings, this study demonstrated that SphK2 is an important regulator of hepatic fatty acid metabolism. In addition, SphK2-medaited activation of AKT signaling pathways protects mice against high-fat-diet-induced glucose intolerance and insulin resistance (52).
7. Bile acid receptors in hepatic lipid metabolism
1) Nuclear receptors
Bile acids are important signaling molecules and play multiple physiological functions including nutrient absorption, regulation of cholesterol, glucose and fatty acid metabolism, maintenance of microbiome homeostasis and intestinal barrier integrity by activating nuclear receptors (79). The FXR and small heterodimer partner (SHP; NR0B2) are the most well-characterized nuclear receptors which play crucial roles in bile acid homeostasis (80, 81). It has been reported that global FXR knock out mice develop liver tumors spontaneously (82). Recent study further showed that liver-specific FXR knock out mice are resistant to spontaneous hepatocarcinogenesis, but susceptible to cholic acid-induced tumor formation (83). However, SHP knock out mice are resistant to bile acid-induced liver damage (84). Furthermore, double knock out mice of FXR and SHP develop more severe cholestasis and liver injury associated with dysregulation of steroid biosynthesis (85). In addition, FXR and SHP also play important role in regulating hepatic glucose and fatty acid metabolism as well as inflammatory response (79).
2) G protein coupled receptors, TGR5 and S1PR2
Discovery of TGR5, as the first bile acid-specific GPCR, was an important milestone in bile acid research (86). TGR5 is widely expressed including the liver, but absent in hepatocytes. Extensive studies have been done to identify the physiological functions of TGR5 during the last decade (87, 88). However, the role of TGR5 in regulating hepatic lipid metabolism is limited. Identification of conjugated bile acids as activators of S1PR2 opened a new direction for bile acid research (89). S1PR2 is highly expressed in hepatocytes, cholangiocytes and Kupffer cells and is activated by conjugated bile acids, such as taurocholic acid (TCA) (89). TCA-mediated activation of S1PR2 further activates the ERK1/2 and AKT signaling pathways, which are important cellular pathways involved in regulating lipid and glucose metabolism (89, 90). In a chronic bile fistula rat model, infusion of TCA not only activated AKT and ERK1/2 signaling pathway along with glycogen synthase kinase, but also upregulated the expression of SphK2 (51, 91). It has been shown that ERK1/2-mediated activation of SphK2 results in production of S1P in the nucleus, which specifically binds to HDAC1 and HDAC2 and enhances the histone acetylation and gene transcription (20). These studies suggest that cross-talk between bile acids and S1PR2/SphK/S1P-mediated signaling pathways plays a pivotal role in regulating hepatic lipid metabolism. In addition, recent studies showed that conjugated bile acid-mediated activation of S1PR2 is responsible for invasive growth of cholangiocarcinoma cells and bile duct ligation-induced cholestatic liver injury (92–94).
8. Conclusion and Future Perspectives
There have been significant advances in understanding the role of SphKs/S1P-mediated signaling pathways in various human diseases during the last decade. The current understanding of cross-talk between SphKs/S1P-mediated signaling pathways and bile acid-mediated signaling pathways in the regulation of hepatic lipid and glucose metabolism has opened a new direction for the future of sphingolipid and bile acid research. The development of tissue or cell-specific transgenic mice for SphKs and S1PRs is necessary for elucidating the key mechanisms underlying S1P/bile acid-mediated regulation of hepatic lipid metabolism under different physiological and pathological conditions. In addition, resolution of the crystal structures of SphKs and individual S1PRs will enable us to develop more specific activators/inhibitors with less off-target effects as potential novel therapeutics for metabolic disorders.
Acknowledgments
We would like to acknowledge the funding support by National Institutes of Health Grant R01 DK104893 (to HZ and PBH), R01DK-057543 (to PBH and HZ), VA Merit Award I01BX001390 (to HZ); National Natural Science Foundation of China Grants 81070245 and 81270489 (to H.Z.); Massey Cancer Center pilot grant (to HZ and PBH).
Abbreviations
- ABC
ATP-binding cassette
- AKT
protein kinase B
- ApoE
apolipoprotein E
- ATF4
activating transcription factor 4
- EDG1
endothelial differentiation gene 1
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- FAS
fatty acid synthase
- FXR
farnesoid × receptor
- GPCRs
G-protein coupled receptors
- HDAC
histone deacetylases
- HDL
high density lipoprotein
- HPH
hypoxia-mediated pulmonary hypertension
- LDLR
low-density lipoprotein receptor
- MAPK
mitogen-activated protein kinase
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NPC
Niemann-Pick type C
- ob/ob
leptin-deficient
- PPARγ
proliferator-activated receptor gamma
- S1P
sphingosine 1-phosphate
- SHP
small heterodimer partner
- Sph
sphingosine
- SphKs
sphingosine kinases
- Spns2
spinster homologue 2
- S1PR
sphingosine 1-phosphate receptor
- SREBP-1c
sterol regulating element-binding protein 1
- TCA
taurocholic acid
- TGR5
G-protein coupled bile acid receptor
Footnotes
Disclosure: The authors declare no competing financial interest. All reported studies with animal subjects performed by the authors have been previously published and complied with all applicable ethical standards. All animal study protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
References
- 1.Chen Y, Liu Y, Sullards MC, Merrill AH., Jr An introduction to sphingolipid metabolism and analysis by new technologies. Neuromolecular Med. 2010 Dec;12(4):306–19. doi: 10.1007/s12017-010-8132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008 Feb;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature reviews Immunology. 2011 Jun;11(6):403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetes associated pathologies (Review) International journal of molecular medicine. 2017 Feb;39(2):243–52. doi: 10.3892/ijmm.2017.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Fadel F, Fayyaz S, Japtok L, Kleuser B. Involvement of Sphingosine 1-Phosphate in Palmitate-Induced Non-Alcoholic Fatty Liver Disease. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;40(6):1637–45. doi: 10.1159/000453213. [DOI] [PubMed] [Google Scholar]
- 6.Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. Journal of lipid research. 2016 Sep;57(9):1636–43. doi: 10.1194/jlr.R069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010 Nov;9(11):883–97. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 8.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochimica et biophysica acta. 2003 Jun 10;1632(1–3):16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 9.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nature reviews Endocrinology. 2017 Feb;13(2):79–91. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 10.Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochimica et biophysica acta. 2009 Jul;1791(7):684–91. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Rao RP, Acharya JK. Sphingolipids and membrane biology as determined from genetic models. Prostaglandins & other lipid mediators. 2008 Feb;85(1–2):1–16. doi: 10.1016/j.prostaglandins.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. The Journal of cell biology. 1999 Nov 01;147(3):545–58. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. The Journal of biological chemistry. 2000 Jun 30;275(26):19513–20. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life. 2006 Aug;58(8):467–72. doi: 10.1080/15216540600871126. [DOI] [PubMed] [Google Scholar]
- 15.Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000 Jun 13;251(1):19–26. doi: 10.1016/s0378-1119(00)00205-5. [DOI] [PubMed] [Google Scholar]
- 16.Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. Journal of lipid research. 2009 Apr;50(Suppl):S272–6. doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitson SM, Pebay A. Regulation of stem cell pluripotency and neural differentiation by lysophospholipids. Neurosignals. 2009;17(4):242–54. doi: 10.1159/000231891. [DOI] [PubMed] [Google Scholar]
- 18.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature reviews Cancer. 2010 Jul;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of biological chemistry. 2003 Nov 21;278(47):46832–9. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 20.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009 Sep 04;325(5945):1254–7. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011 Feb;25(2):600–12. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012 Mar 02;148(5):988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. The Journal of biological chemistry. 2003 Oct 10;278(41):40330–6. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 24.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012 Jan;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoffel W, Assmann G. Metabolism of sphingosine bases. XV. Enzymatic degradation of 4t-sphingenine 1-phosphate (sphingosine 1-phosphate) to 2t-hexadecen-1-al and ethanolamine phosphate. Hoppe Seylers Z Physiol Chem. 1970 Aug;351(8):1041–9. doi: 10.1515/bchm2.1970.351.2.1041. [DOI] [PubMed] [Google Scholar]
- 26.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2006 Oct 31;103(44):16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000 Nov;14(14):2255–65. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009 Jan 23;323(5913):524–7. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 29.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. The Journal of clinical investigation. 2012 Apr;122(4):1416–26. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Advances in experimental medicine and biology. 2010;688:141–55. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. The Journal of biological chemistry. 1998 Oct 16;273(42):27104–10. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 32.Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, et al. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Advances in biological regulation. 2016 Jan;60:151–9. doi: 10.1016/j.jbior.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Karimian G, Buist-Homan M, Schmidt M, Tietge UJ, de Boer JF, Klappe K, et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochimica et biophysica acta. 2013 Dec;1832(12):1922–9. doi: 10.1016/j.bbadis.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. British journal of pharmacology. 2014 Aug;171(15):3575–94. doi: 10.1111/bph.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors. 2012 Sep-Oct;38(5):329–37. doi: 10.1002/biof.1030. [DOI] [PubMed] [Google Scholar]
- 36.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochimica et biophysica acta. 2002 May 23;1582(1–3):222–7. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000 Oct;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004 Jan 22;427(6972):355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 39.MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hearing research. 2006 Oct;220(1–2):38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 40.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, et al. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. The European journal of neuroscience. 2001 Jul;14(2):203–9. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 41.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. The Journal of biological chemistry. 2001 Sep 07;276(36):33697–704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 42.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proceedings of the National Academy of Sciences of the United States of America. 2005 Jun 28;102(26):9270–5. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005 Oct;19(12):1731–3. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 44.Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, et al. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116(4):1053–62. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 45.Pyne NJ, Long JS, Lee SC, Loveridge C, Gillies L, Pyne S. New aspects of sphingosine 1-phosphate signaling in mammalian cells. Advances in enzyme regulation. 2009;49(1):214–21. doi: 10.1016/j.advenzreg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012 Feb 17;335(6070):851–5. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SJ, Im DS. Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. Biomolecules & therapeutics. 2017 Jan 01;25(1):80–90. doi: 10.4062/biomolther.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators of inflammation. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Cuenca S, Pellegrinelli V, Campbell M, Oresic M, Vidal-Puig A. Sphingolipids and glycerophospholipids - The "ying and yang" of lipotoxicity in metabolic diseases. Progress in lipid research. 2017 Jan 16;66:14–29. doi: 10.1016/j.plipres.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Wang W, Qi Y, Kaczorowski D, McCaughan GW, Gamble JR, et al. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: Role of PPARgamma. Biochimica et biophysica acta. 2016 Feb;1861(2):138–47. doi: 10.1016/j.bbalip.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015 Apr;61(4):1216–26. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, et al. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015 Jul;62(1):135–46. doi: 10.1002/hep.27804. [DOI] [PubMed] [Google Scholar]
- 53.Kowalski GM, Kloehn J, Burch ML, Selathurai A, Hamley S, Bayol SA, et al. Overexpression of sphingosine kinase 1 in liver reduces triglyceride content in mice fed a low but not high-fat diet. Biochimica et biophysica acta. 2015 Feb;1851(2):210–9. doi: 10.1016/j.bbalip.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Poti F, Ceglarek U, Burkhardt R, Simoni M, Nofer JR. SKI-II--a sphingosine kinase 1 inhibitor--exacerbates atherosclerosis in low-density lipoprotein receptor-deficient (LDL-R−/−) mice on high cholesterol diet. Atherosclerosis. 2015 May;240(1):212–5. doi: 10.1016/j.atherosclerosis.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Poti F, Simoni M, Nofer JR. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P) Cardiovascular research. 2014 Aug 01;103(3):395–404. doi: 10.1093/cvr/cvu136. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. The Journal of clinical investigation. 2010 Nov;120(11):3979–95. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Luk FS, Kim RY, Li K, Ching D, Wong DK, Joshi SK, et al. Immunosuppression With FTY720 Reverses Cardiac Dysfunction in Hypomorphic ApoE Mice Deficient in SR-BI Expression That Survive Myocardial Infarction Caused by Coronary Atherosclerosis. Journal of cardiovascular pharmacology. 2016 Jan;67(1):47–56. doi: 10.1097/FJC.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, Kim RY, Imhof I, Honbo N, Luk FS, Li K, et al. The immunosuppressant FTY720 prolongs survival in a mouse model of diet-induced coronary atherosclerosis and myocardial infarction. Journal of cardiovascular pharmacology. 2014 Feb;63(2):132–43. doi: 10.1097/FJC.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang K, Li SQ, Wang WJ, Liu LS, Jiang YG, Feng PN, et al. Oral FTY720 administration induces immune tolerance and inhibits early development of atherosclerosis in apolipoprotein E-deficient mice. International journal of immunopathology and pharmacology. 2012 Apr-Jun;25(2):397–406. doi: 10.1177/039463201202500209. [DOI] [PubMed] [Google Scholar]
- 60.Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C, et al. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R(−)/(−)) mice. Vascular pharmacology. 2012 Aug 19;57(1):56–64. doi: 10.1016/j.vph.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007 Jan 30;115(4):501–8. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014 Nov 01;190(9):1032–43. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newton J, Hait NC, Maceyka M, Colaco A, Maczis M, Wassif CA, et al. FTY720/fingolimod increases NPC1 and NPC2 expression and reduces cholesterol and sphingolipid accumulation in Niemann-Pick type C mutant fibroblasts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2017 Jan 12; doi: 10.1096/fj.201601041R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2016 Jun;17(6):510–9. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 65.Blachnio-Zabielska AU, Pulka M, Baranowski M, Nikolajuk A, Zabielski P, Gorska M, et al. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. Journal of cellular physiology. 2012 Feb;227(2):550–7. doi: 10.1002/jcp.22745. [DOI] [PubMed] [Google Scholar]
- 66.Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids in health and disease. 2013 Jul 08;12:98. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristensen D, Prats C, Larsen S, Ara I, Dela F, Helge JW. Ceramide content is higher in type I compared to type II fibers in obesity and type 2 diabetes mellitus. Acta diabetologica. 2013 Oct;50(5):705–12. doi: 10.1007/s00592-012-0379-0. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto T, Igarashi J, Kosaka H. Sphingosine kinase is induced in mouse 3T3-L1 cells and promotes adipogenesis. Journal of lipid research. 2009 Apr;50(4):602–10. doi: 10.1194/jlr.M800206-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, et al. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes. 2012 Dec;61(12):3148–55. doi: 10.2337/db12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nature reviews Disease primers. 2015 Dec 17;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 71.Geng T, Sutter A, Harland MD, Law BA, Ross JS, Lewin D, et al. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. Journal of lipid research. 2015 Dec;56(12):2359–71. doi: 10.1194/jlr.M063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. The FEBS journal. 2013 Nov;280(21):5317–36. doi: 10.1111/febs.12314. [DOI] [PubMed] [Google Scholar]
- 73.Xu T, Li L, Huang C, Peng Y, Li J. Sphingosine kinase 2: a controversial role in arthritis. Rheumatology international. 2014 Jul;34(7):1015–6. doi: 10.1007/s00296-013-2831-z. [DOI] [PubMed] [Google Scholar]
- 74.Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget. 2016 Sep 13;7(37):60181–92. doi: 10.18632/oncotarget.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer biology & therapy. 2015;16(8):1194–204. doi: 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer research. 2014 May 15;74(10):2803–15. doi: 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- 77.Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer biology & therapy. 2011 Mar 01;11(5):524–34. doi: 10.4161/cbt.11.5.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding X, Chaiteerakij R, Moser CD, Shaleh H, Boakye J, Chen G, et al. Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget. 2016 Apr 12;7(15):20080–92. doi: 10.18632/oncotarget.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta pharmaceutica Sinica B. 2015 Mar;5(2):135–44. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim KH, Choi S, Zhou Y, Kim EY, Lee JM, Saha PK, et al. Hepatic FXR/SHP Axis Modulates Systemic Glucose and Fatty Acid Homeostasis in Aged Mice. Hepatology. 2017 Apr 05; doi: 10.1002/hep.29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereira-Fantini PM, Lapthorne S, Joyce SA, Dellios NL, Wilson G, Fouhy F, et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. Journal of hepatology. 2014 Nov;61(5):1115–25. doi: 10.1016/j.jhep.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 82.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer research. 2007 Feb 01;67(3):863–7. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 83.Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, et al. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012 Dec;56(6):2344–52. doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. The Journal of biological chemistry. 2003 Nov 07;278(45):44475–81. doi: 10.1074/jbc.M305258200. [DOI] [PubMed] [Google Scholar]
- 85.Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. The Journal of clinical investigation. 2011 Jan;121(1):86–95. doi: 10.1172/JCI42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochemical and biophysical research communications. 2002 Nov 15;298(5):714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 87.Pols TW. TGR5 in inflammation and cardiovascular disease. Biochemical Society transactions. 2014 Apr;42(2):244–9. doi: 10.1042/BST20130279. [DOI] [PubMed] [Google Scholar]
- 88.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014 Apr;46(4):302–12. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012 Jan;55(1):267–76. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y, et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. Journal of lipid research. 2010 Aug;51(8):2234–44. doi: 10.1194/jlr.M004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB, et al. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Molecular pharmacology. 2007 Apr;71(4):1122–8. doi: 10.1124/mol.106.032060. [DOI] [PubMed] [Google Scholar]
- 92.Liu R, Li X, Qiang X, Luo L, Hylemon PB, Jiang Z, et al. Taurocholate Induces Cyclooxygenase-2 Expression via the Sphingosine 1-phosphate Receptor 2 in a Human Cholangiocarcinoma Cell Line. The Journal of biological chemistry. 2015 Dec 25;290(52):30988–1002. doi: 10.1074/jbc.M115.668277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014 Sep;60(3):908–18. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017 Jan 24; doi: 10.1002/hep.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]