Abstract
Objective
To examine the long-term effects of exercise modality during weight loss on body composition and associations between body composition and physical function changes.
Methods
249 older adults (66.9±4.7 years, 71% women, 32% African American, BMI: 34.4±3.7 kg/m2) were randomized to weight loss (WL; n=82), WL plus aerobic training (WL+AT; n=86), or WL plus resistance training (WL+RT; n=81) for 18-months. DXA-acquired body composition, 400-m walk time, and knee extensor strength were measured at baseline, 6-, and 18-months.
Results
Total body mass loss was enhanced when WL was combined with exercise (WL: −5.7±0.7 kg, WL+AT: −8.5±0.7 kg, WL+RT: −8.7±0.7 kg; p<0.01). Total body fat mass loss was significantly greater in WL+AT (−6.8±0.6 kg, −16.4%) and WL+RT (−7.8±0.5 kg, −19.0%) than WL (−4.8±0.6 kg, −10.9%); both p<0.01. Lean mass loss was greatest in WL+AT (−1.6±0.3 kg, −3.1%) compared to WL+RT (−0.8±0.3 kg, −1.5%) or WL (−1.0±0.3 kg; −2.0%); both p≤0.02). Change in 400-m walk time was associated with change in fat mass (β/SD=+6.1 sec; p<0.01), while change in knee extensor strength was associated with change in lean mass (β/SD=+1.6 Nm; p<0.01).
Conclusions
WL+RT results in less lean mass lost than WL+AT; WL plus exercise yields greater fat mass loss than WL alone.
Keywords: aerobic exercise, resistance exercise, body composition, aging, mobility
Introduction
In addition to the well-known cardiometabolic consequences of obesity, excessive adiposity is also a significant contributor to functional limitation in old age.1 Indeed, if left unabated, current trends suggest that the functionally disabled older adult with obesity will soon become the most commonly encountered phenotype of frailty.2 Lifestyle-based interventions in older adults with obesity demonstrate immediate improvement in muscle strength and function with 5–10% weight loss;3–10 yet, widespread enthusiasm to recommend intentional weight loss in advanced age is diminished due to significant loss in lean mass (i.e., 10–50% of total tissue11,12) and uncertainty surrounding implications for long-term functional status as well as other health outcomes.13 Weight loss strategies that maximize fat mass loss - while minimizing lean mass loss - should provide the greatest health benefit for this demographic, although evidence from well-designed trials is needed to guide recommendations.14
Change in body composition with caloric restriction-induced weight loss is modifiable with exercise. Randomized controlled trial (RCT) evidence in older adults with obesity suggests that the addition of moderate intensity aerobic,15,16 progressive resistance,9,17 or combined3,6 exercise programs to caloric restriction results in a more favorable shift in body composition compared to either intervention alone. A direct comparison of the effects of aerobic or resistance exercise during caloric restriction was recently assessed in a short term (i.e., 6-month) study, with results suggestive of a superior ability of resistance training to attenuate weight loss- associated lean mas loss compared to aerobic training;18 however, confirmatory, long-term data are lacking. In addition, while exercise itself does not significantly lower body weight, consideration of the synergistic effects of exercise added to caloric restriction to augment loss of total body mass and alter composition in older (i.e., 60+ years at baseline) adults has only been evaluated in a handful of RCTs.3,9,15,17 Overall, these studies suggest similar weight and fat mass loss between treatment groups, with exercise modestly attenuating lean mass loss. Studies, however, are generally small (n=11–28/treatment group), of relatively short duration (4–12 months), and done under tightly supervised conditions, limiting their external validity. The paucity of data on this topic, coupled with the fact that many commercial weight loss programs focus exclusively on caloric restriction to induced weight loss, create a rationale for further investigation into the long-term effects of caloric restriction with differing exercise modalities to caloric restriction alone on change in body mass and composition in older adults.
Therefore, the primary purpose of this study was to compare the long-term effects of caloric restriction induced weight loss alone (WL) and with aerobic (WL+AT) or resistance training (WL+RT) on change in body composition in older adults with obesity undergoing an 18- month community based weight loss intervention. This analytic plan represents a secondary analysis of a previously reported primary outcome paper,19 and we hypothesized that WL+RT would better preserve lean mass that WL+AT or WL alone. Second, we examined the contributions of change in total body fat and lean masses on change in 400-m walk time and knee extensor strength, as these objectively measured outcomes are clinically relevant and highly predictive of subsequent disability and death.20,21 We hypothesized that change in total body fat mass would be associated with change in 400-m walk time and that change total body lean mass would be associated with change in knee extensor strength. Data from this aim contributes to a growing body of knowledge6,16,22,23 delineating the relative contributions of fat and lean mass lost during intentional weight loss on physical function.
Methods
Study Design
Details of the study design and methods are published.24 Briefly, the Cooperative Lifestyle Intervention Program (CLIP II; NCT: NCT01547182) was a multisite, single-blinded, RCT involving three YMCAs in Forsyth County, North Carolina. Participants were randomized to one of three treatment groups: caloric restriction induced weight loss alone (WL), weight loss plus aerobic training (WL+AT), or weight loss plus resistance training (WL+RT) for 18 months. The primary outcome paper, including protocol compliance and the intervention effect on dual primary outcome measures, time to complete a 400 meter walk and knee extensor strength, is published.19
Study Participants
A total of 249 participants were enrolled in the CLIP II study. Eligibility criteria consisted of men and women aged 60–79 years who engaged in <60 minutes/week of moderately intense physical activity, had a BMI ≥28 kg/m2 and <42 kg/m2, self-reported limitations with mobility, and had documented evidence of CVD or an ATP III diagnosis of MetS. Individuals were excluded if they had a myocardial infarction or cardiovascular procedure in the past 3- months, a fasting blood glucose ≥140mg/dL, a diagnosis of type 1 diabetes or insulin dependent type 2 diabetes, or their primary care physician had concerns regarding their ability to safely participate. All participants provided written informed consent prior to study enrollment.
Intervention Descriptions
Full intervention descriptions can be found in the published design paper;24 briefly:
Weight Loss
The three study arms received the same behaviorally-based WL intervention in three 6-month phases: intensive (months 1–6), transition (months 7–12), and maintenance (months 13–18), with the goal of eliciting a 0.3 kg/week weight loss in the intensive phase (~330 kcal reduction/day) and a total weight loss of 7–10%. During the intensive phase, participants met at the YMCA for 3 group sessions and 1 individual sessions per month (all 60 minutes in duration). Group sessions tapered off to 3 and then 1 per month for the subsequent phases, with individual sessions scheduled as needed. In accordance with the 2010 dietary guidelines,25 the macronutrient breakdown of the diet was 20–25% protein, 25–30% fat, and 45–55% carbohydrate. For the WL-only group, participants were instructed not to begin a formal exercise program while actively enrolled in the study.
Aerobic Training
The primary mode of AT was an individually tailored, supervised over-ground walking program. The program frequency was four days per week, progressing to a duration goal of 45 minutes/day and walking intensity of 12–14 on the Borg Rating of Perceived Exertion (RPE) Scale.26
Resistance Training
The RT intervention was also individually tailored and involved a training frequency of four days/week, progressing to 45 minutes/day with an RPE of 15–18 as a target intensity for each RT exercise. Participants completed three sets of 10–12 repetitions on 8 machines with initial resistance determined from one repetition maximum (1RM) testing (goal of 75% of 1RM). When a participant completed 12 repetitions in the third set for two consecutive days, the resistance was increased to ensure progressive overload. To assist with recovery time, participants rotated exercises on a two-day schedule: day one included a leg press, hip adduction, hip abduction, calf extension, seated-row, pectoral fly, shoulder press, and rotary torso; day two included leg extension, leg curl, lateral pull down, seated chest press, lateral raise, arm curl, triceps extension, and abdominal crunch.
Measurements
Participant age, gender, race/ethnicity, and medical history/co-morbid status were captured via self-report at the baseline assessment visit. Also at baseline, height was assessed without shoes to the nearest 0.25 cm using a stadiometer (Health O Meter® Portrod) and body mass measured to the nearest 0.05 kg using a calibrated and certified digital scale (Health O Meter® Professional 349KLX). All body composition variables were collected at baseline (n=247), 6- (n=223), and 18- (n=189) months. Total body, fat, and lean mass, as well as appendicular lean/fat masses and trunk fat, were assessed using dual-energy X-ray absorptiometry (DXA; GE Medical Systems, iDXA,) following manufacturer recommendations for patient positioning and scanning. Physical function was also assessed at baseline, 6-, and 18- months via a validated 400 m walk test20 (that requires walking 10 laps as quickly as possible on a 20 m course between two cones with the time for completion recorded in seconds) and an isokinetic dynamometer to assess knee extensor strength as peak torque in Newton-meters (Nm; Biodex, System 4).24
Statistical Analyses
Descriptive statistics were calculated overall and by treatment group at baseline. Overall treatment group comparisons were made using contrasts in a mixed model ANCOVA, with baseline value of the outcome, gender, treatment group, time (6- or 18-months), and a treatment group-by-time interaction included as fixed effects and wave included as a random effect. Data are presented as the average follow up mean (95% CI) and changes from baseline at 6- and 18- months (95% CI).
To determine the association between change in total body mass, lean mass, and fat mass and previously published intervention effects on 400-m walk time and knee extensor strength,19 we adjusted the above ANCOVA models for two additional pairs of variables: baseline and follow up total body mass or baseline and follow up total body composition (lean mass or fat mass, respectively) in treatment groups combined. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) and using a Bonferroni-adjusted Type 1 error rate of 0.025.
Results
Participant Characteristics
Table 1 presents baseline demographic and body composition measures, according to treatment group and overall. Briefly, 249 older (66.9±4.7 years) adults (71% women, 32% African American) with a BMI of 34.4±3.7 kg/m2 and CVD and/or MetS participated in this 18- month RCT, of which 247 had complete DXA data at baseline (90% retention at 6 months, 77% retention at 18 months; see Supplementary Figure 1). At baseline, overall total body, fat, and lean masses were 94.4±14.9 kg, 42.4±8.2 kg (45.0±5.7%), and 49.4±10.1 kg (52.1±5.5%), respectively, with no differences between treatment groups.
Table 1.
Baseline demographic and body composition measures, according to treatment group, and overall.
| WL (n=82) |
WL+AT (n=86) |
WL+RT (n=81) |
Overall (n=249) |
|
|---|---|---|---|---|
| Age (years) | 66.3 ± 4.5 | 67.5 ± 5.1 | 66.9 ± 4.4 | 66.9 ± 4.7 |
| Female, n (%) | 59 (72.0) | 62 (72.1) | 56 (69.1) | 177 (71.1) |
| African American, n (%) | 30 (36.6) | 30 (34.9) | 20 (24.7) | 80 (32.1) |
| BMI (kg/m2) | 34.7 ± 4.0 | 33.9 ± 3.5 | 34.8 ± 3.6 | 34.4 ± 3.7 |
| Body Mass & Composition | ||||
| Body Mass (kg) | 95.1 ± 16.7 | 92.4 ± 13.5 | 95.6 ± 14.2 | 94.4 ± 14.9 |
| Fat Mass (kg) | 42.6 ± 8.8 | 41.4 ± 7.6 | 43.2 ± 8.2 | 42.4 ± 8.2 |
| Fat Mass (%) | 44.9 ± 5.6 | 45.0 ± 5.7 | 45.3 ± 6.0 | 45.0 ± 5.7 |
| Lean Mass (kg) | 49.9 ± 10.9 | 48.3 ± 9.4 | 49.7 ± 10.1 | 49.4 ± 10.1 |
| Lean Mass (%) | 52.4 ± 5.3 | 52.2 ± 5.4 | 51.9 ± 5.8 | 52.1 ± 5.5 |
| Appendicular Lean Mass (kg) | 23.4 ± 5.8 | 22.4 ± 4.9 | 23.3 ± 5.5 | 23.0 ± 5.4 |
| Appendicular Fat Mass (kg) | 17.3 ± 4.5 | 16.9 ± 4.1 | 17.6 ± 4.5 | 17.3 ± 4.4 |
| Trunk Fat Mass (kg) | 24.3 ± 5.6 | 23.5 ± 4.9 | 24.4 ± 5.1 | 24.1 ± 5.2 |
Data are presented as means ± SD or n (%). BMI: Body Mass Index
As previously reported,19 median (25th, 75th percentiles) attendance to scheduled WL intervention sessions was 71.1% (40.5, 83.3) for WL only, 83.1% (47.6, 92.9) for WL+AT, and 85.7% (70.7, 92.7) for WL+RT. All three treatment groups lost significant weight from baseline (−6.1% [95%CI: −7.5 to −4.7] for WL only, −8.6% [95%CI: −10.0 to −7.2] for WL+AT, and −9.7% [95%CI: −11.1 to −8.4] for WL+RT) and both WL plus exercise treatment groups had greater improvement in 400-m walk time than WL alone (mean difference 16.9 [95% CI: 9.7 to 24.0] seconds, p<0.01) and experienced a similar change in knee extensor strength (combined WL+AT and WL+RT mean difference −3.6 [95% CI: −7.5 to 0.3] Nm, p=0.07).
Intervention Effect on Body Mass and Composition
Adjusted overall intervention effects on body composition are presented in Table 2. Total body mass was significantly reduced in all treatment groups (WL: −5.7±0.7 kg, WL+AT: −8.5±0.7 kg, WL+RT: −8.7±0.7 kg; all p<0.01). The two WL plus exercise treatment groups had lower follow-up total body mass compared to WL alone (WL+AT: 85.9±0.9 kg and WL+RT: 85.6±0.7 kg vs. WL: 88.6±0.7 kg; both p<0.01) and were not different from each other (p=0.75). Significant overall treatment effects were also observed for total body fat mass (p<0.01) and total body lean mass (p<0.01). As with total body mass, WL+AT and WL+RT treatment groups had similar and greater reductions in fat mass compared to WL alone (both p<0.01). Interestingly, absolute lean mass was lower in WL+AT (47.8±0.3 kg) compared to WL+RT or WL alone (48.5±0.3 kg and 48.4±0.3 kg, respectively; both p<0.01). However, because of a greater reduction in fat mass in WL+AT compared to WL alone, follow-up lean mass as a percentage of total body mass was significantly higher in WL+AT compared to WL alone (55.8±0.3% vs. 54.5±0.3%; p<0.01). Treatment effects for appendicular lean mass, appendicular fat mass, and trunk fat were similar to those observed for total body lean and fat masses, respectively (Table 2).
Table 2.
Overall intervention effects on body composition measures, adjusted for baseline value of the outcome, treatment group, gender, time, and treatment group by time interaction.
| Body Mass and Composition Measures | WL (n=81) |
WL+AT (n=86) |
WL+RT (n=80) |
p-value
|
|||
|---|---|---|---|---|---|---|---|
| Overall | WL+AT vs. WL | WL+RT vs. WL | WL+AT vs. WL+RT | ||||
| Body Mass (kg) | 88.6 (87.2, 90.0) | 85.9 (84.4, 87.3) | 85.6 (84.3, 87.0) | <.0001 | 0.0002 | <.0001 | 0.7473 |
| Δ 6 Months | −6.2 (−7.9, −4.5) | −7.9 (−9.6, −6.34) | −8.5 (−10.2, −6.9) | ||||
| Δ 18 Months | −5.3 (−7.1, −3.5) | −9.1 (−10.9, −7.3) | −8.9 (−10.7, −7.2) | ||||
| Fat Mass (kg) | 37.6 (36.5, 38.7) | 35.5 (34.4, 36.6) | 34.5 (33.4, 35.6) | <.0001 | 0.0006 | <.0001 | 0.0911 |
| Δ 6 Months | −5.1 (−6.5, −3.7) | −6.4 (−7.7, −5.1) | −7.8 (−9.1, −6.5) | ||||
| Δ 18 Months | −4.4 (−5.8, −3.0) | −7.3 (−8.7, −5.9) | −7.9 (−9.2, −6.5) | ||||
| Fat Mass (%) | 42.6 6 (41.9, 43.3) | 41.1 (40.4, 41.8) | 40.0 (39.4, 40.7) | <.0001 | 0.0003 | <.0001 | 0.0092 |
| Δ 6 Months | −6.0 (−8.2, −3.8) | −8.2 (−10.3, −6.1) | −11.2 (−13.3, −9.1) | ||||
| Δ 18 Months | −4.4 (−7.1, −2.5) | −9.9 (−12.2, −7.6) | −11.3 (−13.5, −9.1) | ||||
| Lean Mass (kg) | 48.4 (47.9, 48.9) | 47.8 (47.2, 48.3) | 48.5 (48.0, 49.0) | <.0001 | 0.0046 | 0.6011 | 0.0006 |
| Δ 6 Months | −1.1 (−1.7, −05) | −1.5 (−2.1, −0.9) | −0.7 (−1.2, −0.1) | ||||
| Δ 18 Months | −0.9 (−1.5, −0.2) | −1.7 (−2.3, −1.1) | −1.2 (−1.6, −0.4) | ||||
| Lean Mass (%) | 54.5 (53.8, 55.1) | 55.8 (55.2, 56.5) | 56.9 (56.2, 57.5) | <.0001 | 0.0004 | <.0001 | 0.0066 |
| Δ 6 Months | 4.7 (3.1, 6.3) | 6.3 (4.7, 7.7) | 8.9 (7.4, 10.5) | ||||
| Δ 18 Months | 4.2 (2.5, 5.8) | 7.7 (6.1, 9.4) | 9.1 (7.5, 10.7) | ||||
| Appendicular Lean Mass (kg) | 22.3 (21.9, 22.8) | 21.9 (21.5, 22.3) | 22.3 (21.9, 22.7) | <.0001 | 0.0182 | 0.8320 | 0.0263 |
| Δ 6 Months | −0.7 (−1.2, −0.3) | −1.1 (−1.5, −0.6) | −0.7 (−1.1, −0.2) | ||||
| Δ 18 Months | −0.7 (−1.1, −0.2) | −1.1 (−1.6, −0.6) | −0.8 (−1.2, −0.3) | ||||
| Appendicular Fat Mass (kg) | 15.5 (15.1, 15.9) | 14.9 (14.5, 15.3) | 14.3 (13.9, 14.7) | <.0001 | 0.0062 | <.0001 | 0.0166 |
| Δ 6 Months | −1.8 (−2.3, −1.3) | −2.2 (−2.7, −1.7) | −3.0 (−3.5, −2.5) | ||||
| Δ 18 Months | −1.7 (−2.2, −1.1) | −2.6 (−3.1, −2.0) | −2.9 (−3.4, −2.4) | ||||
| Trunk Fat Mass (kg) | 21.1 (20.3, 21.8) | 19.6 (18.9, 20.3) | 19.2 (18.5, 19.9) | <.0001 | 0.0003 | <.0001 | 0.3173 |
| Δ 6 Months | −3.3 (−4.2, −2.4) | −4.2 (−5.0, −3.3) | −4.8 (−5.7, −3.9) | ||||
| Δ 18 Months | −2.7 (−3.7, −1.8) | −4.8 (−5.7, −3.8) | −4.9 (−5.8, −4.0) | ||||
Data are presented as mean (95% confidence interval). WL=weight loss; AT=aerobic training; RT=resistance training; kg=kilograms; %=percent.
Model-adjusted change estimates for total body mass, partitioned into total body fat and total body lean compartments and presented by treatment group, are shown in Figure 1. As pictured and in accordance with follow up data, change in absolute lean mass was −0.9±0.3 kg, −1.6±0.3 kg, and −0.8±0.3 kg for the WL, WL+AT, and WL+RT treatment groups, respectively, with significant differences reported between WL and WL+AT and between WL+AT and WL+RT (both p<0.01).
Figure 1.
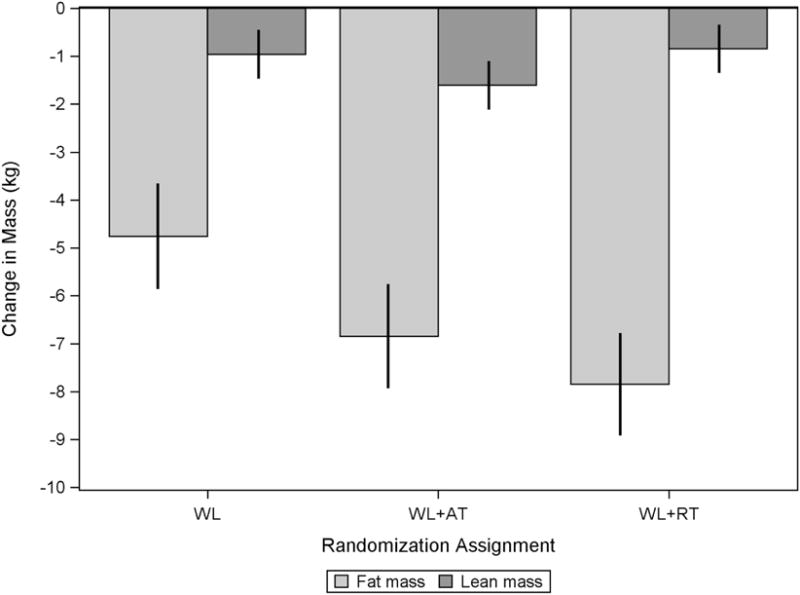
Overall change in total body mass, partitioned into fat and lean compartments, by treatment group and adjusted for baseline value of the outcome, treatment group, gender, time, and treatment group by time interaction.
Relationship between Change in Total Body Mass and Composition and Physical Function
Modeling results for the association between change in total body mass and composition and the physical function outcome measures of 400-m walk time and knee extensor strength are presented in Table 3. No interaction between treatment group and change in total, fat, or lean mass for either functional outcome was observed. In treatment groups combined and after adjustment for baseline value of the outcome, treatment group, gender, time, and treatment group by time interaction, the magnitude of change in total body mass was associated with change in 400-m walk time (p<0.01), with every 1 kg lost associated with a 0.97 second reduction in 400- m walk time. This association was driven primarily by change in fat mass (β±SE: 1.35±0.34 seconds; p<0.01). The magnitude of change in total body mass was not associated with change in knee extensor strength (p=0.06); however, change in knee extensor strength was directly associated with change in lean mass (β±SE: 1.28±0.43 Nm; p<0.01).
Table 3.
Change in 400-meter walk time and knee extensor strength per unit change in body mass, lean mass, and fat mass.
| Δ Body Mass (kg) | Δ Lean Mass (kg) | Δ Fat Mass (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| β | SE | p | β | SE | p | β | SE | p | |
| Δ 400-m Walk Time (sec) | 0.97 | 0.29 | 0.0008 | 0.60 | 0.93 | 0.5210 | 1.35 | 0.34 | 0.0001 |
| Δ Knee Extensor Strength (Nm) | 0.26 | 0.14 | 0.0635 | 1.28 | 0.43 | 0.0033 | 0.19 | 0.17 | 0.2766 |
Kg=kilogram; sec=second; Nm=Newton-meter.
Discussion
Results from this study confirm and extend previous research in older adults, demonstrating the ability of structured exercise to attenuate the proportion of weight lost as lean mass,18 while reporting the novel result of the superior ability of WL+RT to preserve absolute lean mass as compared to WL+AT in the long-term and when executed in a community setting. To date, four RCTs have been published where the additive effect of exercise to WL has been compared to WL alone in older (i.e. 60+ years at baseline) adults, with two studies employing RT,9,17 one study employing AT,15 and one using a combined approach.3 In general agreement with trials employing some form of RT in combination with WL, we observed a significant reduction in the percentage of total body mass lost as lean mass when RT is added to WL (10%) compared to WL alone (16%).3,9,17 Original findings presented here show half as much lean mass is lost with WL+RT compared to WL+AT (−0.8 kg versus −1.6 kg, respectively), despite a similar overall reduction in total body mass, when interventions are directly compared. Although novel, given that RT is a well-established stimulus for muscle growth and maintenance in weight stable older adults,27 results are not overly surprising. What is surprising, however, is that we did not find a lean mass sparing effect of WL+AT compared to WL alone. This is in contrast to several tightly controlled studies conducted in middle-aged and older adults,28 including results from the similarly aged (i.e. 60+ years at baseline, mean: 67.2 years) and designed (i.e. WL+ moderate- intensity walking, three to five day per week for 35–45 minutes per day versus WL alone) study by Chomentowski et al.15 Differences in study duration (4 months15 versus 18 months in the present study), setting (clinical15 versus community-based), or total WL achieved in the WL only arms (9.2%15 versus 6.1% in the present study) may contribute to this discrepancy and warrant further exploration.
Provocative results from this community-based trial also suggest augmented total body mass loss in WL+AT and WL+RT compared to WL alone; our data show an unprecedented near doubling of absolute fat (and therefore, total body) mass loss when exercise is added to caloric restriction induced WL. In contrast, previous trials in older adults report significant, yet similar, weight and fat mass loss between WL only and WL plus exercise arms, with a 5–20% reduction in fat mass, depending on the magnitude of total WL achieved.3,9,15,17 Although the behaviorally-based WL intervention was common to all CLIP II treatment groups, session attendance was lower in the WL only treatment group (71.1%) as compared to the WL plus exercise arms (83.1% and 85.7% for WL+AT and WL+RT, respectively) and may signal reduced dietary compliance. Indeed, the amount of total WL achieved by the WL plus exercise arms (~9%) aligns more closely to what is observed in other trials than the WL only arm (~6%). Nevertheless, this finding is important as it contributes to a growing body of literature examining the effectiveness of single versus multiple health behavior change interventions among older adults,29 and underscores the translational value of combined caloric restriction and exercise interventions to maximize weight and fat mass loss while preserving physical function.
Secondary analyses from this study demonstrate that loss of total body mass is associated with improvement in mobility (as measured by the 400-m walk), driven by reductions in fat mass, and that change in knee extensor strength is directly associated with change in lean mass. Findings contribute to a growing body of data implicating fat, rather than lean, mass as a primary target tissue affecting mobility related tasks6,22,23 and confirm the importance of preserving lean mass for strength during WL in older adults.30 Careful interpretation of these findings, however, necessitates consideration of the magnitude of functional change considered clinically meaningful. Data from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study, for instance, suggest that a 20 second change in 400-meter walk time represents the lower end of the range for clinical significance.31 Using this threshold and results from our modeling approach, a loss of at least 20.6 kg of total body mass or 14.8 kg of fat mass would be needed to elicit a modest improvement in mobility – which are slightly higher than other reported estimates.22 While the baseline walking speed of our sample was much greater than that of the LIFE population,32 calling into question the exact cut-point necessary to achieve clinical significance, it is important to recognize that the degree of weight and fat mass loss necessary to induce a meaningful change in mobility is likely substantial and may be difficult to achieve.
Although clinical cut-points for absolute knee strength have yet to be established, this association should be considered in light of the relatively small percentage of lean mass lost, as well as other known predictors of muscle strength. As presented in the primary outcome paper,19 using normalized values, both WL+RT and WL+AT experienced gains (15% and 14%, respectively) in relative knee extensor strength. Increases in relative strength signal an improvement in muscle quality, which is arguably more important than muscle quantity, and may be driven by reduced fat infiltration33,34 and inflammatory burden,35 as both predict muscle strength independent of mass and are improved with weight loss. Collectively, this framework tempers the concern regarding weight loss associated lean mass loss, although data presented here suggest weight loss interventions that can preferentially reduce fat mass might yield the greatest functional benefit.
Practically, our findings may be used to inform optimal geriatric weight management strategies, as there is a dearth of RCT evidence in this area,14,36 and improve clinical efficacy of diet and exercise recommendations. With regard to the latter, current federal physical activity guidelines are the same for older and younger adults and include 150 minutes/week of moderate to vigorous physical activity plus moderate intensity muscle strengthening activities on two or more days.37 National surveillance data, however, suggest that these ambitious guidelines are less likely to be met by older adults, with just 15% of adults aged 65–75 years meeting goals for both aerobic and strength training activities.38 Moreover, recent findings from a short term clinical trial in older adults with obesity suggest that a combined AT and RT intervention is no better at attenuating weight-loss associated lean mass loss and promoting fat mass loss than WL+RT.18 Thus, a geriatrician managing the older adult with sarcopenic obesity may stress the importance of WL+RT therapy – perhaps even more so than a combined exercise approach, should incorporating both modalities hinder compliance - although, certainly final recommendations must be carefully and individually considered.
Strengths of this study design include the direct comparison of exercise type during weight loss on body composition, inclusion of a large, heterogeneous sample, and long study duration. Moreover, because the intervention was accomplished in a community setting, results are highly translatable and align with the recent National Institutes of Health vision of effectively disseminated clinical intervention research.39 That said, this study does have weaknesses worth noting. First, study findings should only be generalized to older adults with obesity and documented CVD and/or MetS. Second, although DXA-acquired total body lean and fat mass represents the gold standard in total body composition assessment, and can provide some regional estimates, it does not assess change in fat infiltration. Intriguing recent data suggest decreases in visceral and intermuscular adipose tissue are important mechanisms underlying improved function with WL and exercise interventions23 and should be explored further. Third, while we present associations between change in body composition and two clinically meaningful functional endpoints, these analyses are by no means comprehensive. Future work formally exploring the mediating effect of change in total body composition and change physical function would significantly add to this area of inquiry.
Conclusion
The primary findings from this 18-month community based RCT demonstrate: (1) WL+RT results in less lean mass lost than WL+AT and (2) WL plus RT or AT results in greater overall reductions in total body mass than WL alone, driven by augmented fat mass loss. Second, fat mass loss is primarily responsible for weight loss associated improvements in mobility, whereas lean mass loss is primarily responsible for weight loss associated declines in strength. Collectively, these results indicate that the combination of WL+RT may yield the greatest weight loss and most favorable shift in body composition compared to WL+AT or WL alone, thereby maximizing potential functional benefit.
Supplementary Material
What is already known about the subject
The addition of regular exercise to caloric restriction can reduce lean mass loss in comparison to caloric restriction alone.
Data from several structured randomized controlled trials in middle-aged adults suggest significant, yet similar, weight and fat mass loss between weight loss alone and weight loss plus exercise.
One short-term (i.e., 6-month) study in older adults with obesity reports reduced lean mass loss when resistance training is added to caloric restriction induced weight loss as compared to aerobic training added to caloric restriction induced weight loss.
What this study adds
Resistance training added to caloric restriction induced weight loss attenuates long term (i.e., 18-month) lean mass loss when compared to aerobic training added to caloric restriction induced weight loss.
Provocative findings suggest the addition of either resistance or aerobic training to caloric restriction induced weight loss significantly enhances total body and fat mass loss in a community-based setting.
Acknowledgments
Funding: This study was funded by a grant from National Institutes of Health/National Heart, Lung and Blood Institute, R18 HL076441, awarded to Drs. Rejeski and Marsh. Partial support was also provided by a National Institutes on Aging grants, P30 AG021332 and K01 AG047921.
Footnotes
Clinical Trial Registration: NCT01547182
Disclosure: The authors declared no conflicts of interest.
References
- 1.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010 Jan;13(1):46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 2.Alley DE, Ferrucci L, Barbagallo M, Studenski SA, Harris TB. A research agenda: the changing relationship between body weight and health in aging. J Gerontol A Biol Sci Med Sci. 2008 Nov;63(11):1257–1259. doi: 10.1093/gerona/63.11.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar 31;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006 Apr 24;166(8):860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 5.Rejeski WJ, Brubaker PH, Goff DC, Jr, Bearon LB, McClelland JW, Perri MG, Ambrosius WT. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011 May 23;171(10):880–886. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, Newman AB. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015 May;101(5):991–999. doi: 10.3945/ajcn.114.105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016 Jan 5;315(1):36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008 Jul;40(7):1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton SD, Manini TM, Milsom VA, Dubyak P, Cesari M, Cheng J, Daniels MJ, Marsiske M, Pahor M, Leeuwenburgh C, Perri MG. Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clin Interv Aging. 2011;6:141–149. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007 May;31(5):743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 12.Singh MA. Combined exercise and dietary intervention to optimize body composition in aging. Ann N Y Acad Sci. 1998 Nov 20;854:378–393. doi: 10.1111/j.1749-6632.1998.tb09918.x. [DOI] [PubMed] [Google Scholar]
- 13.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp Gerontol. 2016 Dec 15;86:4–13. doi: 10.1016/j.exger.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batsis JA, Gill LE, Masutani RK, Adachi-Mejia AM, Blunt HB, Bagley PJ, Lopez-Jimenez F, Bartels SJ. Weight Loss Interventions in Older Adults with Obesity: A Systematic Review of Randomized Controlled Trials Since 2005. J Am Geriatr Soc. 2017 Feb;65(2):257–268. doi: 10.1111/jgs.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chomentowski P, Dube JJ, Amati F, Stefanovic-Racic M, Zhu S, Toledo FG, Goodpaster BH. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009 May;64(5):575–580. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavers KM, Beavers DP, Nesbit BA, Ambrosius WT, Marsh AP, Nicklas BJ, Rejeski WJ. Effect of an 18 month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity. 2013 Aug 20;22(2):325–31. doi: 10.1002/oby.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005 Dec;16(12):1703–1712. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- 18.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community Weight Loss to Combat Obesity and Disability in At-Risk Older Adults. J Gerontol A Biol Sci Med Sci. 2017 Jan 6; doi: 10.1093/gerona/glw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006 May 3;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjöström M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008 Jul 1;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat Mass Loss Predicts Gain in Physical Function With Intentional Weight Loss in Older Adults. J Gerontol Biol Sci Med Sci. 2013;68(1):80–6. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn NW. Effects of Changes in Regional Body Composition on Physical Function in Older Adults: A Pilot Randomized Controlled Trial. J Nutr Health Aging. 2015 Nov;19(9):913–921. doi: 10.1007/s12603-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh AP, Janssen JA, Ambrosius WT, Burdette JH, Gaukstern JE, Morgan AR, Nesbit BA, Paolini JB, Sheedy JL, Rejeski WJ. The Cooperative Lifestyle Intervention Program-II (CLIP-II): Design and methods. Contemp Clin Trials. 2013;36(2):382–93. doi: 10.1016/j.cct.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.2010 Dietary Guidelines - health.gov [Internet] [cited 2016 Nov 9]. Available from: https://health.gov/dietaryguidelines/2010/
- 26.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 27.Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995 Nov;50:147–150. doi: 10.1093/gerona/50a.special_issue.147. Spec No. [DOI] [PubMed] [Google Scholar]
- 28.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010 Jul;68(7):375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 29.Nigg CR, Long CR. A systematic review of single health behavior change interventions vs. multiple health behavior change interventions among older adults. Transl Behav Med. 2012 Jun;2(2):163–179. doi: 10.1007/s13142-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005 Nov;13(11):1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 31.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009 Jun;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD, investigators L study Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 Jun 18;311(23):2387–96. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008 Nov;105(5):1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol Biol Sci Med Sci. 2005 Mar;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Older Adults - 2008 Physical Activity Guidelines - health.gov [Internet] [cited 2017 Jan 4]. Available from: https://health.gov/paguidelines/guidelines/older-adults.aspx.
- 38.FastStats [Internet] [cited 2017 Jan 4 ]. Available from: http://www.cdc.gov/nchs/fastats/exercise.htm.
- 39.Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning Clinical Science: Unifying the Discipline to Improve the Public Health. Clin Psychol Sci J Assoc Psychol Sci. 2014 Jan 1;2(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


