Abstract
Increased varus–valgus laxity has been reported in individuals with knee osteoarthritis (OA) compared to controls. However, the majority of previous investigations may not report truly passive joint laxity, as their tests have been performed on conscious participants who could be guarding against motion with muscle contraction during laxity evaluation. The purpose of this study was to investigate how a measure of passive knee laxity, recorded when the participant is under anesthesia, is related to varus–valgus excursion during gait, clinical measures of performance, perceived instability, and self-reported function in participants with severe knee OA. We assessed passive varus–valgus knee laxity in 29 participants (30 knees) with severe OA, as they underwent total knee arthroplasty (TKA). Participants also completed gait analysis, clinical assessment of performance (6-min walk (6 MW), stair climbing test (SCT), isometric knee strength), and self-reported measures of function (perceived instability, Knee injury, and Osteoarthritis Outcome Score (KOOS) a median of 18 days before the TKA procedure. We observed that greater passive varus–valgus laxity was associated with greater varus–valgus excursion during gait (R2 =0.34, p =0.002). Significant associations were also observed between greater laxity and greater isometric knee extension strength (p =0.014), farther 6 MW distance (p =0.033) and shorter SCT time (p =0.046). No relationship was observed between passive varus–valgus laxity and isometric knee flexion strength, perceived instability, or any KOOS subscale. The conflicting associations between laxity, frontal excursion during gait, and functional performance suggest a complex relationship between laxity and knee cartilage health, clinical performance, and self-reported function that merits further study.
Keywords: osteoarthritis, knee, tibiofemoral, laxity, instability
More than 50 million adults in the United States report doctor-diagnosed osteoarthritis (OA), which includes almost 50% of all people over the age of 65.1 Knee OA is regularly characterized a loss of cartilage, formation of osteophytes, and a reduction in joint space.2,3 Clinical symptoms associated with severe knee OA are muscle weakness,4,5 perceived instability,6,7 increased knee pain2; and reduced function.8 Increased varus–valgus laxity has also been reported in individuals with knee OA compared to controls,9–16 and this increased laxity may influence disease progression and overall function.
Knee joint laxity is a quantitative measure of knee stability that measures the passive tibiofemoral motion under load. Increased laxity may result in greater motion between articular surfaces of the femur and tibia, which could increase shear stress and alter articular loading patterns.17 These changes may result in the cartilage being loaded in ways it is not adapted to withstand and cause irreversible cartilage damage. Along with the potential for passive varus–valgus knee laxity to alter knee kinematics during gait and initiate a cycle of cartilage degeneration and OA, laxity may also directly influence clinical and self-reported function. van der Esch et al. found greater varus–valgus laxity to be moderately associated with faster 100 m walking time and less lower extremity strength,18 yet no direct association was found between varus–valgus laxity and self-reported function. Alternately, Sharma et al. found greater varus–valgus laxity significantly increased the likelihood of poor self-reported function on the Western Ontario and McMaster Universities physical function (WOMAC-PF) scale; however, varus–valgus laxity was not related to chair stand rate.19 Quadriceps strength has also been linked to disease progression in OA patients with increased laxity,19 possibly due to an increased reliance on active knee stability from muscle contractions which may alter joint contact mechanics. Previous investigations on varus–valgus laxity were performed on conscious participants, who may be guarding against varus–valgus motion with muscle contraction during laxity evaluation.9,20,21 The devices used to quantify laxity also vary in measurement method and load applied, and it can be difficult to discern anatomical varus–valgus displacement from internal to external rotation. These issues could confound examinations of the relationship between varus–valgus laxity and function, leading to conflicting conclusions.
Identifying the factors that are associated with degraded function in people with severe OA is an essential step in understanding the specific characteristics underlying knee function. Quantifying strictly passive varus–valgus laxity may give insight into the effect of laxity on knee function and assist in the development of novel treatment strategies to improve function, slow disease progression, and postpone joint replacement. The purpose of this study was to investigate how passive varus–valgus knee laxity, recorded when the participant is under anesthesia, is related to varus–valgus excursion during gait, clinical measures of performance, perceived instability, and self-reported function in participants with severe OA. Specifically, we hypothesized that larger passive varus–valgus knee laxity would be associated with more varus–valgus excursion during the weight acceptance phase of gait, poorer clinical performance, a greater perception of instability, and reduced self-reported function.
METHODS
Participants
We conducted an analytic, prospective cross-sectional cohort study (Level III). Three orthopaedic surgeons (MB, AG, and JG) identified potential participants following a consultation on total knee arthroplasty at The Ohio State Wexner Medical Center, and with permission had research staff (GF and JL) contact them via telephone to explain the study in detail and enroll those interested. Thirty-three participants (34 knees) enrolled in this study after providing IRB-approved consent. Participants had predominantly medial compartment tibiofemoral osteoarthritis and were scheduled for primary TKA within the following 8 weeks. The exclusion criteria for this study were: Body Mass Index (BMI) >45; inability to walk a short distance (20 m) without an assistive device; predominantly lateral compartment OA; or previous TKA or osteotomy. Four participants were dropped from the study due to sterilization errors prohibiting intra-operative data collection (n =2) and technical difficulties during motion analysis testing resulting in a lack of available data (n =2). The demographics of the 29 included participants (30 knees) are shown in Table 1. Gait, clinical assessments, and self-report surveys were administered a median of 18 days prior to TKA (Interquartile Range IQR = 7.75–35.75 days). Varus–valgus laxity was measured intra-operatively during the TKA procedure on the osteoarthritic joint before any bone cuts were made.
Table 1.
Characteristics of Participants With Knee Osteoarthritis
| Male | Female | Overall | |
|---|---|---|---|
| Number of participants | 10 | 19 | 29 |
| Age, years | 58.6 ± 8.7 | 58.7 ± 7.0 | 58.6 ± 7.46 |
| Height, m | 1.79 ± 0.06 | 1.61 ± 0.06 | 1.67 ± 0.10 |
| Mass, kg | 100.9 ± 16.7 | 88.9 ± 12.9 | 93.0 ± 15.2 |
| BMI, kg/m2 | 31.6 ± 4.8 | 34.2 ± 4.3 | 33.3 ± 4.6 |
| Number of knees analyzed | 10 | 20 | 30 |
| Involved limb, no. (right/left) | 6/4 | 7/13 | 13/17 |
| Standing alignment, deg (+varus) | 6.8 ± 3.4 | 4.7 ± 3.4 | 5.4 ± 3.5 |
| KL grade, no. (I/II/III/IV) | 0/0/4/6 | 0/2/13/5 | 0/2/17/11 |
Values are the mean ± standard deviation unless otherwise noted. BMI, body mass index; KL, Kellgren–Lawrence.
Motion Analysis
Marker data were collected at 150 Hz using 10 Vicon MX-F40 cameras (Vicon; Oxford, UK) and filtered using a 4th order Butterworth filter at 6 Hz. Ground reaction forces were recorded at 1,500 Hz from Bertec 4060-10 force plates (Bertec Corp; Columbus, OH) and used to identify heel contact and toe-off time points in the gait cycle. A modified point-cluster technique marker set22 was used with additional iliac crest and upper body Plug-In Gait markers (Fig. 1A). A functional hip joint center was estimated using the markers on the thigh during a star-arc motion.23 Custom Bodybuilder (Vicon Motion Systems, Ltd.) and MATLAB (Mathworks, Inc., Natick, MA) scripts calculated full body kinematics (Fig. 1B and C).
Figure 1.
(A) Movement analysis was completed using a modified point-cluster marker set. (B) A static calibration pose estimated the location of the femur and tibia utilizing a functional hip joint center and anatomical markers placed on the following bony landmarks; medial and lateral femoral epicondyles, medial and lateral tibial plateaus, and medial and lateral malleoli. (C) Tracking marker clusters on the thigh and shank were used to quantify joint kinematics during dynamic activity. Varus–valgus excursion was calculated as the difference between peak varus and valgus knee angle during the weight acceptance phase of gait.
Standing, frontal-plane knee alignment was found during the motion analysis calibration trial. An estimated functional hip joint center,23 the midpoint of markers on medial and lateral femoral epicondyles markers and the midpoint of markers on the medial and lateral malleoli were used to estimate frontal plane mechanical knee alignment. Positive values indicate a varus knee angle. Similar methods have shown good correlation with standing radiographic alignment (R2 = 0.83, p < 0.0001).24 Self-selected gait speed was calculated in the middle of the 10 m walkway during motion analysis from the mean velocity of markers on the pelvis to be included in statistical analyses as a potential covariate.
Frontal plane excursion was calculated as the difference between peak knee varus and peak knee valgus angle during the weight acceptance phase of gait. Weight acceptance was defined as the time period between initial contact and peak knee flexion during stance phase of the involved limb. Ensemble averages for each variable were calculated over four trials of gait at a self-selected speed.
Clinical Assessment
The primary clinical performance measures were self-selected gait speed, isometric knee strength, the 6-min walk test25,26 (6 MW) and the timed stair climbing test27,28 (SCT). Self-selected gait speed was measured during motion analysis. Knee extension and flexion strength were quantified with a Biodex System 3 dynamometer (Biodex Medical Systems; Shirley, NY) during a maximal voluntary isometric contraction (MVIC). Each participant was seated upright with the involved limb at 60 degrees of knee flexion. Two maximal contractions for each knee extension and knee flexion were separated by 30 s. The maximal torque produced during extension and flexion were recorded and normalized by each participant’s mass (Nm/kg). About 6 MW was measured as the distance a participant could walk around a 90 m indoor track in the 6 min time limit.25,29 Participants were instructed to walk as far as possible in a safe manner. They were encouraged to not take any breaks or use a walking aid unless necessary to complete 6 min of walking. SCT was measured as the time necessary to ascend and descend a 12-step staircase.30,31 The participants were instructed to complete the task as quickly as possible in a safe manner. They were encouraged to not use the handrail unless necessary to complete the test.
Tibiofemoral radiographic severity was assigned using the Kellgren–Lawrence (KL) grading system.3 Two fellowship-trained, musculoskeletal radiologists (JP and AR; see acknowledgements) graded each participant by consensus. All radiographs were obtained at the same facility and consisted of bilateral anterior, bilateral posterior, lateral, and axial patellar views.
Self-Reported Evaluations
Participants’ perceived knee instability was assessed from a question in the Knee Outcome Survey—Activities of Daily Living Scale,32 which has been used previously in OA populations.33–36 The question read “To what degree does giving way, buckling, or shifting of the knee affect your daily activity?” and was scored on a 6-point scale: 0, instability prevented all activity; 1, instability affected activity severely; 2, instability affected activity moderately; 3, instability affected activity slightly; 4, instability did not affect activity; and 5, no instability.
Self-reported function was assessed from four subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS).37 These subscales included multiple-choice questions focused on pain, symptoms, activities of daily living, and knee-related quality of life. The subscale related to sport and recreation was not applicable to the majority of participants in this cohort, and therefore not reported. Responses are assigned a score from 0 to 4. These scores are normalized for each subscale, with a higher score indicating better function.
Intra-Operative Data Collection
To characterize passive knee joint laxity under anesthesia, we quantified the load-displacement relationship of the knee in the frontal plane. Joint laxity of the native osteoarthritic knee was assessed with minimal disruption to the joint structures by performing the measurements after exposing the distal femur and proximal tibia with a standard medial para-patellar approach but prior to any bone, ligamentous, or meniscal alterations associated with a standard total knee arthroplasty. Passive knee kinematics were collected intra-operatively utilizing a validated custom navigation system during the total knee arthroplasty procedure using a technique described previously.38,39 Retro-reflective marker clusters were rigidly attached to the distal femur and proximal tibia with cortical bone screws. The hip joint center was estimated from the femoral cluster motion during hip circum-duction.40 Anatomical landmarks on the femur and tibia were located using a stylus integrated with the motion capture system by the orthopaedic surgeon (MB and JG). The custom surgical navigation system recorded relative tibiofemoral kinematics from the motion of the marker clusters and their relationship to the previously defined anatomical coordinate systems.
Intra-operative laxity was characterized with our navigation system and a custom stability device.39 The orthopaedic surgeon applied loads to a modified Alvarado boot, which rested in a low-friction track of a custom knee stability testing device, using a force application handle (Fig. 2). The handle was instrumented with a tension-compression load cell and linked to the custom surgical navigation system. The orthopaedic surgeon alternately applied load in the varus and valgus direction until firm end points in motion were reached, while manually restricting motion of the femur. The maximal load was determined qualitatively by the surgeon on an individual basis to ensure patient safety. Varus and valgus moments were calculated about the previously defined knee joint center, using the data from the load cell and the surgical navigation system. Maximal moment was not reported to the surgeon, but typically exceeded 10 Nm. This procedure was repeated until a minimum of three trials were completed, each with an applied varus and valgus load. Continuous measurement of tibiofemoral kinematics and load were collected.
Figure 2.

Passive varus–valgus testing completed intra-operatively with a custom knee stability device and surgical navigation system. The orthopaedic surgeon applies load in the varus and valgus direction with an instrumented handle, while manually restricting motion of the femur. Marker clusters screwed into the femur and tibia track kinematics with a motion capture camera.
A varus–valgus load-displacement curve was calculated for three trials of alternating varus and valgus load (Fig. 3). A third order polynomial was fit to the raw data for each trial. Laxity was calculated as the difference in varus–valgus knee angle when the knee was loaded with 10 Nm of varus and 10 Nm of valgus moment. Each participant’s overall varus–valgus laxity is determined from the average of these three trials. Figure 3 illustrates how laxity was defined by presenting the recorded load-displacement data for a single trial of one participant. This participant’s varus–valgus excursion was not centered on mechanical zero alignment, which can be expected in participants with predominantly medial compartment OA and a varus deformity.
Figure 3.
Example case of intra-operative load-displacement data and varus–valgus laxity calculation. Moment applied by the surgeon (Y axis) and frontal plane knee angle (X axis) were measured with a surgical navigation system and knee stability device. A 3rd order polynomial was then fit to the raw data for each trial. Varus–valgus laxity was calculated as the angular difference between a 10 Nm varus and 10 Nm valgus applied load. Each participant’s overall varus–valgus laxity was the average of the laxity calculated in three intra-operative load-displacement trials. Varus–valgus laxity is generally not centered on zero degrees, due to the varus deformity observed in our participant population.
Statistical Analyses
Pearson correlations were used to describe the relationship between varus–valgus laxity and the gait, clinical or self-reported variables with proper data transformation as needed for some variables (e.g., SCT) to satisfy the normal distribution assumption. Spearman correlations were used when variables were categorical (ordinal data, e.g., Perceived instability levels). Univariate and multivariate general linear regression models were used to investigate whether variables were associated with laxity.
RESULTS
The means and standard deviations for the variables of interest in the 30 knees (29 participants) are summarized in Table 2. Correlation coefficients are shown to identify the relationship between varus–valgus laxity and the gait, clinical, and self-reported variables. SCT time was transformed using the natural logarithm to fit normality constraints. Spearman correlations were used for testing the association of varus–valgus laxity with perceived instability and radiographic OA severity, due to the categorical and non-normal distribution of these variables.
Table 2.
Results for Variables of Interest and Association to Passive Varus–Valgus Laxity
| Mean ± Standard Deviation | Correlation Coefficient | p-Value | |
|---|---|---|---|
| Intra-operative knee stability | |||
| Varus–valgus laxity, deg | 5.0 ± 2.6 | n/a | n/a |
| Gait analysis | |||
| Varus–valgus excursion, deg | 5.2 ± 2.1 | 0.582 | 0.001*** |
| Clinical measures | |||
| Self-selected gait speed, m/s | 1.0 ± 0.2 | 0.364 | 0.048* |
| Knee ext strength, Nm/kg | 1.0 ± 0.5 | 0.444 | 0.014* |
| Knee flex strength, Nm/kg | 0.6 ± 0.3 | 0.225 | 0.233 |
| Six min walk, m | 385.8 ± 90.2 | 0.389 | 0.033* |
| Stair climbing test, s | 24.7 ± 12.7 | −0.367a | 0.046* |
| OA radiographic severity (KL grade) | 3.3 ± 0.6 | 0.012b | 0.948 |
| Self-reported evaluation | |||
| Perceived instability, no. | 2.3 ± 1.3 | 0.113b | 0.558 |
| KOOS pain | 47.1 ± 20.2 | 0.099 | 0.604 |
| KOOS symptoms | 42.5 ± 9.2 | −0.219 | 0.246 |
| KOOS ADL | 56.1 ± 23.0 | 0.079 | 0.678 |
| KOOS QOL | 24.7 ± 20.2 | −0.015 | 0.938 |
OA, osteoarthritis, KL, Kellgren–Lawrence, ext, extension; flex, flexion; KOOS, Knee injury and Osteoarthritis Outcome Score; ADL, activities of daily living; QOL, quality of life. Asterisks indicate significant association (*p <0.05, **p <0.01, ***p <0.001).
Indicates data were transformed.
Indicates Spearman correlation.
Gait Analysis
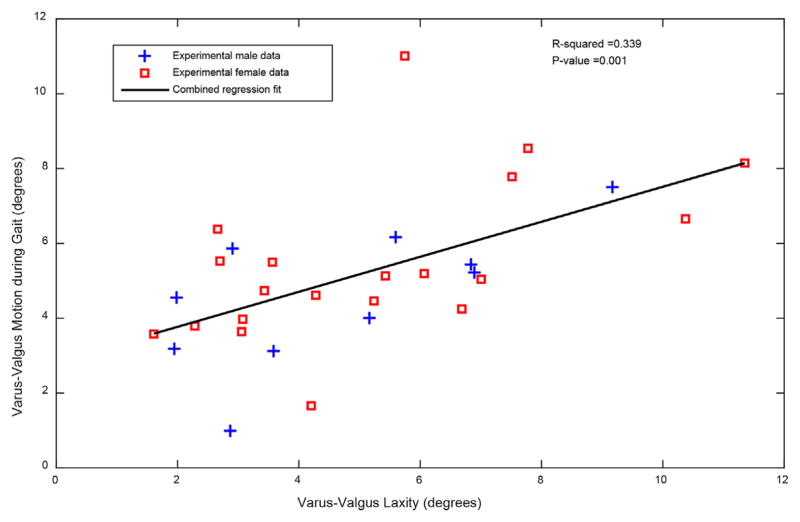
Greater varus–valgus laxity correlated with more varus–valgus excursion during weight acceptance phase of gait (R2 = 0.34, p = 0.001). A scatterplot of this result can be seen in Figure 4, with experimental male data shown as blue crosses and female data shown as red squares. This result confirms our hypothesis that greater passive laxity would result in larger varus–valgus motion during gait in participants with severe OA.
Figure 4.
Varus–valgus laxity was significantly associated with varus–valgus motion during gait.
Clinical Performance Measures
A significant association was observed between greater varus–valgus laxity and faster self-selected gait speed (R2 = 0.13, p = 0.048). Larger varus–valgus laxity was also correlated to greater normalized isometric knee extension strength (Fig. 5 and Table 2, R2 = 0.20, p = 0.014). In contrast, there was no relationship between varus–valgus laxity and normalized isometric knee flexion strength in this cohort (Table 2). Significant associations were also observed between larger varus–valgus laxity and farther 6 MW distance (R2 = 0.15, p = 0.033) and faster SCT time (R2 = 0.13, p = 0.046). To further investigate the relationship between varus–valgus laxity and clinical performance measures, we executed a linear regression analysis with varus–valgus laxity as the response and 6 MW, SCT (log transformed), knee extension strength, and knee flexion strength as predictors. Only knee extension strength showed a trend with varus–valgus laxity, (R2 = 0.29, adj R2 = 0.17, p = 0.053). Self-selected gait speed was not included in the multiple linear regression as 6 MW was chosen as a more distinguishing measure of clinical performance. Radiographic severity was also left out of the multiple linear regression analysis, as there was a skewed distribution in our population toward severe OA, which was expected for patients scheduled for TKA.
Figure 5.
Scatter plot of the correlation between increased varus–valgus laxity and increased knee extension strength (R2 = 0.20, p = 0.014).
Perceived Instability and Self-Reported Function
Perceived instability was not normally distributed, so a Spearman correlation was used to test for an association. No relationship was observed between varus–valgus laxity and perceived instability (Table 2, R2 = 0.03, p = 0.385). There was also no relationship observed between laxity and any of the four KOOS subscales (Table 2).
DISCUSSION
The aims of this study were to identify how passive varus–valgus knee laxity associated with varus–valgus excursion during gait, clinical performance, instability and self-reported function in participants with severe medial knee OA. Varus–valgus excursion during gait was significantly associated with passive varus–valgus laxity, which conflicts with previous findings by van der Esch et al. that found no correlation between varus–valgus laxity and varus–valgus excursion during the loading response phase of gait.41 We recorded comparatively less laxity measured in the current study, 5.0 (2.6) degrees versus 7.58 (3.25) degrees; however, we observed larger varus–valgus excursion during gait, 5.2 (2.1) degrees versus 3.24 (1.73) degrees. These contradictory findings may be due to key differences in the OA severity and laxity testing methodology between studies. Our participants all had severe OA and were awaiting a TKA, while a large percentage of the knees studied by van der Esch (67%) were diagnosed with mild OA (KL grade 1). We performed our knee laxity assessment at full extension while van der Esch et al. measured laxity at 20 degrees of knee flexion. Previous work has found varus–valgus laxity to increase with increasing flexion angle,20 so this flexion angle difference likely influences the magnitude of varus–valgus laxity more than the differences in applied load (10 vs. 7.7 Nm) or level of consciousness (anesthetized vs. conscious). One reason we quantified passive laxity at full extension is because the largest compressive loads during gait are seen shortly after heel strike, when the knee is at approximately 10 degrees of flexion.42,43 Surgeons also typically assess knee stability in full extension, so laxity testing of the knee in this position quantified their subjective assessment and did not add considerable time to the surgical procedure. Our device also accounted for the three-dimensional rotations of the knee during laxity testing and calculated the applied moment using the anatomical coordinate system of the femur and tibia.39 Other laxity measurement devices that do not use three dimensional motion capture and anatomical defined coordinate systems have the potential for artifact when calculating the applied varus or valgus load. We also eliminate the potential for soft-tissue artifact during laxity measurement, by utilizing motion capture trackers screwed directly into the bone. Differences in OA demographics, knee flexion angle during laxity testing, and 3D laxity calculations during measurement may explain why our varus–valgus laxity magnitude was smaller than van der Esch et al.18 even while varus–valgus excursion during gait was larger. Future studies may be able to further investigate these differences in order to identify what measures of laxity are related to varus–valgus excursion in populations with varying levels of OA severity.
It has been theorized that altered joint laxity may increase shear stress on cartilage and shift joint contact patterns during dynamic activity.17 Our observation that greater laxity is associated with larger varus–valgus excursion is consistent with this theory. While we did not explicitly measure knee contact patterns, differences in varus–valgus excursion and alignment likely influence cartilage loading patterns. These changes may load areas of cartilage in a way not previously conditioned to and incite a degenerative process. Contributors to active knee stability may also play an important role in constraining the knee joint in this population, specifically muscle activation patterns for muscles which cross the knee joint. However, additional work is necessary to identify any relationship between active stability and varus–valgus excursion or passive laxity.
Varus–valgus laxity also exhibited positive correlations with greater knee extension strength, better 6 MW distance and faster SCT time, as well as with the potential covariate, self-selected gait speed. Gait speed, 6 MW and SCT scores have been previously linked to knee extension strength, so we performed a multiple linear regression analysis with knee extension strength, knee flexion strength, 6 MW and SCT all entered as factors and self-selected gait speed as a covariate, to identify which of these variables were independently associated with laxity. Only knee extension strength showed a trend in this multi-factor analysis, suggesting that strength may be driving the other trends between clinical performance and laxity, but additional work with a larger population is warranted to investigate these relationships further.
The literature on laxity and self-reported function is conflicting, but our results showed no relationship between laxity and any measured self-reported function variable in OA participants immediately prior to joint replacement. Schmitt et al. and van der Esch et al. also found no relationship between laxity and self-reported outcomes.7,44 Holla et al. observed a significant association between greater laxity and better physical function gauged from the Western Ontario and McMaster osteoarthritis index (WOMAC-PF).45 However, Sharma et al. observed better WOMAC-PF scores in the low-laxity group compared to high-laxity group, and a modest association between greater laxity and worse WOMAC-PF (R2 = 0.04).46 These previous studies included participants with varied severity of knee OA, whose results may not be applicable to individuals with severe OA. Our study was the only to include unconscious measurement of varus–valgus laxity, therefore, reducing the possibility of muscle guarding. The custom knee stability device and three-dimensional surgical navigation system also remove soft-tissue artifact and the artifact that tibial internal/external rotation can have on moment calculations.39 This approach allowed for measurement of the true varus or valgus moment applied relative to the anatomical coordinate system of the knee. In our study participants with severe OA, there was no evidence that varus–valgus laxity is associated with the KOOS subscales.
Limitations of this study include the potential influence of soft-tissue artifact during motion analysis testing. In order to match typical demographics of patients with OA electing for TKA, we included those with a relatively high BMI. To mitigate the issues of soft-tissue artifact during gait analysis, we utilized a point cluster technique marker set22 and functional hip joint center algorithm23 when calculating joint kinematics during gait. Combined, these techniques reduced the effect of individual marker motion and eliminated the reliance on regression equations to calculate hip joint centers from pelvis markers. Moreover, as shown in Table 1, our average BMI was 31.6 for males and 34.2 for females in spite of the broader inclusion criteria. BMI did not affect the laxity measurement, because intra-operatively motion was measured with markers rigidly attached to the tibia and femur. Another limitation is the heterogeneity of our participant’s contralateral limb. One patient had both knees replaced and both sets of data were included in this analysis, while other subjects had previous contralateral knee replacements or varying degrees of OA in the contralateral limb. This heterogeneity may influence our measures of function and make it difficult to assess the impact of only passive laxity on biomechanical, clinical, and self-reported outcomes. Another potential limitation was the choice to use self-selected walking speed rather than a single standard speed across participants. However, in this cohort it would have been difficult to standardize speed due to the variation of function and pain in the participants before TKA. Moreover, in this cohort gait speed was unrelated to varus–valgus excursion (R2 = 0.02, p = 0.41). We also lack a control group due to the invasive nature of our laxity testing; however, our group has published previous laxity research on cadavers without OA. Varus–valgus laxity of the cadaveric knee was only 2.7 (0.5) degrees,47 compared to 5.0 (2.6) degrees in our OA participants. These results are consistent with previous work indicating more varus–valgus laxity in OA populations compared to controls. Also, the degree of active stability from muscle activation patterns was not investigated in this analysis. Muscle forces likely play an important role in knee joint stability, given the large frontal plane moments measured during walking. Lastly, our sample size did not provide sufficient power to detect smaller correlations (<0.5), even though these may still be scientifically significant. The current sample size of 30 provided at least 80% power to detect a correlation of 0.5 at significance level of 0.05, and it provides at least 90% power to detect an effect size of 0.62 standard deviation changes (a 1.2 degree change when std =1.9) for any future studies examining changes in laxity with intervention. Future work should attempt to identify the relationship between passive and active knee stabilizers in participants with severe OA.
This study investigated the relationship between passive varus–valgus laxity and varus–valgus excursion, clinical performance measures, and self-reported instability and function. Varus–valgus excursion was significantly related to passive varus–valgus laxity. There were also significant associations between greater laxity and better clinical performance; however, no association was found between laxity and self-reported instability or self-reported function. Increased laxity has been reported in subjects with OA, and it has been theorized that this may shift cartilage loading patterns and lead to OA. Our observation that greater laxity is associated with more varus–valgus excursion is consistent with this theory. Our results indicate that greater varus–valgus excursion is correlated to greater laxity, which may result in cartilage degeneration; however, patients with severe OA and greater laxity exhibited improved clinical performance. These results suggest a complex relationship between laxity and knee cartilage health, clinical performance, and self-reported function. Additional research is necessary to identify a range of laxity that allows for the favorable knee function over time.
Acknowledgments
National Institute of Arthritis and Musculoskeletal and Skin Diseases; Grant number: R01AR056700.
We would like to thank Jason Payne, MD and Alan Rogers, MD for their assistance in grading the radiographic OA severity. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases nor the National Institutes of Health.
Footnotes
Study design: Prospective, analytic, cross-sectional cohort study; Level of Evidence: III.
AUTHORS’ CONTRIBUTIONS
GF contributed to the study design and interpretation and collection of data and drafted the manuscript. EH, JL, JG, AG, MB, XP, LS, RS, and AC contributed to the study design, data collection, data interpretation, and made critical revisions to the manuscript. XP provided biostatistical support. AC coordinated the project and serves as corresponding author.
References
- 1.Barbour KE, Helmick CG, Theis KA, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation-United States, 2010–2012. MMWR. 2013;62:869–873. [PMC free article] [PubMed] [Google Scholar]
- 2.Hurwitz DE, Sharma L, Andriacchi TP. Effect of knee pain on joint loading in patients with osteoarthritis. Curr Opin Rheumatol. 1999;11:422–426. doi: 10.1097/00002281-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messier SP, Loeser RF, Hoover JL, et al. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- 6.Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941–946. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt LC, Fitzgerald GK, Reisman AS, et al. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88:1506–1516. doi: 10.2522/ptj.20060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurley MV, Scott DL, Rees J, et al. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma L, Lou C, Felson DT, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–870. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Pai YC, Rymer WZ, Chang RW, et al. Effect of age and osteoarthritis on knee proprioception. Arthritis Rheum. 1997;40:2260–2265. doi: 10.1002/art.1780401223. [DOI] [PubMed] [Google Scholar]
- 11.Wada M, Kawahara H, Shimada S, et al. Joint proprioception before and after total knee arthroplasty. Clin Orthop Relat Res. 2002;403:161–167. doi: 10.1097/00003086-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Ishii Y, Noguchi H, Matsuda Y, et al. Preoperative laxity in osteoarthritis patients undergoing total knee arthroplasty. Int Orthop. 2009;33:105–109. doi: 10.1007/s00264-007-0467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar D, Manal KT, Rudolph KS. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:298–305. doi: 10.1016/j.joca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph KS, Schmitt LC, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007;87:1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57:1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andriacchi TP, Mundermann A, Smith RL, et al. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 18.van der Esch M, Steultjens M, Knol DL, et al. Joint laxity and the relationship between muscle strength and functional ability in patients with osteoarthritis of the knee. Arthritis Rheum. 2006;55:953–959. doi: 10.1002/art.22344. [DOI] [PubMed] [Google Scholar]
- 19.Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 20.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee-the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg. 1976;58:583–594. [PubMed] [Google Scholar]
- 21.Lim BW, Hinman RS, Wrigley TV, et al. Varus malalignment and its association with impairments and functional limitations in medial knee osteoarthritis. Arthritis Rheum. 2008;59:935–942. doi: 10.1002/art.23820. [DOI] [PubMed] [Google Scholar]
- 22.Andriacchi TP, Alexander EJ, Toney MK, et al. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. J Biomech Eng. 1998;120:743–749. doi: 10.1115/1.2834888. [DOI] [PubMed] [Google Scholar]
- 23.Camomilla V, Cereatti A, Vannozzi G, et al. An optimized protocol for hip joint centre determination using the functional method. J Biomech. 2006;39:1096–1106. doi: 10.1016/j.jbiomech.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Blazek K, Asay JL, Erhart-Hledik J, et al. Adduction moment increases with age in healthy obese individuals. J Orthop Res. 2013;31:1414–1422. doi: 10.1002/jor.22390. [DOI] [PubMed] [Google Scholar]
- 25.Enright PL. The six-minute walk test. Res Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 26.Terwee CB, Mokkink LB, Steultjens MP, et al. Performance-based methods for measuring the physical function of patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Rheumatology. 2006;45:890–902. doi: 10.1093/rheumatology/kei267. [DOI] [PubMed] [Google Scholar]
- 27.Van Nostrand D, Kjelsberg MO, Humphrey EW. Preresectional evaluation of risk from pneumonectomy. Surg Gynecol Obstet. 1968;127:306–312. [PubMed] [Google Scholar]
- 28.Rejeski WJ, Ettinger WH, Jr, Schumaker S, et al. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157–167. doi: 10.1016/s1063-4584(05)80050-0. [DOI] [PubMed] [Google Scholar]
- 29.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 30.Almeida GJ, Schroeder CA, Gil AB, et al. Interrater reliability and validity of the stair ascend/descend test in subjects with total knee arthroplasty. Arch Phys Med Rehabil. 2010;91:932–938. doi: 10.1016/j.apmr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ettinger WH, Burns, Messier R, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 32.Irrgang JJ, Snyder-Mackler L, Wainner RS, et al. Development of a patient-reported measure of function of the knee. J Bone Joint Surg. 1998;80:1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 33.van der Esch M, Knoop J, van der Leeden M, et al. Self-reported knee instability and activity limitations in patients with knee osteoarthritis: results of the Amsterdam osteoarthritis cohort. Clin Rheumatol. 2012;31:1505–1510. doi: 10.1007/s10067-012-2025-1. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt LC, Fitzgerald GK, Reisman AS, et al. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88:1506–1516. doi: 10.2522/ptj.20060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57:1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res. 2008;26:1180–1185. doi: 10.1002/jor.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos EM, Roos HP, Lohmander LS, et al. Knee Injury and Osteoarthritis Outcome Score (KOOS)-development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 38.Siston RA, Giori NJ, Goodman SB, et al. Intra-operative passive kinematics of osteoarthritic knees before and after total knee arthroplasty. J Orthop Res. 2006;24:1607–1614. doi: 10.1002/jor.20163. [DOI] [PubMed] [Google Scholar]
- 39.Siston RA, Maack TL, Hutter EE, et al. Design and cadaveric validation of a novel device to quantify knee stability during total knee arthroplasty. J Biomech Eng. 2012;134:115001. doi: 10.1115/1.4007822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siston RA, Delp SL. Evaluation of a new algorithm to determine the hip joint center. J Biomech. 2006;39:125–130. doi: 10.1016/j.jbiomech.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 41.van der Esch M, Steultjens M, Harlaar J, et al. Knee varus-valgus motion during gait—a measure of joint stability in patients with osteoarthritis? Osteoarthritis Cartilage. 2008;16:522–525. doi: 10.1016/j.joca.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 43.Mundermann A, Dyrby CO, D’Lima DD, et al. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. J Orthop Res. 2008;26:1167–1172. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 44.van der Esch M, Knoop J, van der Leeden M, et al. Self-reported knee instability and activity limitations in patients with knee osteoarthritis: results of the Amsterdam osteoarthritis cohort. Clin Rheumatol. 2012;31:1505–1510. doi: 10.1007/s10067-012-2025-1. [DOI] [PubMed] [Google Scholar]
- 45.Holla JF, van der Leeden M, Peter WF, et al. Proprioception, laxity, muscle strength and activity limitations in early symptomatic knee osteoarthritis: results from the CHECK cohort. J Rehabil Med. 2012;44:862–868. doi: 10.2340/16501977-1029. [DOI] [PubMed] [Google Scholar]
- 46.Sharma L, Hayes KW, Felson DT, et al. Does laxity alter the relationship between strength and physical function in knee osteoarthritis? Arthritis Rheum. 1999;42:25–32. doi: 10.1002/1529-0131(199901)42:1<25::AID-ANR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 47.Hutter EE, Granger JF, Beal MD, et al. Is there a gold standard for TKA tibial component rotational alignment? Clin Orthop Relat Res. 2013;471:1646–1653. doi: 10.1007/s11999-013-2822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]