Abstract
A recent taxonomic revision of Microascaceae with an emphasis on synnematous fungi enabled re-identification of previously isolated indoor strains of Cephalotrichum. All available Cephalotrichum strains from the culture collection of the Westerdijk Institute were studied, 20 originating from the built environment. Phylogenetic relationships were inferred from DNA sequence data from the internal transcribed spacer 1 and 2 and intervening 5.8S nrDNA (ITS), and parts of β-tubulin (tub2) and translation elongation factor 1-α (tef1) genes. Additionally, herbarium material of 14 Cephalotrichum species described from soil in China was studied, and the taxonomy of C. album, not considered in recent revisions, was reevaluated. Sixteen phylogenetic species in Cephalotrichum are distinguished, five described as new species: C. domesticum, C. lignatile, C. telluricum, C. tenuissimum and C. transvaalense. Five Cephalotrichum species occur in the built environment: C. domesticum, C. gorgonifer (formerly known as Trichurus spiralis), C. microsporum, C. purpureofuscum, and C. verrucisporum. Based on the number of isolates, C. gorgonifer (nine strains) is the most common indoor species. The study of the Chinese herbarium material resulted in the acceptance of three additional Cephalotrichum species: C. casteneum, C. ellipsoideum, and C. spirale. Four species are considered nomena dubia (C. cylindrosporum, C. macrosporum, C. ovoideum, and C. robustum), five are placed in synonymy with other Cephalotrichum species (C. acutisporum, C. inflatum, C. longicollum, C. oblongum, C. terricola) and one species, C. verrucipes, is probably a synonym of Penicillium clavigerum. Cephalotrichum columnare, former Doratomyces columnaris, is transferred to Kernia. Cephalotrichum album, formerly known as Doratomyces putredinis, is transferred to Acaulium and redescribed.
Key words: Doratomyces, Herbarium, Microascaceae, Microascales, Sordariomycetes, Synnematous hyphomycetes
Taxonomic novelties: New combination: Acaulium album (Costantin) Seifert & Woudenb., Kernia columnaris (H.J. Swart) Woudenb. & Samson
New species: Cephalotrichum domesticum Woudenb. & Seifert, C. lignatile Woudenb. & Seifert, C. telluricum Woudenb. & Seifert, C. tenuissimum Woudenb. & Seifert, C. transvaalense Woudenb. & Seifert
Typification: Epitypification (Basionyms): Synpenicillium album Costantin
Introduction
The genus Cephalotrichum is characterised by the formation of dry-spored, indeterminate synnemata and enteroblastic percurrent conidiogenesis. No sexual morph is known. It was first described by Link (1809), for two species, C. rigescens and C. stemonitis. Hughes (1958) chose C. stemonitis as lectotype, anchoring the modern generic concept of Cephalotrichum. Later, Doratomyces was described with D. neesii as its type (Corda 1829, considered a synonym of C. stemonitis by Hughes 1958) and later, Stysanus with S. stemonitis as its type (Corda 1837). Consideration of the type or lectotype species of these three genera, Cephalotrichum, Doratomyces and Stysanus, leads to the conclusion that all are typified by the fungus originally described as Isaria stemonitis (Abbott 2000). A later genus, Trichurus, with T. cylindricus as its type, was distinguished by the presence of sterile setae on the synnemata (Clements & Pound 1896). In the unpublished Abbott (2000) thesis on holomorph studies in the Microascaceae, the synonymies of the three genera Doratomyces, Stysanus and Trichurus under Cephalotrichum were proposed, conclusions followed by Seifert et al. (2011). These synonymies were later confirmed based on analyses of the LSU and ITS rDNA subunits (Sandoval-Denis et al., 2016a, Sandoval-Denis et al., 2016b). Within Cephalotrichum Sandoval-Denis et al. (2016b) described two new species, proposed five new combinations, and designated one neotype specimen, two lectotypes and four epitypes for accepted species. Although this provides a more stable taxonomy for synnematous Microascaceae, the papers also highlighted a large number of taxa that could not be studied because of the absence of living cultures. Their list of uncertain or excluded species included 43 Cephalotrichum spp., and seven Doratomyces spp. These included 14 new Cephalotrichum species described recently from China, mostly based on morphology characters alone (Jiang and Zhang, 2008, Jiang et al., 2011). We were fortunate to obtain herbarium material of these latter species for study, allowing us to evaluate them in the broader context of the Cephalotrichum taxonomy established by Sandoval-Denis et al. (2016b).
Most Cephalotrichum species occur on decaying plant material, straw, dung, wood and in soil (Domsch et al. 2007). They are infrequently reported from the indoor or built environment. Cephalotrichum microsporum (previously known as Doratomyces microsporus) is the species most often reported from the indoor environment (Prezant et al., 2008, Samson et al., 2010, Flannigan et al., 2011), where it is mentioned as occurring especially on wet cellulose-containing substrates like wood. Cephalotrichum purpureofuscum has also been reported from indoor air (Abbott, 2000, Sandoval-Denis et al., 2016b) as has C. gorgonifer (Abbott 2000, as C. spirale). Cephalotrichum species are not regarded as human pathogens, and not known as producers of mycotoxins. Strains have been isolated from clinical origins, mostly human respiratory systems, but are considered passive colonisers or sample contaminants rather than active pathogens (Sandoval-Denis et al. 2016b). Cephalotrichum gorgonifer, for example, has been isolated from human clinical samples and can grow at human body temperatures (Sandoval-Denis et al. 2016b). However such reports are scarce and clinical data is lacking. Given the amount of time we spend indoors, it is important to understand which microorganisms are co-habitants of this environment and what their potential implications may be to human health and to the design of the built environment. For that reason, we re-evaluated the identification of newly isolated strains from house dust and other indoor substrates, and other strains from the built environment in our collections.
The aim of our project was to construct an updated phylogenetic overview of the genus, taking into account the availability of the previously unavailable species described from China, and the strains from the built environment. Cultures and specimens were also examined of an anomalous coprophilous white species, included by Morton & Smith (1963) as Doratomyces putredinis then later renamed as Cephalotrichum album (De Beer et al. 2013), allowing us to complete the phylogenetic analysis of the classical species of this complex that are available in pure culture.
Materials and methods
Isolates and herbarium specimens
Seventy-two strains belonging to the genera Acaulium, Cephalotrichum, Graphium, Kernia and Wardomyces were included in this study (Table 1). They were obtained from the culture collection of the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, the Netherlands and the working collection of the Applied and Industrial Mycology department (DTO) at the Westerdijk Institute. Strains were grown on oatmeal agar (OA) (Samson et al. 2010).
Table 1.
Isolates used in this study and their GenBank accession numbers. Bold accession numbers were generated in other studies.
| Name | CBS Database | Strain number1 | Substrate/host | Location | GenBank accession number |
|||
|---|---|---|---|---|---|---|---|---|
| ITS | tub2 | tef1 | LSU | |||||
| Acaulium acremonium | CBS 104.65ET; ATCC 16282; DSM 1987; MUCL 8274 | Wheat field soil | Germany | KY852468 | LN851109 | LN851056 | KY852479 | |
| A. albonigrescens | CBS 109.69ET; ATCC 18841; IHEM 18560 | Litter, treated with urea | Japan | KY852469 | LN851111 | LN851058 | KY852480 | |
| A. album comb. nov. | Graphium putredinis | CBS 378.64 | Queen of bumble-bee | Denmark | KY852470 | KY852481 | ||
| G. putredinis | CBS 212.73 | Soil | Netherlands | KY852471 | KY852482 | |||
| G. putredinis | CBS 257.82, ATCC 46569 | Decaying Coprinus micaceus | Canada | KY852472 | KY852483 | |||
| G. putredinis | CBS 539.85ET | Hair in dung in pole cat | Netherlands | KY852473 | KY852484 | |||
| A. caviariforme | CBS 536.87T; TRTC 50940 | Decaying meat | Belgium | LM652392 | LN851112 | LN851059 | LN851005 | |
| Cephalotrichum asperulum | Doratomyces stemonitis | CBS 127.22; DTO 170-B5; IMI 086947; LSHB Sc177; MUCL 4031 | Seed | Netherlands | LN850959 | LN851113 | LN851060 | |
| CBS 215.49; DTO 334-G8; ATCC 11259 | Unknown | Indonesia | KY249250 | KY249291 | KY249329 | |||
| D. asperulus | CBS 582.71IT; DTO 104-B7; ATCC 26885; LCP 73.2231 | Soil | Argentina | LN850960 | LN851114 | LN851061 | KX924027 | |
| C. brevistipitatum | D. purpureofuscus | CBS 157.57T; DTO 334-H7; MUCL 4036 | Solanum tuberosum | Netherlands | LN850984 | LN851138 | LN851084 | |
| C. cylindricum | Trichurus terrophilus | CBS 646.70; DTO 335-A2 | Soil | France | KY249251 | KY249292 | KY249330 | |
| T. terrophilus | CBS 587.77; DTO 335-A7 | Soil | Turkey | KY249252 | KY249293 | KY249331 | ||
| CBS 127136; DTO 335-C5; RMF 7618 | Soil | USA | KY249253 | KY249294 | KY249332 | |||
| T. cylindricus | UAMH 1348ET | Sorghum seed | USA | LN850965 | LN851119 | LN851066 | ||
| C. dendrocephalum | T. dendrocephalus | CBS 528.85IT; DTO 170-H4; MUCL 28855; NHL 2927 | Cultivated soil | Iraq | LN850966 | LN851120 | LN851067 | |
| C. domesticum sp. nov. | D. purpureofuscus | CBS 139.42; DTO 334-G6; IFO 7677; MUCL 4025 | Manure | Netherlands | KY249277 | KY249315 | KY249357 | |
| D. purpureofuscus | CBS 255.50; DTO 334-G9; MUCL 4037 | Mushroom compost | Netherlands | KY249278 | KY249316 | KY249358 | ||
| D. purpureofuscus | CBS 395.67; DTO 336-C5 | Indoor, plaster | Netherlands | KY249279 | KY249317 | KY249359 | ||
| CBS 142035T; DTO 077-D6 | Indoor air, house | Netherlands | KY249280 | KY249318 | KY249360 | |||
| C. gorgonifer | T. spiralis | CBS 131.08; DTO 336-C2 | Unknown | USA | LN850974 | LN851128 | KY249333 | |
| T. spiralis | CBS 104.15; DTO 338-G1; MUCL 9831 | Unknown | UK | KY249254 | KY249295 | KY249334 | ||
| T. terrophilus | CBS 368.53; DTO 334-H6 | Treated wood | South Africa | LN850976 | LN851130 | LN851076 | ||
| T. spiralis | CBS 496.62; DTO 338-G2; MUCL 9830 | Compost ground domestic waste | Italy | KY249255 | KY249296 | KY249335 | ||
| T. spiralis | CBS 877.68; DTO 334-I7; ATCC 16231 | Wheat field soil | Germany | KY249256 | KY249297 | KY249336 | ||
| T. spiralis | CBS 635.78ET; DTO 170-G9 | Human hair | Netherlands | LN850977 | LN851131 | LN851077 | ||
| T. spiralis | CBS 120011; DTO 335-C2 | Soil | South Africa | KY249257 | KY249298 | KY249337 | ||
| CBS 124434; DTO 335-C3 | Human foot | Denmark | KY249258 | KY249299 | KY249338 | |||
| D. stemonitis | CBS 125064; DTO 335-C4 | Mouldarray fungi | Denmark | KY249259 | KY249300 | KY249339 | ||
| DTO 005-E7 | Indoor | Germany | KY249260 | KY249301 | KY249340 | |||
| DTO 054-I8 | Indoor | Germany | KY249261 | KY249302 | KY249341 | |||
| DTO 054-I9 | Indoor | Germany | KY249262 | KY249303 | KY249342 | |||
| DTO 055-C1 | Indoor | Germany | KY249263 | KY249304 | KY249343 | |||
| DTO 055-C2 | Indoor | Germany | KY249264 | KY249305 | KY249344 | |||
| DTO 055-D6 | Indoor | Germany | KY249265 | np | KY249345 | |||
| DTO 090-A7 | Indoor air, house | Netherlands | KY249266 | KY249306 | KY249346 | |||
| DTO 164-D4 | Indoor air, bakery | Netherlands | KY249267 | KY249307 | KY249347 | |||
| DTO 240-B2 | Indoor swab, archive | Netherlands | KY249268 | KY249308 | KY249348 | |||
| UAMH 3585 | Mushroom compost | Canada | LN850978 | LN851132 | LN851078 | |||
| C. hinnuleum | D. stemonitis | CBS 289.66T; DTO 334-I1; IFO 8314 | Dung of deer | Australia | LN850985 | LN851139 | LN851085 | |
| C. lignatile sp. nov. | D. microsporus | CBS 209.63T; DTO 170-D5 | Timber in cave | Belgium | KY249269 | KY249309 | KY249349 | |
| C. microsporum | D. purpureofuscus | CBS 523.63ET; DTO 103-I7; ATCC 16224; IFO 31240; MUCL 4041 | Wheat field soil | Germany | LN850967 | np | LN851068 | |
| D. microsporus | CBS 132.68; DTO 055-I1 | Ligustrum vulgare, dead twig | Netherlands | KY249270 | KY249310 | KY249350 | ||
| DTO 152-C1 | Indoor | Unknown | KY249271 | KY249311 | KY249351 | |||
| DTO 152-D4 | Indoor | Unknown | KY249272 | np | KY249352 | |||
| DTO 207-C6 | Indoor | Germany | KY249273 | KY249312 | KY249353 | |||
| UAMH 9365T | Indoor air | Canada | LN850968 | LN851122 | LN851069 | |||
| C. nanum | D. nanus | CBS 188.60; DTO 103-H8; DTO 103-H9; MUCL 4042 | Unknown | Italy | KY249274 | KY249313 | KY249354 | |
| D. nanus | CBS 191.61ET; DTO 334-H8; IFO 8180; IFO 8184; IMI 068394; LSHB Sc14; LSHB Sc142; MUCL 4038 | Dung of deer | UK | LN850969 | LN851123 | LN851070 | ||
| D. nanus | CBS 882.68; DTO 104-B1; ATCC 16219; IFO 31239 | Wheat field soil | Germany | KY249275 | np | KY249355 | ||
| D. nanus | CBS 139532; DTO 335-D3; WSF 5700 | Forest soil | USA | KY249276 | KY249314 | KY249356 | ||
| UAMH 9126 | Dung of bison | Canada | LN850970 | LN851124 | LN851071 | |||
| C. purpureofuscum | D. purpureofuscus | CBS 174.68; DTO 055-I5 | Zea mays, grain | Unknown | KY249281 | KY249319 | KY249361 | |
| D. stemonitis | CBS 116683; DTO 055-I3 | Tunnelwall containing cellulose | Netherlands | KY249282 | KY249320 | KY249362 | ||
| DTO 054-I1 | Indoor | Germany | KY249283 | KY249321 | KY249363 | |||
| DTO 055-H8 | Indoor | Germany | KY249284 | KY249322 | KY249364 | |||
| UAMH 9209 | Indoor air | Canada | LN850971 | LN851125 | LN851072 | |||
| C. stemonitis | D. stemonitis | CBS 103.19NT; DTO 170-B3; MUCL 6960 | Seed | Netherlands | LN850951 | LN850954 | LN850953 | LN850952 |
| D. stemonitis | CBS 180.35; DTO 334-F9; IMI 086946; LSHB Sc124 | Unknown | Unknown | LN850972 | LN851126 | LN851073 | ||
| D. stemonitis | CBS 127788; DTO 335-C6; RMF H 423 | Soil | USA | KY249285 | KY249323 | KY249365 | ||
| UAMH 1532 | Unknown | Unknown | LN850973 | LN851127 | LN851074 | |||
| C. telluricum sp. nov. | T. spiralis | CBS 336.32T; DTO 334-F7; MUCL 9829; UAMH 8882 | Soil | Cyprus | KY249287 | KY249325 | KY249367 | |
| T. terrophilus | CBS 568.50; DTO 334-H1 | Soil | Canada | KY249288 | KY249326 | KY249368 | ||
| C. tenuissimum sp. nov. | D. microsporus | CBS 127792T; DTO 335-C7; RMF H 318 | Soil | USA | KY249286 | KY249324 | KY249366 | |
| C. transvaalense sp. nov. | T. terrophilus | CBS 448.51T; DTO 170-C1; IFO 7660; IMI 046251; LSHB B344 | Eucalyptus saligna timber, in cellar | South Africa | LN850964 | LN851118 | LN851065 | |
| C. verrucisporum | D. asperulus | CBS 512.72; DTO 104-B9; DTO 104-C1; DTO 104-C2 | Agricultural soil | Netherlands | KY249289 | KY249327 | KY249369 | |
| D. asperulus | CBS 187.78; DTO 336-C6 | Sand dune soil | Netherlands | LN850986 | LN851140 | LN851086 | ||
| DTO 055-D7 | Indoor | Germany | KY249290 | KY249328 | KY249370 | |||
| Graphium penicillioides | CBS 102632ET; JCM 10498 | Populus nigra | Czech Republic | KY852474 | KY852485 | |||
| Kernia columnaris comb. nov. | C. columnare | CBS 159.66T; IMI 116691 | Dung of hare | South Africa | KY852475 | KY852477 | KY852478 | KY852486 |
| K. nitida | CBS 282.52; IFO 8200 | Chrysolina sanguinolenta | France | KY852476 | KY852487 | |||
| Wardomyces inflatus | CBS 367.62NT; DTO 170-D2; DAOM 84715; MUCL 669 | Greenhouse soil | Belgium | LN850994 | LN851153 | LN851099 | ||
First strain number is of the examined isolate. ATCC: American Type Culture Collection, Manassas, VA, USA; CBS: Culture Collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands; DAOM: Canadian National Mycological Herbarium, Agriculture and Agri-Food Canada, Ottawa, Canada; DSM: Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; DTO: Working Collection of the Applied and Industrial Mycology Group of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands; IFO: Institute for Fermentation Culture Collection, Osaka, Japan; IHEM: Biomedical Fungi and Yeast Collection of the Belgian Co-ordinated Collections of Micro-organisms (BCCM), Brussels, Belgium; IMI: Culture Collection of CABI Europe-UK, Egham, UK; JCM: Japan Collection of Microorganisms, Microbe Division, RIKEN-BioResource Center, Koyadai, Tsukuba, Ibaraki, Japan; LCP: Laboratory of Cryptogamy, National Museum of Natural History, Paris, France; LSHB: London School of Hygiene and Tropical Medicine, London, UK; MUCL: (Agro)Industrial Fungi and Yeast Collection of the Belgian Co-ordinated Collections of Micro-organisms (BCCM), Louvain-la Neuve, Belgium; NHL: National Institute of Hygienic Sciences, Tokyo, Japan; RMF: Rocky Mountain Herbarium, Fungi, Univeristy of Wyoming, Laramie, WY, USA; TRTC: Royal Ontario Museum Fungarium, Toronto, Canada; UAMH: University of Toronto, UAMH Centre for Global Microfungal Biodiversity, Toronto, Canada; WSF: Wisconsin Soil Fungi Collection, Madison, WI, USA. Ex-epitype, -isotype, -type, and -neotype isolates are indicated with ET, IT, T and NT, respectively.
Portions of fourteen holotype herbarium specimens, originally accessioned in the Plant Pathology Herbarium of the Shandong Agricultural University, China (HSAUP) were recently donated to the herbarium of the Westerdijk Institute (CBS-H) and were re-examined as part of this study (Table 2). For the holotypes of these species, we have indicated the original accession numbers for holotypes from the protologue, and consider the portions deposited in CBS-H to be isotypes, for which new accession numbers are published here with the following form: “holotype HSAUP xxxxx → isotype CBS-H yyyyy.” Additional isotype were listed in the protologues in HMAS; we have not examined these, but include the accession numbers as listed by the authors.
Table 2.
Herbarium specimens studied with their GenBank accession numbers.
| Original name | Collection # | ITS | After examination | |
|---|---|---|---|---|
| C. acutisporum | HSAUPII042724 | CBS H-22780 | – | C. purpureofuscum |
| C. castaneum | HSAUPII051034 | CBS H-22781 | FJ914681 | C. castaneum |
| C. cylindrosporum | HSAUPII052414 | CBS H-22782 | FJ914686 | Nomen dubium |
| C. ellipsoideum | HSAUPII074053 | CBS H-22783 | – | C. ellipsoideum |
| C. inflatum | HSAUPII050918 | CBS H-22784 | FJ914676 | C. microsporum |
| C. longicollum | HSAUPII050802 | CBS H-22785 | FJ914672 | C. purpureofuscum |
| C. macrosporum | HSAUPII050878 | CBS H-22786 | FJ914675 | Nomen dubium |
| C. oblongum | HSAUPII042723 | CBS H-22787 | FJ914667 | C. purpureofuscum |
| C. ovoideum | HSAUPII050846 | CBS H-22788 | FJ914662 | Nomen dubium |
| C. robustum | HSAUPII050875 | CBS H-22789 | FJ914674 | Nomen dubium |
| C. spirale | HSAUPII074033 | CBS H-22790 | FJ914705 | C. spirale |
| C. terricola | HSAUPII050924 | CBS H-22791 | FJ914677 | C. purpureofuscum |
| C. verrucipes | HSAUPII050849 | CBS H-22792 | – | Penicillium clavigerum |
| C. verrucisporum | HSAUP051029 | CBS H-22793 | FJ914680 | C. verrucisporum |
DNA sequences from six strains maintained at the UAMH Centre for Global Microfungal Biodiversity, University of Toronto, Canada were obtained from GenBank (Table 1).
DNA isolation, PCR and sequencing
DNA extractions were performed using the Ultraclean® Microbial DNA Isolation Kit (MoBio laboratories, Carlsbad, CA, USA), following manufacturer's instructions. The internal transcribed spacer 1 and 2 and intervening 5.8S nrDNA (ITS), and parts of the β-tubulin (tub2) and translation elongation factor 1-α (tef1) genes were amplified and sequenced as described in Woudenberg et al. (2017). Consensus sequences were assembled from forward and reverse sequences using Bionumerics v. 4.61 (Applied Maths, St-Martens-Latem, Belgium). All sequences generated were deposited in GenBank (Table 1).
Alignments and phylogenetic analyses
Individual sequence alignments of the ITS, tub2 and tef1 datasets were generated with MAFFT v. 7.271 (http://mafft.cbrc.jp/alignment/server/index.html) using the L-INS-i method. The best nucleotide substitution models were determined with Findmodel (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html). For both the single gene sequence alignments and the concatenated alignment, Bayesian and Maximum-likelihood analyses were performed as described in Woudenberg et al. (2017). An additional phylogenetic tree was constructed based on the ITS sequences of a broader selection of isolates representing all species recognized by Sandoval-Denis et al. (2016b) and in this study, together with ITS sequences from the Chinese herbarium specimens available in GenBank (Table 2). To demonstrate the placement of two species initially classified in Cephalotrichum outside the genus, an alignment and phylogenetic tree based on the ITS and LSU sequences of representative strains of the genera Acaulium, Cephalotrichum, Kernia and Graphium was assembled based on the sampling of Sandoval-Denis et al. (2016a). The resulting trees were printed with TreeView v. 1.6.6 (Page 1996) and, together with the alignments, deposited in TreeBASE (http://www.treebase.org).
Morphology
Cultures were incubated on oatmeal agar (OA, which favours synnema development), malt extract agar (MEA) and dichloran 18 % glycerol agar (DG18) plates (recipes from Samson et al. 2010) at 25 °C in the dark. After 14 d, growth rates were measured and colony characters noted. Colony colours were rated following the charts of Rayner (1970). Dried herbarium material was rehydrated in sterile water, which was then replaced by Shear's mounting media for photomicroscopy (Crous et al. 2009). Measurements and descriptions of microscopic structures were made from cultures grown on synthetic nutrient agar (SNA, Samson et al. 2010) at 25 °C in the dark for 14 d, mounted in 85 % lactic acid. Macroscopic photographs were made with a Nikon SMZ25 stereo microscope equipped with a Nikon DS-Ri2 high-definition colour camera head. Photomicrographs of diagnostic structures were made with a Zeiss Axio Imager A2 microscope equipped with a Nikon DS-Ri2 high-definition colour camera head, using differential interference contrast (DIC) optics and the Nikon software NIS-elements D v. 4.50.
Results
Phylogeny
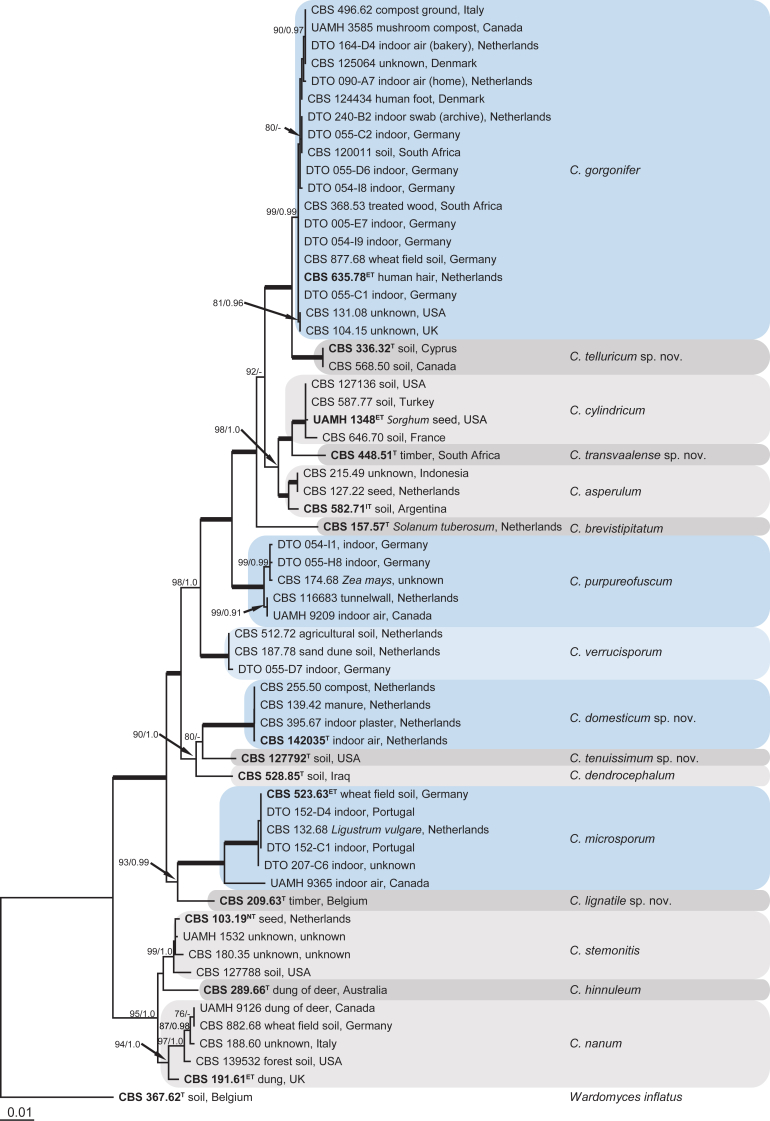
The concatenated, multi-gene Cephalotrichum phylogeny alignment included sequences of 62 strains and was 1 979 bp long, with the partitions being 566 characters for ITS (67 informative or unique), 884 for tef1 (103) and 529 for tub2 (227). The TrN model with a gamma-distributed rate variation was suggested as the best model for the ITS and tub2 alignments, and the GTR model with a gamma-distributed rate variation as the most suitable model for the tef1 alignment. After discarding the burn-in phase trees, the multi-gene Bayesian analysis resulted in 2 020 trees from both runs, from which the majority rule consensus tree and posterior probabilities were calculated.
The multi-gene analysis divided the isolates among 16 species clades (Fig. 1) of which five are proposed as new and described in the Taxonomy section: C. domesticum, C. lignatile, C. telluricum, C. tenuissimum and C. transvaalense. The 20 strains isolated from indoor environment are distributed among five Cephalotrichum species (Fig. 1, blue coloured boxes), namely C. gorgonifer (n = 9), C. microsporum (n = 4), C. domesticum (n = 2), C. purpureofuscum (n = 4) and C. verrucisporum (n = 1). All 16 species can be identified with either tef1 or tub2 partial gene sequences. The only exception is strain CBS 191.61, which based on its tef1 sequence clusters separately from the other C. nanum isolates (data not shown; all single gene phylogenies submitted to TreeBase). Based on ITS barcodes alone, C. cylindricum and C. transvaalense sp. nov. cannot be distinguished (Fig. 2).
Fig 1.
Maximum likelihood tree based on the ITS, tub2 and tef1 sequences of 62 isolates. The RAxML bootstrap support values ≥75 % (BS) and Bayesian posterior probabilities ≥0.95 (PP) are given at the nodes. Thickened lines indicate a BS of 100 % and a PP of 1.0. Ex-type strain numbers are in bold face and indicated with T (or ET,IT, NT, when ex-epitype, ex-isotype or ex-neotype respectively). The blue boxes indicate species which occur in the indoor environment. The tree was rooted to Wardomyces inflatus (CBS 367.62).
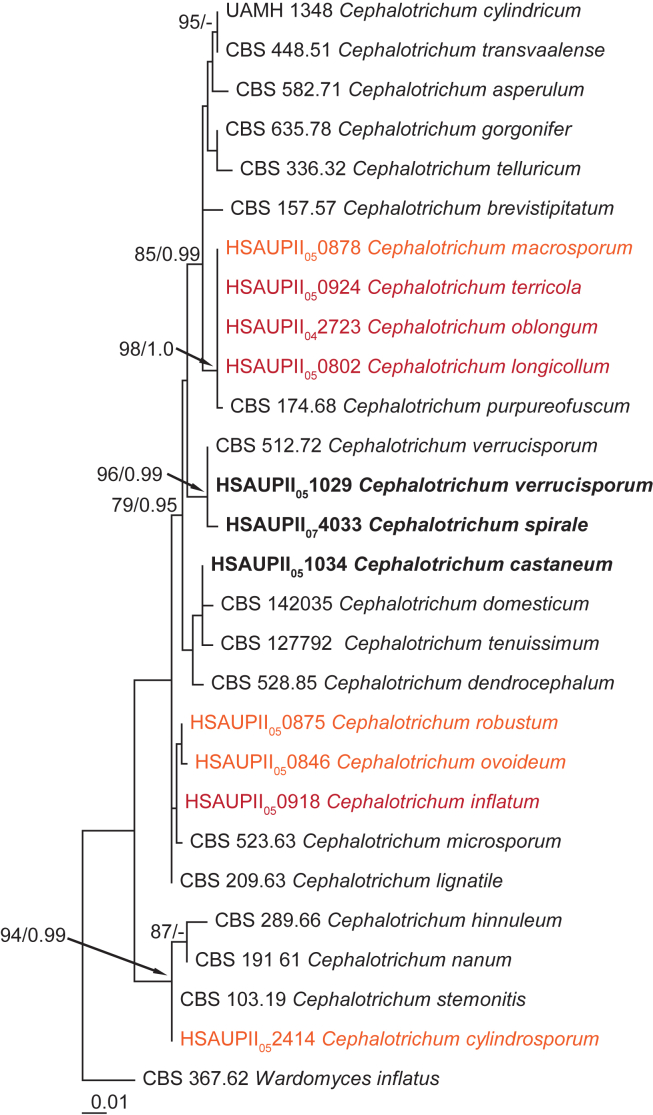
Fig. 2.
Maximum likelihood tree based on the ITS sequences of 28 isolates. The RAxML bootstrap support values ≥75 % (BS) and Bayesian posterior probabilities ≥0.95 (PP) are given at the nodes. Thickened lines indicate a BS of 100 % and a PP of 1.0. Names in bold face represent accepted species names, names in orange are nomen dubium, names in red are names which are synonymised. The tree was rooted to Wardomyces inflatus (CBS 367.62).
A second ITS analysis included reference sequences for accepted species combined with sequences obtained from the herbarium specimens received from China, resulting in 28 sequences with a total length of alignment length of 564 bases, with 66 informative or unique sites. The TrN model with a gamma-distributed rate variation was suggested as the best model. After discarding the burn-in phase trees, the multi-gene Bayesian analysis resulted in 1 202 trees from both runs, from which the majority rule consensus tree and posterior probabilities were calculated (Fig. 2). Results of the phylogenetic analyses and data derived from the morphological observations of the herbarium species are discussed in the section, “Additional notes on Cephalotrichum” below.
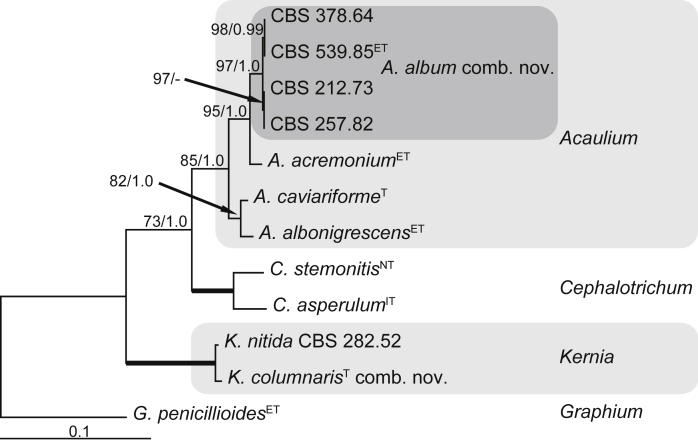
A third phylogeny, based on LSU and ITS sequences, was used to demonstrate the placement of C. album and C. columnare outside Cephalotrichum. The alignment contained sequences from 12 isolates and had a total length of 1 453 characters, with respectively 78 informative or unique characters in the LSU, and 183 in the ITS. The TrN model with a gamma-distributed rate variation was suggested as the best model for the LSU and the GTR model with a gamma-distributed rate variation for the ITS. After discarding burn-in phase trees, the multi-gene Bayesian analysis resulted in 1 502 trees from both runs, from which the majority rule consensus tree and posterior probabilities were calculated (Fig. 3). As a result, new combinations are proposed below for these two species in the “Taxonomy” section.
Fig. 3.
Maximum likelihood tree based on the LSU and ITS sequences of 12 isolates. The RAxML bootstrap support values ≥75 % (BS) and Bayesian posterior probabilities ≥0.95 (PP) are given at the nodes. Thickened lines indicate a BS of 100 % and a PP of 1.0. Ex-type strain numbers are indicated with T (or ET,NT, when ex-epitype or ex-neotype respectively). The tree was rooted to Graphium penicillioides (CBS 102632).
Morphology
In most Cephalotrichum species, both mononematous conidiophores and synnemata occur, either in equal abundance or with one more prevalent, with the distinction between them not always clear. Mononematous conidiophores tend to be more highly branched than those in synnemata, but vary from (i) single, lateral conidiogenous cells to, (ii) monoverticillate conidiophores to, (iii) irregularly biverticillate to terverticillate (i.e. generally penicillate) structures with metulae and/or branches, or (iv) verticillate conidiophores with 2–4 levels of whorls of conidiogenous cells. In the branched conidiophores, conidiogenous cells tend to occur in whorls of 3–7 conidiogenous cells. In synnemata, the conidiophores are usually less branched than in the mononematous form, often arising in a palisade directly from the stipe of the hyphae, or more often with 2–3 conidiogenous cells arising from a lateral metula, and rarely with more levels of branching. Although we provide some observations on conidiophores in our descriptions of new species below, we have no evidence that the branching patterns of either mononematous or synnematous conidiophores have diagnostic value for species. As is common with many synnematous hyphomycetes, some strains have a reduced ability to produce well-developed synnemata with repeated transfer, and sometimes stop producing them completely.
Taxonomy
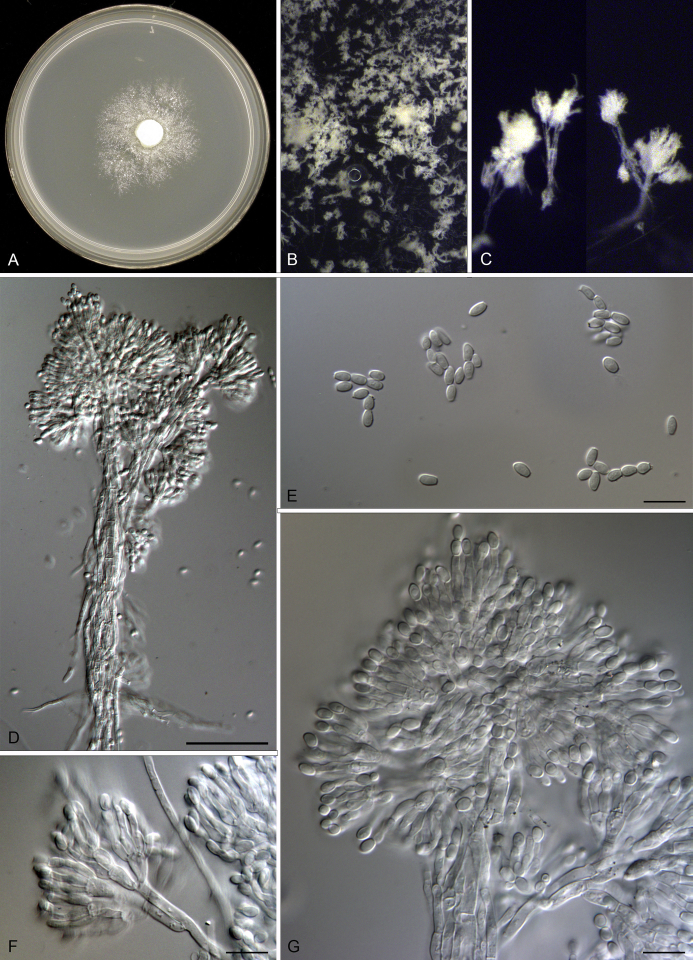
Acaulium album Seifert & Woudenb., comb. nov. MycoBank MB821421. Fig. 4, Fig. 5.
Fig. 4.
Acaulium album DAOMC 226656. A. Four weeks old colony on cornmeal agar. B. Colony surface showing white conidial columns. C. Individual synnemata in side view. D. Synnema with apical conidiophores and broader central hypha. E. Conidia. F. Individual conidiophore on side of synnema. G. Top of synnema showing divergent conidiophores. Scale bars: D. 50 μm. E–G. 10 μm.
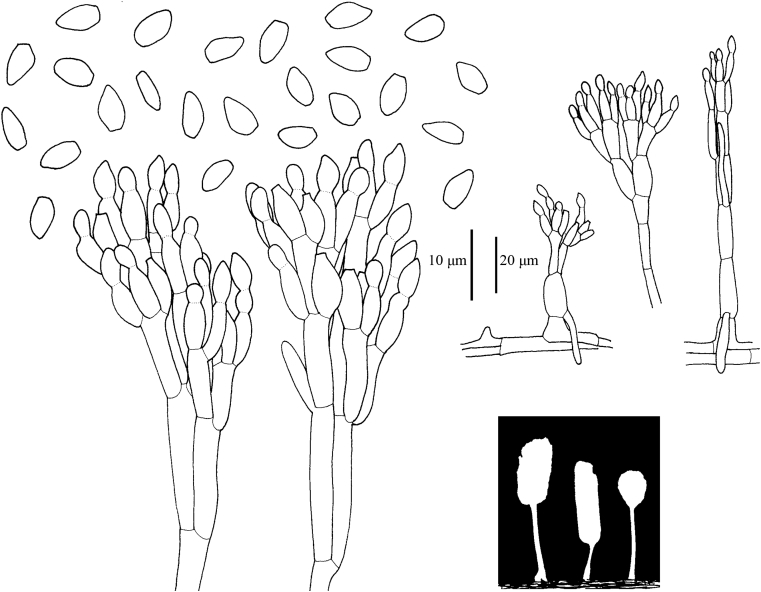
Fig. 5.
Line drawings illustrating conidiophores and conidia of Acaulium album.
Basionym: Synpenicillium album Costantin, Bull. Soc. Mycol. Fr. 4: 62. 1888.
≡ Coremium album (Costantin) Sacc. & Traverso, Syll. fung. 22: 1444. 1913.
≡ Cephalotrichum album (Costantin) Seifert, CBS Biodiversity Series 12: 309. 2013.
Synonyms: Penicillium costantini Bainier, Bull. Soc. Mycol. Fr. 4: 67. 1888. [Non Penicillium album Preuss 1851] ≡ Scopulariopsis costantini (Bainier) Dale, Ann. Mycol. 12: 57. 1914.
Stysanus putredinis Corda, Icon. fung. (Prague) 3: 12. 1839 fide Morton & Smith 1963 ≡ Doratomyces putredinis (Corda) F.J. Morton & G. Sm., Mycol. Pap. 86: 83. 1963. Non Graphium putredinis (Corda) S. Hughes, Can. J. Bot. 36: 770. 1958 ≡ Parascedosporium putredinis (Corda) Lackner & de Hoog, IMA Fungus 2: 44. 2011.]
Acaulium fulvum Sopp, Skr. VidenskSelsk. Christiania, Kl. I, Math.-Natur., no. 11: 67. 1912 (fide Morton & Smith 1963, but synonymy rejected by Abbott (2000) because of discrepancies in spores sizes, and in the absence of a type specimen).
Conidiophores often mononematous in vitro, astipitate, or with a short stipe up to 250 μm tall, then monoverticillate, or irregularly biverticillate or terverticillate, or reduced to single conidiogenous cells; structures with similar dimensions to those in synnemata. Synnemata on the natural substrate scattered or caespitose, up to 500–700(–1 000) μm tall, stipes white, cream-coloured or eventually very pale brown, 10–45 μm wide, unbranched or with 1–3 side branches, conidial heads hyaline to white, divergent or feathery about 20–65 μm wide and tall. Hyphae of stipe hyaline, smooth walled, in two zones: an outer region of parallel hyphae 2.5–4.5 μm wide; surrounding a central broader hypha 7–11 μm wide, with individual cells (10–)20–45 μm long. Setae absent. Conidiophores in synnemata irregularly biverticillate or terverticillate, branches 16–22 × 3–4 μm, metulae 9–13 × 3–3.5 μm. Conidiogenous cells percurrent, ampulliform, hyaline, smooth-walled, 6–8.5 μm long, 2.5–3 μm broad at the widest part, with a distinct shoulder tapering to a cylindrical annellated zone 1.5–2.5 μm wide, up to 6.5 μm long, annellations inconspicuous; in terminal whorls of 3–6. Conidia obovoid, ellipsoidal to irregularly fusiform with a truncate base and rounded or bluntly pointed apex, 4.5–6.5(–7) × 2–3(–4) μm, hyaline, smooth and slightly thick-walled, in long, dry, basipetal chains, sometimes sticking laterally and forming columns up to 1 mm long. Chlamydospores abundant in culture, sometimes also in the synnema stipe, globose to ellipsoidal, 4–8 × 4–5 μm, single or in pairs, cyanophilous. Sexual morph not observed.
Culture characteristics: Colonies on OA 46–47 mm diam after 14 d at 25 °C, planar to low convex, powdery, white to cream-coloured centre with hyaline outer ring, margin discrete, undulate. On MEA 44–45 mm diam, planar to low convex, powdery, white to cream with inconspicuous concentric rings about 1 mm apart, margin undulate.
Specimens and cultures examined: Canada, British Columbia, Vancouver, University of British Columbia (UBC), near University Golf Club, from decaying Coprinus micaceus, July 1981, J. A. MacKinnon, CBS 257.82 = ATCC 46569 (DAOM 230530); British Columbia, Vancouver, UBC Campus, 22 Oct. 1980, R.J. Bandoni, CBS H-3873; Same location, UBC chicken coop, 19 Jan. 1980, on chicken droppings, R.J. Bandoni & T. Thompson, CBS H-3874. Same location, UBC Experimental Garden, 2 Feb. 1981, on rotting potato, J.A. MacKinnon, CBS H-3872. Ontario, Ottawa-Carleton Twp, Bell's Corners, 9 May 1998, on bear dung (Ursus americanus), Keith A. Seifert no. 521 (DAOM 226656). Czech Republic, Prague, on rotting stems of Echium sp., 1838, Fieber (holotype of Stysanus putredinis, PR-C 155673). Denmark, from bumble-bee queen, collection date unknown, J.P. Skou, CBS 378.64. Netherlands, Hoogland, near Amersfoort, from hair in dung of pole cat (Mustela putorius), March 1984, H.A. van der Aa, (epitype designated here CBS H-12128, MBT376922, culture ex-epitype CBS 539.85); Wageningen, from soil, collection date unknown, J.H. van Emden, CBS 212.73. USA, Maine, Kittery Point, from decaying seaweed, 1918, R. Thaxter (FH).
Notes: De Beer et al. (2013, p. 309) briefly reviewed the history of the epithet ‘putredinis’ and its contradictory use in Graphium and Doratomyces, which need not be repeated in detail here. Because the epithet putredinus is now used in Parascedosporium but previously was being used for two distinct species (one by Hughes 1958, the other by Morton & Smith 1963), it was necessary to transfer the next available epithet, i.e. from Synpenicillium album, to Cephalotrichum. The recent treatment by Sandoval-Denis et al. (2016b) noted morphological similarities between this species (as C. album) and other asexual species included in the Acaulium clade by molecular evidence, but did not redescribe or reclassify this white species. The phylogenetic analysis presented here (Fig. 3) shows that this species does not belong to Cephalotrichum, and is best classified as a synnematous species of Acaulium as suggested by Sandoval-Denis et al. (2016b).
Acaulium album is a relatively infrequently reported asexual fungus, but is broadly distributed in Europe and North America on heavily decayed organic material and various kinds of (often carnivore) dung. Apart from the white synnemata and spores, it is distinctive because of the broad hypha in the centre of the synnema stipe. Developmentally, the synnemata are rather odd. The cells of the broad central hypha produce narrower hyphae growing upward near the top of the cells or hyphae growing downward from the bottom of the same cells, all appressed to the central cylinder to make up the stipe. The downward growing hyphae anchor the conidiomata to the substrate; the upward growing hyphae branch to become the conidiophores. Similar downward growing hyphae, and broader core hyphae, are sometimes seen in the synnema stipes of true Cephalotrichum species (Lodha, 1963, Swart, 1964) but are more difficult to see because of the pigmentation of the cells.
Costantin (1888) described his fungus from panther dung from an unreported location, but probably from a zoo in Paris. Morton & Smith (1963) did not locate a type, but considered the identity of the fungus clear from the published illustration. There is a discrepancy in spore sizes, Costantin (1888) reporting dimensions of 7–13 × 3–6 μm, roughly double the size reported here, but given our observations of several specimens of the relatively common species described above, suspect this is probably a measurement error by Costantin (1888). We designate CBS H-12128, isolated from a hair in pole cat dung, as epitype above to further stabilise the species name.
Synpenicillium is an older name than Acaulium but has rarely been used after its original publication in 1888 (Costantin). Acaulium has generally been considered a synonym of Scopulariopsis but recently was re-instated as an accepted genus of Microascaceae with three species (Sandoval-Denis et al. 2016b). Although both names are relatively obscure, we see no reason to resurrect Synpenicillium for this clade. We will propose protection of Acaulium be added to the list of protected generic names now being discussed by the nomenclatural community.
Cephalotrichum domesticum Woudenb. & Seifert sp. nov. MycoBank MB819314. Fig. 6.
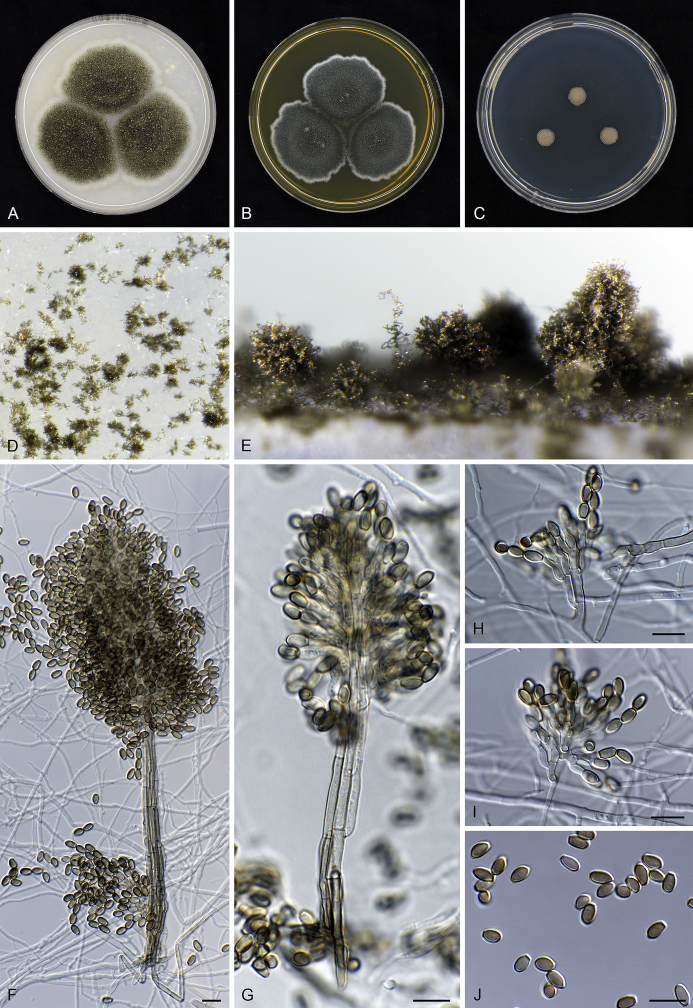
Fig. 6.
Cephalotrichum domesticum sp. nov. CBS 142035. A–C. Fourteen day old colonies on OA (A), MEA (B) and DG18 (C). D–G. Synnemata. H–I. Conidiophores, conidiogenous cells and conidia. J. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the usual occurrence of this species in the built environment, or other environments manipulated by humans, such as farms.
Mononematous conidiophores abundant among and intergrading with synnemata, hyaline to pale brown, monoverticillate, or irregularly biverticillate to terverticillate, sometimes 3–4- level verticillate, with a short stipe 7–25 μm tall, terminating in 3–5(–7) annellides on cylindrical, clavate or swollen metulae 5–8 × 2.5–3.5 μm; branches appressed or divergent, 10–12 × 2–3 μm. Synnemata 130–225(–245) μm tall, stipes pale brown, 7–10 μm wide, composed of rather loosely attached hyphae; conidial heads brown, subglobose to ellipsoidal. Hyphae of stipe parallel, 2–4 μm wide, pale-brown, slightly thick-walled. Setae absent. Conidiophores in synnemata monoverticillate, irregularly biverticillate or conidiogenous cells arising directly from the stipe hyphae, with all elements tightly appressed, metulae 5.5–8.5 × 2–2.5 μm. Conidiogenous cells ampulliform, (5.5–)6–7.5(–8) μm long, 2.5–3(–3.5) μm broad at the widest part, tapering gradually to a cylindrical annellate zone 1.5–2 μm wide, hyaline to pale brown, smooth-walled. Conidia ellipsoidal to cylindrical with truncate base and rounded or pointed apex, 5.5–6(–6.5) × 3–3.5(–4) μm, pale brown to brown, smooth and thick-walled, in basipetal chains. Sexual morph not observed.
Culture characteristics: Colonies on OA 52–55 mm diam after 14 d at 25 °C, low convex, felty, white with iron grey synnemata, margin crenate. On MEA 45–50 mm diam, low convex, finely felty, white with olivaceous grey to iron grey centre, margin uneven. On DG18 13–15 mm diam, planar, finely felty, white with iron grey zones, margin undulate.
Specimens examined: Netherlands, Limburg, from manure, Mar. 1942, P.J. Bels, CBS 139.42 = IFO 7677 = MUCL 4025; Utrecht, dried culture of strain isolated from indoor air of home (kitchen), 28 Aug. 2008, J. Houbraken, (holotype CBS H-22856, culture ex-type CBS 142035); from plaster, before Sept. 1967, H.J. Hueck, CBS 395.67; from mushroom compost, 1950, H.C. Bels-Koning, CBS 255.50 = MUCL 4037.
Notes: Only the ex-type culture CBS 142035 produced synnemata in culture. This phenomenon has also been reported for C. purpureofuscum, where several colonial variants can be obtained by spontaneous sectoring (Domsch et al. 2007). Although morphologically C. domesticum resembles C. purpureofuscum, it can easily be separated from that based on any of the three genes studied here (see Discussion section notes regarding C. purpureofuscum). Given the similar morphology, frequently defined by lack of distinctive characters, examination of a larger number of isolates for C. domesticum and C. purpureofuscum is needed to adequately assess whether any consistent morphological features could be used to identify the species that are clearly distinct based on molecular data. Phylogenetically C. domesticum is closely related to C. tenuissimum. The smaller synnemata of C. domesticum (130–245 μm tall vs. 495–900 μm tall) and faster growth on OA (52–55 mm diam vs. 40 mm) and MEA (45–50 mm diam vs. 30 mm) at 25 °C can be used to distinguish the two species.
Cephalotrichum lignatile Woudenb. & Seifert sp. nov. MycoBank MB819309. Fig. 7.
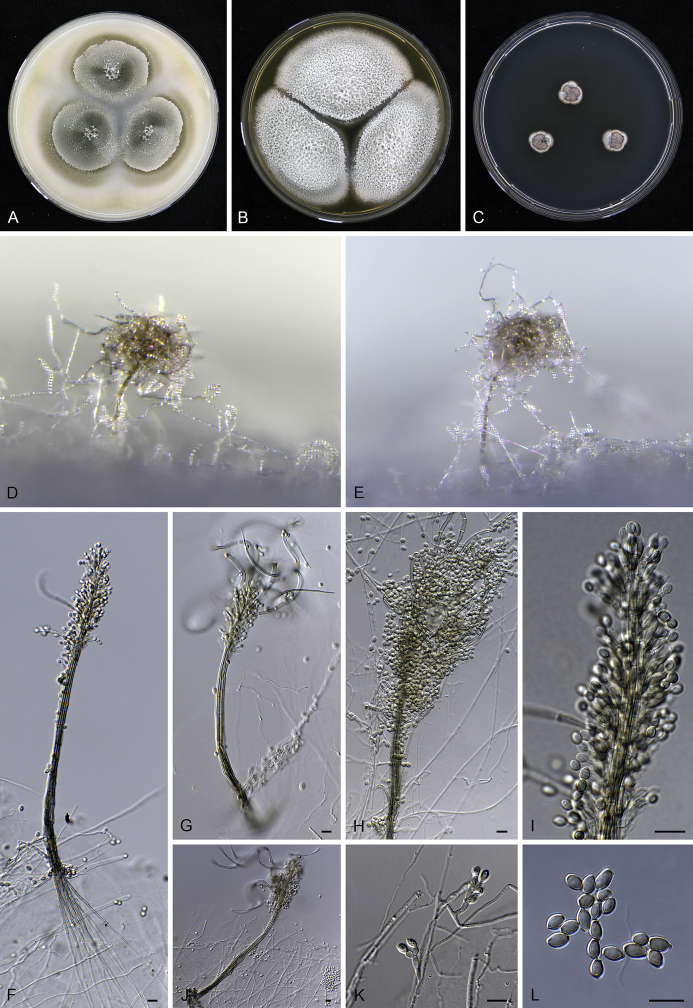
Fig. 7.
Cephalotrichum lignatile sp. nov. CBS 209.63. A–C. Fourteen day old colonies on OA (A), MEA (B) and DG18 (C). D–F. Synnemata. G. Detail of the apical portion of a synnema. H–I. Conidiophores, conidiogenous cells and conidia. J. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the substrate of isolation, timber.
Mononematous conidiophores moderately abundant among synnemata, pale brown, mostly monoverticillate or biverticillate with a short stipe 4–10(–14) × 2–3 μm, sometimes 2-level verticillate, with (2–)3–5 annellides in compact whorls on slightly divergent, cylindrical to slightly clavate metulae 7–8 × 1.5–2.5 μm. Synnemata (280–)300–445(–465) μm tall, stipes pale brown to brown, (5.5–)6–8.5 μm wide, unbranched, conidial heads hyaline to pale brown, ellipsoidal or cylindrical. Hyphae of stipe parallel, 2–2.5 μm wide, pale-brown, slightly thick-walled. Setae absent. Conidiophores in synnemata irregularly monoverticillate, biverticillate or 2–3(–4)-level verticillate with all elements tightly appressed, metulae 6–8 × 2–3(–3.5) μm. Conidiogenous cells ampulliform, hyaline to pale brown, smooth-walled, (5.5–)6–7(–7.5) μm long, 2.5–3 μm broad at the widest part, tapering gradually to a cylindrical annellate zone (1–)1.5(–2) μm wide, annellations inconspicuous. Conidia obovoid to irregularly fusiform with truncate base and rounded or bluntly pointed apex, (4.5–)5–6(–6.5) × (2.5–)3–3.5(–4) μm, pale brown to brown, smooth and thick-walled, in basipetal chains. Chlamydospores absent. Sexual morph not observed.
Culture characteristics: Colonies on OA 36 mm diam after 14 d at 25 °C, planar to low convex, finely felty, dull green centre with white outer ring, margin uneven. On MEA 35 mm diam, low convex, felty, white to buff with pale olivaceous grey zones, radially striate, margin undulate. On DG18 14 mm diam, low convex, finely felty, buff with rosy buff centre, radially striate, margin uneven.
Specimen examined: Belgium, Han-sur-Lesse, dried culture of strain isolated from timber in a cave, 1959, G.L. Hennebert, (holotype CBS H-22852, culture ex-type CBS 209.63).
Notes: The sequences of CBS 209.63, which we describe here as C. lignatile, are identical to the sequences previously published for CBS 159.66 as C. columnare (Sandoval-Denis et al. 2016b). However, the morphology of CBS 209.63 does not match CBS 159.66. We also sequenced older batches of CBS 159.66 from the CBS collection to exclude the possibility of a mix-up, but all gave identical sequence results, placing C. columnare in the genus Kernia, where it is transferred below. The sequences associated with this strain by Sandoval-Denis et al. (2016b) seem to contain an error that we can't explain.
Cephalotrichum telluricum Woudenb. & Seifert sp. nov. MycoBank MB819318. Fig. 8.
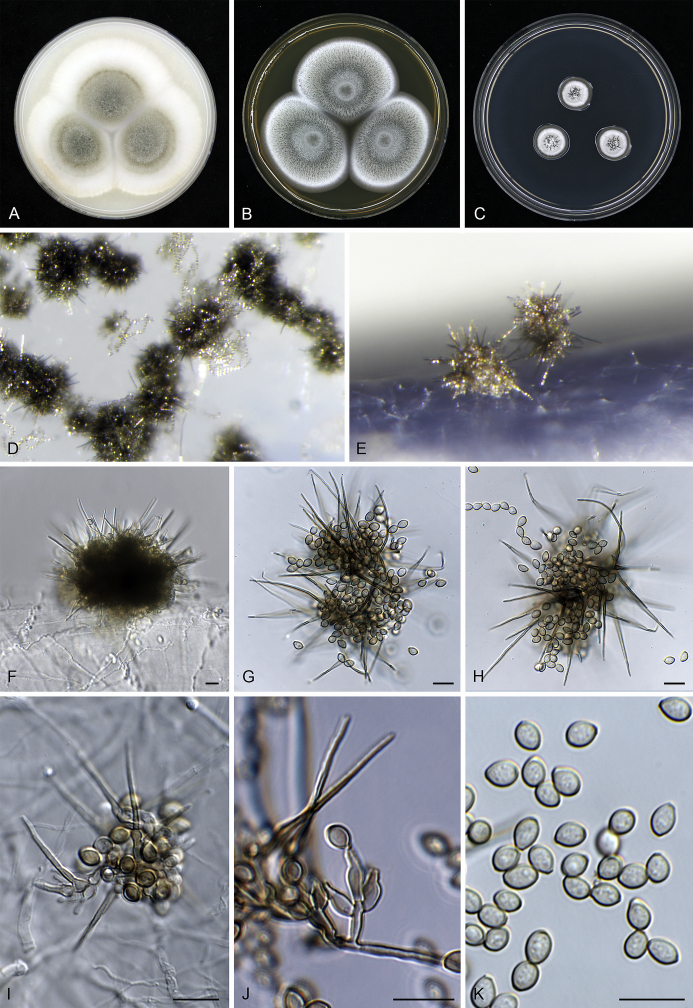
Fig. 8.
Cephalotrichum telluricum sp. nov. CBS 336.32. A–C. Fourteen day old colonies on OA (A), MEA (B) and DG18 (C). D–H, J. Synnemata. I. Detail of the apical portion of a synnema. K. Conidiophores, Conidiogenous cells and conidia. L. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the substrate of isolation, soil.
Mononematous conidiophores sparse among synnemata, hyaline, unbranched to monoverticillate or irregularly biverticillate, bearing 1–3 annellides on cylindrical or swollen metulae 7–12 × 2.5–3 μm. Synnemata 260–424(–490) μm tall, stipes pale brown to brown, 8.5–11.5(–12.5) μm wide, conidial heads pale brown to brown, subglobose or ellipsoidal. Hyphae of stipe parallel, 2–4 μm wide, pale-brown, slightly thick-walled, wider hyphae in the centre of the stipe near the base, downward growing branches occurring near the base of the synnemata. Setae coiled, simple, septate, pale brown, individually up to about 180 μm long, 3–4.5 μm wide, extending about 70–100 μm beyond the level of the conidiogenous cells with a rounded or acute apex. Conidiophores in synnemata solitary and lateral on stipe hyphae, or monoverticillate, metulae 6–8 × 2–3 μm. Conidiogenous cells ampulliform, (5.5–)6.5–8(–8.5) μm long, 2.5–3.5(–4) μm broad at the widest part, tapering gradually to a cylindrical annellate zone (1–)1.5–2 μm wide, hyaline, smooth-walled. Conidia ovoid to broad fusiform with truncate base, (5.5–)6–7.5(–8) × (3.5–)4–4.5 μm, pale brown, smooth, thick-walled, in basipetal chains. Sexual morph not observed.
Culture characteristics: Colonies on OA 65 mm diam after 14 d at 25 °C, planar, finely felty with tufts of mycelium, grey olivaceous with pale greenish grey mycelium and (pale) olivaceous grey sectors in the centre and a white outer ring, margin entire. On MEA 60–62 mm diam, planar to low convex, floccose, white with pale greenish grey ring and lavender grey to smoke grey regions, margin entire. On DG18 10–12 mm diam, raised, finely felty, cinnamon with olivaceous grey zones and white outer ring, margin undulate.
Specimens examined: Canada, Ontario, Vineland, from soil, Sep. 1949, R.F. Cain, CBS 568.50 = TRTC 12269. Cyprus, Nicosia, dried culture of strain isolated from soil, before Jan. 1932, R.M. Nattrass (holotype CBS H-22853, culture ex-type CBS 336.32 = MUCL 9829 = UAMH 8882).
Notes: Based on morphology and phylogeny, C. telluricum is closely related to C. gorgonifer. Both species have spirally coiled setae, but the synnemata of C. telluricum (<500 μm tall) are shorter than those of C. gorgonifer (500–1 000 μm). Based on sequence data C. telluricum can be distinguished from C. gorgonifer by all three genes, with ITS having 5 nt differences, tub2 15 nt, and tef1 3 nt between C. telluricum and the ex-epitype isolate of C. gorgonifer, CBS 635.78.
Cephalotrichum tenuissimum Woudenb. & Seifert sp. nov. MycoBank MB819317. Fig. 9.
Fig. 9.
Cephalotrichum tenuissimum sp. nov. CBS 127792. A–C. Fourteen day old colonies on OA (A), MEA (B) and DG18 (C). D–G. Synnemata. H. Detail of the apical portion of a synnema. I–J. Conidiophores, conidiogenous cells and conidia. K. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the slender synnemata.
Mononematous conidiophores fairly frequent among synnemata, biverticillate to terverticillate but often irregular, bearing 3–5 annellides on cylindrical or swollen metulae 5–8.5 × 2–3 μm; branches divergent, 7.5–12.5 × 2–3 μm; stipe 5–20(–70) × 2–3 μm. Synnemata (495–)630–895(–900) μm tall, stipes pale brown to brown, (14–)15–21(–24.5) μm wide, conidial heads pale brown, obclavate. Hyphae of stipe parallel, brown, 1.5–2.5 μm wide. Setae absent. Conidiogenous cells ampulliform, (5–)6–8(–8.5) μm long, 2.5–3.5 μm broad at the widest part, tapering gradually to a cylindrical annellated zone 1–1.5(–2) μm wide, hyaline, smooth-walled, usually singly and arising at +/− right angles from hyphae of the stipe, sometimes in groups of 2–3 on short metulae. Conidia ellipsoidal with truncate base and rounded apex, (4.5–)5–6(–6.5) × (3–)3.5–4 μm, hyaline to pale green-brown, smooth, thick-walled, single or in short chains. Sexual morph not observed.
Culture characteristics: Colonies on OA 40 mm diam after 14 d at 25 °C, planar, felty, colourless with olivaceous grey synnemata, margin uneven. On MEA 30 mm diam, low convex to umbonate, felty, olivaceous grey with thin pale olivaceous grey ring, radially striate, margin crenated. On DG18 15 mm diam, crateriform, pale olivaceous grey with buff edge, radially striate, margin uneven.
Specimen examined: USA, Wyoming, Hanna, dried culture of strain isolated from soil, 1976, M. Christensen (holotype CBS H-22855, culture ex-type CBS 127792 = RMF H 318).
Notes: Based our phylogenies, C. tenuissimum is closely related to C. domesticum and C. dendrocephalum. Morphologically it is easily distinguished from C. domesticum (see notes under C. domesticum) and C. dendrocephalum, which forms characteristic, undulating, branched setae. The ex-type isolate of C. tenuissimum (CBS 127792) was originally identified in CBS as C. microsporum. However, C. tenuissimum can be distinguished from C. microsporum by the size of the conidia, 5–6 × 3.5–4 for C. tenuissimum vs. 3.5–5 × 2–3 μm for C. microsporum.
Cephalotrichum transvaalense Woudenb. & Seifert sp. nov. MycoBank MB819312. Fig. 10.
Fig. 10.
Cephalotrichum transvaalense sp. nov. CBS 448.51. A–C. Fourteen day old colonies on OA (A), MEA (B) and DG18 (C). D–H. Synnemata. I. Detail of synnema setae. J. conidiophores, conidiogenous cells and conidia K. Conidia. Scale bars = 10 μm.
Etymology: The name refers to the Transvaal Province in South Africa, where the ex-type strain was first isolated.
Mononematous conidiophores monoverticillate to irregularly biverticillate, or 2–3-level verticillate. Synnemata 500–1 500 μm tall, stipes brown, 15–35 μm wide, unbranched, conidial heads pale brown to brown, ellipsoidal; sessile conidiomata lacking a stipe present in some transfers, forming brown to black, subglobose conidial tufts. Hyphae of stipe parallel, 2–4 μm wide, pale-brown, slightly thick-walled. Setae straight, aseptate, brown, (40–)70–110 μm long, 1–1.5 μm wide, base sometimes swollen to 2–2.5 μm wide, unbranched, bifurcate (90–120°) or with several layers of basal branching; sometimes arising from the same hyphae or metulae as conidiogenous cells. Conidiophores in synnemata mostly biverticillate, sometimes monoverticillate, metulae divergent, 5–8 × 2–3 μm. Conidiogenous cells ampulliform, 6–8(–10) μm long, 2–3 μm broad at the widest part, tapering abruptly to a cylindrical annellate zone 1–1.5 μm wide, pale brown, smooth-walled. Conidia ovoid to ellipsoidal with small truncate base and rounded apex, (4.5–)5–6.5 × 3–4.5 μm, pale brown, smooth, thick-walled, in basipetal chains. Sexual morph not observed.
Culture characteristics: Colonies on OA 53 mm diam after 14 d at 25 °C, planar, felty, white with olivaceous grey ring and pale olivaceous grey centre, margin uneven. On MEA 48 mm diam, low convex, wooly, white to (pale) olivaceous grey, margin entire. On DG18 15 mm diam, crateriform, planar, olivaceous grey with white to pale olivaceous grey centre, radially striate, margin entire.
Specimen examined: South Africa, Transvaal, dried culture of strain isolated from Eucalyptus saligna timber in cellar, 1951, leg. Bekker (holotype CBS H-22854, culture ex-type CBS 448.51 = IFO 7660 = IMI 046251 = LSH BB344 = UAMH 8848).
Notes: With the straight setae arising from the conidial head, C. transvaalense morphologically resembles C. cylindricum and the holotype strain was identified as this species in the past. IMI 46251 was used as the basis for the description of C. cylindricum (as Trichurus terrophilus) by Lodha (1963) and Swart (1964); their illustrations and descriptions indicate well-developed synnemata and some aspects of our description are adapted from these sources. Because the strain no longer makes well-developed synnemata, we have adapted measurements and details from these descriptions in our technical description above. Cephalotrichum transvaalense and C. cylindricum are closely related but distinct based on molecular data. The ITS sequences have no differences, but 20 nt differences in tub2 and 9 nt differences in tef1 sequences clearly distinguish the two species.
Kernia columnaris (H.J. Swart) Woudenb. & Samson comb. nov. MycoBank MB820690.
Basionym: Doratomyces columnaris H.J. Swart, Acta Bot. neerl. 15: 521. 1967 ≡ Cephalotrichum columnare (H.J. Swart) S.P. Abbott, Stud. Mycol. 83:206. 2016.
Descriptions and illustrations: Swart, 1967, Abbott, 2000, Sandoval-Denis et al., 2016a, Sandoval-Denis et al., 2016b.
Specimen examined: South Africa, Johannesburg, Melville Koppies Nature Reserve, from dung of hare, 1964, H.J. Swart (culture ex-type CBS 159.66 = IMI 116691).
Notes: As noted, sequences previously published for CBS 159.66 (Sandoval-Denis et al. 2016b) match sequences of CBS 209.63, which we describe above as C. lignatile. To exclude the possibility of mislabelling in the CBS collection, we also studied older preservation batches of CBS 159.66 from the CBS collection, which all yielded identical sequence results that convincingly place this species in the genus Kernia (Fig. 3). The affinity of this species with the latter genus rather than Cephalotrichum was already suggested by Abbott (2000), based on significantly discordant morphological characters recorded from several isolates, including its reduced conidiophores (50–700 μm), mostly mononematous or more rarely synnematous with poorly developed, hyaline stipes, which resemble more to those of the synnematous anamorphs of Kernia hippocrepida and K. pachypleura (Malloch & Cain 1971). Other relevant features of K. columnaris are: annellides subcylindrical to ampulliform, conidia ellipsoid, often slightly asymmetrical, apex rounded or bluntly pointed, smooth, 5–6 × 2.5–4 μm, commonly 5.5 × 3 μm, colonies grey to brown, slow growing (20–30 mm in 14 d at 25 °C). The mentioned characteristics match with the morphological treatment of the species by Sandoval-Denis et al., 2016a, Sandoval-Denis et al., 2016b, suggesting that their illustrations are based on the actual ex-type strain of K. columnaris, while the sequence discrepancy most likely respond to a sequencing overlap.
Additional notes on Cephalotrichum
Recently, 14 new Cephalotrichum species were described based on isolates from soil from China (Jiang and Zhang, 2008, Jiang et al., 2011). The status of those species, only known from their holotypes, could not be thoroughly evaluated by Sandoval-Denis et al. (2016b) because of the unavailability of material. Recently, portions of the holotypes materials were donated to the CBS herbarium collection by the authors of these names, and the species could be re-evaluated in our study. The conclusions derived from the morphological analysis of these specimens, and associated DNA sequences, are as follows:
Accepted species
Cephalotrichum castaneum (Y.L. Jiang & T.Y. Zhang) Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 224. 2011. Fig. 11.
Fig. 11.
Cephalotrichum castaneum isotype CBS H-22781. A–F. Synnemata. G. Detail of the apical portion of a synnema. H. Conidiophore, conidiogenous cells and conidia. I. Conidia. Scale bars = 10 μm.
Synonym: Doratomyces castaneus Y.L. Jiang & T.Y. Zhang, Mycotaxon 104: 131. 2008.
Specimen examined: China, Guizhou Province, Guiyang, Huaxi Park, dried culture isolated from grassland soil, Oct. 6. 2005, Y.L. Jiang (holotype of D. castaneus HSAUPII051034 → isotype CBS H-22781).
Notes: This species is morphologically very similar to C. microsporum. It produces synnemata of almost identical size and shape, and conidia of similar size. However, C. castaneum is easily identifiable by its dark brown, spherical to subspherical conidia, which contrast to the green-brown, oval to ellipsoidal conidia of C. microsporum. Our gene tree based on ITS sequences (Fig. 2) showed C. castaneum to be genetically distant from C. microsporum, clustering without strong bootstrap support as a sister species of C. dendrocephalum, C. domesticum and C. tenuissimum. However, significant morphological differences exist among these species. The taller synnemata and conidial size and shape distinguish C. castaneum from C. domesticum and C. tenuissimum, while the absence of setae in the synnemata of C. castaneum differentiates it from C. dendrocephalum.
Cephalotrichum ellipsoideum H.Q. Pan & T.Y. Zhang, Mycotaxon 117: 211. 2011. Fig. 12.
Fig. 12.
Cephalotrichum ellipsoideum isotype CBS H-22783. A–D. Synnemata. E. Detail of the apical portion of a synnema. F. Conidiogenous cells and conidia. G. Conidia. Scale bars = 10 μm.
Specimen examined: China, Qinghai Province, Maduo, dried culture isolated from grassland soil, Jun. 8. 2007, H.Q. Pan (holotype HSAUPII074053 → isotype CBS H-22783, additional isotype HMAS196224).
Notes: The dimensions of synnemata given in the protologue do not correlate with those observed in the type material. The length and robustness of the synnematal stipe and the shape of the conidial head are the main characters that distinguish this species from other Cephalotrichum spp. In C. ellipsoideum, the synnemata are much more robust (<2 500 μm tall, with stipes <125 μm wide) with an obclavate and elongated conidial head, often tapering towards the apex. Other Cephalotrichum spp. with synnemata of similar size are C. stemonitis and C. verrucisporum; C. ellipsoideum differs from C. stemonitis by the absence of echinobotryum-like morph, and C. verrucisporum by its smooth conidia, in contrast to the markedly verrucose and pointed conidia of C. verrucisporum.
Cephalotrichum spirale H.M. Liu, H.Q. Pan & T.Y. Zhang, Mycotaxon 117: 220. 2011. Fig. 13.
Fig. 13.
Cephalotrichum spirale isotype CBS H-22790. A–C. Synnemata. D. Detail of the apical portion of a synnema. E. Conidiophores, conidiogenous cells and conidia. F. Conidia. Scale bars = 10 μm.
Specimen examined: China, Qinghai Province, Dari County, dried culture isolated from grassland soil, Jun. 12. 2007, H.Q. Pan (holotype HSAUPII074033 → isotype CBS H-22790, additional isotype HMAS196233).
Notes: The type material contains two fungi, the synnematous C. spirale and a Cladosporium spp., the second probably a culture contaminant judging from its sparse presence. Morphologically, C. spirale resembles C. asperulum and C. nanum, and all species have distinctly verrucose conidia. Cephalotrichum spirale differs from C. asperulum mainly by the conidial shape, with rounded apices in C. spirale vs. pointed apices in C. asperulum, and the degree of conidial roughness, which is not as pronounced in C. asperulum, which sometimes has conidia that are smooth. Cephalotrichum spirale differs from C. nanum by the size of its synnemata (<850 μm tall in C. spirale vs. <2 000 μm tall in C. nanum) as well as by conidial size and shape (5–7 × 3–4.5 μm, broadly ovoid to broadly ellipsoidal in C. spirale vs. 6–8 × 4.5–7.5 μm, subspherical to oval in C. nanum). Cephalotrichum verrucisporum is the closest relative genetically (ITS 2 nt difference, Fig. 2) but the two species are easily differentiated morphologically (see note under C. verrucisporum below). Note that this species is different from the well-known fungus described as Trichurus spiralis, but the coincidental epithets in Cephalotrichum led to the adoption of the later species name C. gorgonifer for the latter fungus.
Cephalotrichum verrucisporum (Y.L. Jiang & T.Y. Zhang) Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 223. 2011.
Synonym: Doratomyces verrucisporus Y.L. Jiang & T.Y. Zhang, Mycotaxon 104: 133. 2008.
Specimens examined: China, Guizhou Province, Guiyang, Huaxi Park, dried culture isolated from mountain soil, Oct. 6. 2005, Y.L. Jiang (holotype of D. verrucisporus HSAUP051029 → isotype CBS H-22793); Germany, from indoor environment, 2007, DTO 055-D7; Netherlands, Katwijk, from sand dune (50 cm depth), Mar. 1978, W. Gams, CBS 187.78; Wageningen, from agricultural soil, Jul. 1972, J.W. Veenbaas-Rijks, CBS 512.72.
Notes: This species was recently accepted as distinct and was illustrated by Sandoval-Denis et al. (2016b) on the basis of the identity of an ITS derived from the type with an available culture (CBS 187.78). Our study of type material confirms that application of this name (Fig. 2), showing this species to be a closely related lineage to C. spirale. Both species produce verrucose conidia. Cephalotrichum verrucisporum can be differentiated by its taller synnemata (<3 000 μm tall vs. <850 μm tall in C. spirale) and its somewhat larger and pointed conidia (6–9 × 3–5.5 μm vs. 5–7 × 3–4.5 μm, with a rounded apex in C. spirale). Cephalotrichum verrucisporum has ovoid conidia that are a bit darker than the oval to ellipsoidal pale brown conidia of C. asperulum, and synnemata that are longer than those of C. asperulum, which are usually ∼1 000 μm tall (Sandoval-Denis et al. 2016b).
Doubtful and excluded species
Cephalotrichum acutisporum J.J Xu & T.Y. Zhang, Mycotaxon 117: 208. 2011.
Specimen examined: China, Fujian Province, Zhangping, dried culture isolated from soil of a park, Oct. 22. 2004, J.J. Xu (holotype HSAUPII042724 → isotype CBS H-22780, additional isotype HMAS196222).
Notes: This species is a synonym of C. purpureofuscum. Synnemata and conidiogenous cells in the isotype material are much larger than indicated in the protologue. According to our observations synnemata are (600–)640–955 μm tall, with conidiogenous cells (8.5–)9.5–10.5 × (2.5–)3–4 μm. Also, the conidial shape, originally described and illustrated as markedly pointed is not a consistent character; nearly half of the conidia observed in the isotype have rounded apices, a pattern commonly observed in C. purpureofuscum.
Cephalotrichum cylindrosporum Y.L. Zhang & T.Y. Zhang, Mycotaxon 117: 209. 2011.
Specimen examined: China, Hainan Province, Tunchang, dried culture isolated from rice field soil, Nov. 1. 2005, Y.L. Jiang (holotype HSAUPII052414 → isotype CBS H-22782, additional isotype HMAS196223).
Notes: The fungus on the isotype is morphologically indistinguishable from C. purpureofuscum. As noted by Sandoval-Denis et al. (2016b), this is a serious discrepancy from the ITS sequence derived from the ex-type (FJ914686), which matches the epitype of C. stemonitis, and hence cannot represent C. purpureofuscum (Fig. 2). It is probable that mislabelling or cross contamination of the original culture occurred, and the name must be regarded as a nomen dubium.
Cephalotrichum inflatum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
Specimen examined: China, Sichuan Province, Mianyang, dried culture isolated from mountain soil, Aug. 8. 2005, Y.L. Jiang (holotype HSAUPII050918 → isotype CBS H-22784, additional isotype HMAS196226).
Notes: This species is a synonym of C. microsporum. The main distinctive feature of C. inflatum was the presence of distinctly swollen cells at the top of the synnemata, from which the conidiogenous cells arise. When cultures were grown on PDA, this feature was also observed in our C. microsporum isolates. Although the ITS sequence of C. inflatum has 1 nt difference from the ex-type strain of C. microsporum (CBS 523.63), it is identical to that of C. microsporum strain DTO 207-C6.
Cephalotrichum longicollum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
Specimen examined: China, Sichuan Province, Emei Mountain, dried culture isolated from soil, Aug. 9. 2005, Y.L. Jiang (holotype HSAUPII050802 → isotype CBS H-22785, additional isotype HMAS196228).
Notes: This species is a synonym of C. purpureofuscum. The original description is inaccurate, according to our observations, with conidiogenous cells 8–11(–12.5) × (2.5–)3–4 μm, and conidia (4–)5–6 × 3–3.5(–4) μm. The isotype material contains only short synnemata 475–500 μm tall; however, synnemata of this stature are not uncommon in C. purpureofuscum. Morton & Smith (1963) examined isolates of C. purpureofuscum with reduced synnemata, as little as 50 μm tall. The morphological identity with C. purpureofuscum is confirmed by DNA data. The ITS sequence from the ex-type of C. longicollum has 1 nt difference from the reference strain of C. purpureofuscum (CBS 174.68, Fig. 2), but is identical to the ITS sequences of the other C. purpureofuscum isolates included in this study (data not shown).
Cephalotrichum macrosporum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 214. 2011.
Specimen examined: China, Sichuan Province, Jiuzhaigou, dried culture isolated from forest soil, Aug. 18. 2005, Y.L. Jiang (holotype HSAUPII050878 → isotype CBS H-22786, additional isotype HMAS196229).
Notes: The isotype is in poor condition. Only fragments of synnema stipes and conidia can be seen, and the fungus is unidentifiable. The conidial dimensions, (4–)5.5–7.5(–10) × (2.5–)3.5–4(–4.5) μm, and unusual conidial shapes, cylindrical, irregularly cylindrical, curved or cashew-nut shaped, reflect the degraded state of the material. The ITS sequence from the ex-type has 1 nt difference with the reference strain of C. purpureofuscum (CBS 174.68, Fig. 2), but is identical to the ITS sequences of the other C. purpureofuscum isolates included in this study (data not shown). Provisionally, C. macrosporum seems to be a synonym of C. purpureofuscum. Fresh isolations of this fungus may clarify this status, or the remainder of the holotype will need to be examined to observe undamaged synnemata.
Cephalotrichum oblongum J.J. Xu & T.Y. Zhang, Mycotaxon 117: 216. 2011.
Specimen examined: China, Yunnan Province, Pingbian County, dried culture isolated from soil, Oct. 11. 2004, J.J. Xu (holotype HSAUPII042723 → isotype CBS H-22787, additional isotype HMAS196230).
Notes: This is a synonym of C. purpureofuscum. The isotype material contains elements of two fungi, synnemata of C. purpureofuscum and numerous mesoconidia and macroconidia of a Fusarium spp. The original description deviates from our observations of the synnemata on the type material. Synnemata are taller than reported, (305–)325–650 μm tall, the conidiogenous cells are shorter and wider, 5.5–8 × 3–4 μm, and the conidia are larger, (4.5–)5–6(–6.5) × (2.5–)3–3.5(–4) μm, all dimensions that fit well with C. purpureofuscum. The ITS sequence has 1 nt difference from the reference strain of C. purpureofuscum (CBS 174.68, Fig. 2), but is identical to the ITS sequences of the other C. purpureofuscum isolates included in this study (data not shown).
Cephalotrichum ovoideum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 217. 2011.
Specimen examined: China, Sichuan Province, Jiuzhaigou, dried culture isolated from forest soil, Aug. 18. 2005, Y.L. Jiang (holotype HSAUPII050846 → isotype CBS H-22788, additional isotype HMAS196231).
Notes: Based on the original publication, this species could be synonymised with C. microsporum. However, the isotype material is in poor condition. Only conidia were observed, while synnema stipes and conidial heads were not well preserved. The ITS phylogeny suggests a close affinity with both C. microsporum and C. robustum. Until the remainder of the holotype can be examined, or a fresh isolate obtained, C. ovoideum should be considered a provisional synonym of C. microsporum.
Cephalotrichum robustum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 218. 2011.
Specimen examined: China, Sichuan Province, Jiuzhaigou, dried culture isolated from forest soil, Aug. 18. 2005, Y.L. Jiang (holotype HSAUPII050875 → isotype CBS H-22789, additional isotype HMAS196232).
Notes: This fungus morphologically resembles C. microsporum, C. purpureofuscum and C. ovoideum, but conclusive comparisons are impossible because of the poor condition of the isotype material (see notes on C. ovoideum above). The ITS phylogeny shows a relationship with C. microsporum, from which C. robustum differs morphologically by its shorter synnemata and longer conidia. This species is provisionally synonymised with C. microsporum until the holotype material of fresh isolations can be examined.
Cephalotrichum terricola Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 221. 2011.
Specimen examined: China, Sichuan Province, Panzhihua, dried culture isolated from mountain soil, Aug. 13. 2005, Y.L. Jiang (holotype HSAUPII050924 → isotype CBS H-22791, additional isotype HMAS196227).
Notes: This is a synonym of C. purpureofuscum. The isotype contains two fungi, synnemata of Cephalotrichum and second hyaline fungus, probably an Aspergillus sp. The synnemata show some differences from the original description. The conidial surface is not smooth as reported but presents some fine roughness. Most conidia are finely pointed, but conidia with rounded apices were also commonly observed. The synonymy is supported by sequence data. The ITS sequence from the ex-type of C. terricola has 1 nt difference with the reference strain of C. purpureofuscum (CBS 174.68), but is identical to ITS sequences of other C. purpureofuscum isolates included in this study (data not shown).
Cephalotrichum verrucipes Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 223. 2011.
Specimen examined: China, Sichuan Province, Jiuzhaigou, dried culture isolated from forest soil, Aug. 19. 2005, Y.L. Jiang (holotype HSAUPII050849 → isotype CBS H-22792, additional isotype HMAS196234).
Note: This is a synnematous Penicillium species closely matching morphologically with P. clavigerum. Apart from the reported percurrent rather than phialidic conidiogenesis, the protologue of C. verrucipes is more or less consistent with this synonymy.
Discussion
This study presents a molecular phylogenetic study of species of the genus Cephalotrichum, clarifying the identity of species recently described from China, reclassifying the white synnematous species as Acaulium album, and confirming the identity of the species occurring in the built environment.
Cephalotrichum purpureofuscum has a worldwide distribution and is mainly isolated from soil, dung and wood (Domsch et al. 2007) and as noted below is also common indoors. The absence of clear diagnostic morphological characters (e.g. smooth conidia, absence of setae) can be used to identify an isolated as belonging to the C. purpureofuscum species complex (Sandoval-Denis et al., 2016a, Sandoval-Denis et al., 2016b). The newly described C. domesticum morphologically resembles C. purpureofuscum, but molecular data can easily separate the two species, using any of the three genes studied here. All C. domesticum isolates were initially identified as C. purpureofuscum at CBS based on their morphology. Several of the recently described species from China fall into the broad concept of C. purpureofuscum, and are synonymised here (C. acutisporum, C. longicollum, C. oblongum and C. terricola, with C. cylindrosporum and C. macrosporum possible synonyms). Cephalotrichum microsporum also resembles C. purpureofuscum and C. domesticum, with smooth conidia and the lack of setae, but can be distinguished but its smaller conidia and synnemata. Morphologically, the group of species that would previously have been included in Trichurus are easily recognized, with two species with coiled setae being distinguished by the length of the synnemata, 500–1 000 μm for C. gorgonifer and <500 μm for the newly described C. telluricum. The two species with straight setae, C. cylindricum and the newly described C. transvaalense, are morphologically very similar but easily distinguished by DNA sequences.
All 16 phylogenetic species of Cephalotrichum recognised can be identified with tef1 and tub2 partial gene sequences. One anomaly is the strain C. nanum CBS 191.61, which does not group with other C. nanum isolates based on tef1 (data not shown, all single gene phylogenies are submitted to TreeBase). Based on ITS alone, C. cylindricum and C. transvaalense cannot be distinguished (Fig. 2), but morphology and tub2 and tef1 sequences clearly differentiate them. The lack of discriminating power of ITS barcodes, in combination with the poor quality of some of the isotype herbarium specimens examined here, prevented us from conclusively characterizing four of the recently described Chinese species, leaving them as nomena dubia. This highlights the importance of depositing living material in internationally accessible culture collections for taxonomic and biodiversity studies.
In this study, five Cephalotrichum spp. were confirmed for the built environment, the newly described C. domesticum, C. gorgonifer (previously reported by Abbott 2000), C. microsporum (Prezant et al., 2008, Samson et al., 2010, Flannigan et al., 2011), C. purpureofuscum (Abbott, 2000, Sandoval-Denis et al., 2016b), and newly reported C. verrucisporum. Cephalotrichum gorgonifer (formerly mainly known as Trichurus spiralis) seems to be the most common species in the indoor environment, with C. purpureofuscum and C. microsporum as other commonly isolated species. Cephalotrichum gorgonifer and C. microsporum both have a worldwide distribution, and have mostly been isolated from cultivated soils (Domsch et al. 2007). They both degrade cellulose (Domsch et al. 2007), and the latter may decay wood (Nilsson 1973). Some isolates of C. microsporum also decompose xylan (Domsch & Gams 1969), and may produce extracellular keratinase capable of hydrolysing keratinous materials such as wool, hair, nails and skin (Gradišar et al. 2000).
Only one indoor strain of C. verrucisporum was isolated (DTO 055-D7), for which we unfortunately do not have more information other than that it was isolated from the indoor environment in Germany. The species is morphologically similar to C. asperulum and C. spirale, as all have rough-walled conidia. Both C. verrucisporum isolates were originally identified as Doratomyces asperulus (C. asperulum) at CBS. Additional isolates are necessary to determine whether C. verrucisporum actually occurs consistently in the indoor environment, or whether this was just an incidental, single isolation.
During the publication process of this manuscript, three new Cephalotrichum species are described from a carbonate cave in China (Jiang et al. 2017). Phylogenetic comparison places the new species C. oligotriphicum and C. laeve as sister species of C. verrucisporum. The new species C. guizhouense is closely related to C. dendrocephalum and our newly described species C. tenuissimum and C. domesticum. With the now up-to-date phylogeny of the genus Cephalotrichum, identification of (new) Cephalotrichum species is made much easier. We expect more Cephalotrichum species to be discovered and described.
Acknowledgements
This research was supported by a grant from the Alfred P. Sloan Foundation Program on the Microbiology of the Built Environment. Martin Meijer (CBS-KNAW) is thanked for help with the molecular work.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Abbott S.P. Department of Biological Sciences, University of Alberta; Canada: 2000. Holomorph studies of the Microascaceae. Ph.D. dissertation. [Google Scholar]
- Clements F.E., Pound R. New species of fungi. Botanical Survey of Nebraska. 1896;4:5–23. [Google Scholar]
- Corda, A. C. J. (1829). In J. Sturm (Ed.), Deutschlands Flora, III (Die Pilze Deutschlands) 2, heft 7.
- Corda A.C.J. Czech Republic; Prague: 1837. Icones fungorum huscusque cognitorum 1. [Google Scholar]
- Costantin Observations sur la fascination des Mucédinées. Bulletin de la Société Mycologique de France. 1888;4:62–68. [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., editors. Fungal biodiversity. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2009. (CBS laboratory manual series 1). [Google Scholar]
- De Beer Z.W., Seifert K.A., Wingfield M.J. A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. CBS Biodiversity Series. 2013;12:245–322. [Google Scholar]
- Domsch K.H., Gams W. Variability and potential of a soil fungus population to decompose pectin, xylan, and carboxymethyl-cellulose. Soil Biology and Biochemistry. 1969;1:29–36. [Google Scholar]
- Domsch K.H., Gams W., Anderson T.-H. 2nd edn. IHW-Verlag; Eching, Germany: 2007. Compendium of soil fungi. [Google Scholar]
- Flannigan B., Samson R.A., Miller J.D., editors. Microorganisms in home and indoor work environments. Diversity, health impacts, investigation and control. 2nd edn. CRC press, Taylor & Francis Group; Boca Raton, FL, USA: 2011. [Google Scholar]
- Gradišar H., Kern S., Friedrich J. Keratinase of Doratomyces microspores. Applied Microbiology and Biotechnology. 2000;53:196–200. doi: 10.1007/s002530050008. [DOI] [PubMed] [Google Scholar]
- Hughes S.J. Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Jiang J.-R., Cai L., Liu F. Oligotrophic fungi from a carbonate cave, with three new species of Cephalotrichum. Mycology. 2017 [Google Scholar]
- Jiang Y.-L., Xu J.-J., Wu Y.-M. Studies on Cephalotrichum from soils in China – twelve new species and two new combinations. Mycotaxon. 2011;117:207–225. [Google Scholar]
- Jiang Y.-L., Zhang T.-Y. Two new species of Doratomyces from soil. Mycotaxon. 2008;104:131–134. [Google Scholar]
- Link H.F. Observationes in ordines plantarum naturales. Berlinische Magazin. 1809;3:3–42. [Google Scholar]
- Lodha B.C. Notes on two species of Trichurus. Journal of the Indian Botanical Society. 1963;42:135–142. [Google Scholar]
- Malloch D., Cain R.F. The genus Kernia. Canadian Journal of Botany. 1971;49:855–867. [Google Scholar]
- Morton F.J., Smith G. The genera Scopulariopsis Bainier, Microascus Zukal, and Doratomyces Corda. Mycological Papers. 1963;8:1–96. [Google Scholar]
- Nilsson T. Studies on wood degradation and cellylolytic activity of microfungi. Studia Forestalia Suecica. 1973;104:1–40. [Google Scholar]
- Page R.D.M. TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Prezant B., Weekes D.M., Miller J.D., editors. Recognition, evaluation, and control of indoor mold. American Industrial Hygiene Association; Fairfax, VA, USA: 2008. [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute; Kew, UK: 1970. A mycological colour chart. [Google Scholar]
- Samson R.A., Houbraken J., Thrane U. Food and indoor fungi. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2010. (CBS laboratory manual series 2). [Google Scholar]
- Sandoval-Denis M., Gené J., Sutton D.A. Redefining Microascus, Scopulariopsis and allied genera. Persoonia. 2016;36:1–36. doi: 10.3767/003158516X688027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M., Guarro J., Cano-Lira J.F. Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Studies in Mycology. 2016;83:193–233. doi: 10.1016/j.simyco.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K.A., Morgan-Jones G., Gams W. The genera of Hyphomycetes. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2011. (CBS biodiversity series 9). [Google Scholar]
- Swart H.J. A study of the production of coremia in three species of the genus Trichurus. Antonie van Leeuwenhoek. 1964;30:257–260. doi: 10.1007/BF02046731. [DOI] [PubMed] [Google Scholar]
- Swart H.J. Doratomyces columnaris sp. nov. Acta Botanica Neerlandica. 1967;15:521–523. [Google Scholar]
- Woudenberg J.H.C., Meijer M., Houbraken J. Scopulariopsis and scopulariopsis-like species from indoor environments. Studies in Mycology. 2017;88:1–35. doi: 10.1016/j.simyco.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]