Abstract
Objective
To evaluate whether a behavioral weight management program combined with a smoking cessation program delivered via interactive technology could prevent post-cessation weight gain.
Methods
330 young adult smokers age 18 to 35 were randomized to a smoking cessation program alone (Comparison group) that included behavioral counseling and nicotine replacement or to a behavioral weight management program adapted from the Look AHEAD trial plus the same smoking cessation program (Intervention group).
Results
TARGIT randomized 164 to the Comparison and 166 to the Intervention group respectively. On average the participants gained +0.91 kg after 24 months in the trial (Comparison group +1.45 kg and Intervention group +0.32, p = 0.157). The only variable systematically affecting weight change over time was smoking abstinence, where those that were abstinent on average gained 0.14 kg more per month compared to those who continued to smoke (p < 0.001). In exploratory analyses, the Intervention participants who were abstinent at 6 months had numerically smaller weight gains compared to abstinent Comparison participants, but these differences were not statistically significant.
Conclusions
Providing an intensive weight gain prevention program combined with a smoking cessation program via interactive technology was not associated with greater long-term weight gain prevention.
Keywords: Weight gain, smoking cessation, interactive technology
Introduction
In the United States, the rates of overweight and obesity continue at epidemic levels despite the well-known adverse health consequences and public health awareness efforts to reverse these trends.1–3 Continued weight gain commonly occurs throughout adulthood and the age group with the fastest rate of weight gain has been shown to be young adults.4,5 Many research studies have shown that behavioral weight loss interventions combining both dietary modification and increased physical activity are most effective in achieving weight loss.6–8 Moreover, studies of new modes of delivery of behavioral weight loss or weight maintenance interventions using interactive technology have shown promise. 9–12,13 Unfortunately, many previous studies in young adults have not identified interventions with long term effects on weight gain.14
Cigarette smoking is the leading preventable cause of morbidity and mortality in the United States today.15 The prevalence of current smoking is higher for young adults 18 –34 years old than for all adults.16 While quitting smoking is associated with numerous health benefits, one unwanted “side effect” of quitting smoking is unwanted weight gain which occurs in about 80% of quitters.17 In a study of young adults, weight gain attributable to smoking cessation was found to be 4.2 – 6.6 kg over a seven year follow-up.18 In addition to actual weight gain following smoking cessation, concerns about post-cessation weight gain appear to be a deterrent to smoking cessation attempts and an incentive for younger persons to initiate smoking.19
Approaches to reducing post-cessation weight gain have used lifestyle modification, but studies utilizing this approach generally have met with limited to no success.20,21 Although, more recent attempts have shown that longer-term reductions in post-cessation weight gain can indeed be sustained, and smoking cessation can be enhanced.22 However, few controlled randomized trials have been conducted to test behavioral weight loss/weight gain prevention interventions using interactive technologies in young adults who are attempting to stop smoking.
Therefore, the purpose of this study is to examine whether an intensive behavioral weight management program (i.e. weight gain prevention or weight loss) combined with a smoking cessation program delivered with interactive technologies can prevent or attenuate the weight gain normally associated with a quit attempt. This study targeted recruitment of young adult smokers ages 18–35 attempting to quit smoking as a high risk group in need of a weight management intervention and likely to be responsive to use of technology-based interventions.
Methods
The TARGIT study was designed to test the hypothesis that a smoking cessation program plus a behavioral weight loss/weight gain prevention intervention delivered through interactive technology (Intervention Group) would significantly attenuate or prevent weight gain associated with smoking cessation at 24 months after enrollment compared to the smoking cessation program alone (Comparison Group) in young adult smokers. The University of Tennessee Health Science Center (UTHSC), Department of Preventive Medicine, clinical trials research center in Memphis, TN was the study site. TARGIT is also part of a UO1 cooperative group called EARLY (Early Adult Reduction of weight through LifestYle intervention) that is funded by the National Heart Lung and Blood Institute (NHLBI) to study weight loss and weight gain prevention in young adults using interactive technology over a 24 month period.23 The project was approved by the Institutional Review Board (IRB) at UTHSC and all participants provided informed consent and received a small IRB approved stipend for time and travel to attend clinic visits.
All randomized participants in the TARGIT study were required to be a young adult smoker (18–35 years), who reported smoking at least 10 cigarettes per day, had a BMI < 40 kg/m2, and were willing to stop smoking and wanted to not gain weight. The lower boundary eligibility criteria for BMI was initially ≥ 22, but was decreased to ≥ 20 during the recruitment phase in an effort to increase enrollment (IRB approved change became effective 08/08/11; this change did not affect the baseline balance in the two study arms). Additionally, TARGIT participants were required to have access to a telephone and the internet, demonstrate ability to access a specific web site, and demonstrate the ability to receive and respond to email. Further, interested persons had to be willing to accept random assignment and be able to participate in a 24 month behavioral lifestyle change intervention. A full list of inclusion and exclusion criteria has been previously published.24
Recruitment for the TARGIT study took place over a two-year period from December 2010 to August 2012 and included both traditional- and technology-based methods. During a phone screen, the TARGIT study was explained and eligibility criteria were assessed. Persons who were eligible and interested were scheduled for an onsite screening visit, where written informed consent was obtained and participants were evaluated for additional study eligibility requirements. Eligible and willing participants were asked to record dietary intake online at the study website and were scheduled for a randomization visit. Only those eligible who demonstrated successful dietary recording were randomized as self-monitoring of dietary intake was part of the Intervention group activities. Participants were individually randomized in blocks of 4 (allocation ratio 1:1).25 Neither the participants, nor the study staff who provided the intervention, were blinded to group assignment. However, study personnel who were collecting and assessing outcome data such as weight and the investigators, including the PI, were blinded to treatment assignment (did not know a participant’s group assignment status). All participants were asked not to reveal their study group assignment to blinded clinic personnel.
At study visits, participants completed questionnaires regarding smoking status, dietary intake (NCI Diet History Questionnaire), physical activity (Global Physical Activity Questionnaire) and mood (CES-D).26–28 Weight was measured in the study clinic using a standardized protocol created by the EARLY consortium on a calibrated scale.
Participants randomized to the smoking cessation alone program (Comparison group) received a smoking cessation handbook, 6 weeks of nicotine patches dosed according to reported level of smoking, access to the interactive study website for smoking cessation, and 1 in-person session and 5 intervention calls via a proactive Quit line over a 6 month period. Text and email messages were also used for delivery of the smoking cessation program for 24 months. All participants also received a smoking cessation app on their iPod designed by TARGIT for use.
Participants randomized to the smoking cessation plus weight management program (Intervention group) received the same smoking cessation program plus a behavioral weight loss/weight gain prevention intervention (depending on baseline weight category) program delivered via interactive technology. The dietary, physical activity and behavioral components of the weight management program for the Intervention group were adapted from the Look AHEAD behavioral weight loss program and tailored to young adult smokers.29 The frequency of scheduled contacts varied from weekly during the most intensive phase, to monthly and then quarterly to transition to maintenance and occurred throughout the 24 month study period. The primary goal of the intervention was to provide participants with the core knowledge and behavioral skills necessary to help achieve and maintain their desired reduction in body weight or prevention of weight gain during smoking cessation. Participants were provided with fundamental information about modifying energy intake and increasing energy output, and learned how to monitor dietary intake and physical activity. In addition, weight management sessions presented specific content and application of behavior modification, stimulus control, problem-solving, and cognitive restructuring techniques to promote weight loss or weight gain prevention.
The interactive technology for both groups consisted of use of an iPod Touch plus content delivered via podcasts, email and text messages. Live online webinars for the weight loss or weight gain prevention sessions were available to the Intervention group only. All participants received instruction on how to use the iPod and their personalized interactive study website including accessing additional information and resources on smoking cessation (both groups) and weight loss or weight gain prevention (Intervention only). Reminders to use the study interactive technology were delivered by text message, email message, and reminder phone calls. Engagement data shown represents only active engagement with the interactive technology or the interventionists and does not include passive reminders by text, email or phone message without a verified response from the participant.
The study had a targeted sample size of 330 participants and was powered for a mean difference in absolute weight change (in kg) between baseline and 24 months follow up of 3.5 kg (80% power, type I error rate of α = 5%, assuming a 20% loss to follow-up). The primary outcome was body weight change as measured by blinded trained staff on a calibrated weight scale using a standardized protocol at baseline (BL), 6 month, 12 month, and 24 month follow up study clinic visits.
The secondary outcome, smoking cessation, was assessed as biochemically verified 7-day point prevalence abstinence. Abstinence was only concluded if a participant self-reported not smoking and their exhaled carbon monoxide (CO) was <= 10 ppm. Salivary cotinine was collected only at the 24 month visit and values >= 100 ng/mL were considered to be consistent with smoking.
Statistical Methods
Participants that reported being pregnant at any study visit had weight measured but that weight data was not used during the analysis of the primary outcome at that visit. One participant in each group was pregnant at 12 months and 3 and 4 participants were pregnant in Comparison and Intervention, respectively, at 24 months. The primary analysis of weight change is an intent-to-treat analysis (with every enrolled participant included in the group of random assignment) based on a linear mixed effects model for longitudinal data using SAS PROC MIXED (SAS Institute Inc., Cary, NC; version 9.4 with SAS/STAT 13.2) as well as R (The R Foundation for Statistical Computing; version 3.2.3) with package lme4, focusing on significant fixed effects that can “explain” the different slopes (weights as participants progress) once (independent) random effects on intercept and slope are in the model.30 In addition, a sensitivity analysis was conducted where missing 24 month weight outcomes for all participants where imputed based on the observed outcomes in the control group as well as a hot-deck multiple imputation. Informative missingness was investigated by determining whether known covariates are related to loss-to-follow-up status based on (marginal) statistical significance (on 5% level) of the covariates in a multivariable logistic regression model with the binary outcome being “24-month visit attended yes/no.” Exploratory analyses elaborating on smoking status and weight change used subgroup analyses utilizing the primary outcome model and graphical exposition with pairwise comparisons at various time points. Additional detail about the statistical analyses can be found in the Supplemental Material.
Smoking cessation was a secondary outcome and used biochemically verified 7 day point prevalence.
Results
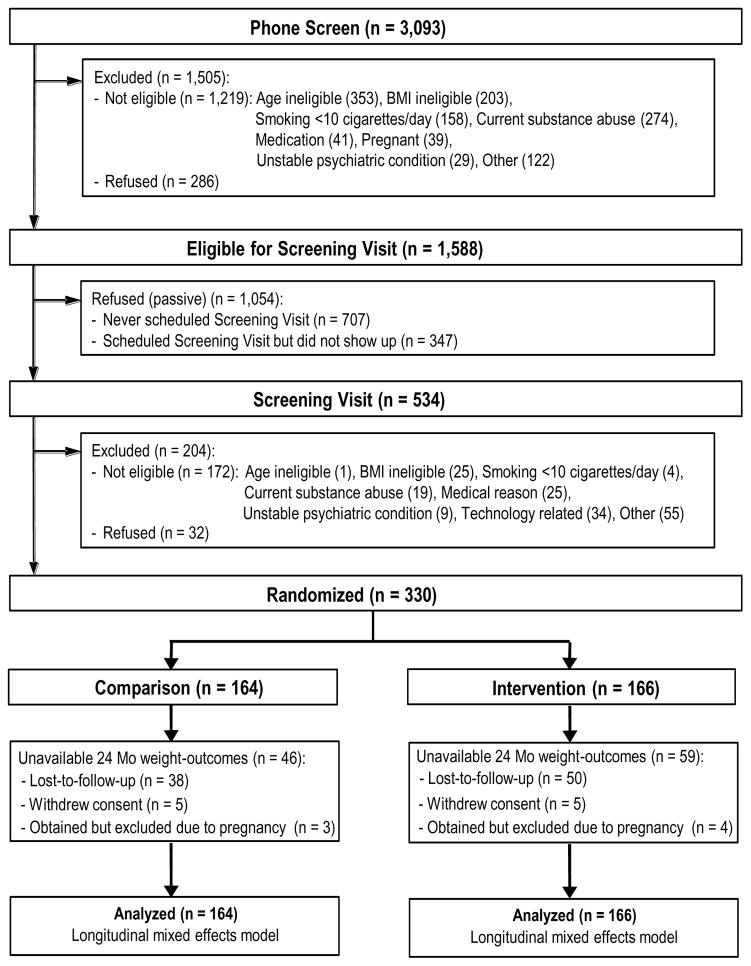
TARGIT enrolled and randomized all planned 330 participants (see Consort Diagram Figure 1).24 Table 1 shows the demographics and baseline characteristics in the Comparison (n = 164) and the Intervention (n = 166) groups. Participants were on average 30 years old, 51% were males, the majority were either Caucasian (57%) or African American (37%) and the majority had some educational training after high school (67%). The mean BMI of TARGIT participants at baseline was 29 with over 76% falling in the overweight or obese category. Systematic differences in baseline weight were present by gender, race and signs of depressed mood. These differences were included as fixed effects variables (intercept) in the primary analysis model (males were on average 13 kg heavier, p < 0.001), (blacks were on average 4.9 kg heavier compared to whites, p = 0.025), (CESD-score >= 16, p < 0.001). All TARGIT participants were cigarette smokers and on average smoked 18 cigarettes per day and had smoked for an average of 12 years. The majority of TARGIT participants (57%) had attempted to quit in the past 12 months and 47% had gained weight during their previous quit attempt. As expected, the randomization procedure successfully balanced those features between the study arms (all p-values > 0.05).
Figure 1.
TARGIT Consort Diagram
Table 1.
Baseline Characteristics by Treatment Assignment
| Variable | All (N = 330) | Comparison (N = 164) | Intervention (N = 166) | p-value |
|---|---|---|---|---|
| Age Mean (std) | 29.70 (4.18) | 30.01 (3.94) | 29.39 (4.40) | 0.309 |
| Gender N (%) | ||||
| Female | 161 (48.79) | 78 (47.56) | 83 (50.00) | 0.658 |
| Male | 169 (51.21) | 86 (52.44) | 83 (50.00) | |
| Hispanic or Latino N (%) | 11 (3.33) | 6 (3.66) | 5 (3.01) | 0.770 |
| Race N (%) | ||||
| Black or African American (only selection) | 123 (37.27) | 67 (40.85) | 56 (33.73) | 0.422 |
| White (only selection) | 189 (57.27) | 89 (54.27) | 100 (60.24) | |
| Other (incl. multiple) | 18 (5.45) | 8 (4.88) | 10 (6.02) | |
| Education N (%) | ||||
| High school graduate or GED | 108 (32.73) | 55 (33.54) | 53 (31.93) | 0.756 |
| At least some vocational or training school after high school | 222 (67.27) | 109 (66.46) | 113 (68.07) | |
| Personal Income (past 12 month) N (%) | ||||
| < $16,000 | 144 (43.64) | 70 (42.68) | 74 (44.58) | 0.960 |
| $16,000 – $49,999 | 142 (43.03) | 73 (44.51) | 69 (41.57) | |
| >= $50,000 | 31 (9.39) | 15 (9.15) | 16 (9.64) | |
| Don’t know | 13 (3.94) | 6 (3.66) | 7 (4.22) | |
| Cigarettes/day Mean (std) | 17.89 (7.73) | 18.42 (8.87) | 17.36 (6.41) | 0.416 |
| Years smoked Mean (std) | 11.86 (4.92) | 12.32 (4.79) | 11.41 (5.02) | 0.070 |
| Quit Attempt Past 12 Month N (%) (n = 11 missing) | 184 (57.68) | 87 (55.06) | 97 (60.25) | 0.349 |
| Weight change during last quit attempt N (%) | ||||
| Gained weight | 149 (47.15) | 76 (48.10) | 73 (46.20) | 0.704 |
| Lost weight | 8 (2.53) | 5 (3.16) | 3 (1.90) | |
| Stayed the same weight | 159 (50.32) | 77 (48.73) | 82 (51.90) | |
| Weight (kg) Mean (std) | 85.65 (17.30) | 85.52 (17.40) | 85.78 (17.24) | 0.894 |
| BMI Mean (std) | 29.19 (4.97) | 29.23 (5.17) | 29.16 (4.78) | 0.904 |
| BMI group N (%) | ||||
| Normal (18.5 – 24.9) | 76 (23.03) | 42 (25.61) | 34 (20.48) | 0.138 |
| Overweight (25.0 – 29.9) | 124 (37.58) | 53 (32.32) | 71 (42.77) | |
| Obese (> 30) | 130 (39.39) | 69 (42.07) | 61 (36.75) | |
| CES-D Mean (std) | 8.47 (6.28) | 9.05 (6.92) | 7.89 (5.53) | 0.182 |
| Total Calories Mean (std) | 2534 (1989) | 2761 (2424) | 2310 (1411) | 0.298 |
| Total Moderate or Vigorous Physical Activity MET-minutes/week Mean (std) | 5417 (8369) | 5239 (7642) | 5591 (9046) | 0.564 |
Despite intensive retention activities and incentives throughout the trial, attendance at study clinic visits declined over time. Figure 2 lists all available weight measurements at the follow-up study clinic visits. The only baseline characteristic identified as predictive of subsequent loss-to-follow up at 24 months was the baseline BMI group (p = 0.035) where the group in the overweight category (BMI 25.0 – 29.9) was less likely to be lost-to-follow up compared to the group in normal weight category (BMI 18.5 – 24.9) (OR = 0.442, 95% C.I. 0.229 – 0.852). Importantly, however the randomization group assignment was not associated with loss-to-follow up (p = 0.129).
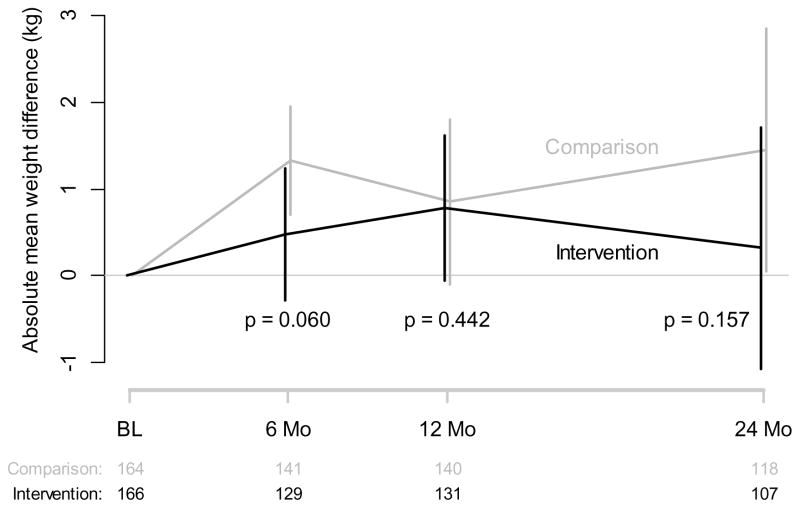
Figure 2.
Weight Change by Treatment Assignment
The average weight gain for all TARGIT participants was 0.007 kg/month (average slope of weight for all participants). The Intervention group gained on average +0.48, +0.78, and +0.32 kg at 6, 12, and 24 months follow-up, whereas the Comparison group gained +1.33, +0.85, and +1.45 kg respectively (6 m p = 0.060, 12 m p = 0.442, and 24 m p = 0.157).(Figure 2 and Supplemental Table S1) In an exploratory analysis we imputed weights for the 105 persons without a measured weight at 24 months (multiple imputation) and our findings of no difference between the randomized groups did not change (See Supplemental Material).
The only variable systematically affecting weight change over time was smoking abstinence during the past 7 days, where those that were abstinent on average gained 0.14 kg per month more compared to those who did smoke (p < 0.001, 95% C.I. 0.04 – 0.26 kg/month). Of note, baseline BMI was not significantly associated with subsequent weight change during follow-up (p = 0.185).
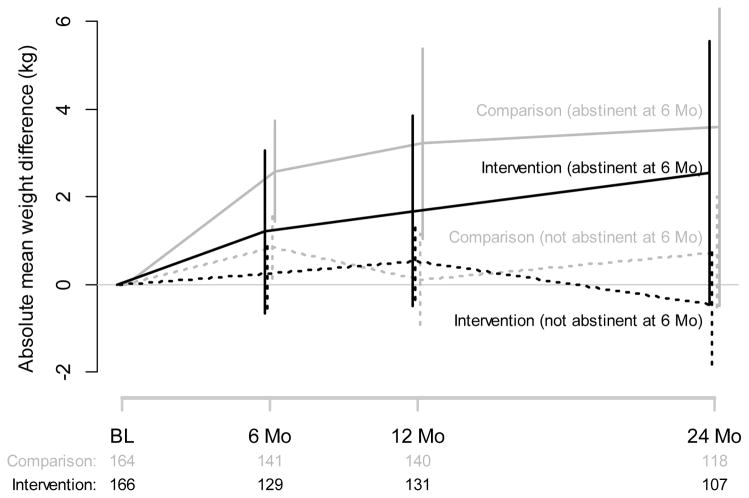
Because of the clear dependence of weight change on smoking status (See Figure 4 and Supplemental Table S3), we performed two exploratory subgroup analyses. First, we fitted the identified mixed-effects model for the subgroup of 64 participants that reported abstinence at 6 months, as well as for the subgroup of 266 participants that were smoking at 6 months. In neither subgroup was the treatment assignment statistically significant for weight change over time (p = 0.975 and p = 0.925, respectively). Second, our graphical analysis (Figure 4) shows that persons who were abstinent at 6 months in both groups (solid lines) gained more weight than persons who were smoking (dotted lines). However, Intervention group participants who were abstinent at 6 months (black solid line) had numerically less weight gain at 6, 12 and 24 months compared to Comparison group participants who were abstinent (gray solid lines) but the difference was not statistically significant at any single time point (p values at 6, 12, and 24 months 0.216, 0.333, and 0.687 respectively).
Figure 4.
Weight Change by 6 Month Smoking Status by Treatment Assignment
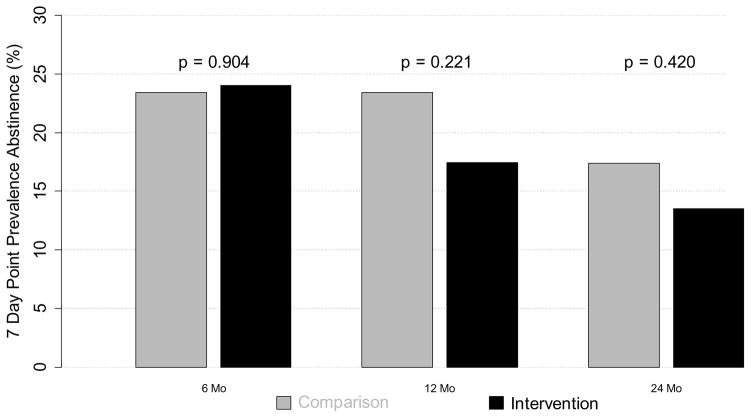
The 7-day point prevalence abstinence rates were the greatest at the 6 month visit in both the Comparison and Intervention groups and gradually decline through 24 months. There was no statistical difference in 7-day point prevalence abstinence rates between the two randomized groups at any follow-up time point (p-values of .90, .22, and .42 for the 6, 12, and 24 month follow-up, respectively, see Figure 3 and Supplemental Table S2). When point prevalence abstinence rates for individuals were followed over time, it was apparent that during the 24 month period persons changed smoking category frequently (see Supplemental Figure S1). For example, of the 64 persons who were not smoking at 6 months, 26% had resumed smoking by 12 months. Whereas 5% of the persons who were smoking at 6 months had quit smoking at 12 month time point.
Figure 3.
Smoking Cessation Rates by Treatment Assignment
Active use of the interactive technology intervention components (including the individualized participant study website, iPod usage, webinar attendance, and phone calls) by the Intervention participants varied over time (see Supplemental Figure S2). During the first month of the trial, all the Intervention participants used some component of the interactive technology. However, over time usage declined to the lowest usage by month 11 of the trial. Encouragement and reminders sent via email, text message, and phone calls for the Intervention participants to engage in usage at 6, 12, 18 and 23 months resulted in significant improvements in usage. For example, usage more than double between months 11 and 12 after the reminders.
Discussion
Overall the intent to treat results of the current investigation do not clearly demonstrate that a behavioral weight loss/weight gain prevention program using interactive technology after smoking cessation results in the prevention of weight gain compared to a smoking cessation program alone after 24 months follow-up. These results are similar to other trials (CITY and IDEA) in young adults that were part of the EARLY consortium which demonstrated mobile based technology may not improve weight loss.31,32 In contrast, recent findings from SNAP another EARLY trial in young adults that used self-regulation approaches delivered initially face-to-face followed by internet-delivered maintenance approaches and frequent self-weighing showed a reduction weight gain.33 Unfortunately, these other EARLY trials in young adults were not primarily conducted with persons attempting to quit smoking and may not be generalizable to the smoking population of young adults.
Previous research has demonstrated that it is common for adults to gain weight and that young adults are at most risk.5 On average young adults gain 0.6 to 0.8 kg per year.4,5 Smoking cessation accelerates weight gain normally associated with aging.18 As seen in the Comparison group in TARGIT, persons who were not smoking at 6 months had gained on average 3.22 kg by 24 months. In contrast, the Intervention group who were not smoking had gained amounts similar to the aging general population and weighed on average 1.06 kg less than the Comparison group. The attenuated weight gain by the Intervention group suggests that the behavioral change program delivered by interactive technology in TARGIT may be of assistance to prevent weight gain when smoking cessation is contemplated. Additional research may be warranted to determine if a face-to-face program (similar to SNAP) combined with an interactive technology delivery approach can be effective in preventing weight gain in young adult smokers trying to quit.
TARGIT also clearly demonstrates the profound effect of smoking on the suppression of weight gain over time in young adults. At all-time points, participants in both groups who were smoking had gained less weight or maintained their weight compared to those who were abstinent. These findings are similar to previous literature demonstrating short and long-term weight gain after smoking cessation.34,35
TARGIT’s smoking cessation rates (24% at 6 months) are similar to published studies using interactive technology as an aid to quitting. For example, in a study using mobile text messaging, 25% of the actively treated group had quit at 26 weeks.36 In a meta-analysis in Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline, a tobacco quit line combined with nicotine replacement therapy resulted in a smoking cessation rate of 28.1%.15 Thus, it appears that the weight gain prevention intervention did not adversely affect smoking cessation rates in TARGIT participants. TARGIT also clearly demonstrates the high recidivism rates seen in young adult smokers. These high recidivism rates in both groups may have affected the ability to detect weight differences between randomized groups as persons who started smoking again did not gain weight.
The TARGIT study had strengths and limitations. The strengths of the study include using the best available evidence to inform the weight gain prevention behavioral intervention and the randomized trial design. Limitations include that the study sample was restricted to only young adults and therefore the findings may not generalize to other age groups. The study was also limited by the lost to follow-up rate for measured weights by 24 months despite intensive retention activities and incentives to participate. The loss of outcome data, while not differential by treatment assignment, most likely resulted in reduced precision of our estimated effects and power to see a difference between the intervention and comparison groups and may in part have been responsible for our null findings. Another limitation of TARGIT is the choice to provide nicotine replacement therapy (NRT) to both groups, as NRT use has been associated with attenuation of post-cessation weight gain.37,38 TARGIT recognized this issue but chose to provide NRT to improve smoking cessation rates in both groups in order to test our primary hypothesis regarding weight gain prevention during smoking cessation. Our approach is consistent with Clinical Practice Guidelines 39 which advocate behavioral and pharmacological treatment for all smokers wishing to quit. The finding that a behavioral change program delivered via interactive technology may not assist with weight loss or weight gain prevention long term may be related to an additional limitation of the TARGIT study – the observed waning use of the interactive technology over time. Thus lower engagement in the intervention over time also could have contributed to our null results. Additional investigation is needed to examine for whom these interactive technologies appear to be the most effective to assist in weight loss or weight gain prevention and how to maintain motivation of use over time.
Conclusion
Providing a weight gain prevention program via interactive technology was not associated with less weight gain long term in smokers attempting to quit. The weight gain prevention program did not appear to adversely affect smoking cessation rates. However, recidivism to smoking was high in this long term study which may have affected the ability to detect weight differences among the randomized groups.
Supplementary Material
What is already known about this subject?
Smoking cessation results in weight gain.
The fastest rate of weight gain occurs during the young adult years.
Behavioral weight gain prevention delivered by interactive technology has shown promise but has not been tested in young adult smokers trying to quit.
What does this study add
Attenuation of weight gain using interactive technology in young adult smokers trying to quit may be possible especially in the subgroup who can maintain smoking abstinence.
Profound effect of smoking on the suppression of weight gain over time in young adults.
A weight gain prevention intervention did not adversely affect smoking cessation rates.
Acknowledgments
Funding Source: Grant funding from National Heart Lung and Blood Institute, National Institutes of Health
Footnotes
Trial Registration: ClinicalTrials.gov Identifier NCT01199185
Disclosure: The authors declared no conflict of interest
Author Contributions: KJ, MC, FT, FT, PR conceived the experiment and assisted in obtaining grant funding. KJ, MC, FT, FT, PR, QT, and DM carried out the experiments. FT and QT analyzed data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. Jama. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Archives of internal medicine. 1990;150(3):665–672. [PubMed] [Google Scholar]
- 5.Ball K, Crawford D, Ireland P, Hodge A. Patterns and demographic predictors of 5-year weight change in a multi-ethnic cohort of men and women in Australia. Public health nutrition. 2003;6(3):269–281. doi: 10.1079/PHN2002431. [DOI] [PubMed] [Google Scholar]
- 6.Wadden TAFG. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–461. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GABC, James WPT, editors. Handbook of Obesity. New York: Marcel Dekker, Inc; 1998. pp. 855–873. [Google Scholar]
- 8.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. Jama. 2001;285(9):1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 10.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. Jama. 2003;289(14):1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 11.Harvey-Berino J, Pintauro S, Buzzell P, Gold EC. Effect of internet support on the long-term maintenance of weight loss. Obesity research. 2004;12(2):320–329. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 12.Harvey-Berino J, Pintauro S, Buzzell P, et al. Does using the Internet facilitate the maintenance of weight loss? Int J Obes Relat Metab Disord. 2002;26(9):1254–1260. doi: 10.1038/sj.ijo.0802051. [DOI] [PubMed] [Google Scholar]
- 13.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. The New England journal of medicine. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 14.Hebden L, Chey T, Allman-Farinelli M. Lifestyle intervention for preventing weight gain in young adults: a systematic review and meta-analysis of RCTs. Obes Rev. 2012;13(8):692–710. doi: 10.1111/j.1467-789X.2012.00990.x. [DOI] [PubMed] [Google Scholar]
- 15.Fiore MCJC, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service; May, 2008. [Google Scholar]
- 16.CDC. Behavioral Risk Factor Surveillance System. National Center for Chronic Disease Prevention and Health Promotion. Centers for Disease Control and Prevention; Aug 25, 2008. Prevalence Data - 2007 - Tennessee vs Nationwide - Tobacco Use. [Google Scholar]
- 17.Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. The Journal of family practice. 1998;46(6):460–464. [PubMed] [Google Scholar]
- 18.Klesges RC, Ward KD, Ray JW, Cutter G, Jacobs DR, Jr, Wagenknecht LE. The prospective relationships between smoking and weight in a young, biracial cohort: the Coronary Artery Risk Development in Young Adults Study. Journal of consulting and clinical psychology. 1998;66(6):987–993. [PubMed] [Google Scholar]
- 19.Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106(2):204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 20.Hall SM, Tunstall CD, Vila KL, Duffy J. Weight gain prevention and smoking cessation: cautionary findings. American journal of public health. 1992;82(6):799–803. doi: 10.2105/ajph.82.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirie PL, McBride CM, Hellerstedt W, et al. Smoking cessation in women concerned about weight. Am J Public Health. 1992;82(9):1238–1243. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danielsson T, Rossner S, Westin A. Open randomised trial of intermittent very low energy diet together with nicotine gum for stopping smoking in women who gained weight in previous attempts to quit. Bmj. 1999;319(7208):490–493. doi: 10.1136/bmj.319.7208.490. discussion 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lytle LA, Svetkey LP, Patrick K, et al. The EARLY trials: a consortium of studies targeting weight control in young adults. Transl Behav Med. 2014;4(3):304–313. doi: 10.1007/s13142-014-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coday M, Richey P, Thomas F, et al. The Recruitment Experience of a Randomized Clinical Trial to Aid Young Adult Smokers to Stop Smoking without Weight Gain with Interactive Technology. Contemp Clin Trials Commun. 2016;2:61–68. doi: 10.1016/j.conctc.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meinert CL, Tonascia S. Clinical Trials: Design, Conduct, and Analysis. New York: Oxford University Press; 1986. [Google Scholar]
- 26.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 27.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1(3):385–401. [Google Scholar]
- 28.Applied Research, Cancer Control and Population Sciences, National Cancer Institute. [Accessed 05/25/2017];Diet History Questionnaire II & Canadian Diet History Questionnaire II (C-DHQII) http://appliedresearch.cancer.gov/dhq2/
- 29.Look ARG, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md. 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 31.Svetkey LP, Batch BC, Lin PH, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring, Md. 2015;23(11):2133–2141. doi: 10.1002/oby.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss: The IDEA Randomized Clinical Trial. JAMA. 2016;316(11):1161–1171. doi: 10.1001/jama.2016.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing RR, Tate DF, Espeland MA, et al. Innovative Self-Regulation Strategies to Reduce Weight Gain in Young Adults: The Study of Novel Approaches to Weight Gain Prevention (SNAP) Randomized Clinical Trial. JAMA Intern Med. 2016;176(6):755–762. doi: 10.1001/jamainternmed.2016.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomerleau CS, Pomerleau OF, Namenek RJ, Mehringer AM. Short-term weight gain in abstaining women smokers. J Subst Abuse Treat. 2000;18(4):339–342. doi: 10.1016/s0740-5472(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 35.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. The New England journal of medicine. 1991;324(11):739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers A, Corbett T, Bramley D, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale LC, Schroeder DR, Wolter TD, Croghan IT, Hurt RD, Offord KP. Weight change after smoking cessation using variable doses of transdermal nicotine replacement. J Gen Intern Med. 1998;13(1):9–15. doi: 10.1046/j.1525-1497.1998.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emont SL, Cummings KM. Weight gain following smoking cessation: a possible role for nicotine replacement in weight management. Addictive behaviors. 1987;12(2):151–155. doi: 10.1016/0306-4603(87)90021-9. [DOI] [PubMed] [Google Scholar]
- 39.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. [Accessed January 13, 2012];Clinical Practice Guideline. 2008 www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.