Abstract
AIM
Previous research investigating outcomes after pediatric intracerebral hemorrhage (ICH) has generally been limited to global and sensorimotor outcomes. This study examined cognitive outcomes after spontaneous ICH in school-aged children with serial assessments over 2 years after stroke.
METHOD
Seven children (age range 6–16y, median 13; six males; 57% white, 43% black) presenting with spontaneous ICH (six arteriovenous malformations) were assessed at 3, 12, and 24 months after stroke. The Pediatric Stroke Outcome Measure (PSOM) quantified neurological outcome and Wechsler Intelligence Scales measured cognitive outcomes: verbal comprehension, perceptual reasoning, working memory, and processing speed.
RESULTS
PSOM scales showed improved neurological function over the first 12 months, with mild to no sensorimotor deficits and moderate overall deficits at 1- and 2-year follow-ups (median 2-year sensorimotor PSOM=0.5, total PSOM=1.5). Changes in cognitive function indicated a different trajectory; verbal comprehension and perceptual reasoning improved over 24 months; low performance was sustained in processing speed and working memory. Age-normed centile scores decreased between 1- and 2-year follow-ups for working memory, suggesting emerging deficits compared with peers.
INTERPRETATION
Early and serial cognitive testing in children with ICH is needed to assess cognitive functioning and support children in school as they age and cognitive deficits become more apparent and important for function.
Previous studies of intracerebral hemorrhage (ICH) and hemorrhagic stroke in childhood have simply reported neurological outcome qualitatively as ‘normal’, ‘good’, or ‘poor’1,2 or have used disability scales or measures of global neurological outcome that are heavily influenced by sensorimotor function.3–7 One larger study by Lo et al. reported neurological outcome as well as quality of life after pediatric ICH.8 From a group of 59 children, there were 39 survivors; follow-up was available in 19 children at a median of 6 years 7 months after ICH. Children tended to have mild to moderate physical deficits, but many had significant impairment in quality of life, specifically in school functioning. Similar findings are also reported in two other patient cohorts.9,10 In addition, a study by Liu et al. of 70 Chinese children found that the level of disability improved significantly between 3 and 6 months after ICH.6 Taken together, findings in previous studies generally suggest improvement in physical disability when assessed months to years after cerebral hemorrhage11 but a significant reduction in quality of life persists.
However, less attention has been given to cognitive outcome after non-traumatic ICH in children. In one study of pediatric hemorrhagic stroke (parenchymal and subarachnoid hemorrhage), 17 out of 31 children demonstrated some level of cognitive impairment (10 mild, seven moderate to severe) 10 years after hemorrhage.11 In a study of neonatal arterial ischemic stroke that tested preschool children and then 18 or more months later as school-aged children (up to 8y after stroke), there was a decline in full-scale IQ with emerging deficits in working memory and processing speed during the school years.12 Other studies of childhood brain injuries, such as survivors of newborn hypoxic ischemic encephalopathy requiring cooling, have reported similar findings, suggesting that deficits may emerge later in development in children.13 However, previous studies have not conducted serial testing to examine the trajectory of cognitive function in children with non-traumatic ICH.
We investigated cognitive outcome after spontaneous ICH in school-aged children with serial assessments over time, 3, 12, and 24 months after stroke. We expected that cognitive function would slowly improve at each time point in parallel with improvements in sensorimotor function and global neurological outcome.
METHOD
Procedure
Children born at term and aged greater than 28 days to 16 years at the time of spontaneous ICH, including those with primary intraparenchymal hemorrhage and/or intraventricular hemorrhage, were prospectively, consecutively enrolled from 2011 to 2015 at a single institution. The setting was a tertiary care center, located in a metropolitan area in the southeast of the USA. The Institutional Review Board of Vanderbilt University Medical Center approved this study. Consent was obtained from the participants’ parents and assent from children 7 years or older. Children were excluded if hemorrhage was related to brain tumor or trauma, or if they died before hospital discharge. Of eligible patients approached, only two refused: one lived too far away to return for follow-up assessment, the other because of lack of interest. Cognitive assessments were only administered to children who were 6 years and older at time 1 (3mo after ICH) and native English speakers; children were excluded from this cognitive study if they were younger than 6 (n=6) or if they were not native English speakers (n=3). Two raters measured total brain volume and intraparenchymal hemorrhage volume using the validated ABC/XYZ method.14 Outcome was assessed with the Pediatric Stroke Outcome Measure and a cognitive testing battery at 3 months (time 1, hereafter T1), 12 months (time 2, T2), and 24 months (time 3, T3) after ICH.
Measures
Neurological function
The Pediatric Stroke Outcome Measure (PSOM) is based on neurological examination and includes five subscale scores: sensorimotor left, sensorimotor right, expressive language, receptive language, and cognition/behavior. The subscales are scored as 0 (no deficit), 0.5 (mild deficit that does not interfere with function), 1 (moderate deficit that interferes with function), and 2 (severe deficit). In the current study, we report the PSOM sensorimotor average for the affected side, either right or left (no child had bilateral sensorimotor dysfunction) as well as total PSOM (range 0–10). Moderate disability has been defined as PSOM greater than or equal to 1 and severe disability as PSOM greater than or equal to 2.15
The Pediatric ICH Score is a clinical grading scale for outcome prediction in the acute period after ICH.16 These scores range from 0 to 4; scores greater than 1 have been shown to be predictive of moderate disability at 3 months after ICH.
Cognitive function
The Wechsler Abbreviated Scale of Intelligence (2nd edition)17 and select subtests of the Wechsler Intelligence Scale for Children (4th edition)18 or the Wechsler Adult Intelligence Scale (3rd edition)19 were administered to all participants. These are widely used and well-validated measures of cognitive function and intelligence. For current analyses, subtests that were not strongly dependent on graphomotor function were used. The vocabulary subtest was used as an index of verbal comprehension and the matrix reasoning subtest was used as an index of perceptual reasoning from the Wechsler Abbreviated Scale of Intelligence; the symbol search subtest was used as a measure of processing speed and the digit span subtest was used as measure of working memory from the Wechsler Intelligence Scale for Children or the Wechsler Adult Intelligence Scale. The same tests were given at each time point because parallel tests are not available for these Wechsler instruments. Cognition scores are reported as both raw scores and age-normed centiles.
Statistical analyses
Statistical analyses were conducted with R (RStudio, Inc., Boston, MA, USA). Mean, median, and interquartile range (IQR) were calculated. Power for a sample size of 7 was 0.20, much lower than the recommended 0.80.20 Therefore, no formal statistical testing was conducted due to the relatively small sample size.
RESULTS
Participants included seven children, ages 6 to 16 years (median 13), six males, one female, 57% white, 43% black. See Table I for demographic and clinical data for the sample. Two participants had pure ICH and five had both ICH and intraventricular hemorrhage. One child had two re-bleeds between the 12- and 24-month follow-ups (Table I, participant 2). Brain arteriovenous malformation was the cause of ICH in six out of the seven participants. In one child, etiology could not be determined with careful evaluation. Brain arteriovenous malformation was suspected on the basis of hemorrhage pattern, mixed ICH and intraventricular hemorrhage,21 but could not be confirmed. Cerebral angiography was performed in all children; in the child with etiology not determined, angiography was completed during the acute hospital stay as well as 3 and 12 months after ICH. Angiography was negative for a vascular cause at all of these time points. Initial Glasgow Coma Scale values ranged from 3 to 14 (median 9). Median hemorrhage volume as a percentage of brain volume was 1.96, IQR 1.17 to 3.74. A hemorrhage of 2% of total brain volume represents a moderate-sized ICH.22,23 All but one child had a Pediatric ICH Score of greater than or equal to 1. Total PSOMs improved over time from median of 3 (IQR 2.25–3.75) at 3 months to 1.5 (IQR 0.75–2.75) at 24 months. However, median PSOM did not change between 12 and 24 months, indicating that improvement may slow after 6 months.
Table I.
Demographics and clinical information for children with spontaneous intracerebral hemorrhage (ICH)
| Subject | Age at ICH (y:mo) |
Hemorrhage cause |
Hemorrhage location |
Hemorrhage volume (% of brain volume) |
GCS at presentation |
Pediatric ICH score |
Intracranial pressure concerns? |
Treatment | Epilepsy | Total PSOM at 3mo |
Total PSOM at 12mo |
Total PSOM at 24mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13:2 | Idiopathic | Right parietal, IVE, subcortical | 1.09 | 8 | 2 | Yes, severe, EVD, hemicraniectomy required | Emergency surgery, hematoma evacuation | No | 3 | 1 | 1 |
| 2 | 6:10 | AVM | Left parietal, basal ganglia, IVE and recurrent ICH, subcortical | 1.24 | 9 | 1 | Yes, EVD required | Embolization ×2, radiation ×2 | Yes, well controlled | 5 | 3 | 2.5 |
| 3 | 16:11 | AVM | Left parietal, large IVE, subcortical | 0.06 | 6 | 1 | Yes, EVD required | Surgery | No | 3 | 1.5 | 2.5a |
| 4 | 6:6 | AVM | Left frontal, IVE, subcortical | 1.96 | 14 | 0 | No | Surgery | No | 0 | 0 | 0 |
| 5 | 12:0 | AVM | Right frontal, cortical and subcortical | 6.50 | 11 | 1 | Yes | Embolization, surgery ×2 | No | 3 | 2.5 | 1.5 |
| 6 | 14:7 | AVM | Left parietal, occipital, cortical and subcortical | 2.48 | 14 | 1 | Yes, EVD required | Surgery | Yes, well controlled. | 1.5 | 0.5 | 0.5 |
| 7 | 12:11 | AVM | Left parietal, basal ganglia, cortical and subcortical | 4.99 | 3 | 4 | Yes, severe, hemicraniectomy | Emergency surgery | No | 4.5 | 4 | 3 |
PSOM increased at 24mo on the basis of the PSOM behavior/cognitive subscale.
GCS, Glasgow Coma Scale; PSOM, Pediatric Stroke Outcome Measure; IVE, intraventricular extension; EVD, extraventricular drain; AVM, arteriovenous malformation of the brain.
Sensorimotor and cognitive function
Table II shows median and IQRs for sensorimotor function (as measured by the PSOM) and cognitive function (as measured by Wechsler tests with age-normed centiles) over three time points.
Table II.
Neurological outcome and cognition scores at 3, 12, and 24mo after intracerebral hemorrhage
| Median (IQR) at T1 (3mo) | Median (IQR) at T2 (12mo) | Median (IQR) at T3 (24mo) | Change T1–T3 (3–24mo) | |
|---|---|---|---|---|
| PSOM total | 3.00 (2.25–3.75) | 1.50 (0.75–2.75) | 1.50 (0.75–2.75) | −1.50 |
| PSOM sensorimotor subscales | 1.00 (0.75–1.50) | 0.50 (0.25–0.75) | 0.50 (0.25–1.25) | −0.50 |
| PSOM expressive language | 1.00 (0–1.50) | 0 (0–0.50) | 0 (0–1.00) | −1.00 |
| PSOM receptive language | 0 (0–0.50) | 0 (0–0) | 0 (0–0) | 0 |
| PSOM cognitive/behavioral | 1.00 (0.25–1.00) | 1.00 (0.25–1.00) | 0 (0–0.50) | −1.00 |
| Full-scale IQa | 18.0 (13.0–24.0) | 34.0 (14.0–55.5) | 45.0 (28.0–58.0) | +27 |
| Verbal comprehensiona | 13.0 (6.5–22.0) | 50.0 (23.0–58.0) | 34.0 (34.0–72.5) | +21 |
| Perceptual reasoninga | 45.0 (12.0–69.0) | 47.0 (20.0–70.0) | 63.00 (48.5–68.0) | +18 |
| Working memorya | 16.0 (1.5–37.5) | 25.0 (12.5–60.5) | 9.00 (9.0–37.0) | −7.00 |
| Processing speeda | 5.0 (3.5–9.0) | 25.0 (16.0–31.0) | 37.00 (20.5–43.5) | +32 |
Expressed as centile on the basis of age norms.
Verbal comprehension was measured with the Wechsler Abbreviated Scale of Intelligence (2nd edition) (WASI-II) vocabulary subtest; perceptual reasoning with the WASI-II matrix reasoning subtest; working memory with the Wechsler Intelligence Scale for Children (4th edition) (WISC-IV) or Wechsler Adult Intelligence Scale (3rd edition) (WAIS-III) digit span subtest; processing speed with the WISC-IV or WAIS-III symbol search subtest. IQR, interquartile range; PSOM, Pediatric Stroke Outcome Measure.
Results from PSOM scores generally reflected improvement between the 3- and 12-month assessments, and then stability between the 12- and 24-month assessments. Sensorimotor performance largely improved over time, with a median of 0.5 at 24 months indicating mild deficit that did not interfere with function. The child who had an arteriovenous malformation that re-bled twice showed worsened sensorimotor function from 0.5 to 1.0 at 24 months; the median PSOM sensorimotor score for all study participants was 0.5 at both 12 and 24 months but the IQR increased due to this child’s worsening PSOM. PSOM expressive language, receptive language, and cognitive/behavioral each had a median of 0 at 24 months.
While results of serial cognitive testing with Wechsler tests also suggested general improvements between T1 and T3 (Table II), this was not the case for each domain. Median verbal comprehension scores improved from the 13th to the 34th centile over time and median perceptual reasoning scores improved from the 45th to the 63rd centile over time. Upper and lower bounds of the IQR for both verbal comprehension and perceptual reasoning contained the published normative mean for age (50th centile) at T3.
However, there was sustained low performance in working memory and processing speed as the IQR remained below the 50th centile at T3 for both domains. Median processing speed scores improved from the 5th to the 37th centile over time, while median working memory scores declined from the 16th to the 9th centile over time. It is noteworthy that, for both verbal comprehension and working memory, there was a trend for T2 scores to be higher than T3 scores. However, no changes were tested for statistical significance.
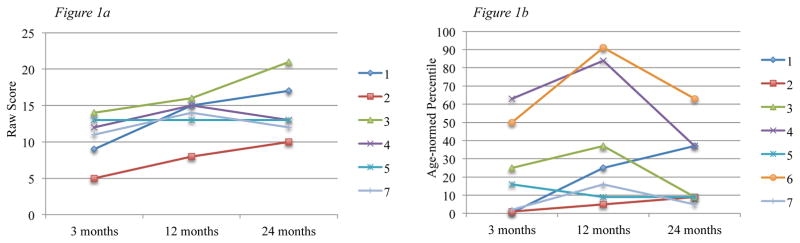
Given that working memory remained the most impaired at the 24-month follow-up, we examined both raw scores and age-normed centiles (Fig. 1). Results in the figure indicate that children’s raw scores improved over a 2-year period, yet this was not reflected in age-normed centiles, which declined over time.
Figure 1. Working memory raw scores and age-normed percentiles in children with ICH.
Figure 1a shows raw scores at 3-, 12- and 24-months for 7 study participants as measured by the Wechsler Intelligence Scale for Children -IV Digit Span subtest; Figure 1b shows age-normed percentiles at 3-, 12- and 24-months for these participants for the Digit Span subtest.
Note: Participant 6 was administered the WISC-IV at 3- and 12-months, then the WAIS-III at T3. For this reason, raw scores are not displayed in Figure 1a.
DISCUSSION
This study presents, to the best of our knowledge, the first serial cognitive testing over three points in time after spontaneous ICH in school-aged children. The total PSOM and PSOM sensorimotor data fit the typical clinical impression and previous research in children with ischemic and hemorrhagic stroke, which is global improvement in physical disability during the first 12 months24 or at 6-year follow-up.11 However, serial cognitive testing suggests a different trajectory. Testing at 24 months indicated sustained low performance in processing speed and working memory. Results indicated that although individual performance continued to improve, children were not able to keep up with increasing demands of working memory, and remained behind peers in age comparisons at the 12- and 24-month follow-ups. These findings are similar to work in children with ischemic stroke showing that cognitive impairments may be more significant than motor impairments over time.25 Further, in support of the fact that motor function is not a sensitive indicator of cognitive function, PSOM motor scores are not significantly correlated with intelligence measures for preschool or school-aged children with a history of neonatal arterial ischemic stroke.12 Therefore, children with good motor function cannot always be assumed to have good cognitive function; formal cognitive testing is required.
Results from the current sample also indicate heterogeneity both across participants and across domains of cognitive function. This further demonstrates that a prognosis of gradual, slow improvement over time may not be the case for every child. Additionally, five of the seven children still had moderate deficits or greater according to the total PSOM score at 2 years after stroke.
In the current study, verbal comprehension and perceptual reasoning improved from T1 to T3, with median centile scores in the average range compared with same-age peers at 24 months. This is similar to results from a previous study of pediatric hemorrhagic stroke that found that verbal and perceptual IQ were in the average range 10 years after stroke.11 Median scores for processing speed were also within the average range, but IQR remained below the 50th centile, indicating sustained low performance but not worsening performance from 3 months to 24 months after ICH (Table II).
Unlike other domains, working memory failed to improve over time, with median score at the 9th centile at 24-month follow-up. This is similar to a study of children with ischemic stroke that found increasing deficits in this domain as children aged.12 Although participants with ICH generally showed improvements in raw scores at each time point, age-corrected scores showed declines between 12 and 24 months. This suggests that children with brain injury may not keep up with peers as cognitive demands increase developmentally with age. This phenomenon has been studied in pediatric cancer and congenital heart disease conditions where both subtle brain injury and stroke may occur, and is known as ‘growing into deficits’.26,27 Results from the current study show that although children with ICH may maintain individual levels of cognitive functioning over time, the performance gap with peers does not improve and worsens over time between 12 and 24 months. Therefore, this phenomenon of growing into deficits could potentially be relevant to the current sample. This is especially salient to aspects of executive function, such as working memory, which continue to develop during the periods of adolescence and early adulthood.28 The present findings that suggest emerging deficits in working memory may reflect an interaction between insults associated with brain injury related to ICH and normative developmental processes in brain maturation.29 This may suggest a sensitive period for development of working memory that is not similarly reflected in domains of verbal comprehension or perceptual reasoning.
Limitations of this work include the small sample size. Non-traumatic ICH has an incidence of 0.8 per 100 000.30 To conduct more careful quantitative analyses, large multisite studies will be needed to achieve the necessary sample size to increase generalizability. To minimize bias, we approached all eligible children about study participation; however, we did not have the ability to test non-native English speakers. In addition, owing to the small sample size, we did not exclude the participant with the recurrent hemorrhage. The patient’s total PSOM improved from 2.5 to 3 despite recurrent small hemorrhages. While serial testing over three points in time is a notable strength, practice effects may have inflated cognitive scores at 12 months as children were tested at both 3 and 12 months after ICH. Most cognitive tests are not given more frequently than every 12 months, in order to avoid improvement in scores due to practice or familiarity with the test. It is our experience that children with ICH or other brain injuries typically receive cognitive testing at 2 to 3 months to guide educational placement as they return to school as well as about 1 year after ICH to reassess educational placement. This study design was therefore meant to mirror common clinical practice. Future studies should consider alternative, parallel tests. However, it is interesting to note that even with the potential for practice effects, the overall patterns of results still suggested sustained low performance in processing speed and in working memory. Indeed, results from parallel tests may find more prominent deficits.
In conclusion, this study suggests that neurological improvement occurs primarily during the first 12 months and becomes stable afterwards; however, trajectories of cognitive domains differ, as some improve while others remain stable or decline over time. The clinical implications of these findings include the need for early and serial cognitive testing to assess cognitive difficulties as children with a history of ICH age. Motor skills are visible and easy to assess in clinic, while cognitive function is subtler and requires neuropsychological assessment. Clinicians may see improvements over time during subsequent clinic visits, but neuropsychological testing is needed to better understand how children compare with peers. However, as obtaining insurance coverage for cognitive testing is increasingly difficult, many children are being assessed not by neuropsychologists but by school psychologists who may have limited experience with ICH or similar brain injuries.
These results suggest that although children with a history of ICH may have the capacity to perform as well as same-aged peers, as reflected in verbal and perceptual reasoning skills, their cognitive proficiency is limited, as indicated by sustained low performance in processing speed and worsening working memory compared with peers. This suggests that school-based supports may be needed, such as individual learning plans and additional time on tests, which is consistent with a previous study of ICH indicating that, at 12 months after ICH, over 50% of children were receiving educational supports.10 Results of serial neuropsychological testing can inform referrals for and coordination of services, so that children can maximize their potential after ICH.
Supplementary Material
What this paper adds.
In children with intracerebral hemorrhage (ICH), motor function improved between 3 and 24 months.
Improvements in cognitive function were variable between 3 and 24 months.
Working memory centiles declined, suggesting emerging deficits compared with peers.
Processing speed improved but remained significantly below the 50th centile.
Cognitive impact of ICH may increase with age in children.
Acknowledgments
This study was supported by K23-NS062110 from the National Institute of Neurological Disorders and Stroke/National Institutes of Health (to LCJ) and UL1 TR000445 from National Center for Advancing Translational Sciences/National Institutes of Health (to Vanderbilt University). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
ABBREVIATIONS
- ICH
Intracerebral hemorrhage
- IQR
Interquartile range
- PSOM
Pediatric Stroke Outcome Measure
References
- 1.Eeg-Olofsson O, Ringheim Y. Stroke in children. Clinical characteristics and prognosis. Acta Paediatr Scand. 1983;72:391–5. doi: 10.1111/j.1651-2227.1983.tb09734.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15:284–9. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25:416–21. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 4.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40:1666–71. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinman JT, Beslow LA, Engelmann K, et al. Evaluation of intraventricular hemorrhage in pediatric intracerebral hemorrhage. J Child Neurol. 2012;27:526–31. doi: 10.1177/0883073811422272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Wang D, Lei C, et al. Etiology, clinical characteristics and prognosis of spontaneous intracerebral hemorrhage in children: a prospective cohort study in China. J Neurol Sci. 2015;358:367–70. doi: 10.1016/j.jns.2015.09.366. [DOI] [PubMed] [Google Scholar]
- 7.Nair AP, Kumar R, Mehrotra A, Srivastava AK, Sahu RN, Nair P. Clinical, radiological profile and outcome in pediatric Spetzler-Martin grades I-III arteriovenous malformations. Child Nerv Syst. 2012;28:593–8. doi: 10.1007/s00381-011-1668-6. [DOI] [PubMed] [Google Scholar]
- 8.Lo WD, Hajek C, Pappa C, Wang W, Zumberge N. Outcomes in children with hemorrhagic stroke. JAMA Neurol. 2013;70:66–71. doi: 10.1001/jamaneurol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abecassis IJ, Nerva JD, Barber J, et al. Toward a comprehensive assessment of functional outcomes in pediatric patients with brain arteriovenous malformations: the Pediatric Quality of Life Inventory. J Neurosurg Pediatr. 2016;18:611–22. doi: 10.3171/2016.6.PEDS16103. [DOI] [PubMed] [Google Scholar]
- 10.Hawks C, Jordan LC, Gindville M, Ichord RN, Licht DJ, Beslow LA. Educational placement after pediatric intracerebral hemorrhage. Pediatr Neurol. 2016;61:46–50. doi: 10.1016/j.pediatrneurol.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2003;45:233–9. doi: 10.1017/s001216220300046x. [DOI] [PubMed] [Google Scholar]
- 12.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009;40:2012–9. doi: 10.1161/STROKEAHA.108.533976. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beslow LA, Ichord RN, Kasner SE, et al. ABC/XYZ estimates intracerebral hemorrhage volume as a percent of total brain volume in children. Stroke. 2010;41:691–4. doi: 10.1161/STROKEAHA.109.566430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure. Stroke. 2012;43:1602–8. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 16.Beslow LA, Ichord RN, Gindville MC, et al. Pediatric Intracerebral Hemorrhage Score. Stroke. 2014;45:66–70. doi: 10.1161/STROKEAHA.113.003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D, Hsiao-pin C. WASI-II: Wechsler Abbreviated Scale of Intelligence. 2. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 18.Wechsler D. WISC-IV Wechsler Intelligence Scale for Children. 4. London: Pearson; 2003. [Google Scholar]
- 19.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 20.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40:400–5. doi: 10.1161/STROKEAHA.108.518761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41:313–8. doi: 10.1161/STROKEAHA.109.568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40:1666–71. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeggel Simonetti B, Cavelti A, et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurol. 2015;84:1941–7. doi: 10.1212/WNL.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 25.Gordon AL, Ganesan V, Towell A, Kirkham FJ. Functional outcome following stroke in children. J Child Neurol. 2002;17:429–34. doi: 10.1177/088307380201700606. [DOI] [PubMed] [Google Scholar]
- 26.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41:1057–66. doi: 10.1007/s00134-015-3789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106:396–402. doi: 10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]
- 28.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81:1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14:189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–94. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.