Abstract
Objective
A relationship between reduced brain tissue oxygenation (PbtO2) and poor outcome following severe traumatic brain injury (TBI) has been reported in observational studies. We designed a Phase II trial to assess whether a neurocritical care management protocol could improve PbtO2 levels in patients with severe TBI and the feasibility of a Phase III efficacy study.
Design
Randomized prospective clinical trial
Setting
Ten ICUs in the United States
Patients
One hundred nineteen severe TBI patients
Interventions
Patients were randomized to treatment protocol based on intracranial pressure (ICP) plus PbtO2 monitoring versus ICP monitoring alone. PbtO2 data were recorded in the ICP-only group in blinded fashion. Tiered interventions in each arm were specified and impact on ICP and PbtO2 measured. Monitors were removed if values were normal for 48 hours consecutively, or after 5 days. Outcome was measured at 6 months using the Glasgow Outcome Scale–Extended.
Measurements and Main Results
A management protocol based on PbtO2 and ICP monitoring reduced the proportion of time with brain tissue hypoxia after severe TBI (0.45 in ICP-only group, 0.16 in ICP+PbtO2 group; p<0.0001). ICP control was similar in both groups. Safety and feasibility of the tiered treatment protocol was confirmed. There were no procedure related complications. Treatment of secondary injury after severe TBI based on PbtO2 and ICP values was consistent with reduced mortality and increased proportions of patients with good recovery compared to ICP-only management; however, the study was not powered for clinical efficacy.
Conclusions
Management of severe TBI informed by multimodal ICP and PbtO2 monitoring reduced brain tissue hypoxia with a trend towards lower mortality and more favorable outcomes than ICP-only treatment. A Phase III randomized trial to assess impact on neurologic outcome of ICP plus PbtO2-directed treatment of severe TBI is warranted.
Keywords: Traumatic Brain Injury, Randomized Clinical Trial, Brain Oxygenation, Glasgow Outcome Scale, Hypoxia, Brain/*diagnosis/physiopathology, ICU monitoring
Introduction
Traumatic brain injury (TBI) remains a significant public health burden, with severe TBI (Glasgow Coma Scale (GCS) score ≤8) contributing to 30% of all injury-related deaths in the U.S. and costing more than $76 billion in 2010 (1). The magnitude of this problem has led to multiple clinical trials attempting to improve survival and functional outcomes with few effective therapies identified.
Acute management of severe TBI focuses on addressing intracranial mass lesions and minimizing secondary brain injury, including increased intracranial pressure (ICP). Although ICP-guided therapy has not been validated in randomized trials, most clinicians believe that monitoring ICP and treating elevations may improve outcome after TBI (2); as reflected in the most current Guidelines for the Management of Severe Brain Injury (3). ICP elevations may be an insensitive and late indicator of secondary brain injury, and monitoring and treating other physiological parameters may enhance patient care (4). One physiological parameter of particular interest is brain tissue oxygenation (PbtO2), since the brain depends on an uninterrupted supply of oxygen and glucose to maintain cellular metabolism and viability. Additionally, observational studies demonstrate that brain tissue hypoxia may occur even when ICP or cerebral perfusion pressure (CPP) is normal and result from diffusion rather than perfusion defects (5–6). This raises the question whether medical interventions based on PbtO2 may reduce secondary injury and improve outcomes.
The average normal PbtO2 is 23 ± 7 mm Hg (7). Several observational studies have noted that reduced PbtO2 is common after TBI; PbtO2 values <20mmHg may occur in >70% of monitored patients within the first few days after injury, including when ICP and CPP are normal (8–11). Reduced PbtO2 has been associated with a poor outcome after TBI in several observational clinical studies (8, 10, 12–14). Several observational studies suggest the addition of PbtO2-directed care to conventional ICP/CPP based management may be associated with improved outcome after severe TBI (15–18). However clinical equiposie remains due to the absence of randomized controlled trials. This prompted the Brain Oxygen Optimization in Severe TBI Phase II (BOOST-II) study. The primary hypothesis was that a management protocol informed by PbtO2 and ICP values would reduce the total burden of brain hypoxia.
Materials and Methods
Study Design
The BOOST-II study was a two-arm, single-blind, prospective randomized controlled multicenter phase II trial assessing safety and efficacy of a management protocol optimizing PbtO2 following severe TBI (ClinicalTrials.gov registration NCT 00974259). The study also obtained data required for design of a definitive phase III study, including evidence of physiologic efficacy, feasibility of implementing a complex management protocol at multiple centers, and confirming non-futility of PbtO2-directed interventions.
Participants
Patients with severe TBI who required ICP monitoring were screened at ten Level 1 trauma centers with experience in PbtO2 monitoring. Inclusion criteria and exclusion criteria are described in Supplemental Table 3.
Patients admitted with an initial GCS >8 who deteriorated neurologically (within 48 hours of injury) from a presumptive intracranial cause and met criteria for ICP monitoring could also be enrolled provided randomization and ICP monitor placement occurred within 12 hours of deterioration.
This study was performed under a site-specific IRB approved protocol, and a proxy informed consent was obtained before any research procedures. Continued participation consent was obtained at or before the 6-month follow-up if the patient regained cognitive capacity.
Randomization and Masking
Randomization (ICP-only or ICP+PbtO2-guided management) was performed using a secure website (Data Coordinating Center (DCC), University of Washington) after inclusion and exclusion criteria confirmation. To reduce likelihood of imbalance of important prognostic factors between groups, a stratified blocked randomization scheme was used consisting of clinical site and severity of TBI (GCS 3–5 or, if intubated, motor GCS 1–2 vs. GCS 6–8, or, if intubated, motor GCS 3–5).
Study Procedures
Intracranial Monitoring Placement and Management
Patients had both intraparenchymal ICP and PbtO2 monitors (Integra LifeSciences, Plainsboro, NJ) placed. PbtO2 probes were inserted into brain parenchyma approximately 2cm from the cortical surface to sample primarily sub-cortical white matter in the least trauma-affected frontal lobe. Probe position was confirmed by a CT scan and function by a brief oxygen challenge. Following randomization, the control group (ICP-only management) was medically managed with a standard-of-care stepwise intervention strategy triggered by an ICP ≥20 mmHg for > 5 minutes. The intervention group (ICP and PbtO2 management) was medically managed with step-wise treatments to correct either an ICP increase or a reduction in PbtO2 (≤20mHg, > 5 minutes). Patients randomized to the control group also had PbtO2 probes inserted; however, after device calibration by the study coordinator, a locked cover was placed over the PbtO2 display, so values were accessible only to unblinded study coordinators. Digital data recorders (Moberg ICU Solutions, Inc., Ambler, PA) continuously recorded physiologic data for both groups.
The PbtO2 treatment protocol was a set of physiologic interventions that addressed isolated intracranial hypertension, isolated brain hypoxia, or simultaneous occurrence of both. The treatment protocol was tiered in a hierarchical fashion, with less aggressive interventions attempted before more aggressive maneuvers. In the ICP-only group, interventions administered as part of medical care that could affect PbtO2 such as transfusion, ventilator adjustments, or treatment of CPP, were recorded in case report forms.
PbtO2 monitors were kept in place a minimum of 48 hours if no abnormalities were found, until the patient awakened from coma, or for a maximum of 5 days. Removal was at the discretion of treating physicians (Figure 1A for Study Schematic). At randomization each control patient was assigned a pre-specified duration of PbtO2 monitoring known only by the study coordinators (range: 48 hours to 5 days). The unblinded study coordinator could direct the treatment team to continue monitoring up to 5 days if PbtO2 was <20mmHg to balance monitoring duration between groups.
Figure 1.
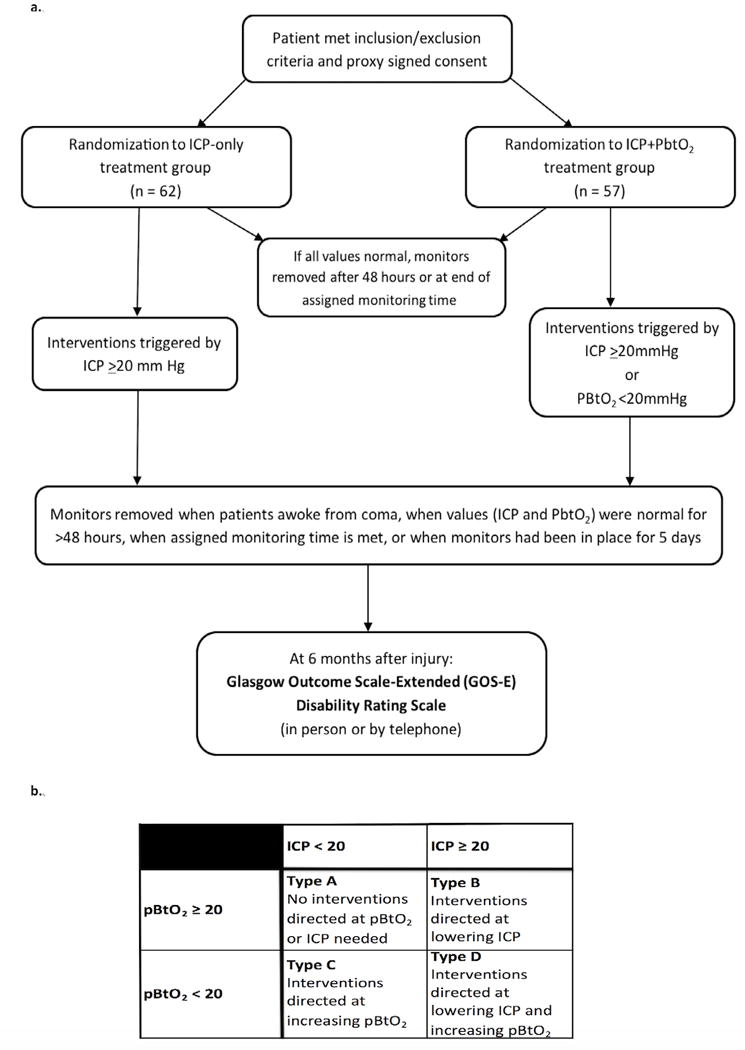
Panel 1A: Study Schematic Panel 1B: Treatment Scenarios
Patient Medical Management
Each enrolled patient, independent of randomization assignment, was medically managed according to the BOOST-II manual of operating procedures (MOP), adapted from the 3rd edition Brain Trauma Foundation Guidelines for the Management of Severe Traumatic Brain Injury (19). This management included measures to maintain: 1) euvolemia or slight hypervolemia and CPP of 50 – 70 mmHg, using vasopressors if necessary; 2) PaCO2 35 – 40mmHg and SaO2 ≥90%; 3) serum Na+ ≥135 mEq, serum glucose > 60 and < 150 mg/dL, 4) normal PT and PTT per local laboratory; 5) normothermia; and 6) timely evacuation of intracranial mass lesions.
Randomized control patients were medically managed for ICP ≥20mmHg. The intervention group, (patients randomized to both ICP and PbtO2 monitoring), were managed with a treatment strategy to correct one or both parameters, defined by 4 types of management (types A, B, C and D; Figure 1B). For patients with PbtO2 <20mmHg, a hierarchical treatment algorithm (see Web Appendix) was instituted. Treatment was directed to each individual episode.
Study protocol algorithms were developed through a combination of evidence-based data and best practices in neurocritical care to limit center-to-center variability. Treatments in Tier 1 were started within 15 minutes of an episode start, and if ineffective after 60 minutes, an additional treatment option was started from the next tier. Initiation of at least one Tier 1 treatment option was required before escalating care to Tier 2. Tier 3 treatments were optional at the physician’s discretion. To insure protocol compliance, in-service training was conducted at each site.
Outcomes
The primary outcome measure, physiologic efficacy of PbtO2 treatment, was obtained from continuous PbtO2 monitoring records. Patient safety was assessed through review of adverse events by site PIs, the Independent Medical Monitor and Data Safety Monitoring Committee (DSMC). For each occurrence of increased ICP and decreased PbtO2, variables collected included: 1) time episode was recognized; 2) type and time of initial treatment, and 3) additional treatments required when less invasive interventions proved ineffective. The treating physician had discretion in following the tiered interventions if, in their opinion, it was indicated for patient safety.
A blinded, trained examiner assessed 6-month neurological outcome in person or by telephone interview. The Glasgow Outcome Scale – Extended (GOS-E) score (primary outcome measure) (20) and Disability Rating Scale (DRS) were obtained (21).
Statistical Analysis
Patient demographics in each group were compared using Mann-Whitney tests for ordinal or interval variables or Fisher’s exact tests for nominal categorical variables. PbtO2 and ICP values were recorded multiple times per minute using a CNS multimodal neuromonitor (Moberg). Seven subjects were excluded from physiologic data analysis because of missing data or unknown monitor insertion times. Data were summarized into 1-minute averages for analysis and further summarized into: 1) proportion of time PbtO2 was < 20mmHg or ICP was > 20mmHg, 2) average depth of brain tissue hypoxia (sum of the number of mmHg PbtO2 was < 20mmHg divided by number of minutes monitored times when PbtO2 was >20mmHg contribute 0 to the sum, but are included in the denominator), and 3) area over the curve (defined as sum of the amount by which PbtO2 was <20mmHg multiplied by the number of minutes it was at that value divided by 60min) (Figure 2A). Physiologic outcomes were compared using t-tests on the log of the variable, with zeros replaced by the power of 10 to make the distribution nearly normal (0.001 for proportion of time, 0.01 for average depth, and 10 for area over the curve). Six-month GOS-E and DRS scores were compared by Mann-Whitney tests.
Figure 2.
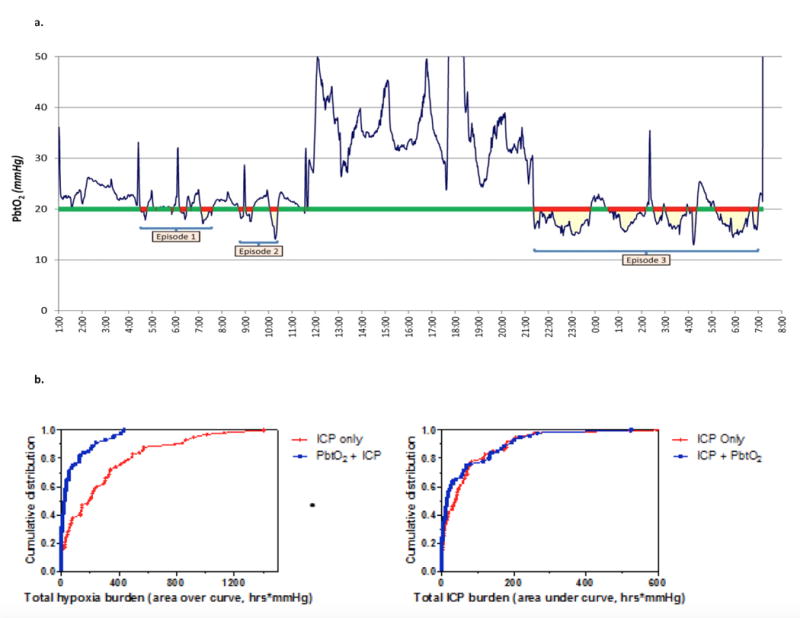
Panel 2A: Sample trace of continuous PbtO2, illustrating how time of brain tissue hypoxia (red bars) and area over the curve (yellow) were assessed over time (x-axis). Panel 2B: Left Panel: Cumulative distribution of total hypoxia burden (area over the curve in hrs*mmHg) for each participant in BOOST-II. Mean hypoxia burden in 55 patients in PbtO2 and ICP treatment arm was 74.9 hrs*mmHg (95% CI 43.9 – 105.9), while for the 58 patients in the ICP only treatment arm mean hypoxia burden was 285.8 hrs*mmHg (95% CI 202.0 – 369.7), p < 0.0001. Right Panel: Cumulative distribution of total intracranial hypertension burden (area under the curve in hrs * mm Hg) for each participant in BOOST-II. Mean hypertension burden in 55 patients in PbtO2 and ICP treatment arm was 61.1 hrs*mmHg (95% CI 35.0 – 87.9) while for the 59 patients in the ICP only treatment arm mean hypertension burden was 67.9 hrs*mmHg (95% CI 42.5 – 93.4), p = 0.21.
Role of the Funding Source
The National Institutes of Health (National Institute of Neurological Disorders and Stroke- NINDS) funded the study; however played no role in study design, data collection, data analysis, data interpretation, composition of manuscript, or decision to submit the paper for publication.
Results
Study population
One hundred nineteen patients were enrolled; 62 randomized to the ICP-only treatment group and 57 to the ICP+PbtO2 treatment group. Patient demographics and injury severity were similar in each treatment arm (Table 1).
Table 1.
Demographics and injury characteristics of the study population.
| Characteristic | Overall | ICP Only | PbtO2 + ICP | p-value |
|---|---|---|---|---|
| Subjects | 119 | 62 | 57 | |
| Age [Mean,(SD)] | 37.0 (17.3) | 36.2 (17.5) | 37.8 (17.2) | .613 |
| Male Sex [n (%)] | 92 (79%) | 46 (74%) | 46 (84%) | .262 |
| Race | ||||
| White | 100 (86%) | 53 (85%) | 47 (87%) | .359 |
| Black | 12 (10%) | 7 (11%) | 5 (9%) | |
| Other | 4 (4%) | 2 (4%) | 2 (4%) | |
| Unknown | 3 | 0 | 3 | |
| Injury Type | ||||
| Closed | 118 (99%) | 62 (100%) | 56 (98%) | .479 |
| Penetrating | 0 (0%) | 0 (0%) | 0 (0%) | |
| Blast | 0 (0%) | 0 (0%) | 0 (0%) | |
| Crush | 1 (1%) | 0 (0%) | 1 (2%) | |
| GCS Motor [Mean (SD)] | 3.7 (1.5) | 3.7 (1.5) | 3.6 (1.5) | .858 |
| CT scan results | ||||
| Contusions [n (%)] | 20 (38.4) | 26 (56.5) | .104 | |
| Midline Shift (n (%) | 28 (53.8) | 26 (56.5) | .841 | |
| Midline shift [mm, Mean + SD, Median)] | 3.38 ± 4.68 2 |
2.98 ± 4.56 1 |
.918 | |
| IVH [n (%)] | 17 (32.7) | 15 (32.6) | 1 | |
| Basal Cisterns compressed or absent | 42 (80.7) | 27 (58.7) | .566 | |
| Craniectomy | 18 (35) | 12 (24) | .285 |
Primary Outcome
Tiered management for episodes of PbtO2<20mmHg resulted in significantly less brain tissue hypoxia in the ICP+PbtO2 group than the ICP group. This result demonstrates that treatment of reduced PbtO2 decreased total duration of hypoxia by 66%, and average depth of hypoxia by 72%. The area over the curve (hrs × mm Hg) was reduced by 77% (Table 2 and Figures 2B). ICP was similar between groups. (Figure 2B, right panel).
Table 2.
PbtO2 and Intracranial Pressure parameters by study group.
| PbtO2 metric | ICP Only (N=58) |
PbtO2 + ICP (N=55) |
p-value |
|---|---|---|---|
| Proportion of time below 20 mmHg | .44 (.31) Median .45 |
.15 (.21) Median .07 |
.0000147 |
| Average depth (mmHg) | 3.6 (3.9) Median 2.3 |
1.0 (2.0) Median 0.2 |
.0000005 |
| Area [over] the curve (mmHg*hrs)** | 255 (291) Median 187 |
58 (97) Median 14 |
.0000002 |
| Intracranial Pressure metric | ICP Only (N=57) |
PbtO2 + ICP (N=55) |
p-value |
|---|---|---|---|
| Proportion of time above 20mmHg | .15 (.19) Median .10 |
.12 (.19) Median .04 |
.115 |
| Average depth (mmHg) | 1.6 (6.9)* Median 0.4 |
0.7 (1.3)* Median 0.3 |
.194 |
| Average depth (mmHg) (excluding the 2 extreme outliers) |
0.7 (0.9) Median 0.4 |
0.6 (0.9) Median 0.2 |
.195 |
| Area under the curve (mmHg*hrs)** | 103 (408)* Median 36 |
50 (88)* Median 17 |
.113 |
| Area under the curve (mmHg*hrs)** (excluding the 2 extreme outliers) |
50 (56) Median 34 |
41 (59) Median 15 |
.115 |
One extreme outlier in each group is dominating the Mean(SD) estimate
The “area under the curve” analysis does not adjust for inconsistent monitoring durations, thus a low AUC value could be due to normal PbtO2 values or a short duration of monitoring. (This pitfall is avoided with the “average depth” analysis, in which the AUC value is subsequently divided by the duration of monitoring. It also yields a more interpretable metric.)
Secondary Outcomes
Safety
The management protocol to optimize PbtO2 and control ICP was safe. Incidence of SAEs was low and similar between groups (Supplementary Table 5). There were no cases of hemorrhage or infection related to placement of monitoring catheters.
Feasibility
Time from injury to insertion of monitors was 9.05 (SD 5.22) hours. Time from insertion of monitors to onset of data analysis was 5 hours, to allow for equilibration and performing the FiO2 challenge. Valid PbtO2 data was obtained from an average of 80.3 (SD 42.6) hours, with unreliable data only 3% of monitoring time. Monitors were disconnected for an average of 4.1 hours while patients traveled for procedures, and an additional 2.5 hours data was considered invalid. There was no difference between treatment groups in total time of monitoring or the fraction of time with usable data.
The BOOST-II management protocol was complex (see Web Appendix). Our results show that the management protocol was feasible: treatment-related protocol deviations related to ICP management occurred with similar frequency between groups; ICP-only group 13%; ICP+PbtO2 group 11% (Table 6). Nineteen percent of patients in the ICP+PbtO2 group had untreated PbtO2<15mmHg for more than 30 minutes and a similar percent had an episode where PbtO2 of 15–19mm Hg was untreated (both classified as protocol deviations). Five patients had both a deviation and a violation of the PbtO2 treatment protocol.
There were substantial differences between the types of treatments instituted in each group. The ICP only group received an aggregate of 933 interventions, while the ICP+PbtO2 group received an aggregate of 867 interventions, 334 of those were during episodes of isolated low PbtO2, while another 122 interventions were directed at both low PbtO2 and high ICP. Additional details are provided in Supplementary Table 4.
Outcomes
6-month GOS-E scores were obtained in 106 patients (ICP-only group =53; ICP+PbtO2 group =53) and trended towards lower mortality and better outcomes in the ICP+ PbtO2 management group, though the difference did not reach statistical significance due to sample size (Figure 3A). Mortality in the ICP-only treatment group was 34% compared to 25% in ICP+PbtO2 group. Furthermore, in the ICP+PbtO2 group, 11% more patients had favorable outcomes (GOSE 5–8) than the ICP only group, and more than twice as many patients in the ICP+PbtO2 group achieved the highest outcome category of GOS-E 8 (ICP+PbtO2 group 13%; ICP-only group 6%). This outcome difference exceeded the protocol-specified non-futility threshold needed to advance the intervention strategy to a Phase III trial. DRS results demonstrated similar trends with better scores in the ICP+PbtO2 arm (medians 5 ICP+PbtO2 group and 6 ICP-only group, p=.217) (Figure 3B).
Figure 3.
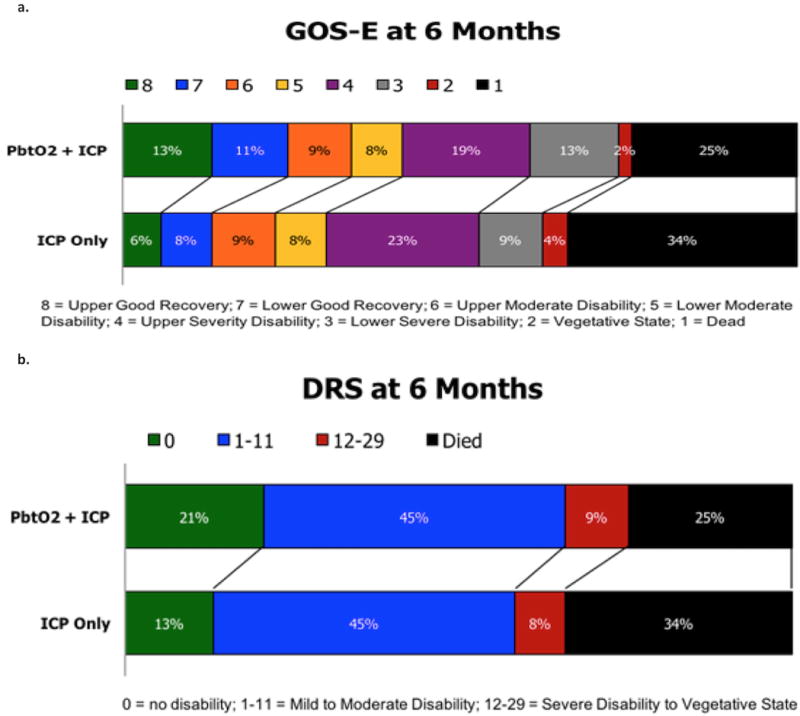
Panel 3A: GOS-E at 6 months after injury in each study treatment group. Panel 3B: DRS at 6 months after injury in each study treatment group.
The trial was stopped early by the DSMC after successful demonstration of the primary outcome in a smaller sample size than originally proposed.
Discussion
In BOOST-II, management of severe TBI based on multimodal ICP and PbtO2 monitoring compared to ICP monitoring alone reduced brain tissue hypoxia. The safety and feasibility of a PbtO2 directed treatment protocol was confirmed; protocol violations were low (11–16%, and equal between groups) and adverse events were in the anticipated range for this patient population. The trend towards more favorable outcomes and lower mortality with ICP+PbtO2-guided treatment exceeded the pre-specified non-futility threshold.
An important goal in TBI care is prevention, identification and treatment of secondary brain injury that can worsen patient outcome. ICP management has been at the center of this approach but there is still no level I evidence to support this concept. Meta-analytic studies suggest ICP based care, particularly when Brain Trauma Foundation guidelines are followed, is associated with improved outcome (22–25). However, in recent years there has been a conceptual shift in how ICP is managed to include inclusion of other clinical, radiologic and physiologic variables to better individualize therapy (4).
The management algorithm was not linear, offering multiple options within a given tier. This empowered two simultaneous goals: protocol-driven care to reduce variability with personalization of care based on clinical findings. The BOOST-2 trial represents one of the first targeted TBI management trials, i.e. precision medicine, a departure from the longstanding practice of treating all TBI as a uniform diagnosis. Overall, successful performance of the BOOST-II study indicates generalizability of multi-modal, goal-directed therapy into broad clinical practice, should a Phase III trial be successful.
Physiologic efficacy of PbtO2 based care
Management of severe TBI patients is premised on end-organ preservation and support to enable recovery. Brain tissue hypoxia following TBI is associated with worse outcomes clinically and with adverse pathophysiologic consequences in the experimental TBI literature (11–17). In the BOOST-II study, goal-directed therapy to maintain PbtO2 >20mmHg produced significant reductions in brain tissue hypoxia. Patients in the ICP+PbtO2 group had, on average, 15% of ICP+PbtO2 values consistent with brain hypoxia compared to 44% of patients in the ICP-only group. The results of BOOST-II support the hypothesis that PbtO2-directed therapy can reduce secondary brain injury following TBI.
While recently published studies suggest that a PbtO2 threshold of < 15 mm Hg is more highly correlated with brain ischemia (26), we believe that a treatment threshold of 20 mm Hg is appropriate, due to the association with increased risk of unfavorable outcome in observational studies (27). We believe that treating PbtO2 below 20 mm Hg is appropriate, since waiting until PbtO2 falls below 15 mm Hg may not allow an adequate safety margin for therapy to reverse hypoxia.
Safety
Therapies to enhance PbtO2 have potential risks, similar to ICP management, and respiratory complications are of particular concern. Respiratory SAEs were observed in only 4% of patients, were similar between groups, and consistent with those usually observed in severe TBI patients. Of the four respiratory SAE’s observed in the ICP+PbtO2 group, two were pneumonia and two were respiratory failure, and none were thought to be specifically associated with PbtO2 therapy.
Conclusions
BOOST-II was designed to demonstrate feasibility and safety of a treatment protocol. The DSMC stopped the trial early following successful demonstration of the primary outcome in a smaller sample size than originally proposed. 6-month neurological outcome indicated that PbtO2-directed therapy is not futile. Indeed, patients in the ICP+PbtO2 group showed a trend towards improved outcome with less mortality and more favorable outcomes. The planned BOOST-III study will assess impact on neurologic outcome of multimodal ICP plus PbtO2-directed management of severe TBI.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Neurological Disorders and Stroke (R01 NS061860). The investigators thank Karen March and Jason Marzuola for device education and technical support.
Footnotes
*Conflicts of Interest and Source of Funding
Dick Moberg has a proprietary interest in Moberg ICU Solutions, which manufactures the data collection device used in the study. No other potential conflict of interest relevant to this article was reported. Dr. Le Roux receives research funding from Integra Lifesciences. He is a consultant for Integra Lifesciences, Codman, Depuy-Synthes, and Neurologica and a member of the scientific advisory board of Cerebrotech and Edge Therapeutics. The remaining authors have disclosed that they do not have any conflicts of interest.
Copyright form disclosure: Drs. Okonkwo, Shutter, Moore, Temkin, Puccio, Madden, Chesnut, McGregor, Weaver, LeRoux, and Diaz-Arrastia received support for article research from the National Institutes of Health (NIH). Dr. Okonkwo disclosed off-label product use of ICP monitors and PbtO2 monitors (Integra LifeSciences, Plainsboro, NJ). Dr. Shutter’s institution received funding from the NIH; she received funding from legal firms for expert testimony on cases not related to this study; and she disclosed that her spouse was briefly an independent contractor for medical sales with Raumedic, Inc, who makes a medical device that measures brain tissue oxygen. The Raumedic device was not used in this study (total compensation was $3,500). Dr. Temkin’s institution received funding from the NIH/National Institute of Neurological Disorders and Stroke; she disclosed that her salary through her institution comes primarily from grants and contracts with various US federal agencies; and she received funding from various pharmaceutical companies and academic institutions (data and safety monitoring boards and statistical consulting); and from reviewing grants for various federal agencies and for non-profits. Dr. Chesnut disclosed that he holds the Integra Endowed Professorship in Neurotrauma at the University of Washington which was established in total, with no further contributions, prior to the origin of this work. Dr. Grant received funding from honorarium for talk about brain oxygen monitoring from Integra Life Sciences in 2016, 2 years after completion of Boost II Trial. Dr. McGregor’s institution received funding from the NIH. Dr. LeRoux’s institution received funding from the NIH, and he received other support from consulting for Integra and Codman. Mr. Moberg’s institution received funding from the NIH, and he disclosed he is the founder, CEO, and shareholder of a company that provided data collection equipment and expertise for the project. Dr. Diaz-Arrastia’s institution received funding from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
David O. Okonkwo, University of Pittsburgh School of Medicine.
Lori A. Shutter, University of Pittsburgh School of Medicine.
Carol Moore, Uniformed Services Univ. of the Health Sciences – 4301 Jones Bridge Rd., Bethesda, MD 20814.
Nancy R. Temkin, University of Washington.
Ava M. Puccio, University of Pittsburgh School of Medicine.
Christopher J. Madden, UT Southwestern Medical Ctr – 5323 Harry Hines Blvd., Dallas, TX 75390.
Norberto Andaluz, Univ. of Cincinnati College of Medicine-234 Goodman St., Cincinnati, OH 45219.
Randall M. Chesnut, University of Washington.
M. Ross Bullock, U of Miami, Miller School of Medicine – 1600 NW 10th Ave., Miami, FL 33136.
Gerald A. Grant, Stanford University – 300 Pasteur Drive, Stanford, CA 94305.
John McGregor, Ohio State University College of Medicine – 370 W. 9th Ave., Columbus, OH 43210.
Michael Weaver, Lewis Katz School of Medicine – 3500 N. Broad St., Philadelphia, PA 19140.
Jack Jallo, Thomas Jefferson University – 909 Walnut Street, Suite 200, Philadelphia, PA 19107.
Peter D. LeRoux, Lankenau Medical Center – 100 E. Lancaster Ave., Wynnewood, PA 19096.
Dick Moberg, Moberg Research, Inc. – 224 S. Maple Street, Ambler, PA 19002.
Jason Barber, University of Washington.
Christos Lazaridis, Baylor St. Luke’s Medical Center – 6720 Bertner Ave, Houston, TX 77030.
Ramon R. Diaz-Arrastia, University of Pennsylvania.
References
- 1.Injury Prevention and Control: Traumatic Brain Injury & Concussion. http://www.cdc.gov/traumaticbraininjury/severe.html. Accessed 8-29-16.
- 2.Chesnut R, Videtta W, Vespa P, et al. Intracranial pressure monitoring: Fundamental considerations and rationale for monitoring. Neurocrit Care. 2014;21(Suppl 2):S64–84. doi: 10.1007/s12028-014-0048-y. [DOI] [PubMed] [Google Scholar]
- 3.Carney N, Totten AM, OʼReilly C, et al. Guidelines for the management of severe traumatic brain Injury, Fourth Edition. J Neurosurg. 2016 doi: 10.1227/NEU.0000000000001432. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Le Roux P, Menon DK, Citerio G. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A list of recommendations and additional conclusions: A statement for healthcare professionals from the Neurocritical Care Society and European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S297–361. doi: 10.1007/s12028-014-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon DK, Coles JP, Gupta AK, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32(6):1384–1390. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- 6.Vespa PM, O’Phelan K, McArthur D, et al. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007;35(4):1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- 7.Pennings FA, Schuurman PR, van den Munckhof P, et al. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: The assessment of normal values. J Neurotrauma. 2008;25(10):1173–1177. doi: 10.1089/neu.2007.0402. [DOI] [PubMed] [Google Scholar]
- 8.van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. J Neurosurg. 1996;38:21–31. doi: 10.1097/00006123-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Valadka AB, Gopinath SP, Contant CF, et al. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26(9):1576–1581. doi: 10.1097/00003246-199809000-00029. [DOI] [PubMed] [Google Scholar]
- 10.van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. J Neurosurg. 2000;46(4):868–878. doi: 10.1097/00006123-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Gracias VH, Guillamondegui OD, Stiefel MF, et al. Cerebral cortical oxygenation: A pilot study. J Trauma Acute Care Surg. 2004;56(3):469–474. doi: 10.1097/01.ta.0000114274.95423.c0. [DOI] [PubMed] [Google Scholar]
- 12.Artru F, Jourdan C, Perret-Liaudet A, et al. Low brain tissue oxygen pressure: incidence and corrective therapies. J Neurol Res. 1998;20(Suppl 1):S48–51. doi: 10.1080/01616412.1998.11740610. [DOI] [PubMed] [Google Scholar]
- 13.Valadka AB, Goodman JC, Gopinath SP, et al. Comparison of brain tissue oxygen tension to microdialysis-based measures of cerebral ischemia in fatally head-injured humans. J Neurotrauma. 1998;15(7):509–519. doi: 10.1089/neu.1998.15.509. [DOI] [PubMed] [Google Scholar]
- 14.Bardt TF, Unterberg AW, Härtl R, et al. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153–156. doi: 10.1007/978-3-7091-6475-4_45. [DOI] [PubMed] [Google Scholar]
- 15.Kiening KL, Härtl R, Unterberg AW, et al. Brain tissue pO2-monitoring in comatose patients: implications for therapy. J Neurol Research. 1997;19(3):233–40. doi: 10.1080/01616412.1997.11740805. [DOI] [PubMed] [Google Scholar]
- 16.Bohman LE, Heuer GG, Macyszyn L, et al. Medical management of compromised brain oxygen in patients with severe traumatic brain injury. Neurocrit Care. 2011;14(3):361–369. doi: 10.1007/s12028-011-9526-7. [DOI] [PubMed] [Google Scholar]
- 17.Pascual JL, Georgoff P, Maloney-Wilensky E, et al. Reduced brain tissue oxygen in traumatic brain injury: are most commonly used interventions successful? J Trauma Acute Care Surg. 2011;70(3):535–546. doi: 10.1097/TA.0b013e31820b59de. [DOI] [PubMed] [Google Scholar]
- 18.Nangunoori R, Maloney-Wilensky E, Stiefel M, et al. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: a systematic literature review. Neurocrit Care. 2012;17(1):131–138. doi: 10.1007/s12028-011-9621-9. [DOI] [PubMed] [Google Scholar]
- 19.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2008;25(3):276–278. [Google Scholar]
- 20.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 21.Rappaport M, Hall KM, Hopkins K, et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 22.Stein SC, Georgoff P, Meghan S, et al. Relationship of aggressive monitoring and treatment to improved outcomes in severe traumatic brain injury. J Neurosurg. 2010;112(5):1105–1112. doi: 10.3171/2009.8.JNS09738. [DOI] [PubMed] [Google Scholar]
- 23.Alali AS, Fowler RA, Mainprize TG, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30(20):1737–1746. doi: 10.1089/neu.2012.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talving P, Karamanos E, Teixeira PG, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg. 2013;119(5):1248–1254. doi: 10.3171/2013.7.JNS122255. [DOI] [PubMed] [Google Scholar]
- 25.Gerber LM, Chiu YL, Carney N, et al. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119(6):1583–1590. doi: 10.3171/2013.8.JNS13276. [DOI] [PubMed] [Google Scholar]
- 26.Veenith TV, Carter EL, Geeraerts T, et al. Pathophysiologic Mechanisms of Cerebral Ischemia and Diffusion Hypoxia in Traumatic Brain Injury. JAMA Neurol. 2016 May;73(5):1. 542–50. doi: 10.1001/jamaneurol.2016.0091. [DOI] [PubMed] [Google Scholar]
- 27.Chang JJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009 Jan;37(1):283–90. doi: 10.1097/CCM.0b013e318192fbd7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


