Introduction
Chronic disease management is the biggest health care problem facing the United States today. In 2012, nearly 1 in 2 Americans (117 million) had at least one chronic condition1 and 26% of the population had multiple chronic conditions. These numbers are expected to steadily increase over the next 30 years.2 Chronic diseases especially affect older adults3 in whom it is widely recognized that exacerbations result in dramatic changes and decline in health status, hospitalization, complex treatment interventions, and high cost4. Recognition of small changes in health conditions are essential for early interventions when treatment is most effective, prevention of dramatic decline is still possible, and costs can be controlled. Early illness recognition and early treatment is not only a key to improving health status with rapid recovery after an exacerbation of a chronic illness or acute illness, but also a key to reducing morbidity and mortality in older adults.5,6,7,8
This randomized prospective intervention study was conducted to measure the clinical effectiveness and cost effectiveness of using sensor data from an environmentally embedded sensor system for early illness recognition. This sensor system has demonstrated in pilot studies to measure functional ability in older adults and actually detected changes in chronic diseases or acute illnesses on average 10 days to 2 weeks before usual assessment methods or self-reports of illness9,10. Inexpensive sensors are embedded in the environment, so subjects do not “have to use” any equipment or “wear” any devices. Motion sensors monitor subjects continuously while they go about daily activities in their homes. Unobtrusive bed sensors collect data about the subjects’ pulse, breathing, and restlessness while they sleep. A gait sensor monitors increasing fall risk and alerts when people fall within the view of the sensor. The sensor system automatically detects changes in functional activities, normal sleeping patterns and walking to alert health care providers of potential health problems. 9,10 The purpose of this prospective intervention study was to measure the clinical and cost effectiveness of using sensor data to detect early signs of illness or functional decline in a randomized sample of older adults (n=87) living in assisted living (AL) communities as compared to usual health assessment methods of older adults living in those same AL communities (n=85).
Design and Methods
A prospective intervention study of assisted living residents randomly assigned to intervention or control groups was conducted. Based on the data from pilot work, minimum sample size for 80% power and 0.7 effect size was calculated to be 55 older adults; 65 per group was our initial target. We planned for rolling enrollment into both groups over 2.5 years to accomplish adequate numbers of participants. We were able to increase numbers into both groups to assure exposure to the intervention as we experienced sensor data transmission interruptions due to network infrastructure problems within the AL communities. This enabled each participant one year of experience living with the sensor system, which we estimated in the study plan was an adequate minimum duration of the intervention based on our prior work.9 Inclusion criteria included the ability to walk a minimum of 20 feet without staff assistance, although using a cane or walker was permissible; ability to grip with hands (as grip strength was a measure collected); willing to have sensor systems installed in apartments; willing to participate in baseline and quarterly data collections lasting a few minutes; sensor data transmission for an average of one year for intervention participants as well as continuous enrollment for control group for an average of one year.
Theoretical Model
Figure 1 is our theoretical model of early detection guiding the sensor system development and outcomes expected from its use. The outcome logic is that if changes in function/health status are detected earlier using the sensor information, like bed restlessness and vital signs, then they are managed at an earlier stage, therefore preventing emergency room (ER) visits, hospitalizations, and nursing home admissions. We have successfully measured most components in the Early Detection Model in prior work.9,11,12
Figure 1.
Sample
Thirteen AL communities were recruited from a large and reputable long term care corporation located in Missouri. Sites were selected based on driving radius of about 100 miles of the research team conducting the study. Facilities ranged in size from 16 to 68 residents; most of the study participants lived in private rooms with private baths. Facilities were located in both urban and rural areas. Subjects were recruited from all 13 AL communities. A total of 171 people were enrolled and then randomly assigned to the intervention or control group. During the rolling enrollment, 86 were assigned to the intervention group and 85 to the control. It was necessary to continue enrollment beyond targeted numbers to reach the duration for sensor data transmission defined for exposure to the intervention for the intervention subjects. Demographic descriptors are displayed in Table 1.
Table 1.
Demographics
| Characteristic | Intervention N=86 (%) Mean (Std dev) |
Control N=85 (%) Mean (Std dev) |
|---|---|---|
| Race | ||
| African American | 3 (3.5%) | 1 (1.2%) |
| Caucasian | 83 (96.5%) | 84 (98.8%) |
| Ethnicity | ||
| Hispanic or Latino | 0 (0%) | 1 (1.2%) |
| Non-Hispanic or Latino | 86 (100%) | 84 (98.8%) |
| Sex | ||
| Male | 22 (25.6%) | 23 (27.1%) |
| Female | 64 (74.4%) | 62 (72.9%) |
| Days enrolled in study | 50.6 (212.6) | 382.4 (198.0) |
| Age | 83.6 (9.4) | 86.0 (8.0) |
Figure 2 displays the dose of the study in months (intervention group on the left and control group on the right) and Table 2 displays the dose of the intervention in days. Intervention group was living with the sensors and control group was exposed to usual health assessment methods.
Figure 2.
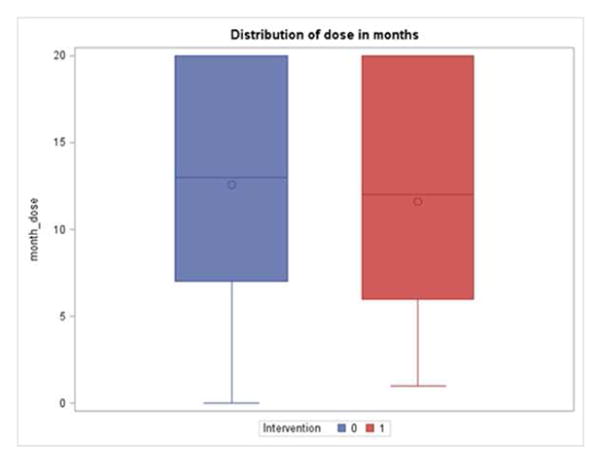
Table 2.
Intervention Dose in Days
| Group | N | Mean | Std dev | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| Control | 85 | 382.39 | 198.01 | 7 | 397 | 607 |
| Intervention | 86 | 350.56 | 212.57 | 18 | 358 | 607 |
Intervention
The sensor system deployed in this intervention consists of a “standard” suite of environmentally embedded (non-wearable) sensors to unobtrusively and automatically monitor functional status of older adults, detect potential changes in health or functional status, and send early alerts to healthcare providers. 10 Sensors include motion sensors to measure overall activity, an under mattress bed sensor to capture respiration, pulse, and restlessness as people sleep, and a gait sensor. The gait sensor is a small depth image sensor that uses non-identifiable, shadow-like images to continuously measure gait speed, stride length and time, and automatically assess for increasing fall risk. Continuously running computer algorithms are applied to the sensor data and send alerts to staff at the time changes in sensor data patterns are detected, which may be days/weeks before typical signs or symptoms are recognized by the study participant, family members, or providers. Health alerts are sent to AL nurses via email, and each alert contains an electronic hyperlink that displays the content of the health alert in the web-based sensor data interface. The AL nurses would then determine, based on their knowledge of the resident and his/her current health conditions, if further assessment was necessary. In this way, the sensor system is designed to serve as a clinical decision support tool, augmenting the assessment of individual residents.
The gait sensor also sends immediate alerts to staff when a fall occurs via an email to their cell phone or in the case of these study sites, iPod touch devices that were configured specifically to receive these alerts for AL staff. Each alert features a short video clip of privacy-protecting shadow-like images of the alert trigger event. Viewing this clip, staff can determine if an actual fall has occurred and respond accordingly.13–15 In the case of a false alarm, the video clip allows staff to dismiss the alert without disturbing the study participant.
AL staff at each participating site received orientation to the sensor system and the alerts, as well as instruction (both in person and written guide) in how to use the sensor data interface, alerts, and typically how to interpret and respond to alerts. This training was conducted by webinar and in person after the sensor system was installed and operational in each facility. Over the course of the study, follow-up staff training was conducted at each facility by the project coordinator. The project coordinator conducted research site visits every 1–2 months for the duration of the study. Other research staff working with the technology were on-site as problems occurred that could not be addressed remotely. For example, occasionally a computer would need to be rebooted or motion sensors would need to be repositioned.
Difficulties in the intervention implementation interfered with subjects receiving the intervention as planned. While the sensors themselves functioned as expected, the network infrastructure within the AL communities was unable to consistently transmit the data so that real time use of the data (as in our pilot work) could be accomplished. Weeks and months of working with technology staff of the corporation operating the participating communities could never quite resolve all the issues so that each sensor and the sensor interface viewed by the nurses for the health alerts could be quickly viewed and analyzed.
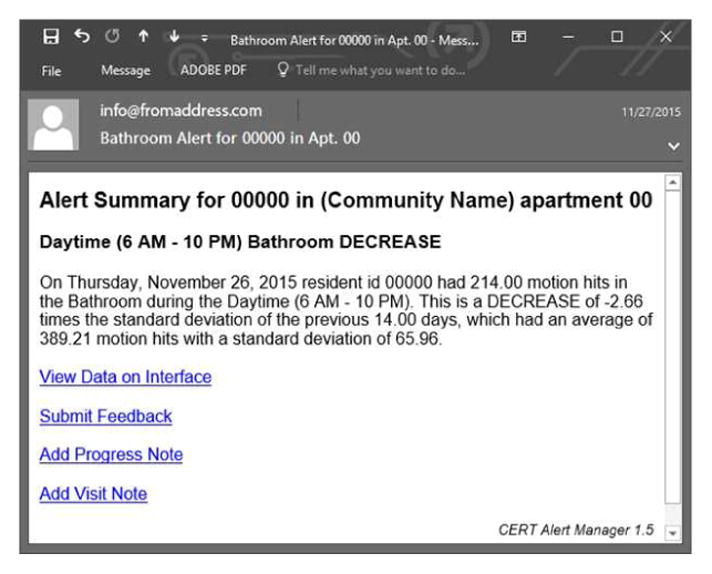
The nurses received the health alerts via email, but did not consistently have access to the interface to actually view the data displays and understand the changes in decline of activity, or increases or decreases in bed restlessness, heart rate and respiration. Nurses received health alerts every morning for the prior 24 hours as they occurred. These were simplistic emails, such as “Resident #---, apartment number---, increase in bed restlessness during the night” or “increase in bathroom frequency during the night” (See Figure 3 Health Alert). Details of the alerts were only visible on the website which they were not able to consistently access, as explained above.
Figure 3.
Real time fall alerts did function well in each facility, so staff could respond quickly when people in the intervention group fell. These data and alerts were electronically processed on-site within the sensor system in each facility and bypassed the problematic portions of the network and website infrastructure in the facility. Staff carried iPod Touch devices that immediately alerted them and displayed an electronic hyper-link directing them to a short video clip of the fall sensor images (shadow-like, privacy protecting images) so they could determine if a real fall occurred and if they needed to respond, or if the alert by the sensor system was a false alarm that they could ignore. Staff did consistently use the real time fall alerts of the sensor system throughout the duration of the intervention displayed in the dose Figure 2.
Data Collected
Quantitative data for outcome measures from all intervention and control subjects included: Short form 12 (SF12), Geriatric Depression Scale (GDS), Mini Mental State Exam (MMSE), activities of daily living (ADL) and instrumental activities of daily living (IADL), gait speed (resident walks 10′ and time is measured with a stop watch), GaitRite (resident walks across the GaitRite Mat) (automatic measurement of velocity, step time left and right, step length left and right, stride length left and right, and calculation of the GaitRite Functional Ambulation Profile (FAP)), hand grips (left and right hand grip measured with digital dynamometer). Each of these instruments are known to be valid and reliable measures16–21 and we have used them with success in several studies.9,22,23 The instruments are simple to complete with less than 15 items each. Also, falls, emergency room (ER) visits, hospitalizations (number and length of stay), nursing home stays, and physician visits were tracked. Demographics, including medical diagnoses and medications were collected for description and co-variation as needed in analyses.
Data Analysis
Several preliminary analyses were conducted to examine the data and understand the potential group sizes at various doses of the intervention. Analyses were conducted at monthly intervals, quarterly, and then at the beginning and end of the study for each subject to consider impacts of early effects, latent effects, and overall effectiveness of the intervention.
The original analytic plan for determining intervention effectiveness was to test beginning and ending outcome measures for each group. After preliminary analyses, this was determined to be the most appropriate approach to explain the final results of the quantitative outcome analysis. Repeated measures general linear models testing fixed effects for dose of the intervention (controlling for time spent in the intervention or control group) at beginning and end time points were used to determine effectiveness of the intervention for each continuous outcome measured in both groups. The independent variables investigated were group (intervention/control), time (beginning/end), dose (time spent in the intervention), and the group by time interaction term.
Results
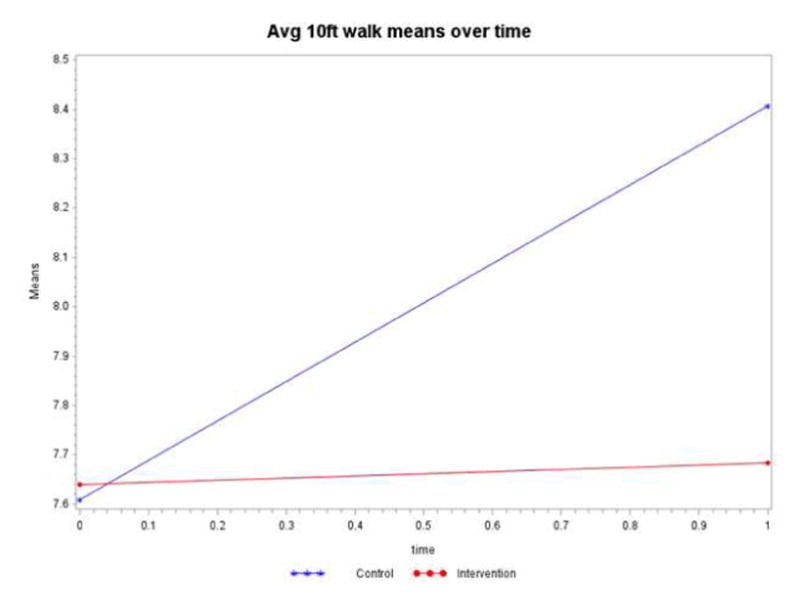
Walking speed in seconds (Average 10-foot walk means over time)
Walking speed was measured by research staff for both intervention and control groups throughout the study for each subject, on average, two times per year. Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term is not statistically significant (p=0.1384) (Table 3). The mean walking speeds displayed in Figure 4 shows the intervention group has a stable slope (essentially no increase in walking time) compared to the control group. The control group’s walking speed increased by 0.80 sec., whereas the intervention group increased only by 0.04 sec, indicating a more rapid decline for the control group than the intervention group. However, the groups started off as statistically equivalent (p=0.9689), and ended up as statistically equivalent (p=0.3370).
Table 3.
Model Results Walking Speed of 10ft Walks
| Type 3 Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| dose | 1 | 112 | 0.55 | 0.4595 |
| time | 1 | 113 | 2.77 | 0.0986 |
| intervention | 1 | 112 | 0.24 | 0.6243 |
| intervention*time | 1 | 113 | 2.23 | 0.1384 |
Figure 4.
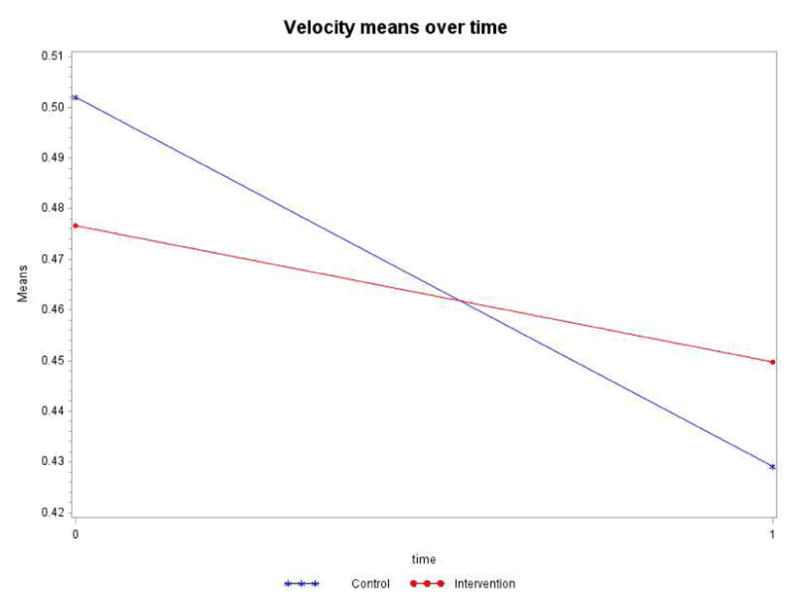
Velocity (measured by GaitRite in meters per second)
Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term is not statistically significant (p=0.0894) although velocity decline was statistically significant for both groups (Table 4). As Figure 5 shows, the intervention group has a more stable slope (less of a drop in velocity) than the control group. The control group’s decline of 0.073m/s was more pronounced than the intervention group’s decline of 0.027m/s over the one-year study.
Table 4.
Model Results Velocity (Gaitrite)
| Type 3 Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| dose | 1 | 111 | 0.00 | 0.9852 |
| intervention | 1 | 111 | 0.00 | 0.9483 |
| time | 1 | 112 | 13.86 | 0.0003 |
| intervention*time | 1 | 112 | 2.94 | 0.0894 |
Figure 5.
Stride length right (measured by GaitRite in meters)
Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term is not statistically significant (p=0.1637). Both groups significantly declined over time (Table 5). However, as shown in Figure 6, the control group decline of 0.0494m was more pronounced than the intervention group decline of 0.0111m during the study.
Table 5.
Model Results Stride Length Right
| Type 3 Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| dose | 1 | 111 | 0.64 | 0.4269 |
| intervention | 1 | 111 | 0.45 | 0.5019 |
| time | 1 | 112 | 4.88 | 0.0292 |
| intervention*time | 1 | 112 | 1.96 | 0.1637 |
Figure 6.
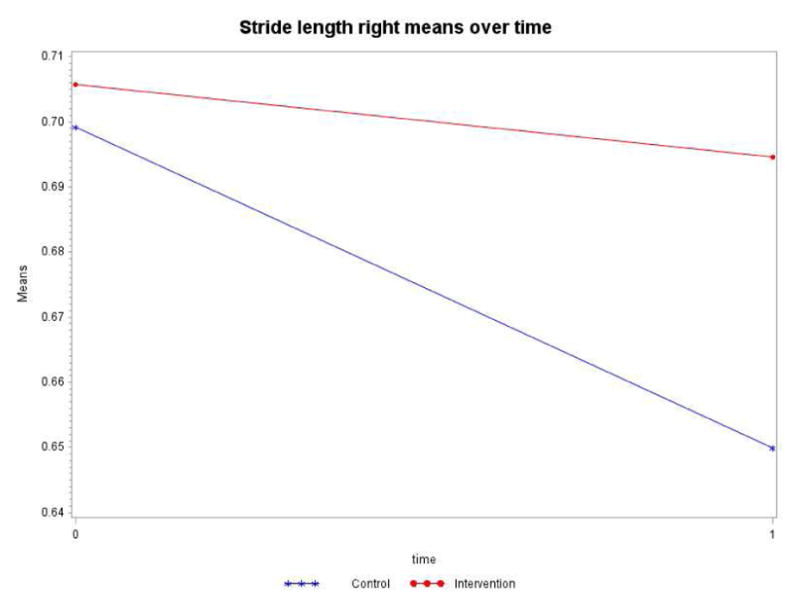
Stride length left (measured by GaitRite in meters)
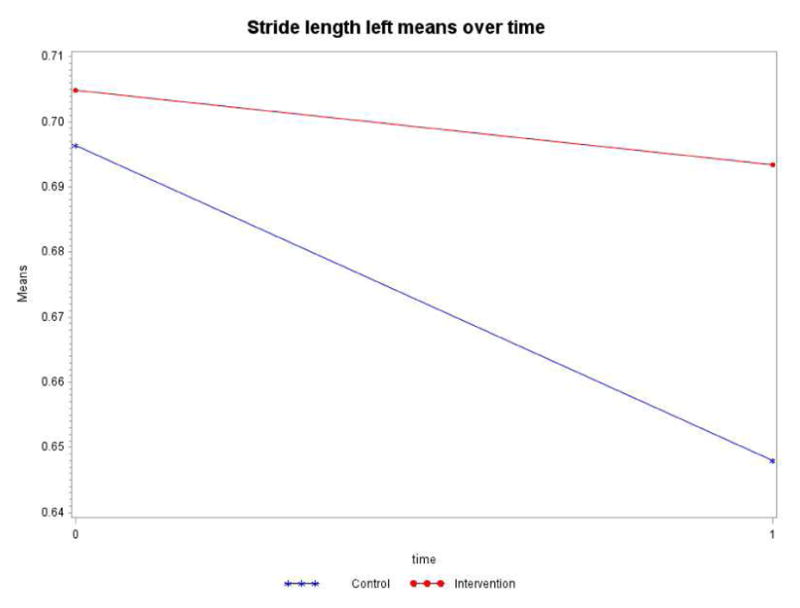
Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term is not statistically significant (p=0.1680). As in stride length right, both groups significantly declined over time (Table 6). However, as displayed in Figure 6, the control group decline of 0.0484m was more pronounced than the intervention group decline of 0.0114m.
Table 6.
Model Results Stride Length Left
| Type 3 Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| dose | 1 | 111 | 0.51 | 0.4780 |
| intervention | 1 | 111 | 0.51 | 0.4777 |
| time | 1 | 112 | 5.02 | 0.0270 |
| intervention*time | 1 | 112 | 1.93 | 0.1680 |
Step length right and left (measured by GaitRite in meters)
Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term is not statistically significant (p=2318 for right and p=0.4602 for left). Similar to stride length and velocity, step length for both right and left for both groups significantly declined over time (p=0.01, respectively). However, the control group step length right decline of 0.0255m was more pronounced than the intervention group decline of 0.0091m. Similarly, step length left decline of 0.0245m for the control group was more pronounced than the intervention group decline of 0.0133m.
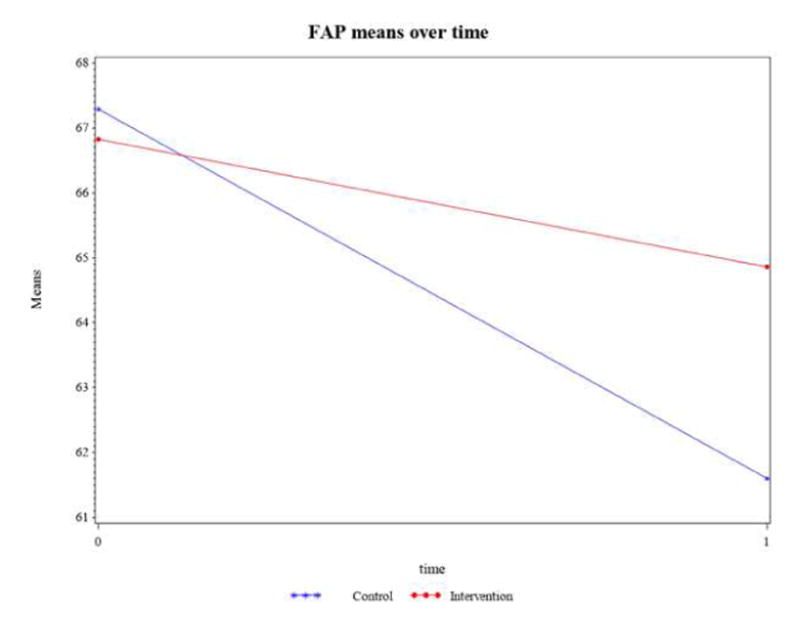
Functional Ambulation Profile (FAP) (measured by GaitRite), a performance index composite score; (range 30–100 for disabled, 95–100 for non-disabled people).24
Controlling for the time spent in the intervention or control group (dose), the intervention by time interaction term in Table 7 is not statistically significant (p=0.0792). As shown in Figure 8, the intervention and control groups do not have similarly declining slopes. The control group declined by a score of 5.69, and the intervention group declined by 1.96. Similar to the other reported gait measures, clinically, this decrease for the control group more than the intervention group is an important clinically relevant indicator of increasing fall risk.24
Table 7.
Model Results Functional Ambulation Profile (FAP) (Gaitrite)
| Type 3 Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| dose | 1 | 111 | 0.00 | 0.9752 |
| intervention | 1 | 111 | 0.24 | 0.6279 |
| time | 1 | 112 | 13.25 | 0.0004 |
| intervention*time | 1 | 112 | 3.14 | 0.0792 |
Figure 8.
Other outcomes of health, healthcare use, and cost
There were multiple other measures of health measured to analyze differences between the intervention and control groups to potentially explain the results of the primary outcome measures (presented above). These health measures included SF12, GDS, MMSE, ADL and IADL, grip strength (left and right hand grip measured with dynamometer), and falls. These were collected on average twice yearly and analyzed for significant differences between intervention and control groups using the same analytic methods as in the primary outcomes presented above. No significant differences of group comparisons were measured.
Also measured were falls, emergency room (ER) visits, hospitalizations and nursing home rehabilitation (number and length of stay in days), and physician visits; these were also analyzed using the same analytic methods as the primary outcomes; none were significantly different between groups or over time. There were more falls in the control group than intervention (85 subjects for 8.3 mean vs 78 subjects for 6.5 mean, respectively) but not significantly different (p=0.12). Similarly, ER visits, hospitalizations, nursing home stays, and physician visits were not significantly different across groups. Means were very similar: Hospital days (58 control subjects for 1.5 days, 63 intervention subjects for 1.57, p=0.78), ER visits (58 control subjects for 1.5 visits, 63 intervention subjects for 1.98, p=0.02), and Physician Visits (86 control subjects for 3.97, 78 intervention subjects for 4.35, p=0.64). Nursing Home Rehabilitation days were measured in medians due to small sample size and similar (5 subjects in control for median 27 days, 2 subjects in intervention for median 29 days, p=0.25).
An important part of this study was a cost analysis that included a large number of variables that are representative of health care costs. Medicare files were not analyzed due to the high costs required for obtaining Medicare files for analysis. Instead, our study plan for the cost analysis was to estimate costs based on the primary data collected from the sites: number of residents in each group, falls, fractures, ER visits, hospitalizations, hospitalization days, rehabilitation days, mental health facility days, number not returning to AL community, ER visits resulting in hospitalization, and average length of stay of ER visit. Costs for these analyses were estimated using Kaiser State Health Facts25 information of average cost of hospitalizations and average hospital cost per resident for state/local, nonprofit, and for profit status. Since the primary data collection did not include the name of the hospital where each subject was admitted, hospital status could not be determined for each hospitalization. Therefore, all data were analyzed using the three possible hospital status information for the state in which the study was conducted. No significant differences in costs were measured for any variable.
The perspectives of the clinician users of the sensor interface and alerts throughout the study was measured. It is critical that clinicians find the sensor information easy to use and clinically relevant. A 7-question visual analog evaluation tool (possible range 0–100) was collected monthly from research staff and clinicians in the AL communities who were using the web-based interface in the intervention study. The instrument was collected during the prospective intervention study.
Overall, the average score increased by a mean of 8 total points (improved) during the study. The greatest improvement was rating clinical relevance of the sensor system which improved 29 points (from 61 to 90). The question read “The intelligent sensor system displays clinically relevant sensor data summarizing activity, bed restlessness, pulse and respiration.” However, the average score on the question rating if “The intelligent sensor system is readily available” declined from 68.6 to 62. This is a direct reflection of the networking problems which made the system unreliable in the facilities.
Discussion
There are important results in this prospective intervention study designed to measure the clinical and cost effectiveness of using sensor data to detect early signs of illness or functional decline in a randomized sample of older adults living in AL communities. The randomized comparison group functionally declined more rapidly than the intervention group. Walking speed and several measures from GaitRite, velocity, step length left and right, stride length left and right, and the fall risk measure of functional ambulation profile (FAP) all had clinically significant changes. The walking speed increase (worse) and velocity decline (worse) of 0.073m/s for the comparison group exceeds 0.05 m/s, a value considered to be a minimum clinically important difference.26 Similarly, the comparison group’s decline of almost 5cm in both right and left stride length compared to the intervention group’s decline of just over 1cm bilaterally suggests the comparison group is at greater risk of falling than the intervention group. 11 Prior research examining in-home gait parameters found that a one-week change in stride length of 0.0254m was associated with a 6.78 odds of falling in the next three weeks. These findings demonstrate that sensor data with health alerts and fall alerts sent to AL nursing staff can be an effective strategy to detect and intervene in early signs of illness or functional decline. There was a finding that may indicate the nursing staff may have intervened with functional decline, as more subjects were referred from the AL community to nursing home rehabilitation (5 intervention vs 2 control subjects). While a small number, this finding may be a reflection of attempts to refer for rehabilitation due to detection of functional decline alerted by the sensors.
Other outcomes of health, measured by SF 12, GDS, MMSE, ADL or IADL scales, or grip strength, did not reveal clinical differences between the two groups. There were fewer falls in the intervention group than the comparison group, but not significantly fewer. Similarly, results of the cost analysis of ER visits, hospitalizations, nursing home stays, and physician visits were not different between the groups. One contributor to these results may be that these measures were not affected because the AL communities were unable to receive the full dose of the intervention due to the networking problems with the facilities. Networking basic service problems of internet connections, slow speeds, interruptions in service for sometimes days or weeks, and connection losses within the networking infrastructure negatively impacted the intervention. These problems were not experienced in the pilot study site, but affected every community in the sample made available for this study by the same parent company as the pilot site. The AL communities were located in both urban and rural locations, even in the same large metro area as the pilot site, but the infrastructures were each different and had fundamental networking problems that were not readily solved by research staff working cooperatively with corporate staff.
Proper internet connections and networking are essential for the correct operation of not only the sensor data collection and transmission, but importantly, the speed of displaying the visual data interface to the clinicians receiving health alerts. When clinicians receive the health alert, they should be able to click on the electronic hyper-link in the email and within 3–5 seconds see the visual displays of the sensor data for them to interpret the changes detected by the automated system. With the overwhelming networking problems, more often than not, clinicians were unable to access the interface in under a minute or even longer, which was a disincentive to using the displays. A typical pattern would be that they would move from the email alert with a cursory description of the sensor change to considering if the change could be relevant, perhaps paying attention to the resident a bit more than usual, but not examining the sensor data for actual or patterns of changes. It is likely the interface delays and periods of internet unavailability that occurred frequently in each of the sites resulted in a lower than anticipated dose of the intervention. This conclusion is supported by the decline in the average score on the question on sensor system availability (from 68.6 at the start of the study to 62 at the end) that clinician users answered throughout the study.
Despite the technical difficulties with the interface, the clinicians still found the sensor system to be a valuable tool in alerting them to a potential change in a resident’s status. They perceived summarizations of activity, bed restlessness, pulse and respiration to be clinically relevant data. Nurses use trends over time as cues for potential deterioration.27 Indeed, trends in physiological parameters, such as heart rate and respiratory rate, have been found to be independent predictors of illness and deterioration.28, 29
In addition to the health alerts, as explained earlier in methods, fall alerts were sent immediately to the nursing staff in the AL communities. Fall alerts bypassed the networking infrastructure in the communities required by the health alerts and these alerts functioned properly throughout the study. The dose of the intervention for fall alerts was consistently received by staff when falls were detected. With the alert received by staff on I-pods set up specifically for this purpose by research staff, clips of the images could be viewed by staff to assess if the alert was indeed a fall or a false alarm. Viewing the clip, staff could determine the legitimacy of the fall. If the alert registered a false alarm such as a blanket falling to the floor from a resident’s lap, staff could determine that a fall had not occurred. This feature avoided unnecessary room checks and interruption of privacy that could disturb a resident who had not fallen. If the person did fall, staff immediately responded. The alerts provided an opportunity for staff to attend to the fall and treat any injury that might have occurred within a shorter time frame. Residents also avoided extended periods of time on the floor. This knowledge that staff responded quickly provided the residents with a higher sense of security and safety. Residents commented to research staff that knowing someone was watching over them was a relief. Staff also commented about the helpfulness of the fall alerts to notify them of the fall and for them to see how the resident actually fell and what potential body areas were likely affected in a fall.
While study results did not find significant differences in costs, there is some evidence of potential cost savings using technology in pilot studies of this sensor system and the care coordination delivery model in the facility where the pilot studies were conducted. For example, in a five-year longitudinal analysis of length of stay (LOS) for all residents living in this setting, the residents who lived with sensors (n=52) had an average LOS of 4.3 years, significantly longer (p=0.0006) than those who lived without sensors (n=81, LOS of 2.6 years). Both groups were comparable based on admission age, gender, number of chronic illnesses, SF12 physical health, SF12 mental health, Geriatric Depression Scale (GDS), activities of daily living, independent activities of daily living, and mini-mental status examination scores. 12 In two other evaluations, the model of care coordination with the services of a professional nurse and social worker demonstrated cost effectiveness as compared to care in traditional long term care providing services to people with similar health problems and care needs.30, 31
Study strengths are the adequate sample size for the outcome measures used, the randomization of subjects, and multiple AL sites where the study was conducted in both urban and rural regions. The limitations include the unanticipated networking problems encountered that likely interfered with the subjects receiving the full dose of the intervention, limiting the study to one state and one corporation, and possible self-selection bias. These participants were all willing to participate, and although the group of willing participants were randomized, all participants may have agreed to participate for reasons (such as creating a legacy, being exposed to research at a younger age, wishing to help others) which set them apart from persons who did not chose to participate.32 Finally, although racial/ethnic diversity of the sample is lacking, the racial/ethnic composition of the sample reflects the overwhelmingly non-Hispanic white population residing in a 100-mile radius of the research institution. Despite these limitations, there are important contributions to health care learned in this study and the efforts to develop new strategies to effectively respond to rising health care costs and demand for acute nursing home care for the expanding aging population.
Detecting functional decline, early illness, and chronic illness changes are keys to promoting health, independence, and function of older adults, assisting them to age in place.11,12,30,33,34 Ultimately with this new technology, the research team believes costly hospitalizations and relocation to assisted living or nursing homes can be reduced. Without new solutions to the old challenges of promoting health, independence, and function, the service demand of older adults, who will represent 20% of our population in 2030,35 has the potential to overwhelm our health care system and our country’s economic future.36
Hospitalizations for older people occur about three times more than for persons of all ages, and their average length of stay is longer.42 Accidents, acute illnesses, and acute conditions related to chronic illnesses precipitate hospitalizations. The management of chronic illnesses may be the single most effective strategy to manage the burgeoning demand for health care services. Nearly 50% of the population (approximately 175 million) has a chronic condition and 26% have multiple chronic conditions.1 Importantly, these numbers are expected to steadily increase over the next 30 years.2 Chronic illnesses especially affect older adults; among adults age 65–74 years, 63% have two or more chronic conditions, increasing to 83% for people ages 85 and older age.37
Timely and appropriate care can prevent exacerbations of chronic illness that result in major changes in health status, hospitalization, complex treatment interventions, and high cost.30,31 Recent estimates suggest 86% of U.S. health care costs are attributable chronic disease treatment. 38 At this time, Medicare, the payer for most 65 and over, is the single largest payer for all hospitalizations, responsible for 46% of all inpatient costs, over $175 billion. 39 In 2011, Medicare per capita spending for traditional Medicare beneficiaries was $15,732 for 95-year olds compared to $5,562 for 66-year olds. 40 When chronic illnesses are considered, Medicare fee for service beneficiaries with no chronic conditions incurred about $2025 annually while those with six or more conditions incurred $32,658 in 2010.37
With the innovative technological solutions like those we tested in this study, elders can benefit from early detection and recognition of small changes in health conditions and get help early when treatment is the most effective and when prevention of costly hospital or nursing home care is still possible. Most importantly, function can be restored, so they can continue living independently at home or in the housing community of their choice, where they want to be.41
Finding ways to prompt early intervention will be essential to help the increasing numbers of people aging with chronic diseases remain as independent as possible for as long as possible. If we can help older adults remain healthier, active, and control their chronic illnesses with early detection of changes in health status and early intervention by health care providers, millions can remain independent as they age, avoiding or reducing debilitating and costly hospital stays, and for many, avoiding or delaying the move to a nursing home.
Figure 7.
Acknowledgments
This project was supported by grant number R01NR014255 from the National Institute of Nursing of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINR. Authors wish to acknowledge the gracious cooperation of the AL communities participating in this study, staff of Americare Systems, Inc., of Sikeston, MO, and the residents and families who participated in this research.
The Institutional Review Board of the University of Missouri approved the study protocol. All participants signed informed consent to participate in the research project.
Conflicts of interest: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marilyn Rantz, University of Missouri, S406 Sinclair School of Nursing, Columbia, MO 65211.
Lorraine J. Phillips, University of Missouri, Sinclair School of Nursing
Colleen Galambos, University of Missouri, School of Social Work.
Kari Lane, University of Missouri, Sinclair School of Nursing.
Gregory L. Alexander, University of Missouri, Sinclair School of Nursing
Laurel Despins, University of Missouri, Sinclair School of Nursing.
Richelle J. Koopman, University of Missouri, School of Medicine, Department of Family and Community Medicine
Marjorie Skubic, University of Missouri, Electrical and Computer Engineering.
Lanis Hicks, University of Missouri, Health Management and Informatics, School of Medicine.
Steven Miller, University of Missouri, Sinclair School of Nursing.
Andy Craver, University of Missouri, Health Management and Informatics, School of Medicine.
Bradford H Harris, University of Missouri, Electrical and Computer Engineering.
Chelsea B. Deroche, University of Missouri, Biostatistics & Research Design Unit, Health Management & Informatics
References
- 1.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: A 2012 update. Prev Chronic Dis. 2014;11:130389. doi: 10.5888/pcd11.130389. http://dx.doi.org/10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119:263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. The state of healthy aging in America 2013. 2013 Available at https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Retrieved May 1, 2017.
- 4.Kane RL. A new model of chronic care. Generations. 1999;23:35–37. [Google Scholar]
- 5.Boockvar K, Brodie HD, Lachs M. Nursing assistants detect behavior changes in nursing home residents that precede acute illness: Development and validation of an illness warning instrument. J Am Geriatr Soc. 2000;48:1086–1091. doi: 10.1111/j.1532-5415.2000.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 6.Boockvar KS, Lachs MS. Predictive value of nonspecific symptoms for acute illness in nursing home residents. J Am Geriatr Society. 2003;51:1111–1115. doi: 10.1046/j.1532-5415.2003.51360.x. [DOI] [PubMed] [Google Scholar]
- 7.Hogan J. Why don’t nurses monitor the respiratory rates of patients? British J Nurs. 2006;15:489–492. doi: 10.12968/bjon.2006.15.9.21087. [DOI] [PubMed] [Google Scholar]
- 8.Ridley S. The recognition and early management of critical illness. Ann R Col Surg Engl. 2005;87:315–322. doi: 10.1308/003588405X60669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rantz MJ, Skubic M, Koopman RJ, et al. Automated technology to speed recognition of signs of illness in older adults. J Gerontol Nurs. 2012;38:18–23. doi: 10.3928/00989134-20120307-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skubic M, Guevara R, Rantz M. Automated health alerts using in-home sensor data for embedded health assessment. IEEE J Translational Eng Health Med. 2015;3:1–11. doi: 10.1109/JTEHM.2015.2421499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips LJ, Deroche C, Rantz M, et al. Using embedded sensors in independent living to predict gait changes and falls. West J Nurs Res. 2016;39:78–94. doi: 10.1177/0193945916662027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantz MJ, Lane KR, Phillips LJ, et al. Enhanced RN care coordination with sensor technology: Impact on length of stay and cost in Aging in Place housing. Nurs Outlook. 2015;63:650–655. doi: 10.1016/j.outlook.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Stone E, Skubic M. Unobtrusive, continuous, in-home gait measurement using the Microsoft Kinect. IEEE Trans Biomedical Eng. 2013;60:2925–2932. doi: 10.1109/TBME.2013.2266341. [DOI] [PubMed] [Google Scholar]
- 14.Stone E, Skubic M. Testing real-time in-home fall alerts with embedded depth video hyperlink. In: Bodine C, Helal S, Gu T, Mokhtari M, editors. Smart homes and health telematics: 12th International Conference, ICOST 2014; Denver, CO, USA. June 25–27, 2014; Cham, Switzerland: Springer International; pp. 41–48. Revised papers. [Google Scholar]
- 15.Stone E, Skubic M. Fall detection in homes of older adults using the Microsoft Kinect. IEEE J Biomed Health Informatics. 2015;19:290–301. doi: 10.1109/JBHI.2014.2312180. [DOI] [PubMed] [Google Scholar]
- 16.Almeida OP, Almeida SA. Short versions of the Geriatric Depression Scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Outcome and Assessment Information Set. 2012 Available at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/OASIS/index.html?redirect=/OASIS/ Retrieved May 15, 2017.
- 19.Resnick B, Nahm ES. Reliability and validity testing of the revised 12-item Short-Form Health Survey in older adults. J Nurs Measurement. 2001;9:151–161. [PubMed] [Google Scholar]
- 20.Sheikh VI, Yesavage VA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York, NY: Haworth Press; 1986. pp. 165–174. [Google Scholar]
- 21.Ware J, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Marek K, Popejoy L, Petroski G, et al. Clinical outcomes of aging in place. Nurs Res. 2005;54:202–211. doi: 10.1097/00006199-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Marek KD, Popejoy L, Petroski G, Rantz MJ. Nurse care coordination in community-based long-term care. J Nurs Scholarship. 2006;38:80–86. doi: 10.1111/j.1547-5069.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Gouelle A. Use of Functional Ambulation Performance Score as measurement of gait ability: Review. J Rehabil Res Dev. 2004;51:665–674. doi: 10.1682/JRRD.2013.09.0198. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser State Health Facts. Hospital adjusted expenses per inpatient day by ownership. 2014 Available at http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0 Retrieved May 15, 2017.
- 26.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 27.Gazarian PK, Henneman EA, Chandler GE. Nurse decision making in the prearrest period. Clin Nurs Res. 2010;19:21–37. doi: 10.1177/1054773809353161. [DOI] [PubMed] [Google Scholar]
- 28.Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation. 2016;102:1–5. doi: 10.1016/j.resuscitation.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar GJ, La Guardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: Development of predictive models using data from an automated electronic medical record. J Hospital Med. 2012;7:388–395. doi: 10.1002/jhm.1929. [DOI] [PubMed] [Google Scholar]
- 30.Rantz MJ, Phillips L, Aud M, et al. Evaluation of aging in place model with home care services and registered nurse care coordination in senior housing. Nurs Outlook. 2011;59:37–46. doi: 10.1016/j.outlook.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Rantz M, Popejoy LL, Galambos C, et al. The continued success of registered nurse care coordination in a state evaluation of Aging in Place in senior housing. Nursing Outlook. 2014;62:237–246. doi: 10.1016/j.outlook.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Lavrakas PJ. Encyclopedia of survey research methods. Thousand Oaks, CA: Sage; 2008. [DOI] [Google Scholar]
- 33.Stone E, Skubic M, Rantz MJ, et al. Average in-home gait speed: Investigation of a new metric for mobility and fall risk assessment of elders. Gait Posture. 2015;41:57–62. doi: 10.1016/j.gaitpost.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Rantz M, Skubic M, Abbott C, et al. Automated in-home fall risk assessment and detection sensor system for elders. Gerontologist. 2015;55:S78–S87. doi: 10.1093/geront/gnv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2010: Key indicators of well-being. Washington DC: US Government Printing Office; 2010. [Google Scholar]
- 36.Kirn TF. Baby boomers with chronic conditions may overwhelm system. Clinical Psychiatry News. 2005;33:2. [Google Scholar]
- 37.Centers for Medicare and Medicaid Services. Chronic conditions among Medicare beneficiaries, Chartbook, 2012 edition. Baltimore, MD: Author; 2012. [Google Scholar]
- 38.National Center for Chronic Disease Prevention and Health Promotion. Chronic disease overview. 2016 Available at https://www.cdc.gov/chronicdisease/overview/index.htm Retrieved May 1, 2017.
- 39.Torio C, Moore B. National inpatient hospital costs: The most expensive conditions by payer, 2013. 2016 May; (HCUP Statistical Brief #204). Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf Retrieved April 28, 2017. [PubMed]
- 40.Neuman T, Cubanski J, Huang J, Damico A. The rising cost of living longer: Analysis of Medicare spending by age for beneficiaries in traditional. Medicare. 2015 Available at: http://kff.org/medicare/report/the-rising-cost-of-living-longer-analysis-of-medicare-spending-by-age-for-beneficiaries-in-traditional-medicare/ Retrieved May 1, 2017.
- 41.Stafford D. Living out your years at home can be challenging. Kansas City Star. 2011 Apr 16; Available at http://www.kansascity.com Retrieved May 1, 2017.
- 42.Administration on Aging. [Accessed on April 15, 2017];A profile of older Americans: 2010. 2010 Available at https://aoa.acl.gov/Aging_Statistics/Profile/2010/2.aspx.



